Abstract
The formation of advanced glycation endproducts (AGEs) on collagen within the arterial wall may be responsible for the development of diabetic vascular injury. This study was to examine the role of aminoguanidine (AG), an inhibitor of AGEs formation, in the prevention of arterial stiffening and cardiac hypertrophy in streptozotocin (STZ) induced diabetes in rats.
Diabetes was induced in animals by a single tail vein injection with 65 mg kg−1 STZ. After confirmation of the development of hyperglycemia (2 days later), rats were treated for 8 weeks with AG (daily peritoneal injections of 50 mg kg−1) and compared with the age-matched untreated diabetic controls.
After exposure to AG, the STZ-diabetic rats showed no alterations in cardiac output, aortic pressure profiles, total peripheral resistance, and aortic characteristic impedance.
By contrast, treatment of this experimental diabetes with AG resulted in a significant increase in wave transit time (τ), from 20.4±0.6 to 24.7±0.5 ms (P<0.05) and a decrease in wave reflection factor (Rf), from 0.78±0.04 to 0.53±0.02 (P<0.05). The decreased Rf associated with the increased τ suggest that AG may retard the diabetes-induced augmentation in systolic load of the left ventricle coupled to its arterial system.
Meanwhile, the diminished ratio of left ventricular weight to body weight suggests that prevention of the diabetes-related cardiac hypertrophy by AG may correspond to the drug-induced decline in aortic stiffening.
Glycation-derived modification on aortic collagen was also found to be enhanced in rats with diabetes (+65.3%, P<0.05) and the advanced glycation process was retarded by AG treatment.
We conclude that long-term administration of AG to the STZ-treated rats imparts significant protection against the diabetes-derived deterioration in vascular dynamics, at least partly through inhibition of the AGEs accumulation on collagen in the arterial wall.
Keywords: Advanced glycation endproducts, aminoguanidine, aortic input impedance, streptozotocin-diabetic rats, pulse wave reflection
Introduction
Diabetes mellitus is a complex metabolic disorder, which involves redox imbalance and increased oxidative stress (Oberley, 1988). Increased levels of reactive oxygen species (Gillery et al., 1989) and the substrate stress, for example glucose (Dyer et al., 1993) are suggested to play a crucial role in the development of the disease and its late complications (Baynes, 1991; Wolff, 1993; Tesfamariam, 1994; Baynes & Thrope, 1999) through nonenzymatic glycation of proteins, formation of advanced glycation endproducts (AGEs) (Brownlee et al., 1988). AGEs are a complex and heterogeneous group of compounds that have been shown to accumulate slowly in vascular and renal tissues with age, and at a more rapid rate in diabetes (Brownlee et al., 1988; Vlassara et al., 1994). Despite their complexity and widespread pathological distribution, AGEs produce the formation of covalent crosslinks between proteins, which are thought to be one of the central underlying processes by which they cause damage (Bucala, 1997). The pathological crosslinking of long-lived proteins such as collagen can affect tissue remodeling and result in loss of elasticity. Thus, the diabetes-related increase in AGEs accumulation on collagen in arterial walls may contribute to the development of certain physical changes of the diabetic blood vessels.
Aminoguanidine (AG) is a prototype of scavenging agents that inhibit AGEs formation and protein–protein crosslinking (Brownlee et al., 1986). It has been shown to decrease methylglyoxal-mediated formation of AGEs implicated as a downstream consequence of oxidative stress induced by mitochondrial dysfunction in hyperglycemia (Edelstein & Brownlee, 1992; Brownlee, 2001). AG therapy of rats administered streptozotocin (STZ) reduced diabetes-related disorders: glomerulosclerosis (Parving, 2001), mesenteric vascular hypertrophy (Soulis et al., 1999) and impaired relaxation in aorta (Ozyazgan et al., 2000). AG is also reported to prevent cardiac hypertrophy in STZ-diabetic rats by blocking protein carbonylation in the hearts (Stadler et al., 2005). However, little attention has been given to the lowering effect of AG on arterial load imposed on the diabetic ventricle. Thus, this study was to examine the role of AG in the prevention of arterial stiffening and cardiac hypertrophy in STZ-induced diabetes in rats, using the aortic impedance analysis (Milnor, 1989; Nichols & O'Rourke, 1998). Glycation-derived modification on aortic collagen was also detected by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE).
Methods
Animals and catheterization
Male Wistar rats at age 2 months were randomly divided into four groups (n=17 in each group) as follows: (i) normal controls (NC); (ii) STZ-diabetic rats (DM); (iii) NC treated with AG (NC+AG); (iv) DM treated with AG (DM+AG). Diabetes was induced in animals by a single tail vein injection with 65 mg kg−1 STZ in 0.1 M citrate buffer (pH 4.5) (Sigma Chemical Co., St Louis, MO, U.S.A.). After comfirmation of the development of hyperglycemia (2 days later) by blood glucose determination using a SURESTEP Test Strip (Lifescan Inc., Milpitas, CA, U.S.A.), rats were randomized into a vehicle-treated diabetic group, and a treatment group receiving daily injections with 50 mg kg−1 AG (Sigma Chemical Co., St Louis, MO, U.S.A.). Animals were studied 8 weeks after induction of diabetes to detect the effects of diabetes and AG on the physical properties of the vasculature. Rats were allowed free access to Purina chow and water and housed two to three per cage in a 12-h light/dark cycle animal room. The animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals, and were approved by the Animal Care and Use Committee of the National Taiwan University.
