Abstract
The role of the delta opioid receptor in regulating anxiety-like behavior in male Sprague–Dawley rats was examined.
Using an elevated plus maze, the effects of the selective delta opioid receptor antagonist naltrindole (1 or 5 mg kg−1) and agonist SNC80 (1, 5 or 20 mg kg−1) on anxiety-like behavior were measured. Anxiety was also measured following administration of diazepam (3 mg kg−1) and amphetamine (1 mg kg−1) and compared to the effects of SNC80. Locomotor activity following administration of naltrindole, SNC80, diazepam, and amphetamine was measured. Finally, the defensive burying paradigm was used to confirm the findings from the elevated plus maze.
Results demonstrated that SNC80 produced dose-dependent anxiolytic effects similar to that of the classical antianxiety agent, diazepam. Administration of naltrindole caused anxiogenic behavior in rats further supporting the involvement of the delta opioid receptor system in regulating anxiety. Naltrindole also blocked the anxiolytic effects of SNC80. Amphetamine had no effect on anxiety-like behavior. SNC80 induced hyperactivity similar to amphetamine at the doses tested, while naltrindole and diazepam did not significantly affect locomotor activity.
Although SNC80 can increase locomotor activity, control experiments reported herein indicate that hyperlocomotion is not sufficient to produce an anxiolytic response on the elevated plus maze. Together with the results from the defensive burying paradigm, this suggests that the effects of SNC80 on reducing anxiety are independent of its effects on locomotion. Collectively these data show that the delta opioid receptor system can regulate anxiety-like behavior in an anxiolytic (agonist) and anxiogenic (antagonist) manner.
Keywords: Delta opioid receptor, SNC80, naltrindole, anxiety, locomotion, elevated plus maze, defensive burying paradigm, rat
Introduction
Several lines of investigation support the involvement of the opioid receptor system in the regulation of anxiety. The most compelling evidence for the involvement of the delta opioid receptor system in anxiety comes from a study on delta opioid receptor knockout mice. Specifically, delta opioid receptor deficient mice exhibit an anxiogenic-like phenotype measured in a light dark box and an elevated plus maze (Filliol et al., 2000).
To explore the hypothesis that delta opioid receptors are involved in tonic and/or active control of anxiety-like behavior in the rat, the present study utilized the selective delta opioid receptor agonist SNC80 and antagonist naltrindole. SNC80 {(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide} is a nonpeptide delta opioid receptor agonist that is active after systemic administration. It is a methyl ether analog of a group of compounds known as diarylmethylpiperazines (Calderon et al., 1994; Bilsky et al., 1995; reviewed by Calderon & Coop, 2004). The calculated Ki ratios for SNC80 at mu/delta and kappa/delta sites are 495- and 248-fold, respectively, exemplifying the high selectivity for delta opioid receptors (Bilsky et al., 1995). SNC80 binds to cloned delta opioid receptors to initiate GTPγS binding (Plobeck et al., 2000) and inhibit adenylyl cyclase activity (Knapp et al., 1996). Furthermore, several studies have characterized the antinociceptive, antidepressant, stimulant and convulsant activities of SNC80 (Bilsky et al., 1995; Broom et al., 2002a, 2002b). Naltrindole {17-cyclopropylmethyl-6,7-dehydro-4,5α-epoxy-3,14-dihydroxy-6,7,2′,3′-indolomophinan} is a highly selective, nonpeptide delta opioid receptor antagonist that crosses the blood–brain barrier. Portoghese et al. (1988) first reported it as a potent antagonist, and it has been used extensively both in vitro and in vivo due to its high selectivity for the delta opioid receptor.
Previous studies indicate that SNC80 has effects on depression-like behavior. Unlike typical antidepressants, delta opioid receptor agonists are effective following a single dose in the forced swim test (Broom et al., 2002b). Furthermore, it has been shown that the hyperactivity and convulsant properties of SNC80 are not required for its antidepressant-like effects (Broom et al., 2002a, 2002b), and the antidepressant-like behaviors are independent of SNC80's disruptive effects on learning (Jutkiewicz et al., 2003). The majority of behavioral research that has been done on SNC80 and other nonpeptide delta opioid receptor agonists has focused on antinociception (Bilsky et al., 1995; Pacheco et al., 2005), antidepressant-like effects and convulsant properties (reviewed in Broom et al., 2002c). A focused study examining the effects of delta opioid receptor agonists on anxiety-like behavior has not been done heretofore.
Here, the role of delta opioid receptors in anxiety using two behavioral models, the elevated plus maze and the defensive burying paradigm, was examined. Both models have been well characterized and have common appeal due to their ability to detect novel and proven therapeutic agents that reduce anxiety, thus making them pharmacologically robust models with high-predictive validity (Dawson & Tricklebank, 1995; De Boer & Koolhaas, 2003). The elevated plus maze was chosen as the main rodent model because it is a noninvasive test that does not involve a painful stimulus since it measures the tendency for a rat to avoid the open arms (Pellow et al., 1985; Handley & McBlane, 1993; Dawson & Tricklebank, 1995). The defensive burying paradigm measures the instinctual behavior of a rat to bury something that poses a threat or danger (De Boer & Koolhaas, 2003).
The goals of the present study were to evaluate the potential anxiolytic effects of the delta opioid receptor agonist SNC80 and to elucidate the role of the endogenous delta opioid receptor system in modulating anxiety. The elevated plus maze was used to thoroughly explore this aim and the defensive burying paradigm was used to confirm results. Finally, the potential confounding effect of hyperactivity on the anxiolytic effects of SNC80 as measured by the elevated plus maze was examined.
Methods
Animals
Male Sprague–Dawley rats were purchased from Charles River Laboratories (Wilmington, MA, U.S.A.) and housed on a 12 h light–dark cycle (07:00–19:00) with food and water available ad libitum. The weight range of animals upon arrival was 150–200 g and the weight range upon testing was 275–375 g. Animals were allowed to acclimate for a minimum of two weeks prior to experimentation. During this time, animals were weighed and handled daily to reduce stress due to human contact. All behavioral tests were performed between 10:00 and 14:00. Measures were taken to minimize potential pain and discomfort to experimental animals, and all procedures were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by Temple University School of Medicine's or the University of Pennsylvania's Institutional Animal Care and Use Committee.
