Abstract
Glaucoma pathophysiology appears to involve vascular deficits, which may contribute to initiation and progression of the disease.
Anandamide, the endogenous cannabinoid ligand, and WIN55212-2, a synthetic cannabinoid agonist, are able to evoke concentration-dependent relaxations in bovine ophthalmic artery rings, precontracted with 5-hydroxytryptamine (5-HT) (1 μM). Endothelium removal reduces cannabinoid agonist potency and efficacy.
The selective cannabinoid 1 (CB1) receptor antagonists SR141716A (100 nM) and AM251 (100 nM) cause a shift to the right in the concentration–response curves to anandamide and WIN55212-2 in arterial rings both in the presence and in the absence of endothelium.
In endothelium-intact arteries, the nitric oxide synthase inhibitor, NG-monomethyl-L-arginine (L-NMMA, 300 μM), completely blocked the anandamide- and WIN55212-2-relaxant responses; by contrast, the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP, 100 μM) induced an increase in vasorelaxant responses to cannabinoid agonists.
Relaxations to anandamide and WIN55212-2 were inhibited by iberiotoxin (IbTX, 200 nM), a blocker of large conductance, Ca2+-activated K+ channel (BKCa), and by 4-aminopyridine (4-AP; 1 mM), a blocker of delayed rectifier K+ channel, whereas the blockade of KATP channels by glibenclamide (5 μM) and of small conductance Ca2+-activated K+ channels (SKCa) by apamin (100 nM) did not produce any effects.
These data suggest that anandamide and WIN55212-2 relax the bovine ophthalmic artery by involving CB1 the cannabinoid receptor-sensitive pathway. In endothelium-intact arteries, relaxation occurs through activation of nitric oxide synthase cyclic GMP and Ca2+-activated K+ channels. They also cause endothelium-independent relaxation by involving potassium channel opening.
Keywords: Cannabinoid CB1 receptor, anandamide, WIN55212-2, bovine ophthalmic artery, relaxation, nitric oxide, cGMP, potassium channels.
Introduction
Glaucoma is a progressive optic neuropathy, which induces characteristic structural modifications to the optic nerve head and typical alteration of the visual field. Elevated intraocular pressure clearly remains the major risk factor, even if there is an increasing acceptance in the current literature showing that the ischaemic component contributes to the pathophysiology of glaucoma (Flammer, 1994; Chung et al., 1999; Flammer et al., 2002). Subsequent, to various clinical observations, research has recently been oriented towards the study of vascular risk factors that could contribute both to initiation and progression of disease. It has been observed that glaucomatous eyes show an impaired ocular blood flow; the causative factors remain unknown (Flammer et al., 2002). All drugs currently used to treat glaucoma aim at lowering intraocular pressure, therefore an evaluation of the vasoactive effects of drugs utilized in glaucoma pharmacological therapy might be particularly relevant. In addition, glaucoma is a chronic disease and the quest for new ocular hypotensive agents able to improve ocular blood flow is important for its treatment.
The presence of a functional endocannabinoid system in the eye demonstrates an important role for endocannabinoid in ocular physiology. Numerous studies support the intraocular pressure-lowering effects of cannabinoid (Pate et al., 1996; Song & Slowey, 2000; Porcella et al., 2001) through actions that are mostly mediated by cannabinoid 1 (CB1) receptors. Recently, we have found CB1 receptors in bovine ciliary muscle, supporting a direct role for the CB1 receptors in controlling intraocular pressure (Lograno & Romano, 2004).
Cannabinoids and, in particular, the endocannabinoid anandamide are involved in potent dilator responses in various vascular beds (for review see Randall et al., 2002). Some of the most exciting studies show that anandamide is able to mediate vasorelaxant responses, probably via coupling to nitric oxide release, metabolism to vasoactive arachidonic metabolites, prostanoid involvement, endothelium-derived hyperpolarizating factor release and inhibition of calcium channels (Deutsch et al., 1997; Pratt et al., 1998; Liu et al., 2000; Grainger & Boachie-Ansah, 2001; Moezi et al., 2004; O'Sullivan et al., 2004). Indeed, although there are a variety of putative mechanisms that could explain these events, in the literature there is still considerable discrepancy. The anandamide vasorelaxant responses appear to be both endothelium dependent and independent according to the vascular bed (White & Hiley, 1997; Randall & Kendall, 1998). In cat cerebral artery and rat mesenteric artery, ananadamide-induced relaxations were independent of the endothelium (Plane et al., 1997; White & Hiley, 1997; Gebremedhin et al., 1999); by contrast, in rabbit cerebral and aortic arteries, rat renal arterioles and bovine coronary arteries, anandamide-evoked relaxations were endothelium dependent (Deutsch et al., 1997; Pratt et al., 1998; Fleming et al., 1999; Gebremedhin et al., 1999; Mukhopadhyay et al., 2002). In addition, studies in isolated preparations of vascular tissues have also demonstrated that vasorelaxant effects of anandamide were sensitive to the selective cannabinoid antagonist SR141716A, supporting the involvement of the CB1 receptors (White & Hiley, 1997; Gebremedhin et al., 1999).
Still, very little is known on the vascular actions of cannabinoids in ocular tissues; however, cannabinoid vasorelaxant properties might be able to increase ocular blood flow (Tomida et al., 2004). Porcella et al. (1998) have proposed that cannabinoids can act as vasodilators on blood vessels of the anterior uvea, thus improving aqueous humour uveoscleral outflow.