General surgical procedures and measurement of the hemodynamic variables in anesthetized rats have been described (Chang et al., 2003). In brief, rats were anesthetized with sodium pentobarbital (50 mg kg−1, i.p.), placed on a heating pad, intubated, and ventilated with a rodent respirator (Model 131; New England Medical Instruments, Medway, MA, U.S.A.). The chest was opened through the second intercostal space of the right side. An electromagnetic flow probe (model 100 series, internal circumference 8 mm; Carolina Medical Electronics, King, NC, U.S.A.) was positioned around the ascending aorta to measure the pulsatile aortic flow. A high-fidelity pressure catheter (model SPC 320, size 2F; Millar Instruments, Houston, TX, U.S.A.) was used to measure the pulsatile aortic pressure via isolated carotid artery of the right side. The electrocardiogram (ECG) of lead II was recorded with a Gould ECG/Biotach amplifier (Gould Electronics, Cleveland, OH, U.S.A.). The selective pressure and flow signals of 5–10 beats were averaged in the time domain, using the peak R wave of ECG as a fiducial point. Timing between the pressure and flow signals, due to spatial distance between the flow probe and proximal aortic pressure transducer, was corrected by a time-domain approach, in which the foot of the pressure waveform was realigned with that of the flow (Mitchell et al., 1994). The resulting pressure and flow signals were subjected to further vascular impedance analysis.
Aortic input impedance spectra
The aortic input impedance (Zi) could be obtained from the ratio of ascending aortic pressure harmonics to the corresponding flow harmonics, using a standard Fourier series expansion technique (Milnor, 1989; Nichols & O'Rourke, 1998; Chang et al., 2003). Total peripheral resistance of the systemic circulation (Rp) was calculated as mean aortic pressure divided by mean aortic flow. The aortic characteristic impedance (Zc) was computed by averaging high-frequency moduli of the aortic input impedance data points (4th–10th harmonics) (Huijberts et al., 1993; Gaballa et al., 1999). Taking Zc into consideration, we calculated the systemic arterial compliance C at mean aortic pressure Pm by expanding the two-element (Liu et al., 1986) into the three-element Windkessel model, which accounts for a nonlinear exponential pressure-volume relationship:
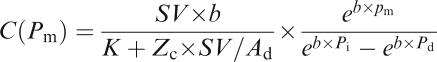 |
SV is the stroke volume; K is the ratio of total area under the aortic pressure curve to the diastolic area (Ad); b is the coefficient in the pressure–volume relation (−0.0131±0.009 in aortic arch); Pi is the pressure at the time of incisura and Pd is the end-diastolic pressure.
The wave transit time (τ) can be computed by the impulse response of the filtered Zi. This was accomplished by the inverse transformation of Zi after multiplication of the first 12 harmonics by a Dolph–Chebychev weighting function with the order 24 (Laxminarayan et al., 1978). Meanwhile, the time domain reflection factor (Rf) can be derived as the amplitude ratio of backward-to-forward peak pressure wave by the method Westerhof et al. (1972) proposed. Therefore, both the wave transit time and the wave reflection factor characterize the wave reflection phenomenon in the vasculature.
Gel electrophoresis
Method for measuring collagen glycation has been proposed by Turk et al. (1999). Collagen samples from aortic walls, previously digested by pepsin, proteinase K, and collagenase, were investigated by SDS–PAGE on Mini PROTEAN® 3 System (Bio-Rad Lab., Hercules, CA, U.S.A.). This was carried out using a 4% stacking and a 10% separating gel, running buffer system (Tris-HCl, pH 8.3/SDS/glycine), and Coomassie blue staining. Each lane was loaded with 20 μg protein from 2 to 3 rats. Protein blotting analysis was carried on PVDF membrane, using anti-AGE antibody 6D12 (Trans Genic Inc., Kumamoto, Japan).
Statistics
Results are expressed as means±s.e. As cardiac output is significantly related to body shape, this variable was normalized to body weight when comparison was made between STZ-diabetic rats and age-matched controls. Other hemodynamic variables derived from blood flow were also normalized to body weight to detect the effects of diabetes on these parameters with or without AG treatment. A two-way analysis of variance (ANOVA) was used to determine the effects of diabetes and AG on the physical properties of the rat arterial system. Simple effect analysis was used when significant interaction between diabetes and AG occurred. Differences among means within levels of a factor were determined by Tukey's honestly significant difference method. Significant differences were assumed at the level of P<0.05.
Results
Table 1 shows the effects of diabetes and AG on blood glucose level, body weight (BW), left ventricular weight (LVW), and aortic pressure profile in rats studied. Blood glucose level showed a significant increase in the STZ-diabetic animals, whereas they did not change in response to AG treatment. Diabetes was associated with a decrease in BW and LVW as a result of the prolonged hyperglycemia. After exposure to AG, the diabetic rats showed a significant fall in LVW, but did not differ in BW from the untreated diabetic animals. The diabetes-related increase in LVW/BW ratio was prevented by administration of AG to rats with insulin deficiency. By contrast, AG exerted no effects on those basic data in NC. Neither diabetes nor AG produced a significant difference in aortic pressure profile, nor was there a diabetes × AG interaction for the arterial blood pressure.