Elevated plus maze
The elevated plus maze (Coulbourn Instruments, Allentown, PA, U.S.A.) was used to measure anxiety-like behavior. The plus maze is made up of four equal-sized runways (45 cm long × 10 cm wide) laid-out in the shape of a plus sign and elevated off the ground by 52 cm (Handley & McBlane, 1993). Two of the arms are enclosed by a solid wall 30 cm high on the long sides of the arms (closed arms), whereas the other two arms have no sides and are thus open (open arms). A ledge (0.5 cm high) is present around the perimeter of the open arms of the maze. The presence of open arm ledges has been shown to increase the pharmacological reliability of the test (Fernandes & File, 1996). Testing room lighting was adjusted from normal level (∼450 lux) to a dimmer setting of 150–210 lux, where open arm light levels were ∼200 lux and closed arm light levels were ∼160 lux. On the test day, drugs or vehicle controls were administered 60 min prior to testing. For behavioral measurement, each rat was placed on an open arm of the maze facing the center and their behavior was recorded on videocassette in real time for 10 min. The experimenter was not blind to the study. However, scoring was confirmed by a rater blind to drug treatment in selected cases. In these cases, the scores of the two raters were averaged for each measure. Following the test, the animal was returned to its home cage and the maze was cleaned with water and dried. Anxiety-like behavior was determined by calculating the amount of time and number of entries each rat made in the open arms and closed arms and reported as a percentage of the total time or number of entries. An arm entry was counted when the superior portion of the rat including the head and neck (cranial to the transverse plane or ventral midline), the shoulders (pectoral region), the forelimbs and forepaws, and the thoracic region (anterior to the rib cage) moved into an open or closed arm. This measure sufficiently improves the reliability of the test by including open arm risk assessment behaviors, namely stretch attend postures (i.e. the rat stretches forward without moving its hindlimbs and hindpaws) and head dippings (i.e. leaning over the edges of the open arms) (Dawson & Tricklebank, 1995). Group sizes were n=7–10.
Defensive burying paradigm
The defensive burying paradigm was used to measure anxiolytic activity of SNC80. Rats were tested in a standard rat housing cage with 5 cm of fresh bedding. The test cage contained a small hole at the end of the cage approximately 7 cm from the bottom. A glass probe (∼1 cm diameter and 9 cm length) was inserted through the hole in the cage, and metal wires with a positive or negative charge were wrapped tightly around the probe in such a way that touching the two wires simultaneously closed the circuit and delivered a shock of 2 A. At 1 day before testing, each rat was acclimated to the test cage for 15 min without the probe. On the test day, saline (1 ml kg−1) or SNC80 (5 mg kg−1) was administered 60 min prior to testing. The rats were placed in the test cage with the electrified probe for 15 min, and the session was videotaped. The video tapes were scored for five measures: latency to bury from first shock, latency to bury from last shock, duration of bury, total number of shocks received and intensity of shock response. The shock response ratings ranged from 1 to 4, with 1 indicating only a small flinch and 4 corresponding to a whole body flinch with the animal immediately moving to the other side of the cage (Treit et al., 1981; Presold & Treit, 1992). The videos were scored by two separate experimenters, where one was blind to the treatment. The scores of the two raters were averaged for each measure. Group sizes were n=9 for the saline group and n=10 for the SNC80 group.
Locomotor activity
Locomotor activity was monitored for 30 min prior to and 120 min after drug administration in separate groups of rats. Behavioral activity was measured by a computerized monitoring system (Digiscan DMicro, Accuscan Inst, Columbus, OH, U.S.A.), which consists of a metal frame containing 16 parallel infrared photobeams and receivers into which a standard plastic rat cage is placed. Photobeam breaks were recorded and stored on a computer linked to the activity monitors. Ambulatory activity was measured by the number of times the animal broke consecutive light beams. Rats were tested under normal lighting conditions. Eight animals (random sampling) were monitored simultaneously. Each testing period contained saline-injected control rats, thus resulting in a larger sample size for the saline group. Group sizes were n=20 for the saline group and n=8 for all other groups.
Drugs
All drugs were injected peripherally at a volume of 1 ml kg−1 rat body weight. Diazepam and diazepam vehicle were injected intraperitoneally, whereas saline, amphetamine, naltrindole, naltrindole vehicle, SNC80, and SNC80 vehicle were administered subcutaneously. Amphetamine was generously supplied by National Institute on Drug Abuse (NIDA) and dissolved in normal saline (0.9% NaCl) at a concentration of 1 mg ml−1. SNC80 was supplied by NIDA and prepared as a 20 mg ml−1 stock in normal saline at pH 4.5–5. A stock of 20 mg ml−1 SNC80 was diluted with normal saline to get 1 and 5 mg ml−1 SNC80 doses. Naltrindole was supplied by NIDA and prepared as a 5 mg ml−1 stock in 20% Tween-20 and 80% normal saline. The stock of 5 mg ml−1 naltrindole was diluted with normal saline for the 1 mg ml−1 naltrindole dose. Diazepam (3 mg ml−1) was purchased from Sigma (St Louis, MO, U.S.A.) and dissolved in 50% water, 40% EtOH and 10% propylene glycol.
Data analysis
Scores from the elevated plus maze and activity/10 min period were analyzed using a one-way analysis of variance (ANOVA) followed by Bonferroni's multiple comparison post hoc analysis. Two-tailed unpaired t-tests were used to analyze the data from the defensive burying paradigm. One-way repeated-measures ANOVA followed by Bonferroni's multiple comparison post hoc analysis was used to analyze activity time course data. Statistical significance was considered when P<0.05. An inter-rater correlation was calculated for data where the scores of two raters were averaged. All data are expressed as the mean±standard error of the mean (s.e.m.) and represent a sample size (n) of 7–20 for each experimental group. Each animal was tested only once.
Results
Delta opioid receptor ligands affect anxiety-like behavior in the rat as measured by the elevated plus maze
The anxiety-like behavior of rats administered delta opioid receptor ligands was measured using the elevated plus maze (Figure 1). Open arm entries and time spent in open arms were recorded and expressed as a percent of total entries and total time spent on the elevated plus maze (respectively).