The aim of the present study was to provide pharmacological evidence for the existence of functional cannabinoid receptors in the bovine ophthalmic artery and to investigate the vasoactive properties of endogenous and synthetic cannabinoid agonists. For this purpose, we have detected the possible mechanism(s) underlying the relaxant effects of cannabinoids by examining the involvement of vascular endothelium, the possible role of nitric oxide and the putative role of potassium channels.
Methods
Myograph studies
Bovine eyes, including the immediate retro-orbital structures, obtained from a slaughterhouse, were enucleated within 5 min after death and immediately put in ice-cold oxygenated modified Krebs solution (composition, mM: NaCl 136.8, KCl 5.4, MgSO4 0.8, NaH2PO4 1.4, NaHCO3 12, CaCl2 2.7, D-glucose 5, Na-ascorbate 0.2, pH 7.4) to transport to the laboratory. The main ophthalmic artery running along the optic nerve to the eye was dissected free of adherent connective and adipose tissue. Two adjacent rings were cut from each artery (0.7–1.2 mm in diameter, 2–3 mm in length) and were mounted on fine tungsten wires (50 μM in diameter) on a myograph system (Fort 10, WPI, Sarasota, FL, U.S.A.). Tension was measured and recorded on a powerLab 4/20 recorder (ADInstruments, Castle Hill, NSW, Australia). Once mounted, samples were kept at 37°C in Krebs solution continuously gassed with 5% CO2 in O2. The ophthalmic arteries were stretched to an optimal passive tension of 5 mN and allowed to equilibrate for at least 90 min during which the Krebs solution was changed several times; all experiments were carried out in the presence of indomethacin (10 μM). In all vessels, the integrity of the endothelium was assessed by precontracting the vessel with 1 μM 5-hydroxytryptamine (5-HT), followed by relaxation with 10 μM carbachol; relaxations greater than 90% were designated as endothelium intact. When endothelium was not required, it was removed by rubbing the intima with a human hair; carbachol-induced relaxation of <10% indicated successful removal. Removal of the endothelium had no significant effect on 5-HT-induced tone in the same preparations (with endothelium, 14.7±0.7 mN; after endothelial removal, 14.5±0.7 mN; n=34).
Experimental protocol
The effects of anandamide and WIN55212-2 on 5-HT-induced tone were examined in paired rings derived from the same arteries. Ophthalmic arteries were contracted with 5-HT (1 μM), and once a stable contraction was achieved and maintained, the vasorelaxant effects of anandamide and WIN55212-2 were assessed as cumulative concentration–response curves by the addition of stock solutions to the buffer. All protocols were carried out in both the presence and the absence of endothelium. The steady-state response to anandamide and WIN55212-2 was taken at each concentration and expressed as a percentage relaxation of the imposed 5-HT contraction. The involvement of cannabinoid receptors was assessed using the selective CB1 receptor antagonists SR141716A and AM251, each at 100 nM (Rinaldi-Carmona et al., 1996; Lan et al., 1999). These antagonists were added to the preparations 15 min before preconstriction and were present during construction of the concentration–response curves.
The vasorelaxant action to cannabinoid agonists was also evaluated in the presence of pertussis toxin (400 ng/ml), the nitric oxide synthase inhibitor NG-monomethyl-L-arginine (L-NMMA; 300 μM, Rees et al., 1990), the nitric oxide donor S-nitroso-N-acetylpenicillamine (SNAP, 100 μM; Ferrero et al., 1999), the guanylyl cyclase inhibitor 1H-[1,2,4] oxadiazolo-[4,3-a] quinoxalin-1-one (ODQ, 1 μM; Garthwaite et al., 1995) and capsaicin (10 μM). All these were added 30 min before and remained present throughout the construction of the concentration–response curves. In line with the findings of White et al. (2001), capsaicin was also added 30 min before determination of concentration–response curves. However, in some of the experiments with capsaicin, a 60 min preincubation was also used to evaluate possible time-dependent effects.
Finally, the potassium channel role in the vasorelaxant effects to cannabinoid agonists was evaluated. A pretreatment for 30 min with potassium channel blockers, such as iberiotoxin (IbTX, 200 nM; Galvez et al., 1990), glibenclamide (5 μM), apamin (100 nM) and 4-aminopyridine (4-AP, 1 mM), was assayed on arterial rings to provide further insight for the identification of the potassium channel(s) involved. Thereafter, rings were precontracted with 5-HT and maximally relaxed to cannabinoid agonists as cited above.
Data and statistical analysis
All relaxation responses are expressed as percentage relaxation of the tone induced by 1 μM 5-HT. Values are given as mean±s.e.m. and n refers to the number of animals. The concentration of vasorelaxation, giving a half-maximal response (EC50), was obtained by fitting four-parameter sigmoidal concentration–response curves using Prism GraphPad (version 3.0, San Diego, CA, U.S.A.), and expressed as its negative logarithm, pEC50. Rmax refers to the maximal response achieved. Statistical analysis was performed by analysis of variance (ANOVA), followed by Bonferroni's post hoc test (Prism 3.0). Student's t-test was used when appropriate. P-values of <0.05 were considered as statistically significant.