Table 1.
Effects of diabetes and AG on blood glucose level, body weight, left ventricular weight, and aortic pressure profile in male Wistar rats
| Variable | NC (n=17) | NC+AG (n=17) | DM (n=17) | DM+AG (n=17) |
|---|---|---|---|---|
| Glucose (ml dl−1) | 102.4±2.8 | 95.7±2.2 | 437.1±13.6† | 428.9±14.9 |
| BW (g) | 447.7±6.8 | 437.9±7.0 | 280.5±10.0† | 283.0±8.6 |
| LVW (g) | 0.853±0.017 | 0.839±0.018 | 0.722±0.027† | 0.590±0.023‡ |
| LVW/BW (mg g−1) | 1.91±0.03 | 1.92±0.03 | 2.58±0.06† | 2.08±0.04‡ |
| Ps (mmHg) | 120.6±2.0 | 124.5±2.2 | 112.3±2.4 | 108.0±2.6 |
| Pd (mmHg) | 95.6±1.7 | 98.2±2.4 | 86.2±2.5 | 83.4±2.7 |
| Pm (mmHg) | 109.1±1.7 | 112.5±2.3 | 100.0±2.3 | 97.4±2.6 |
| PP (mmHg) | 25.0±1.5 | 26.6±0.9 | 26.2±1.1 | 24.6±1.2 |
All values are expressed as means±s.e. BW, body weight; LVW, left ventricular weight; Ps, systolic aortic pressure; Pd, diastolic aortic pressure; Pm, mean aortic pressure; PP, pulse pressure.
Statistical difference (P<0.05) from the control group (NC).
Statistical difference (P<0.05) from the STZ-diabetic group (DM). AG, aminoguanidine.
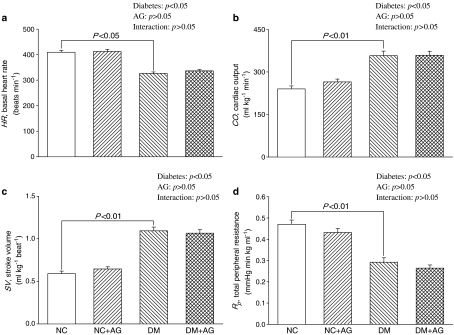
Figure 1 shows the effects of diabetes and AG on the static hemodynamic data, including basal heart rate (HR), cardiac output (CO), stroke volume (SV), and total peripheral resistance (Rp). Diabetes significantly lowered HR, and the diabetes-related changes in HR were not modified by administration of AG to rats treated with STZ (Figure 1a). Both CO (Figure 1b) and SV (Figure 1c) were increased markedly in the diabetic animals as compared with the age-matched controls. An increase in CO in the absence of any significant changes in Pm (Table 1) caused a decline in Rp in rats with insulin deficiency, from 0.47±0.02 to 0.29±0.02 mmHg min kg ml−1 (P<0.01) (Figure 1d). On the contrary, the STZ-diabetic rats showed no significant alterations in CO, SV, as well as Rp in response to AG treatment. Meanwhile, AG treatment exerted no effects on those static hemodynamic variables in NC.
Figure 1.
Effects of diabetes and AG on basal heart rate (HR in a), cardiac output (CO in b), stroke volume (SV in c), and total peripheral resistance (Rp in d). Diabetes had decreased HR and increased CO and SV. An increase in CO in the absence of any significant changes in aortic pressure resulted in a fall in Rp. However, these alterations caused by diabetes were not significantly modified by treatment with AG. AG also exerted no effects on these static hemodynamic variables in the age-matched controls. NC, normal controls; DM, STZ-diabetic rats; AG, aminoguanidine.
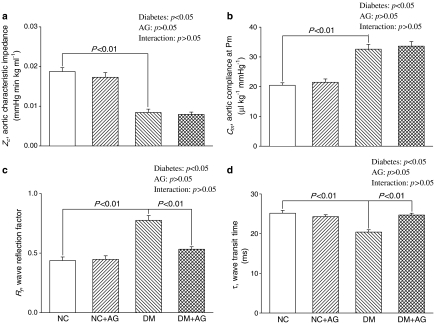
Figure 2 depicts the effects of diabetes and AG on the pulsatile nature of blood flows in arteries in terms of aortic characteristic impedance (Zc), aortic compliance (Cm), wave transit time (τ), and wave reflection factor (Rf). Diabetes contributed to a marked decrease in Zc, from 0.019±0.001 to 0.008±0.001 mmHg min kg ml−1 (P<0.01) (Figure 2a) and an increase in Cm, from 20.5±1.8 to 32.6±1.6 μl kg−1 mmHg−1 (P<0.01) (Figure 2b). However, AG administered to the diabetic rats for 8 weeks produced no significant alterations in Zc and Cm. The STZ-diabetic rats had increased Rf, at 0.44±0.03 versus 0.78±0.04 (P<0.01) (Figure 2c) and decreased τ, at 25.2±0.7 versus 20.4±0.6 ms (P<0.01) (Figure 2d). Early return with the augmented magnitude of the reflected wave from the peripheral circulation was prevented by treatment of the diabetic animals with AG, as evidenced by the increase of 21.0% in τ (P<0.05) and by the reduction of 29.3% in Rf (P<0.05). By contrast, the oscillatory components of the ventricular afterload, including Zc, Cm, τ, and Rf, were not modified by administration of AG to the NC.