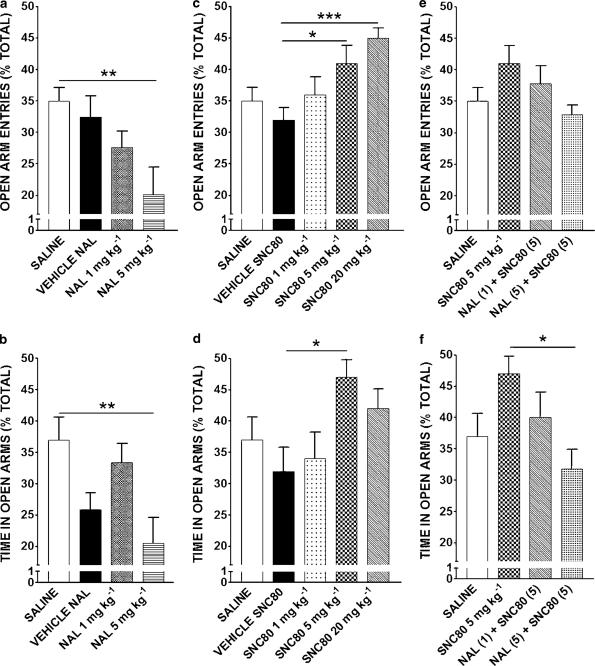
Figure 1.
Effects of naltrindole (NAL, 1 or 5 mg kg−1; (a, b), SNC80 (1, 5 or 20 mg kg−1; (c, d) and SNC80 (5 mg kg−1) antagonism by naltrindole (1 or 5 mg kg−1) (e, f) on the elevated plus maze. Data are expressed as means±s.e.m. of open arm entries (a, c, e) and time on open arms (b, d, f) expressed as a percentage of the total time or number of entries (n=7–10/group). *P<0.05, **P<0.01, and ***P<0.001.
Naltrindole, 1 or 5 mg kg−1 s.c., produced a dose-dependent anxiogenic-like effect on behavior as measured by a decrease in open arm entries (ANOVA, F3,27=4.544, P<0.01; Figure 1a) or time in open arms (ANOVA, F3,27=4.540, P<0.01; Figure 1b). Bonferroni's post hoc analysis of open arm entry data showed that naltrindole 5 mg kg−1 was significantly different from saline (P<0.01; Figure 1a). The post hoc analysis of time in open arms data revealed a significant difference between naltrindole 5 mg kg−1 and saline (P<0.01) (Figure 1b). Post hoc analysis indicated that there were no significant differences between saline and naltrindole vehicle in either measure (P>0.05).
SNC80, 1, 5 or 20 mg kg−1 s.c., produced an anxiolytic-like effect on rat behavior as measured by an increase in open arm entries (ANOVA, F4,41=5.729, P<0.001; Figure 1c) and an increase in time spent in open arms (ANOVA, F4,41=2.745, P<0.05; Figure 1d). No significant difference was observed between saline and SNC80 vehicle in either measure (P>0.05). Bonferroni's multiple comparison post hoc analysis between vehicle and SNC80 revealed a significant difference in SNC80 5 mg kg−1 versus SNC80 vehicle (P<0.05) and SNC80 20 mg kg−1 versus SNC80 vehicle (P<0.001) in open arm entries as a percent of total entries (Figure 1c). Post hoc analysis of time spent in open arms as a percent of total time data showed a significant difference between SNC80 5 mg kg−1 and SNC80 vehicle (P<0.05). SNC80 20 mg kg−1 caused an anxiolytic-like trend, albeit not significant, in time on open arms as compared to SNC80 vehicle (Figure 1d).
The specificity of the anxiolytic effects of SNC80 for delta opioid receptors was tested by the ability of naltrindole to block the effects of SNC80. Results demonstrate a significant difference as measured by time spent in open arms (ANOVA, F3,28=3.133, P<0.05; Figure 1f). Bonferroni's multiple comparison analysis showed no significant difference between saline and naltrindole+SNC80 groups (P>0.05) in either measure, while naltrindole 5 mg kg−1+SNC80 5 mg kg−1 was significantly different from SNC80 5 mg kg−1 in time in open arms (P<0.05; Figure 1f). These data demonstrate that naltrindole can block the effects of SNC80. Inter-rater correlation coefficients were calculated for all data that were scored by two raters and found to be high (0.95–0.98).
SNC80 produces anxiolytic-like effects in a second model of anxiety, the defensive burying paradigm
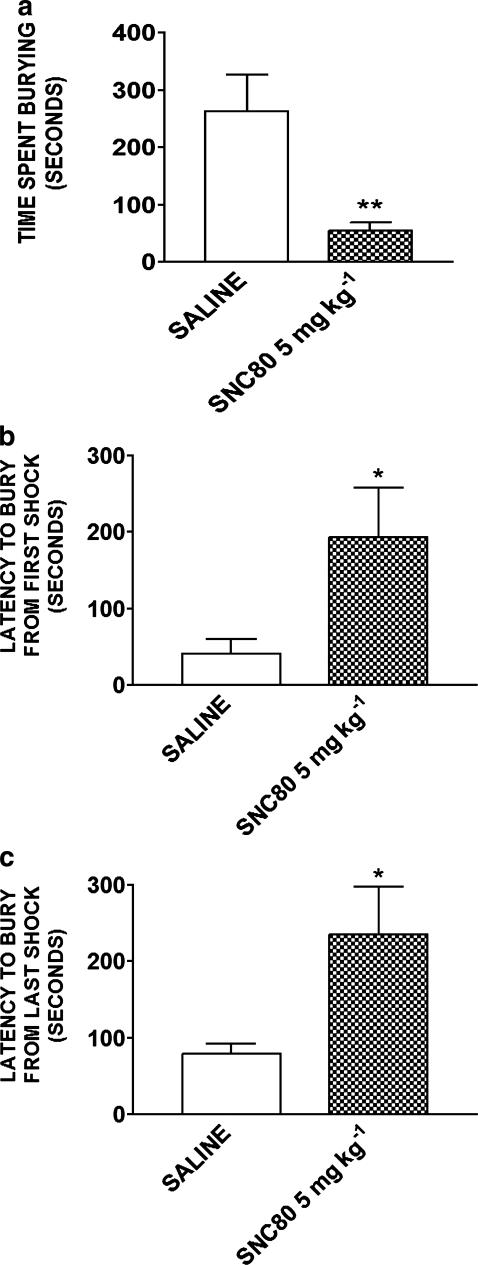
The anxiety-like behavior of rats treated with SNC80 (5 mg kg−1) was also measured using the defensive burying paradigm. Time spent burying, latency to bury from first shock and latency to bury from last shock were recorded and expressed as time in seconds (Figure 2). SNC80 5 mg kg−1 significantly decreased time spent burying compared to saline control (t17=3.32, P<0.01; Figure 2a). In accordance, the latency to bury from first shock and from last shock were significantly increased for the SNC80-injected rats (t17=2.14, P<0.05; Figure 2b and t17=2.31, P<0.05; Figure 2c, respectively). No differences in the intensity of shock response scores (saline 2.13±0.14 versus SNC80 1.83±0.12) or total number of shocks (saline 3.11±1.25 versus SNC80 4.95±1.32) were observed between the groups. Inter-rater correlation coefficients were calculated for all scores and found to be high (0.95–0.98).