Drugs
All drugs were supplied by Tocris Cookson (Bristol, U.K.), except where indicated. 5-HT, creatinine sulphate, IbTX, pertussis toxin (Sigma Aldrich, St Louis, MO, U.S.A.), L-NMMA acetate and SNAP were dissolved in distilled water to a concentration of 10 mM. Anandamide was supplied as a water-soluble emulsion and dissolved in distilled water. SR141716A and SR144528 (granted by Sanofi Recherche, Montpellier, France), AM251, WIN55212-2 and ODQ were dissolved in dimethylsulphoxide (Sigma) to a concentration of 10 mM. Serial dilutions were prepared daily in Krebs solution. All reagents were of analytical grade. The final bath concentration of dimethylsulphoxide did not exceed 0.1%, which we have found elsewhere to have little or no effect on mechanical activity.
Results
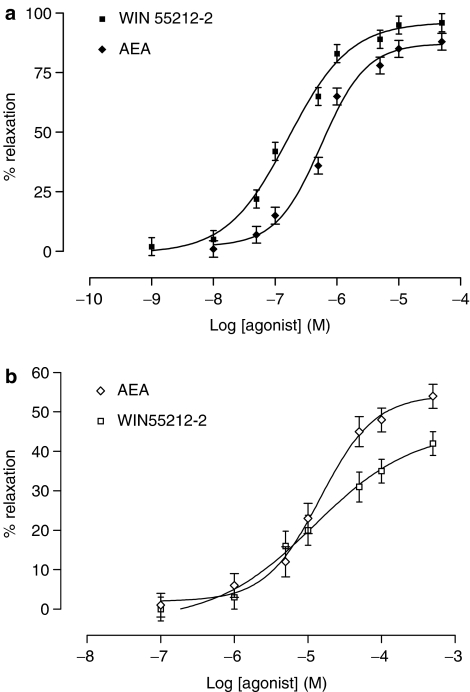
Anandamide (0.01–50 μM) and WIN55212-2 (1 nM–50 μM) evoked concentration-dependent relaxant responses in the bovine ophthalmic arteries precontracted with 5-HT. Both the potency (P<0.001) and reactivity (P<0.001) of cannabinoid agonists were significantly greater in endothelium-intact arterial rings compared with endothelium-denuded (Figure 1, Table 1) rings. Relaxation to cannabinoid agonists was unaffected by the presence or the absence of indomethacin.
Figure 1.
The vasorelaxant responses of anandamide and WIN55212-2 in bovine ophthalmic artery precontracted to 5-HT with (a) endothelium intact and (b) after removal of endothelium. Data are given as means with error bars representing s.e.m.
Table 1.
Effects of cannabinoid receptor antagonists on the vasorelaxation responses to anadamide and WIN55212-2 in the endothelium-intact bovine ophthalmic artery compared with endothelium-denuded precontracted with 5-HT
| Treatment | Endothelium denuded | Endothelium intact | ||||
|---|---|---|---|---|---|---|
| pEC50 | Rmax (%) | n | pEC50 | Rmax (%) | n | |
| Anandamide control | 6.38±0.08 | 87.3±4.1 | 12 | 4.89±0.09 | 54.3±3.9 | 10 |
| SR141716A (100 nM) | 5.23±0.11** | 59.1±5.7* | 10 | 4.08±0.22* | 39.1±7.8* | 10 |
| AM 251 (100 nM) | 5.79±0.12* | 65.4±3.5* | 10 | 4.17±0.14* | 32.9±4.6* | 10 |
| SR144528 (1 μM) | 6.41±0.02 | 86.1±3.6 | 8 | 4.78±0.06 | 54.9±6.8 | 8 |
| WIN55212-2 control | 6.78±0.07 | 96.2±3.0 | 12 | 5.11±0.09 | 46.0±2.6 | 12 |
| SR141716A (100 nM) | 5.13±0.52** | 83.2±29.7 | 10 | 4.26±0.24* | 29.7±8.0* | 8 |
| AM 251 (100 nM) | 5.84±0.17* | 57.4±5.9* | 10 | 4.31±0.08* | 27.7±1.4* | 8 |
| SR144528 (1 μM) | 6.77±0.18 | 94.1±11.0 | 8 | 5.10±0.1 | 49±3.8 | 8 |
All experiments were performed in the presence of 10 μM indomethacin. Data are presented as mean±s.e.m. pEC50 and Rmax values were obtained directly from individual log concentration–response curves.
n indicates the number of animals.
P<0.05,
P<0.001 compared to control values.
The involvement of cannabinoid CB1 receptors
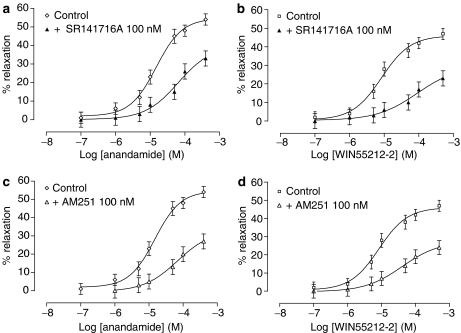
In endothelium-intact arteries, the CB1 receptor antagonist SR141716A (100 nM) produced a significant shift to the right in the concentration–response curve to anandamide (P<0.001; Figure 2a; Table 1). Similarly, SR141716A (100 nM) significantly reduced the vasorelaxant response to WIN55212-2 (P<0.001; Table 1; Figure 2b); again, in the absence of endothelium, SR141716A (100 nM) yielded small but significant inhibition of relaxation to cannabinoid agonists (P<0.05; Table 1; Figure 3a, b). Another CB1 receptor antagonist AM251 (100 nM) inhibited relaxation to anandamide (P<0.05; Table 1; Figure 2c) and WIN55212-2 (P<0.05; Table 1; Figure 2d). Similarly, in the absence of endothelium, AM251 produced slight displacement to the right of the concentration–response curves to anandamide and WIN55212-2 (P<0.05; Table 1; Figure 3c, d). The CB2 receptor antagonist SR144528 (1 μM) was without any effect on relaxation elicited by cannabinoid agonists (Table 1). In endothelium-denuded arteries, pretreatment with capsaicin (for 30 min; 10 μM), which causes functional desensitization of the vanilloid receptor system, had no effect on relaxation evoked by cannabinoid agonists (in the presence of capsaicin: anandamide pEC50=4.91±0.07, Rmax =53.6±2.8; n=6; WIN55212-2 pEC50=5.10±0.06, Rmax=46.2±3.0; n=6). The same lack of effects by capsaicin was observed with preincubation up to 60 min (data not shown).