Figure 2.
Effects of diabetes and AG on aortic characteristic impedance (Zc in a), systemic arterial compliance at mean aortic pressure (Cm in b), wave reflection factor (Rf in c), and wave transit time (τ in d). Zc and Cm were, respectively, decreased and increased with diabetes, and these alterations were not significantly modified by AG treatment. By contrast, the diabetes-derived deterioration in Rf and τ were prevented by administration of AG to rats treated with STZ. AG exerted no effects on the pulsatile nature of blood flows in arteries in the age-matched controls. NC, normal controls; DM, STZ-diabetic rats; AG, aminoguanidine.
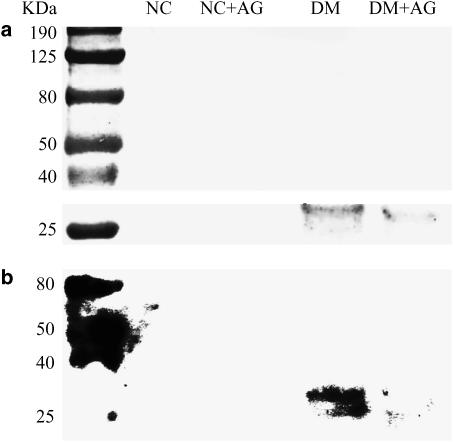
Figure 3 demonstrated the SDS–PAGE electrophoretic profiles of aortic collagen from animals studied. Collagen samples at 8 weeks after being administered STZ displayed molecular weight fragments between 25 and 40 kDa that are similar to those of aortic collagen samples of long-term diabetic rats observed by Turk et al. (1999). There was a 65.3 (±3.6)% increase in collagen AGE content at 8 weeks after induction of diabetes compared with the age-matched NC. The glycated aortic collagen was diminished by administration of AG for 8 weeks to the STZ-diabetic rats.
Figure 3.
(a) SDS–PAGE electrophoretic profiles of aortic collagen from animals studied; (b) the corresponding protein blotting analysis on PVDF membrane, using anti-AGE antibody 6D12. Collagen samples were previously digested by pepsin, proteinase K, and collagenase. Samples were loaded onto 10% separating gel and stained with Coomassie blue. Lane 1: molecular weight marker; lane 2: NC, lane 3: NC treated with AG; lane 4: DM; lane 5: DM treated with AG. Diabetic collagen samples display molecular weight fragments between 25 and 40 kDa that are much higher in amount than those from the age-matched controls. The glycated aortic collagen was diminished by treatment of the STZ-diabetic rats with AG for 8 weeks. NC, normal controls; DM, STZ-diabetic rats; AG, aminoguanidine.
Discussion
We demonstrate that the diabetes-related arterial stiffening and early return of the pulse wave reflection were significantly improved by AG treatment, at least partly through its inhibition of the AGEs accumulation on collagen in the arterial wall. These results are similar to those obtained by Corman et al. (1998), who found that the prevention of age-associated arterial stiffening and cardiac hypertrophy by AG is related to a decrease in the AGE-induced crosslinking of the extracellular matrix.
In this report, the STZ-diabetic rats showed isobaric vasodilatation, an increase in blood flow that occurs in the absence of any significant change in arterial blood pressure, resulting in a decrease in Rp (Figure 1d). It has been shown that oxidative protein modification along with high generation of nitric oxide (NOK) and peroxynitrite are important early events, 3 weeks after the onset of diabetes, in the development of cardiovascular complications in STZ-treated rats (Stadler et al., 2005). These metabolic parameters have the potential to cause contractile dysfunction of the vascular smooth muscle cells in arteries with diabetes (Snyder & Bredt, 1992; Tilton et al., 1993). The contractile dysfunction of the diabetic resistance vessels may lengthen the vascular smooth muscle cells, causing an increase in arteriolar diameter and thus a fall in Rp. This arterial damage in early diabetes may be lasting even at later stage, 8 weeks after induction of diabetes, with impaired NOK-mediated relaxation in the blood vessel walls (Pieper, 1999). Although AG could act as a preventive agent in diabetic cardiovascular complications by both AGEs-blocking and NOS-inhibition pathways (Corbett et al., 1992), no beneficial effect of AG on resistance to blood flow was observed in the STZ-treated rats with prolonged hyperglycemia. The results were also supported by several authors who found no beneficial effect of AG on disordered endothelial-dependent vasodilation in this experimental diabetes (Hill & Ege, 1994; Pieper et al., 1996; Crijns et al., 1998).