Figure 2.
Effect of SNC80 (5 mg kg−1) on the defensive burying paradigm. Time spent burying a shock-probe (a) and latency to begin burying after first (b) and last (c) shock are shown. Data are expressed as the mean±s.e.m. (n=9–10/group). *P<0.05 and **P<0.01.
SNC80 increases activity
SNC80 and other delta opioid receptor agonists increase locomotor activity in a dose- and time-dependent manner (Broom et al., 2002b; Jutkiewicz et al., 2004). When total arm entries were analyzed from the elevated plus maze data described above, SNC80 produced a significant and dose-dependent increase in total arm entries compared to its vehicle or saline. Furthermore, the effect was reduced dose-dependently by naltrindole (data not shown). Total number of arm entries has been suggested to be an index of locomotor activity, albeit a relatively insensitive measure (Dawson & Tricklebank, 1995). For these reasons, SNC80-induced activity was measured using a testing apparatus with greater sensitivity.
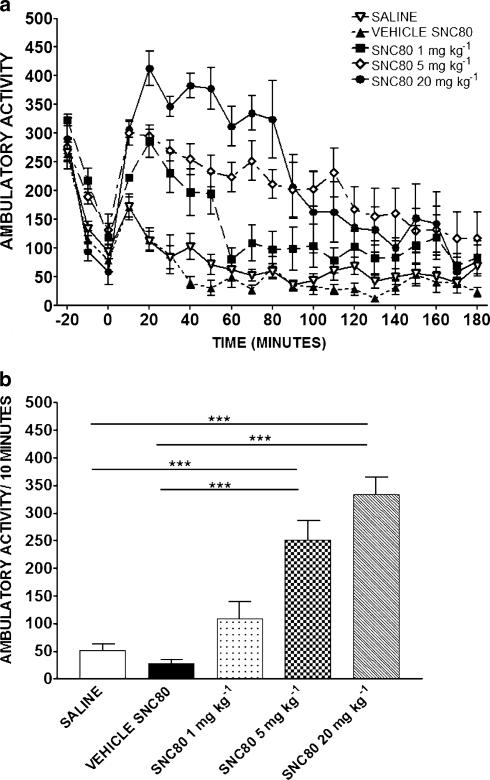
Figure 3a shows the time course for ambulatory activity produced by SNC80 (1, 5 and 20 mg kg−1), SNC80 vehicle and saline. Repeated-measures ANOVA revealed that SNC80 produced a significant increase in activity (F4,20=35.62, P<0.0001) with a sharp rise in locomotor activity that peaked at 20 min postinjection and gradually declined over time. Bonferroni's multiple comparison analysis revealed that 1 (P<0.01), 5 (P<0.001) and 20 (P<0.001) mg kg−1 SNC80 were statistically different from their control and saline. Figure 3b highlights the locomotor activity induced by SNC80 during the 10 min period between 60–70 min postinjection, which is the time when rats were tested for anxiety-like behavior. SNC80 produced a significant and dose-dependent increase in locomotor activity during this time period (F4,47=35.84, P<0.001). Post hoc analysis showed that SNC80 5 mg kg−1 (P<0.001) and 20 mg kg−1 (P<0.001) were significantly different than SNC80 vehicle and saline.
Figure 3.
Effects of SNC80 (1, 5 or 20 mg kg−1) on ambulatory activity. Activity over time (a) and during the 10 min period between 60–70 min postinjection (b) are shown. Data are expressed as the mean±s.e.m. (n=20 for saline group and n=8 for all other groups). ***P<0.001.
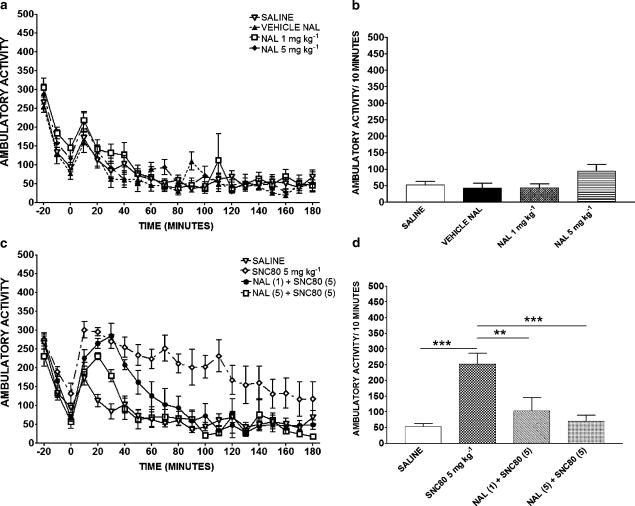
Figure 4 shows the effects of naltrindole alone and naltrindole+SNC80 on ambulatory activity. Ambulatory activity in rats administered 1 or 5 mg kg−1 naltrindole was not different from those administered saline or naltrindole vehicle over the 3 h period postinjection (repeated-measures ANOVA; P>0.05; Figure 4a). There was no difference between naltrindole and vehicle on activity during the 10 min period corresponding to the time when rats were tested for anxiety-like behavior (F3,40=2.298, P>0.05; Figure 4b). As shown in Figure 4c, when naltrindole was administered 15 min before SNC80, a blockade of the effects of SNC80 on activity was seen (repeated-measures ANOVA, F3,60=54.11, P<0.0001) with a significant reversal of 5 mg kg−1 SNC80 with 1 and 5 mg kg−1 naltrindole (P<0.001). Figure 4d shows similar results for the time period corresponding to the time when anxiety-like behaviors were measured. ANOVA revealed that there was a significant overall effect of drug administration on activity (F3,40=13.83, P<0.0001). Post hoc analysis showed that the effects of 5 mg kg−1 SNC80 were blocked by pretreatment with 1 mg kg−1 (P<0.01) and 5 mg kg−1 naltrindole (P<0.001). Naltrindole+SNC80 groups were not significantly different than saline (P>0.05).
Figure 4.
Effects of naltrindole (NAL, 1 or 5 mg kg−1; (a, b) and the combination of SNC80 (5 mg kg−1) and naltrindole (1 and 5 mg kg−1) (c, d) on ambulatory activity. Ambulatory activity over time (a, c) and the activity during the 10 min period between 60–70 min postinjection (b, d) are shown. Data are expressed as the mean±s.e.m. (n=20 for saline group and n=8 for all other groups). **P<0.01 and ***P<0.001.