Figure 2.
Effects of the CB1 receptor antagonists SR141716A 100 nM (a and b) and AM251 100 nM (c and d) on vasorelaxant responses to anandamide and WIN55212-2 in endothelium-intact bovine ophthalmic artery precontracted with 5-HT. Data are given as means with error bars representing s.e.m.
Figure 3.
Effects of the CB1 receptor antagonist SR141716A 100 nM (a and b) and AM251 100 nM (c and d) on vasorelaxant responses to anandamide and WIN55212-2 in endothelium-denuded bovine ophthalmic artery precontracted with 5-HT. Data are given as means with error bars representing s.e.m.
Involvement of the Gi/Go protein
CB1 receptors have been shown to couple to Gi as well as Go protein (Porter & Felder, 2001). To analyse the effector pathway responsible for activation of vasorelaxation to cannabinoid agonists in bovine ophthalmic artery, we determined the effect of pertussis toxin. Bovine ophthalmic arterial rings were incubated with 400 ng ml−1 pertussis toxin for a period of 30 min at 37°C. As shown in Figure 4, vasorelaxation with 50 μM anandamide was completely abolished both in the presence and the absence of endothelium. The same results were obtained with pertussis toxin and WIN55212-2 (50 μM; data not shown), suggesting the involvement of the Gi/o protein-coupled signalling pathway in vasorelaxation to anandamide and WIN55212-2.
Figure 4.
Typical tracing showing one experiment in which the anandamide (50 μM) response was blocked by pretreatment (20 min) with pertussis toxin (400 ng/ml).
Involvement of nitric oxide in mediating cannabinoid vasorelaxations
In endothelium-intact arteries, the nitric oxide synthase inhibitor L-NMMA (300 μM) completely blocked relaxation to anandamide and WIN55212-2 (P<0.001, Figure 5a, b). In endothelium-denuded arteries, L-NMMA (300 μM) had no effect on anandamide and WIN55212-2 vasorelaxations (control anandamide: pEC50=6.16±0.04, Rmax=90.3±3.4, n=6; in the presence of inhibitor: pEC50=6.16±0.08, Rmax=89.1±2.9, n=6; control WIN55212-2: pEC50=6.80±0.08, Rmax=96.3±3.0, n=6; in the presence of inhibitor: pEC50=6.81±0.11, Rmax=94.8±2.9, n=6).
Figure 5.
Effects of L-NMMA (300 μM) and SNAP (100 μM) on (a) anandamide- and (b) WIN55212-2-induced relaxation in endothelium-intact bovine ophthalmic artery precontracted with 5-HT. Data are given as means with error bars representing s.e.m.
The nitric oxide donor SNAP (100 μM) caused a small but significant increase in vasorelaxation to anadamide (pEC50=6.24±0.06, Rmax=112.1±3.9%, P<0.05; n=8; Figure 5a), most notably at high concentrations of anandamide. It is noteworthy that in the presence of SNAP, WIN55212-2 raised maximal relaxation, which was not significant (pEC50=6.63±0.18, Rmax 118.4±9.9%; P>0.05, n=10; Figure 5b).
Involvement of cyclic GMP
To investigate the involvement of guanylyl cyclase in the cannabinoid-evoked response, endothelium-intact ophthalmic arteries were pretreated with the selective guanylyl cyclase inhibitor ODQ (1 μM) for 30 min. ODQ completely abolished the vasorelaxant effects evoked by 50 μM anandamide (Figure 6, n=8) but did not alter the 5-HT response. Interestingly, the same results were obtained with WIN55212-2 (50 μM) in the presence of ODQ (1 μM).
Figure 6.
Typical tracing showing one experiment in which the anandamide (50 μM) response was blocked by pretreatment with ODQ (1 μM) for 30 min.
Role of potassium channel in vasorelaxant responses to cannabinoid agonists
In endothelium-intact arterial rings, the selective blocker of large conductance Ca2+-activated K+ (BKCa) channels IbTX (200 nM) significantly reduced the vasorelaxant effect of anandamide and WIN55212-2 (P<0.01;. Figure 7a, b; n=6). Similarly, 4-AP (1 mM), a delayed rectifier K+ current blocker, slightly attenuated the vasorelaxant responses caused by anandamide and WIN55212-2 (Figure 7a, b; n=5). By contrast, pretreatment of arterial rings with the small conductance Ca2+-activated K+ (SKCa) channel blocker apamin (100 nM) and the selective blocker of ATP-sensitive (KATP) K+ channels glibenclamide (5 μM) failed to modify the relaxation induced by anandamide and WIN55212-2. Interestingly, in endothelium-denuded arteries, IbTX (200 nM) caused a substantial reduction of relaxation induced by anandamide and WIN55212-2 (Figure 8a, b; n=6); likely, the relaxant responses to cannabinoid agonists were also affected by the presence of 4-AP (1 mM), whereas apamin (100 nM) and glibenclamide (5 μM) did not induce any effects (Figure 8a, b; n=6).