To aortic distensibility, the physiological implication of the reduced Zc (Figure 2a) seems to conflict with that of the diminished τ (Figure 2d) in the STZ-diabetic rats. In a hydraulic vascular system, Zc is directly related to the blood density and pulse wave velocity and is inversely related to the lumen radius squared of the tube. For large arteries, pulse wave velocity has an inverse relation to the distensibility of the aortic wall (Milnor, 1989; Nichols & O'Rourke, 1998). As a consequence, Zc has been frequently used as an indicator of aortic stiffness: the higher the aortic characteristic impedance, the stiffer the aortic wall. Meanwhile, being relatively independent to body shape, wave transit time (τ), which is inversely related to pulse wave velocity, could be derived to describe the aortic distensibility: the stiffer the aortic wall, the shorter the wave transit time and vice versa (Milnor, 1989; Chang et al., 2000, 2003).
With unaltered aortic pressure, a decline in Zc suggests that the contractile function of the vascular smooth muscle cells may be impaired in Windkessel vessels (Williamson et al., 1989). With isobaric vasodilatation, the inactivation of the vascular smooth muscle cells has the potential to elevate the elastic modulus of the aortic wall and contribute to a fall in aortic distensibility (Milnor, 1989). A decline in aortic distensibility in diabetes could be reflected in the reduction in τ along the path. Meanwhile, the contractile dysfunction of the diabetic aortas probably lengthens the aortic smooth muscle cells, resulting in an increase in aortic lumen diameter that can cause a fall in Zc. Because the net results of diabetes on Zc depend on the relative influence of those counter-balancing factors, that is pulse wave velocity (indicative of aortic stiffness) and aortic cross-sectional area, there is difficulty using Zc to describe the aortic distensibility in this experimental diabetes.
In addition to the smooth muscle inactivation to elevate the aortic rigidity, the accumulation of AGEs on collagen in the arterial wall may be another one of the important factors responsible for the increased aortic stiffness in rats with diabetes (Figure 3). AG administered to the STZ-treated rats for 8 weeks prevented the diabetes-related fall in aortic distensibility, as evidenced by the increase of 21.0% in τ (Figure 2d). The prevention of diabetes-related aortic stiffness by AG treatment is likely related to inhibition of the AGEs accumulation on collagen in the wall of the elastic reservoir (Figure 3). Although Zc was slightly lower in the AG-treated diabetic rats compared with the untreated diabetic animals, no significant difference in Zc was found between the two groups. However, Huijberts et al. (1993) considered Zc an indicator of aortic stiffness to reach the same conclusion of the important role of AGEs in affecting the vascular dynamics in diabetes, based on the lowering effect of AG on this hemodynamic parameter.
Changes with diabetes in timing and magnitude of the pulse wave reflection do impair the loading condition for the left ventricle coupled to the arterial system (O'Rourke et al., 1987). As mentioned earlier, a reduction in τ was detected in rats with insulin deficiency, suggesting that diabetes may cause an early return of the pulse wave reflection from the peripheral circulation. Administration of AG for 8 weeks prevented this early return of the pulse wave reflection in the STZ-diabetic animals (Figure 2d). Meanwhile, diabetes contributed to a significant rise in Rf (Figure 2c), indicating that the heavy reflection intensity occurs in the STZ-diabetic rats. After exposure to AG, the diabetic animals showed a significant fall of 29.3% in Rf, suggesting that the heavy reflection phenomenon may be alleviated in the vasculature. The observed increase in τ and decrease in Rf indicate that AG, by preventing the AGEs accumulation on collagen in the diabetic arterial wall, can improve the systolic loading condition for the left ventricle coupled to its arterial system. The ratio of LVW to BW was decreased by AG treatment, suggesting that the prevention of diabetes-related cardiac hypertrophy may correspond to the drug-induced decline in arterial load.
Just as the elastic modulus is an expression employed to characterize the material properties, so distensibility is a term used to describe the elastic behavior of a hollow vessel. Compliance and distensibility are quite different, for compliance is equal to distensibility times volume (Guyton, 1992). Herein, the STZ-diabetic rats showed an increase in aortic compliance at Pm (Figure 2b). The decreased distensibility associated with the increased compliance suggests that volume expansion in the arterial system may exist in rats with insulin deficiency. The volume expansion in STZ-diabetic rats is supported by other reports in the literature (Zatz & Brenner, 1986; Tomlinson et al., 1992). AG administered to rats with diabetes for 8 weeks produced no significant alteration in Cm. Thus, the diabetes-derived abnormality in volume expansion was prevented by AG treatment, as manifested by the increased distensibility associated with the unchanged Cm.
Herein, no significant changes in systolic blood pressure and arterial pulse pressure were observed in the STZ-diabetic rats with the increased arterial stiffness (Table 1). Several factors importantly affect the magnitude of the pulse pressure, including the stroke volume and the aortic compliance (West, 1991). The arterial pulse pressure varies directly with the stroke volume but inversely with the arterial compliance. Thus, the increase in SV (Figure 1c) with diabetes may blunt the effect of the augmented Cm (Figure 2b) on the systolic blood pressure and pulse pressure in the STZ-treated rats. After exposure to AG, this experimental diabetes also showed no significant alterations in systolic blood pressure and pulse pressure while SV and Cm remained unchanged.