SNC80-induced anxiolytic effects are not dependent on its stimulant effects
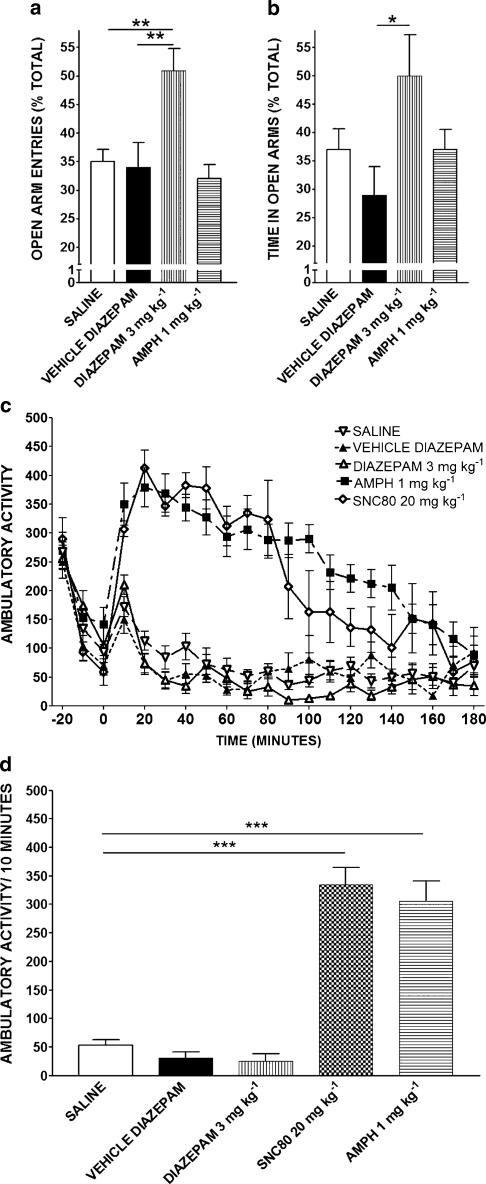
In order to determine if drug-induced increased locomotor activity is sufficient to produce an anxiolytic-like response on the elevated plus maze, another compound with locomotor-stimulating properties was investigated. Figure 5a & b show the effects of saline, amphetamine 1 mg kg−1, diazepam 3 mg kg−1, and diazepam vehicle on the elevated plus maze. ANOVA shows significance in open arm entries (F3,33=7.117, P<0.0001; Figure 5a) and time in open arms (F3,33=2.8, P<0.05; Figure 5b). Bonferroni's post hoc analysis revealed that diazepam produced anxiolytic-like effects, as expected (P<0.05). Amphetamine had no significant affect on anxiety-like behavior (P>0.05).
Figure 5.
Effects of diazepam (3 mg kg−1) and amphetamine (1 mg kg−1) on an elevated plus maze (a, b) and on ambulatory activity (c, d). Data are expressed as the mean±s.e.m. (n=7–10/group for a, b; n=20 for saline group and n=8 for all other groups for c, d). *P<0.05, **P<0.01 and ***P<0.001.
Figure 5c shows the time course of ambulatory activity following saline (1 ml kg−1), amphetamine (1 mg kg−1), diazepam (3 mg kg−1), and diazepam vehicle (1 ml kg−1). The effect of 20 mg kg−1 SNC80 is also shown for comparative purposes. Repeated-measures ANOVA showed a significant difference between treatment groups (F4,80=63.52, P<0.0001). Amphetamine increased locomotor activity (Bonferroni's multiple comparison P<0.001). Figure 5d shows the activity data for the time period corresponding to the time tested on the elevated plus maze or defensive burying paradigm (60–70 min postinjection). There was a significant difference between groups (ANOVA F4,47=39.19, P<0.0001). Amphetamine 1 mg kg−1 significantly increased activity over control (P<0.001). Diazepam 3 mg kg−1 did not alter locomotor activity (P>0.05).
Discussion
Pharmacological research suggests the involvement of the delta opioid receptor system in anxiety. Delta opioid receptor agonists induce anxiolytic-like effects in rodent models of anxiety (Saitoh et al., 2004). Furthermore, studies have shown that the delta opioid receptor antagonist naltrindole abolishes anxiolytic-like effects induced by tetrahydrocannabinol (THC) (Berrendero & Maldonado, 2002) and, when administered alone, induces anxiogenic-like effects in the elevated plus maze (Marin et al., 2003). It is also known that endogenous opioids regulate basal anxiety tone by inhibiting the stress responsive hypothalamic–pituitary–adrenal (HPA) axis (Kreek et al., 2002). While these studies suggest the involvement of the delta opioid receptor system, they have not thoroughly explored it and have not investigated the relationship between the anxiety-like effects and stimulatory effects on activity of delta opioid receptor agonists in modulating levels of anxiety.
The aim of the present study was to investigate the role of delta opioid receptors in anxiety-like behaviors. It was hypothesized that a delta opioid receptor agonist would produce anxiolytic-like effects. This is, in fact, what was found. SNC80 increased the number of open arm visits and time spent in open arms and these effects were reversed by the delta opioid receptor antagonist naltrindole. The anxiolytic effects of SNC80 were similar to the effects of the classical antianxiety therapeutic, diazepam, which also significantly increased both measures (Figure 5a). Furthermore, SNC80 (5 mg kg−1) was effective in reducing anxiety-like measures in a second model of anxiety, the defensive burying paradigm. In contrast, rats injected with naltrindole spent less time and entered the open arms less than their matched controls, indicative of an anxiogenic response. This suggests that basal anxiety states are controlled by endogenous delta opioid receptor system tone and can be regulated in both an anxiolytic-like (agonist) and anxiogenic-like (antagonist) direction.
There are two dependent measures for the elevated plus maze, open arm entries and time in open arms. A change in either dependent measure, or both, is commonly reported to be indicative of a change in anxiety (Dawson & Tricklebank, 1995). Inconsistencies between the two measures for the elevated plus maze are sometimes observed and have been attributed to unknown nonspecific effects, locomotor effects, risk assessment behaviors or other drug-induced behaviors that might be reflected exclusively in a single measure (Dawson & Tricklebank, 1995). In the present study, the results for open arm entries and time in open arms were generally consistent across the two measures, except for the data from the 20 mg kg−1 dose of SNC80. This dose of SNC80 produced a significant increase in open arm entries, but time in open arms did not reach statistical significance. The reason for this difference is unknown. However, our overall results support the notion that SNC80 is an effective anxiolytic agent because 5 mg kg−1 SNC80 significantly increased both the number of open arm entries and time in open arms on the elevated plus maze and showed significant antianxiety effects on all three measures in the defensive burying paradigm.