Figure 7.
Effects of potassium channel blockers on vasorelaxation to (a) anandamide and (b) WIN55212-2 in bovine ophthalmic artery with intact endothelium. Data are given as means with error bars representing s.e.m. *P<0.05, **P<0.001 significance when compared with the control relaxation using one-way ANOVA followed by Bonferroni's post hoc test.
Figure 8.
Effects of potassium channel blockers on vasorelaxation to anandamide (a) and WIN55212-2 (b) in bovine ophthalmic artery with endothelium denuded. Data are given as means with error bars representing s.e.m. *P<0.05 significance when compared with control relaxation using one-way ANOVA followed by Bonferroni's post hoc test.
Role of CB1 receptor in K+ channel activation on relaxation to cannabinoid agonists
To further evaluate the role of CB1 receptor in K+ channel activation in endothelium-independent relaxation evoked by cannabinoid agonists, we have assessed the combination of IbTX (200 nM) with AM251 (100 nM), in endothelium-denuded arteries, which caused complete inhibition of anandamide relaxations (Figure 9; n=6). The same result was obtained in the presence of WIN55212-2 (data not shown).
Figure 9.
Effect of coadministration of IbTX (200 nM) and AM251 (100 nM) on vasorelaxation to anandamide in endothelium-denuded bovine ophthalmic artery precontracted with 5-HT. Data are given as means with error bars representing s.e.m.
Discussion
The major finding in this study is the presence of functional cannabinoid receptors in the bovine ophthalmic artery. Our results show that relaxation to cannabinoid agonists such as anandamide and WIN55212-2 involves activation of K+ channels via a nitric oxide/cyclic GMP-sensitive pathway, which is apparently dependent on the CB1 cannabinoid receptor.
Anandamide was able to affect concentration-dependent dilation of the ophthalmic arteries precontracted with 5-HT. This effect was greater in ophthalmic arterial rings with intact endothelium. In endothelium-denuded arteries, the ability of anandamide to provoke relaxations was greatly reduced; the maximal effect yielded was about 50%, indicating that endothelial integrity plays a significant role in mediating vasorelaxation. Surprisingly, the anandamide-induced vasorelaxant effect was mimicked by WIN55212-2 in arterial rings with both intact and denuded endothelium. These data are consistent with those of a previous study conducted in cat cerebral artery, which has demonstrated the involvement of CB1 cannabinoid receptors in relaxation to anandamide and that WIN55212-2 was able to mimic the relaxant effect (Gebremedhin et al., 1999).
To examine the role of cannabinoid receptors in relaxation to cannabinoid agonists, the effects of anandamide and WIN55212-2 were tested in the presence of cannabinoid receptor antagonists. At a low concentration, SR141716A (100 nM) caused a rightward displacement of the concentration–response curve of anandamide and WIN55212-2, both in endothelium-intact and -denuded arteries, showing that the antagonism was not exclusively through the endothelial receptor. In addition, an alternative CB1 receptor antagonist AM251 produced a small but significant shift to the right of the concentration–response curve to anandamide and WIN55212-2, both in the presence and absence of endothelium. The CB2 cannabinoid receptor antagonist SR144528 did not block the relaxation of ophthalmic arteries induced by anandamide and WIN55212-2, both in the presence and absence of endothelium. This suggests that CB2 cannabinoid receptors were not involved in anandamide- or WIN55212-2-induced relaxation.
The important role of CB1 cannabinoid receptor in cannabinoid agonist-induced relaxation has been supported by investigation with pertussis toxin, which blocked vasorelaxation to anandamide and WIN55212-2 in endothelium-intact and -denuded arteries, indicating that the coupling of cannabinoid receptor to Gi/Go proteins is essential to initiate the vasorelaxant responses. Sugiura et al. (1998) reported that CB1 receptor mRNAs are highly expressed in human aortic smooth muscle cells as well as endothelial cells; although the expression of CB1 receptor proteins in ocular vascular cells has not yet been investigated, one could hypothesize their presence.