In this report, we demonstrated that AG ameliorates vascular complications observed in experimentally induced diabetes probably through inhibition of the AGEs formation. However, when using AG it is important to be aware of the facts that this drug, apart from preventing formation of AGEs, also inhibits diamine oxidase (DAO) (Nilsson, 1999). In the in vivo situation inhibition of DAO might lead to serious vascular and respiratory side effects due to accumulation of histamine in the blood stream. In addition, AG in high doses may bind to S-adenosylmethionine decarboxylase (SAMDC) and thereby might affect polyamine formation. The effects of AG on the mechanical properties of the vasculature due to inhibition of DAO and stabilization of SAMDC remains to be determined in rats treated with STZ.
Our contribution in this endeavor was to provide a path to consider the clinical application of an AGE inhibitor in the prevention of diabetes-related deterioration in vascular dynamics. AG seems to target larger Windkessel vessels and wave reflection phenomena by inhibiting the formation and accumulation of AGEs on collagen in the diabetic arterial walls with insulin deficiency. However, an important limitation of AG and other AGE inhibitors is that they cannot reverse pre-existing AGEs crosslinking, and thus the AGE ‘breakers' are supposed to be noted. Recently, several studies have used the AGE ‘breaker' compound 4,5-dimethyl-3-phenacylthiazolium chloride (also called ALT-711) for animal and human studies (Wolffenbuttel et al., 1998; Kass et al., 2001). The results of ongoing clinical studies will determine if these compounds can become the first specific therapy for diabetes-related cardiovascular disorder.
Taken together, diabetes produces a detriment to the physical properties of the resistance vessels and the Windkessel vessels in rats treated with STZ. The increased τ by AG treatment for 8 weeks suggests that the drug may prevent the diabetes-induced fall in aortic distensibility. Both the increased τ and the decreased Rf indicate that AG can retard the diabetes-derived augmentation in systolic load of the left ventricle coupled to its vasculature. AG, by reducing the LV oscillatory load, may prevent the diabetes-related cardiac hypertrophy in rats with insulin deficiency. Meanwhile, the glycated aortic collagen was diminished by administration of AG to this experimental diabetes. We conclude that AG ameliorates vascular complications observed in this experimentally induced diabetes possibly through inhibition of the AGEs formation and accumulation on collagen in the arterial walls.
Acknowledgments
This study was supported by grants from the National Taiwan University Hospital (NTUH 93-S021) and from the National Science Council of Taiwan (NSC 92-2320-B-002-087 and NSC 93-2320-B-002-062).
Abbreviations
- AG
aminoguanidine
- AGEs
advanced glycation endproducts
- BW
body weight (g)
- C
systemic arterial compliance (μl kg−1 mmHg−1)
- CO
cardiac output (ml kg−1 min−1)
- HR
basal heart rate (beats min−1)
- iNOS
inducible isoform of nitric oxide syntheses
- LVW
left ventricular weight (g)
- NO
nitric oxide
- Pb
magnitude of the forward pressure (mmHg)
- Pd
diastolic aortic pressure (mmHg)
- Pf
magnitude of the forward pressure (mmHg)
- Pm
mean aortic pressure (mmHg)
- Ps
systolic aortic pressure (mmHg)
- Rf
wave reflection factor
- Rp
total peripheral resistance (mmHg min kg ml−1)
- SDS–PAGE
sodium dodecyl sulfate–polyacrylamide gel electrophoresis
- STZ
streptozotocin
- SV
stroke volume (ml kg−1 beat−1)
- Zc
aortic characteristic impedance (mmHg min kg ml−1)
- Zi
aortic input impedance spectra (mmHg min kg ml−1)
- τ
wave transit time (ms)
References
- BAYNES J.W. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- BAYNES J.W., THROPE S.R. Role of oxidative stress in diabetic complications. A new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M., CERAMI A., VLASSARA H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M., VLASSARA H., KOONEY A., ULRICH P., CERAMI A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- BROWNLEE M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- BUCALA R. Lipid and lipoprotein modification by GEA's: role in atherosclerosis. Exp. Physiol. 1997;82:327–337. doi: 10.1113/expphysiol.1997.sp004028. [DOI] [PubMed] [Google Scholar]