Our finding that naltrindole produced anxiogenic-like effects supports the previous finding that delta opioid receptor knockout mice show increased levels of anxiety as measured in a light dark box and elevated plus maze (Filliol et al., 2000) and extends the finding into a rat pharmacological model. Recently, the observation that naltrindole acts as an anxiogenic agent in rats has been confirmed by another group who further demonstrated that the effects are mediated by putative delta2 opioid receptors (Saitoh et al., 2005). Both sets of data suggest that an endogenous opioid tone on anxiety is present, although the mechanism underlying this has not been elucidated. There are two distinct possibilities to explain this phenomenon. One, endogenous release of opioid peptides may modulate a basal level of anxiety that is suppressed by naltrindole. Two, the delta opioid receptor may have constitutive receptor activity and naltrindole may be acting as an inverse agonist to cause increases in anxiety measures. Research has shown that several G-protein coupled receptors have agonist-independent (constitutive) activity and the delta opioid receptor fits this category. Traynor and co-workers determined that delta opioid receptors are constitutively active by measuring the level of [35S]-GTPγS binding in the presence and absence of pertussis toxin (Neilan et al., 1999). Three delta opioid receptor antagonists acted as delta opioid receptor inverse agonists; however, naltrindole was shown to be a neutral antagonist and blocked the activity caused by the inverse agonists (Neilan et al., 1999). In addition, naltrindole and related derivatives had little guanosine 5′-O-(3-thio)triphosphate binding or reporter gene assay activity (Tryoen-Toth et al., 2005). Therefore, it is unlikely that naltrindole is acting as an inverse agonist to produce anxiogenic behavior in rats. Although ligand-independent effects at delta opioid receptors including receptor-G-protein coupling and receptor trafficking (Zaki et al., 2001) have been demonstrated in vitro, no studies to date have shown that this occurs in vivo and it is improbable that ligand-independent effects could modulate an endogenous opioid receptor system tone on anxiety. Thus, the most probable explanation and the one best supported by current literature is that a basal tone of anxiety is modulated by endogenous delta opioid peptides. In rats, endogenous opioids regulate basal anxiety tone by inhibiting the stress responsive HPA axis (Kreek et al., 2002). Recently, a study in mice has provided further evidence that endogenous enkephalins, specifically acting at delta opioid receptors, regulate the homeostasis of emotion and motivation behaviors (Nieto et al., 2005).
The effects of SNC80 and related delta opioid receptor agonists on locomotor activity have been studied. In agreement with the present data, it has been reported that SNC80 produces a dose-dependent effect on activity (Broom et al., 2002b; Jutkiewicz et al., 2004). Thus, a concern for the current study was that SNC80-induced activity might confound antianxiety measures. There are two indications reported here that argue this is not the case. One, the increase in ambulatory active seen in rats injected with SNC80 is unlikely to confound the anxiety measures in the defensive burying paradigm because the dependent measure is a decrease in active behavior (i.e. decreased burying) and not an increase in behavior. Two, by indirectly comparing the actions of amphetamine and diazepam with SNC80 on plus maze performance and ambulatory activity, it is evident that not all drugs which cause hyperactivity induce changes in anxiety measures (i.e. amphetamine) and, vice versa, not all anxiolytics cause hyperactivity (i.e. diazepam). Diazepam has been reported to decrease (Patterson et al., 2005) or produce no change in ambulatory activity (Sipos et al., 1999). The most likely explanation for the differences is due to the lighting conditions when testing was carried out. The data presented here were collected under normal lighting conditions and showed no change in activity following diazepam (3 mg kg−1), similar to the study of Sipos et al. (1999); (0.3–5.6 mg kg−1), whereas the decrease in activity reported by Patterson et al. (2005); (1, 2 or 5 mg kg−1) was measured under low-level lighting conditions in the test room.
The actions of SNC80 and related delta opioid receptor agonists in animal models of depression have been well characterized. Woods and co-workers have shown in a number of elegant studies that delta opioid receptor agonists have robust antidepressant-like effects in rats and these effects are independent of drug-induced hyperactivity, convulsant properties or disruptive effects on learning and memory (Broom et al., 2002a, 2002b; Jutkiewicz et al., 2003). Here, data from two distinct animal models of anxiety illustrate the robust actions of SNC80 on anxiety-like behavior in the rat. It is intriguing to speculate that the close relationship between depression and anxiety may be due to a neurochemical substrate that is common to both of these disorders. Several studies suggest that the serotonergic system may be this common link (see reviews: Stark & Hen, 1999; Zhuang et al., 1999; Sibille & Hen, 2001). However, other studies suggest that this common substrate may be one of the opioid systems acting in part on the delta opioid receptor. Clinically it is well known that classical anxiolytics are not effective treatments for depression and, vice versa, classical antidepressants are not widely used to treat generalized anxiety. However, the delta opioid system has been suggested to play a role in the mechanism of action of the antidepressant imipramine (Varona et al., 2003), and a possible target of action of the benzodiazepines nerisopam and girisopam may be the opioid receptor signal transduction system (Fekete et al., 1997). While not conclusive, these studies agree that the opioid systems play a role in modulating depression-like and anxiety-like behaviors.
In summary, the data presented here, show that the delta opioid receptors are involved in modulation of anxiety-like behavior in the rat. SNC80 causes robust pharmacological effects on anxiolytic-like measures and naltrindole modulates anxiogenic-like behaviors, suggesting that basal anxiety states are under the tonic modulation of the delta opioid system. The modulation of anxiety-like behavior by opioid receptor ligands, specifically delta opioid receptor agonists, may prove to be a useful clinical alternative to treat anxiety disorders that are resistant to typical anxiolytics. Interestingly, human cocaine addicts frequently use opiates to cope with anxiety caused by cocaine withdrawal due to the ineffectiveness of current drugs (Kosten et al., 1986; Gawin, 1991; O'Brien, 2001).
Acknowledgments
We thank Irwin Lucki, Ph.D., for providing the means to conduct the defensive burying paradigm, Tom J. Gould, Ph.D., and Alan Cowan, Ph.D., for their advice and help with the elevated plus maze, and Jordan Trecki, M.S., Michelle Niculescu, Ph.D., Joe A. Schroeder, Ph.D., Imran Sheikh and Kris Guardiario, for their technical assistance. This work was made possible by the support of NIH R01 DA018326, T32 DA07237 and P30 DA13429 and by NIDA for the generous supply of drugs used in this study.