The endothelium-dependent relaxation induced by cannabinoid agonists in bovine ophthalmic artery could be mediated via a cannabinoid agonist-evoked release of endothelium-derived relaxant factors such as nitric oxide. To test this hypothesis, in arteries with endothelium, the nitric oxide synthase inhibitor L-NMMA has been assessed inhibiting the vasorelaxation to anandamide and WIN55212-2; this supports the hypothesis that the cannabinoid agonist effects in bovine ophthalmic artery are mediated via release of nitric oxide from endothelium. These data are consistent with previous results obtained in a variety of vascular tissues (Deutsch et al., 1997; Bilfinger et al., 1998; Maccarrone et al., 2000; Mukhopadhyay et al., 2002). Moreover, the nitric oxide donor SNAP increased relaxation to anandamide, especially at a high concentration; by contrast, in the presence of SNAP, WIN55212-2 raised the maximal relaxant response but not significantly. This is likely after due to the different stereochemistry of WIN55212-2 compared with anandamide, which could activate a synergic process of slower nitric oxide-dependent transduction. It is known that after nitric oxide has been produced, it links to the haeme group of soluble guanylyl cyclase (Ignarro, 1990a) stimulating the production of cyclic GMP. For this reason, the generation of cyclic GMP is widely used as an index of nitric oxide biosynthesis (Ignarro, 1990b). It is intriguing that a guanylyl cyclase inhibitor ODQ almost completely eliminated of vasorelaxation to cannabinoid agonists in bovine ophthalmic artery. These findings clearly indicate that the increase in nitric oxide production/release and the activation of guanylyl cyclase to generate cyclic GMP are involved in CB1 receptor-mediated endothelium-dependent relaxation, emphasizing the role of PLC in the activation of nitric oxide synthase and in the accumulation of cGMP. Indeed, it is observed that cannabinoid CB1 agonists are able to evoke a rapid and transient increase in intracellular Ca2+ by a mechanism whereby a receptor-mediated release of Gi/o protein βγ subunits might activate PLC, leading to inositol-1,4,5-triphosphate release (Sugiura et al., 1998); this, in turn, triggers cascade reactions involving the stimulation of constitutive nitric oxide synthase activity (Maccarrone et al., 2000). It is interesting to note that pretreatment with L-NMMA (300 μM) on endothelium-denuded arteries did not have an impact on the relaxant effects of cannabinoid agonists, suggesting a limited role for nitric oxide. This lack of response is unlikely due to insufficient treatment as we have found the same protocol to be effective at completely inhibiting nitric oxide-dependent relaxation in the presence of endothelium.
In endothelium-intact arteries, the involvement of nitric oxide is known to activate K+ channels. Indeed, we have tested the highly selective blocker of BKCa channels, IbTX (200 nM), which caused potent inhibition of vasorelaxant responses to cannabinoid agonists. In addition, 4-AP (1 mM), glibenclamide (5 μM) and apamin (100 nM) did not cause any inhibition of the relaxation to cannabinoid agonists. This indicates that the activation of Ca2+-activated K+ channels is involved in relaxation mediated by cannabinoid receptors present at the endothelial level. Our results suggest that the endothelial component of cannabinoid agonist-induced relaxation involves a nitric oxide/cyclic GMP-sensitive and Ca2+-activated K+ channel pathway.
In addition to endothelial effects, the cannabinoid agonists can produce endothelium-independent relaxation by stimulating the vanilloid receptor (Zygmunt et al., 1999; White et al., 2001). Our observations have shown that pretreatment of endothelium-denuded arteries with capsaicin, which is an agonist of vanilloid receptors and provokes functional desensitization of capsaicin-sensitive sensory nerves, had no effect on relaxation induced by anandamide or WIN55212-2.
To establish the factors involved in cannabinoid agonist-evoked vasorelaxation in endothelium-denuded bovine ophthalmic arterial rings, we have examined the contribution of K+ channels. IbTX (200 nM) significantly reduced vasorelaxation to the cannabinoid agonists examined. This is in agreement with the work by Plane et al. (1997) who found that in isolated mesenteric arterial segments, the relaxation responses to anandamide were blocked by selective inhibitors of BKCa channels. However, in other vascular tissues, the role of BKCa channel activation in anandamide-induced relaxation is quite controversial (White & Hiley, 1997; Randall & Kendall, 1998). In addition, 4-AP (1 mM) attenuated the vasorelaxation, even if the inhibitor effect was lower when compared with that of IbTX. This might be due to the presence of a minor amount of delayed rectifier K+ current or to the possibility that it could not be the main mechanism underlying the vasorelaxation of cannabinoid agonists in bovine ophthalmic artery.
Apamin (100 nM), a SKCa channel blocker, and glibenclamide (5 μM), a KATP-channel blocker, failed to modify cannabinoid agonist-induced relaxation in bovine ophthalmic arteries, in agreement with various findings in other vascular beds (Randall et al., 1997; White & Hiley, 1997). The role of potassium channels in the vasorelaxant response modulation to cannabinoid agonists is conflictual within the literature. The explanation for these conflicts could be provided by tissue specificity or species specificity, as well as by the varied distribution of cannabinoid receptors in several vascular tissues.
To shed light on the relevance of CB1 receptors in K+ channel activation, the AM251/IbTX combination was tested in denuded arteries. This produced additional inhibitory effects on anandamide relaxation, suggesting that they utilize different pathways. Therefore, it is not speculative to hypothesize the presence of a heterogenous cannabinoid receptor population and/or multiple relaxation mechanisms.
In conclusion, these data demonstrate that the relaxant effects of anandamide and WIN55212-2 in bovine ophthalmic arteries involve CB1 cannabinoid receptors. In particular, endothelium-dependent relaxation involves the CB1 cannabinoid receptor-sensitive pathway that might involve activation of nitric oxide synthase, cyclic GMP and Ca2+-activated K+ channels. Additionally, we have shown that anandamide and WIN55212-2 also cause endothelium-independent relaxation by involving the activation of potassium channels. Exploring the role of cannabinoid agonists in ophthalmic artery is an important goal for understanding the pharmacology of ocular blood flow. It is not speculative to assert that ophthalmic artery vasorelaxation may increase the supply of oxygen to the retina, thus preventing ischaemic injury.
Acknowledgments
We thank Dr Vito Masciopinto, veterinarian of the slaughterhouse of the AUSL BA/5 (Bari, Italy), Dr Yole De Bellis for assistance in the preparation of this manuscript and Sanofi Recherche (Montpellier, France) for having provided SR141716A and SR144528. Support was granted by ‘Ministero dell'Università e della Ricerca Scientifica e Tecnologica (Cofinanziamento 2003).