- CHANG K.C., CHEN T.J., PENG Y.I., LI T.H., TSENG Y.Z. Impaired vascular dynamics in normotensive diabetic rats induced by streptozotocin: tapered T-tube model analysis. J. Ther. Biol. 2000;204:371–380. doi: 10.1006/jtbi.2000.2021. [DOI] [PubMed] [Google Scholar]
- CHANG K.C., HSU K.L., TSENG Y.Z. Effects of diabetes and gender on mechanical properties of the arterial system in rats: aortic impedance analysis. Exp. Biol. Med. 2003;228:70–78. doi: 10.1177/153537020322800110. [DOI] [PubMed] [Google Scholar]
- CORMAN B., DURIEZ M., POITEVIN P., HEUDES D., BRUNEVAL P., TEDGUI A., LÉVY B.I. Aminoguanidine prevents age-related arterial stiffening and cardiac hypertrophy. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1301–1306. doi: 10.1073/pnas.95.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORBETT J.A., TILTON R.G., CHANG K., HASAN K.S., IDO Y., WANG J.L., SWEETLAND M.A., LANCASTER J.R., WILLIAMSON J.R., MCDANIEL M.L. Aminoguanidine, a novel inhibitor of nitric oxide formation, prevents diabetic vascular dysfunction. Diabetes. 1992;41:552–556. doi: 10.2337/diab.41.4.552. [DOI] [PubMed] [Google Scholar]
- CRIJNS F.R., STRUIJKER-BOUDIER H.A., WOLFFENBUTTEL B.H. Arteriolar reactivity in conscious diabetic rats. Influence of aminoguanidine treatment. Diabetes. 1998;47:918–923. doi: 10.2337/diabetes.47.6.918. [DOI] [PubMed] [Google Scholar]
- DYER D.G., DUNN J.A., THORPE S.R., BAILLIE K.E., LYONS T.J., MCCANCE D.R., BAYNES J.W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J. Clin. Invest. 1993;91:2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELSTEIN D., BROWNLEE M. Mechanistic studies of advanced glycosylation end product inhibition by aminoguanidine. Diabetes. 1992;41:26–29. doi: 10.2337/diab.41.1.26. [DOI] [PubMed] [Google Scholar]
- GABALLA M.A., RAYA T.E., HOOVER C.A., GOLDMAN S. Effects of endothelial and inducible nitric oxide synthases inhibition on circulatory function in rats after myocardial infarction. Cardiovasc. Res. 1999;42:627–635. doi: 10.1016/s0008-6363(98)00343-5. [DOI] [PubMed] [Google Scholar]
- GILLERY P., MONBOISSE J.C., MAQUART F.X., BOREL J.P. Dose oxygen free radical increased formation explain long term complications of diabetes mellitus. Med. Hypotheses. 1989;29:47–50. doi: 10.1016/0306-9877(89)90167-9. [DOI] [PubMed] [Google Scholar]
- GUYTON A.C. Human Physiology and Mechanisms of Disease 1992Philadelphia: Saunders; 115p [Google Scholar]
- HILL M.A., EGE E.A. Active and passive mechanical properties of isolated arterioles from STZ-induced diabetic rats. Effect of aminoguanidine treatment. Diabetes. 1994;43:1450–1456. doi: 10.2337/diab.43.12.1450. [DOI] [PubMed] [Google Scholar]
- HUIJBERTS M.S., WOLFFENBUTTEL B.H., BOUDIER H.A., CRIJNS F.R., KRUSEMAN A.C., POITEVIN P., LÉVY B.I. Aminoguanidine treatment increases elasticity and decreases fluid filtration of large arteries from diabetic rats. J. Clin. Invest. 1993;92:1407–1411. doi: 10.1172/JCI116716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASS D.A., SHAPIRO E.P., KAWAGUCHI M., CAPRIOTTI A.R., SCUTERI A., DEGROOF R.C., LAKATTA E.G. Improved arterial compliance by a novel advanced glycation end-product crosslink breaker. Circulation. 2001;104:1464–1470. doi: 10.1161/hc3801.097806. [DOI] [PubMed] [Google Scholar]
- LAXMINARAYAN S., SIPKEMA P., WESTERHOF N. Characterization of the arterial system in the time domain. IEEE Trans. Biomed. Eng. 1978;25:177–184. doi: 10.1109/TBME.1978.326244. [DOI] [PubMed] [Google Scholar]
- LIU A., BRIN K.P., YIN F.C.P. Estimation of total arterial compliance: an improved method and evaluation of current methods. Am. J. Physiol. 1986;251 (Heart Circ. Physiol., 20):H588–H600. doi: 10.1152/ajpheart.1986.251.3.H588. [DOI] [PubMed] [Google Scholar]
- MILNOR W.R. Hemodynamics. Baltimore: Williams & Wilkins Co; 1989. [Google Scholar]
- MITCHELL G.F., PFEFFER M.A., WESTERHOF N., PFEFFER J.M. Measurement of aortic input impedance in rats. Am. J. Physiol. 1994;267 (Heart Circ. Physiol., 36):H1907–H1915. doi: 10.1152/ajpheart.1994.267.5.H1907. [DOI] [PubMed] [Google Scholar]
- NILSSON B. Biological effects of aminoguanidine: an update. Inflamm. Res. 1999;48:509–515. doi: 10.1007/s000110050495. [DOI] [PubMed] [Google Scholar]
- NICHOLS W.W., O'ROURKE M.F. McDonald's Blood Flow in Arteries. London: Arnold; 1998. [Google Scholar]
- OBERLEY L.W. Free radicals and diabetes. Free Radic. Biol. Med. 1988;5:113–124. doi: 10.1016/0891-5849(88)90036-6. [DOI] [PubMed] [Google Scholar]