Abbreviations
- SNC80
(+)-4-[(αR)-α-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide
References
- BERRENDERO F., MALDONADO R. Involvement of the opioid system in the anxiolytic-like effects induced by Delta(9)-tetrahydrocannabinol. Psychopharmacology (Berlin) 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- BILSKY E.J., CALDERON S.N., WANG T., BERNSTEIN R.N., DAVIS P., HRUBY V.J., MCNUTT R.W., ROTHMAN R.B., RICE K.C., PORRECA F. SNC 80, a selective, nonpeptidic and systemically active opioid delta agonist. J. Pharmacol. Exp. Ther. 1995;273:359–366. [PubMed] [Google Scholar]
- BROOM D.C., JUTKIEWICZ E.M., FOLK J.E., TRAYNOR J.R., RICE K.C., WOODS J.H. Convulsant activity of a non-peptidic delta-opioid receptor agonist is not required for its antidepressant-like effects in Sprague–Dawley rats. Psychopharmacology (Berlin) 2002a;164:42–48. doi: 10.1007/s00213-002-1179-y. [DOI] [PubMed] [Google Scholar]
- BROOM D.C., JUTKIEWICZ E.M., FOLK J.E., TRAYNOR J.R., RICE K.C., WOODS J.H. Nonpeptidic delta-opioid receptor agonists reduce immobility in the forced swim assay in rats. Neuropsychopharmacology. 2002b;26:744–755. doi: 10.1016/S0893-133X(01)00413-4. [DOI] [PubMed] [Google Scholar]
- BROOM D.C., JUTKIEWICZ E.M., RICE K.C., TRAYNOR J.R., WOODS J.H. Behavioral effects of delta-opioid receptor agonists: potential antidepressants. Jpn. J. Pharmacol. 2002c;90:1–6. doi: 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- CALDERON S.N., COOP A. SNC 80 and related delta opioid agonists. Curr. Pharm. 2004;10:733–742. doi: 10.2174/1381612043453054. [DOI] [PubMed] [Google Scholar]
- CALDERON S.N., ROTHMAN R.B., PORRECA F., FLIPPEN-ANDERSON J.L., MCNUTT R.W., XU H., SMITH L.E., BILSKY E.J., DAVIS P., RICE K.C. Probes for narcotic receptor mediated phenomena. 19. Synthesis of (+)-4-[(alpha R)-alpha-((2S,5R)-4-allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide (SNC 80): a highly selective, nonpeptide delta opioid receptor agonist. J. Med. Chem. 1994;37:2125–2128. doi: 10.1021/jm00040a002. [DOI] [PubMed] [Google Scholar]
- DAWSON G.R., TRICKLEBANK M.D. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- DE BOER S.F., KOOLHAAS J.M. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur. J. Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- FEKETE M.I., HORVATH K., KEDVES R., MATE I., SZEKELY J.I., SZENTKUTI E. Selective interaction of homophtalazine derivatives with morphine. Eur. J. Pharmacol. 1997;331:175–183. doi: 10.1016/s0014-2999(97)01056-x. [DOI] [PubMed] [Google Scholar]
- FERNANDES C., FILE S.E. The influence of open arm ledges and maze experience in the elevated plus-maze. Pharmacol. Biochem. Behav. 1996;54:31–40. doi: 10.1016/0091-3057(95)02171-x. [DOI] [PubMed] [Google Scholar]
- FILLIOL D., GHOZLAND S., CHLUBA J., MARTIN M., MATTHES H.W., SIMONIN F., BEFORT K., GAVERIAUX-RUFF C., DIERICH A., LEMEUR M., VALVERDE O., MALDONADO R., KIEFFER B.L. Mice deficient for delta- and mu-opioid receptors exhibit opposing alterations of emotional responses. Nat. Genet. 2000;25:195–200. doi: 10.1038/76061. [DOI] [PubMed] [Google Scholar]
- GAWIN F.H. Cocaine addiction: psychology and neurophysiology. Science. 1991;251:1580–1586. doi: 10.1126/science.2011738. [DOI] [PubMed] [Google Scholar]
- HANDLEY S.L., MCBLANE J.W. An assessment of the elevated X-maze for studying anxiety and anxiety-modulating drugs. J. Pharmacol. Toxicol. Methods. 1993;29:129–138. doi: 10.1016/1056-8719(93)90063-k. [DOI] [PubMed] [Google Scholar]
- JUTKIEWICZ E.M., ELLER E.B., FOLK J.E., RICE K.C., TRAYNOR J.R., WOODS J.H. Delta-opioid agonists: differential efficacy and potency of SNC80, its 3-OH (SNC86) and 3-desoxy (SNC162) derivatives in Sprague–Dawley rats. J. Pharmacol. Exp. Ther. 2004;309:173–181. doi: 10.1124/jpet.103.061242. [DOI] [PubMed] [Google Scholar]
- JUTKIEWICZ E.M., RICE K.C., WOODS J.H., WINSAUER P.J. Effects of the delta-opioid receptor agonist SNC80 on learning relative to its antidepressant-like effects in rats. Behav. Pharmacol. 2003;14:509–516. doi: 10.1097/00008877-200311000-00003. [DOI] [PubMed] [Google Scholar]
- KNAPP R.J., SANTORO G., DE LEON I.A., LEE K.B., EDSALL S.A., WAITE S., MALATYNSKA E., VARGA E., CALDERON S.N., RICE K.C., ROTHMAN R.B., PORRECA F., ROESKE W.R., YAMAMURA H.I. Structure-activity relationships for SNC80 and related compounds at cloned human delta and mu opioid receptors. J. Pharmacol. Exp. Ther. 1996;277:1284–1291. [PubMed] [Google Scholar]
- KOSTEN T.R., GAWIN F.H., ROUNSAVILLE B.J., KLEBER H.D. Cocaine abuse among opioid addicts: demographic and diagnostic factors in treatment. Am. J. Drug Alcohol Abuse. 1986;12:1–16. doi: 10.3109/00952998609083739. [DOI] [PubMed] [Google Scholar]
- KREEK M.J., BOR G.L., ZHOU Y., SCHLUGER J.Relationship between endocrine functions and substance abuse syndromes: Heroin and related short-acting opiates in addiction contrasted with methadone and other long-acting opioid agonists used in pharmacotherapy of addiction Hormones, Brain and Behavior 2002Academic Press, San Diego; 781–830.ed. Pfaff, D., pp [Google Scholar]