Abbreviations
- AM251
N-(piperidin-1-yl)-5-(iodophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- 4-AP
4-aminopyridine
- BKCa
large conductance Ca2+-activated K+ channels
- CB1
cannabinoid 1 receptors
- IbTX
iberiotoxin
- L-NMMA
NG-monomethyl-L-arginine
- ODQ
1H-[1,2,4] oxadiazolo-[4,3-a] quinoxalin-1-one
- SKCa
small conductance Ca2+-activated K+ channel
- SNAP
S-nitroso-N-acetylpenicillamine
- SR141716A
N-(piperidin-1-yl)-5-(chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide
- SR144528
N-[(1S)-endo-1,3,3-trimethyl bicyclo [2.2.1] heptan-2-yl]-5-(4-chloro-3-methyl-phenyl)-1-(4-methylbenzyl)-pyrazole-3-carboxamide
References
- BILFINGER T.V., SALZET M., FIMIANI C., DEUTSCH A., TRAMU G., STEFANO G.B. Pharmacological evidence for anandamide amidase in human cardiac vascular tissues. Int. J. Cardiol. 1998;64:S15–S22. doi: 10.1016/s0167-5273(98)00031-x. [DOI] [PubMed] [Google Scholar]
- CHUNG H.S., HARRIS A., EVANS D.W., KAGEMANN L., GARZOZI H.J., MARTIN B. Vascular aspects in the pathophysiology of glaucomatous optic neuropathy. Surv. Ophthalmol. 1999;43 (Suppl 1):S43–S50. doi: 10.1016/s0039-6257(99)00050-8. [DOI] [PubMed] [Google Scholar]
- DEUTSCH D.G., GOLIGORSKY M.S., SCHMID P.C., KREBSBACH R.J., SCHMID H.H.O., DAS S.K., DEY S.K., ARREAZA G., THORUP C., STEFANO G., MOORE L.C. Production and physiological actions of anandamide in the vasculature of the rat kidney. J. Clin. Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERRERO R., RODRIGUEZ-PASCUAL F., MIRAS-PORTUGAL M.T., TORRES M. Comparative effects of several nitric oxide donors on intracellular cyclic GMP levels in bovine chromaffin cells: correlation with nitric oxide production. Br. J. Pharmacol. 1999;127:779–787. doi: 10.1038/sj.bjp.0702607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLAMMER J. The vascular concept of glaucoma. Surv. Ophthalmol. 1994;38:3–6. doi: 10.1016/0039-6257(94)90041-8. [DOI] [PubMed] [Google Scholar]
- FLAMMER J., ORGÜL S., COSTA V.P., ORZALESI N., KRIEGLSTEIN G.K., SERRA L.M., RENALD J.P., STEFÁNSSON E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002;21:359–393. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- FLEMING L., SCHERMER B., POPP R., BUSSE R. Inhibition of the production of endothelium-derived hyperpolarizing factor by cannabinoid receptor agonists. Br. J. Pharmacol. 1999;126:949–960. doi: 10.1038/sj.bjp.0702381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVEZ A., GIMENEZ-GALLENGO G., REUBEN J.P., ROY-CONTACIN L., FEIGENBAUM P., KACZOROWSKI J., GARCIA M.L. Purification and characterization of a unique, potent, peptidyl probe for the high conductance calcium-actived potassium channel from venom of the scorpion Buthus tamulus. J. Biol. Chem. 1990;265:11083–11090. [PubMed] [Google Scholar]
- GARTHWAITE J., SOUTHAM E., BOULTON C.L., NIELSEN E.B., SCHMIDT K., MAYER B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol. Pharmacol. 1995;48:184–188. [PubMed] [Google Scholar]
- GEBREMEDHIN D., LANGE A.R., CAMPBELL W.B., HILLARD C.J., HARDER D.R. Cannabinoid CB1 receptor of cat cerebral arterial muscle functions to inhibit L-type Ca2+ channel current. Am. J. Physiol. 1999;276:H2085–H2093. doi: 10.1152/ajpheart.1999.276.6.H2085. [DOI] [PubMed] [Google Scholar]
- GRAINGER J., BOACHIE-ANSAH G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br. J. Pharmacol. 2001;134:1003–1012. doi: 10.1038/sj.bjp.0704340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGNARRO L.J. Biosynthesis and metabolism of endothelium-derived nitric oxide. Ann. Rev. Pharmacol. Toxicol. 1990a;30:535–560. doi: 10.1146/annurev.pa.30.040190.002535. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J. Nitric oxide. A novel signal transduction mechanism for intracellular communication. Hypertension. 1990b;16:477–483. doi: 10.1161/01.hyp.16.5.477. [DOI] [PubMed] [Google Scholar]
- LAN R., LIU Q., FAN P., LIN S., FERNANDO S.R., MCCALLION D., PERTWEE R., MAKRIYANNIS A. Structure–activity relationships of pyrazole derivatives as cannabinoid receptor antagonists. J. Med. Chem. 1999;42:769–776. doi: 10.1021/jm980363y. [DOI] [PubMed] [Google Scholar]
- LIU J., GAO B., MIRSHAHI F., SANYAL A.J., KHANOLKAR A.D., MAKRIYANNIS A., KUNOS G. Functional CB1 receptors in human vascular endothelial cells. Biochem. J. 2000;346:835–840. [PMC free article] [PubMed] [Google Scholar]
- LOGRANO M.D., ROMANO M.R. Cannabinoid agonists induce contractile responses through Gi/o-dependent activation of phospholipase C in the bovine ciliary muscle. Eur. J. Pharmacol. 2004;494:55–62. doi: 10.1016/j.ejphar.2004.04.039. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., BARI M., LORENZON T., BISOGNO T., DI MARZO V., FINAZZI-AGRO A. Anadamide uptake by human endothelial cells and its regulation by nitric oxide. J. Biol. Chem. 2000;275:13484–13492. doi: 10.1074/jbc.275.18.13484. [DOI] [PubMed] [Google Scholar]