- O'ROURKE M.F., AVOLIO A.P., NICHOLS W.W.Left ventricular-systemic arterial coupling in humans and strategies to improve coupling in disease states Ventricular/Vascular Coupling 1987New York: Springer-Verlag; 1–19.ed. Yin, F.C.P., pp [Google Scholar]
- OZYAZGAN S., UNLUCERCI Y., BEKPINAR S., AKKAN A.G. Impaired relaxation in aorta from streptozotocin-diabetic rats: effect of aminoguanidine treatment. Int. J. Exp. Diabet. Res. 2000;1:145–153. doi: 10.1155/EDR.2000.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARVING H.H. Diabetic nephropathy: prevention and treatment. Kidney Int. 2001;60:2041–2055. doi: 10.1046/j.1523-1755.2001.00020.x. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M. Enhanced, unaltered and impaired nitric oxide-mediated endothelium-dependent relaxation in experimental diabetes mellitus: importance of disease duration. Diabetologia. 1999;42:204–213. doi: 10.1007/s001250051140. [DOI] [PubMed] [Google Scholar]
- PIEPER G.M., MOORE-HILTON G., ROZA A.M. Evaluation of the mechanism of endothelial dysfunction in the genetically-diabetic BB rat. Life Sci. 1996;58:147–152. doi: 10.1016/0024-3205(95)02360-7. [DOI] [PubMed] [Google Scholar]
- SNYDER T., BREDT A. Biological roles of nitric oxide. Sci. Am. 1992;5:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- SOULIS T., SASTRA S., THALLAS V., MORTENSEN S.B., WILKEN M., CLAUSEN J.T., BJERRUM O.J., PETERSEN H., LAU J., JERUMS G., BOEL E., COOPER M.E. A novel inhibitor of advanced glycation end-product formation inhibits mesenteric vascular hypertrophy in experimental diabetes. Diabetologia. 1999;42:472–479. doi: 10.1007/s001250051181. [DOI] [PubMed] [Google Scholar]
- STADLER K., JENEI V., SOMOGYI A., JAKUS J. Beneficial effects of aminoguanidine on the cardiovascular system of diabetic rats. Diabetes Metab. Res. Rev. 2005;21:189–196. doi: 10.1002/dmrr.501. [DOI] [PubMed] [Google Scholar]
- TESFAMARIAM B. Free radicals in diabetic endothelial cell dysfunction. Free Radic. Biol. Med. 1994;16:383–391. doi: 10.1016/0891-5849(94)90040-x. [DOI] [PubMed] [Google Scholar]
- TILTON R.G., CHANG K., HASAN K.S., SMITH S.R., PETRASH J.M., MISKO T.P., MOORE W.M., CURRIE M.G., CORBETT J.A., MCDANIEL M.L., WILLIAMSON J.R. Prevention of diabetic vascular dysfunction by guanidines: inhibition of nitric oxide synthase versus advanced glycation end-product formation. Diabetes. 1993;42:221–232. doi: 10.2337/diab.42.2.221. [DOI] [PubMed] [Google Scholar]
- TOMLINSON K.C., GARDINER S.M., HEBDEN R.A., BENNETT T. Functional consequences of streptozotocin-induced diabetes mellitus, with particular reference to the cardiovascular system. Pharmacol. Rev. 1992;44:103–150. [PubMed] [Google Scholar]
- TURK Z., MIŠUR I., TURK N., BENKO B. Rat tissue collagen modified by advanced glycation: correlation with duration of diabetes and glycemic control. Clin. Chem. Lab. Med. 1999;37:813–820. doi: 10.1515/CCLM.1999.122. [DOI] [PubMed] [Google Scholar]
- VLASSARA H., BUCALA R., STRIKER L. Pathogenic effects of advanced glycosylation: biochemical, biological, and clinical implications for diabetes and aging. Lab. Invest. 1994;70:138–151. [PubMed] [Google Scholar]
- WEST J.B. BEST AND TAYLOR'S physiological basis of medical practice. In: Dynamics of the Peripheral Circulation 1991Baltimore: Williams & Wilkins; 147–149.ed. West, J.B., pp [Google Scholar]
- WESTERHOF N., SIPKEMAM P., VANDEN-BOS G.C., ELZINGA G. Forward and backward waves in the arterial system. Cardiovasc. Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON J.R., CHANG K., TILTON R.G., KILO C.Models for studying diabetic complications Diabetes Mellitus: Pathophysiology and Therapy 1989NewYork: Springer-Verlag; 142–151.eds. Creutzfeldt W. & Lefebvre P., pp [Google Scholar]
- WOLFF S.P. Diabetes mellitus and free radicals. Free radicals, transition metals and oxidative stress in the aetiology of diabetes mellitus and complications. Br. Med. Bull. 1993;49:642–652. doi: 10.1093/oxfordjournals.bmb.a072637. [DOI] [PubMed] [Google Scholar]
- WOLFFENBUTTEL B.H., BOULANGER C.M., CRIJNS F.R., HUIJBERTS M.S., POITEVIN P., SWENNEN G.N., VASAN S., EGAN J.J., ULRICH P., CERAMI A., LÉVY B.I. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc. Natl. Acad. Sci. U.S.A. 1988;95:4630–4634. doi: 10.1073/pnas.95.8.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZATZ R., BRENNER B.M. Pathogenesis of diabetic microangiopathy: the hemodynamic view. Am. J. Med. 1986;80:443–453. doi: 10.1016/0002-9343(86)90719-9. [DOI] [PubMed] [Google Scholar]