- MARIN S., MARCO E., BISCAIA M., FERNANDEZ B., RUBIO M., GAUZA C., SCHMIDHAMMER H., VIVEROS M.P. Involvement of the kappa-opioid receptor in the anxiogenic-like effect of CP 55,940 in male rats. Pharmacol. Biochem. Behav. 2003;74:649–656. doi: 10.1016/s0091-3057(02)01041-9. [DOI] [PubMed] [Google Scholar]
- NEILAN C.L., AKIL H., WOODS J.H., TRAYNOR J.R. Constitutive activity of the delta-opioid receptor expressed in C6 glioma cells: identification of non-peptide delta-inverse agonists. Br. J. Pharmacol. 1999;128:556–562. doi: 10.1038/sj.bjp.0702816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIETO M.M., GUEN S.L.E., KIEFFER B.L., ROQUES B.P., NOBLE F. Physiological control of emotion-related behaviors by endogenous enkephalins involves essentially the delta opioid receptors. Neuroscience. 2005;135:305–313. doi: 10.1016/j.neuroscience.2005.06.025. [DOI] [PubMed] [Google Scholar]
- O'BRIEN C.P.Drug Addiction and Drug Abuse Goodman & Gilman's The Pharmacological Basis of Therapeutics 2001McGraw-Hill, New York; 633–636.10th editioneds. HARDMAN, J.G., LIMBIRD, L.E., GILMAN, A.G., pp [Google Scholar]
- PACHECO D.F., REIS G.M., FRANCISCHI J.N., CASTRO M.S., PEREZ A.C., DUARTE I.D. delta-Opioid receptor agonist SNC80 elicits peripheral antinociception via delta(1) and delta(2) receptors and activation of the l-arginine/nitric oxide/cyclic GMP pathway. Life Sci. 2005;78:54–60. doi: 10.1016/j.lfs.2005.04.032. [DOI] [PubMed] [Google Scholar]
- PATTERSON J.P., MARKGRAF C.G., CIRINO M., BASS A.S. Validation of a motor activity system by a robotically controlled vehicle and using standard reference compounds. J. Pharmacol. Toxicol. Methods. 2005;52:159–167. doi: 10.1016/j.vascn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- PELLOW S., CHOPIN P., FILE S.E., BRILEY M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- PLOBECK N., DELORME D., WEI Z.Y., YANG H., ZHOU F., SCHWARZ P., GAWELL L., GAGNON H., PELCMAN B., SCHMIDT R., YUE S.Y., WALPOLE C., BROWN W., ZHOU E., LABARRE M., PAYZA K., ST-ONGE S., KAMASSAH A., MORIN P.E., PROJEAN D., DUCHARME J., ROBERTS E. New diarylmethylpiperazines as potent and selective nonpeptidic delta opioid receptor agonists with increased in vitro metabolic stability. J. Med. Chem. 2000;43:3878–3894. doi: 10.1021/jm000228x. [DOI] [PubMed] [Google Scholar]
- PORTOGHESE P.S., SULTANA M., TAKEMORI A.E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur. J. Pharmacol. 1988;146:185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- PRESOLD C., TREIT D. Excitotoxic lesions of the septum produce anxiolytic effects in the elevated plus-maze and the shock-probe burying tests. Physiol. Behav. 1992;52:37–47. doi: 10.1016/0031-9384(92)90431-z. [DOI] [PubMed] [Google Scholar]
- SAITOH A., KIMURA Y., SUZUKI T., KAWAI K., NAGASE H., KAMEI J. Potential anxiolytic and antidepressant-like activities of SNC80, a selective delta-opioid agonist, in behavioral models in rodents. J. Pharmacol. Sci. 2004;95:374–380. doi: 10.1254/jphs.fpj04014x. [DOI] [PubMed] [Google Scholar]
- SAITOH A., YOSHIKAWA Y., ONODERA K., KAMEI J. Role of δ-opioid receptor subtypes in anxiety-related behaviors in the elevated plus-maze in rats. Psychopharmacology. 2005;182:327–334. doi: 10.1007/s00213-005-0112-6. [DOI] [PubMed] [Google Scholar]
- SIBILLE E., HEN R. Combining genetic and genomic approaches to study mood disorders. Eur. Neuropsychopharmacol. 2001;11:413–421. doi: 10.1016/s0924-977x(01)00118-3. [DOI] [PubMed] [Google Scholar]
- SIPOS M.L., BURCHNELL V., GALBICKA G. Dose-response curves and time-course effects of select5ed anticholinergics on locomotor activity in rats. Psychopharmacology. 1999;147:250–256. doi: 10.1007/s002130051164. [DOI] [PubMed] [Google Scholar]
- STARK K.L., HEN R. Knockout Corner. Int. J. Neuropsychopharmacol. 1999;2:145–150. doi: 10.1017/S146114579900142X. [DOI] [PubMed] [Google Scholar]
- TREIT D., PINEL J.P., FIBIGER H.C. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol. Biochem. Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- TRYOEN-TOTH P., DECAILLOT F.M., FILLIOL D., BEFORT K., LAZARUS L.H., SCHILLER P.W., SCHMIDHAMMER H., KIEFFER B.L. Inverse agonism and neutral antagonism at wild-type and constitutively active mutant delta opioid receptors. J. Pharmacol. Exp. Ther. 2005;313:410–421. doi: 10.1124/jpet.104.077321. [DOI] [PubMed] [Google Scholar]
- VARONA A., GIL J., SARACIBAR G., MAZA J.L., ECHEVARRIA E., IRAZUSTA J. Effects of imipramine treatment on delta-opioid receptors of the rat brain cortex and striatum. Arzneimittelforschung. 2003;53:21–25. doi: 10.1055/s-0031-1297065. [DOI] [PubMed] [Google Scholar]
- ZAKI P.A., KEITH D.E., JR, THOMAS J.B., CARROLL F.I., EVANS C.J. Agonist-, antagonist-, and inverse agonist-regulated trafficking of the delta-opioid receptor correlates with, but does not require, G protein activation. J. Pharmacol. Exp. Ther. 2001;298:1015–1020. [PubMed] [Google Scholar]
- ZHUANG X., GROSS C., SANTARELLI L., COMPAN V., TRILLAT A.C., HEN R. Altered emotional states in knockout mice lacking 5-HT1A or 5-HT1B receptors. Neuropsychopharm. 1999;21 (2 Suppl):52S–60S. doi: 10.1016/S0893-133X(99)00047-0. [DOI] [PubMed] [Google Scholar]