- MOEZI L., REZAYAT M., SAMINI M., SHAFAROODI H., MEHR S.E., EBRAHIMKHANI M.R., DEHPOUR A.R. Potentiation of anandamide effects in mesenteric beds isolated from bile duct-ligated rats: role of nitric oxide. Eur. J. Pharmacol. 2004;486:53–59. doi: 10.1016/j.ejphar.2003.12.004. [DOI] [PubMed] [Google Scholar]
- MUKHOPADHYAY S., CHAPNICK B.M., HOWLETT A.C. Anandamide-induced vasorelaxation in rabbit aortic rings has two component: G protein dependent and independent. Am. J. Physiol. 2002;282:H2046–H2054. doi: 10.1152/ajpheart.00497.2001. [DOI] [PubMed] [Google Scholar]
- O'SULLIVAN S.E., KENDALL D.A., RANDALL M.D. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistence and conduit rat mesenteric arteries. Br. J. Pharmacol. 2004;142:435–442. doi: 10.1038/sj.bjp.0705810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATE D.W., JÄRVINEN K., URTTI A., JARBO D., FICH M., MAHADEVAN V., JÄRVINEN T. Effects of topical anandamides on intraocular pressure in normotensive rabbits. Life Sci. 1996;21:1849–1860. doi: 10.1016/0024-3205(96)00169-5. [DOI] [PubMed] [Google Scholar]
- PLANE F., HILLARD C.J., WALDRON G.J., GARLAND C.J., BOYLE J.P. Evidence that anandamide and EDHF act via different mechanisms in rat isolated mesenteric arteries. Br. J. Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORCELLA A., CASELLAS P., GESSA G.L., PANI L. Cannabinoid receptor CB1 mRNA is highly expressed in the rat ciliary body: implications for the antiglaucoma properties of mahuana. Mol. Brain Res. 1998;58:240–245. doi: 10.1016/s0169-328x(98)00105-3. [DOI] [PubMed] [Google Scholar]
- PORCELLA A., MAXIA C., GESSA G.L., PANI L. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressare in human glaucoma resistent to conventional therapies. Eur. J. Neurosci. 2001;13:409–412. doi: 10.1046/j.0953-816x.2000.01401.x. [DOI] [PubMed] [Google Scholar]
- PORTER A.C., FELDER C.C. The endocannabinoid nervous system: unique opportunities for therapeutic intervention. Pharmacol. Ther. 2001;90:45–60. doi: 10.1016/s0163-7258(01)00130-9. [DOI] [PubMed] [Google Scholar]
- PRATT P.F., HILLARD C.J., EDGEMOND W.S., CAMPBELL W.B. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 receptor. Am. J. Physiol. 1998;43:H375–H381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., KENDALL D.A. Anandamide and endothelium-derived hyperpolarizing factor act via a common vasorelaxant mechanism in rat mesentery. Eur. J. Pharmacol. 1998;346:51–53. doi: 10.1016/s0014-2999(98)00003-x. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., HARRIS D., KENDALL D., RALEVIC V. Cardiovascular effects of cannabinoids. Pharm. Ther. 2002;95:191–202. doi: 10.1016/s0163-7258(02)00258-9. [DOI] [PubMed] [Google Scholar]
- RANDALL M.D., MCCULLOCH A.I., KENDALL D.A. Comparative pharmacology of endothelium-derived hyperpolarizing factor and anandamide in rat isolated mesentery. Eur. J. Pharmacol. 1997;333:191–197. doi: 10.1016/s0014-2999(97)01137-0. [DOI] [PubMed] [Google Scholar]
- REES D.D., PALMER R.M.J., SCHULZ R., HODSON H.F., MONCADA S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br. J. Pharmacol. 1990;101:746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINALDI-CARMONA M., PIALOT F., CONGY C., REDON E., BARTH F., BACHY A., BRELIERE J., SOUBRIE P., LE FUR G. Characterization and distribution of binding sites for [3 H]-SR 141716A, a selective brain (CB1) cannabinoid receptor antagonist, in rodent brain. Life Sci. 1996;58:1239–1247. doi: 10.1016/0024-3205(96)00085-9. [DOI] [PubMed] [Google Scholar]
- SONG Z.H., SLOWEY C.A. Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J. Pharmacol. Exp. Ther. 2000;292:136–139. [PubMed] [Google Scholar]
- SUGIURA T., KODAKA T., NAKANE S., KISHIMOTO S., KONDO S., WAKU K. Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator. Biochem. Biophys. Res. Commun. 1998;243:838–843. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- TOMIDA I., PERTWEE R.G., AZUARA-BLANCO A. Cannabinoids and glaucoma. Br. J. Ophthalmol. 2004;88:708–713. doi: 10.1136/bjo.2003.032250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HILEY C.R. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE R., HO W.-S.V., BOTTRILL F.E., FORD W.R., HILEY C.R. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br. J. Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SOGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]