Abstract
The liver modulates insulin sensitivity through a prandial-dependent mechanism that requires activation of the hepatic parasympathetic nerves, hepatic nitric oxide (NO) and hepatic glutathione (GSH). We tested the hypothesis that co-administration of GSH and NO to the liver enhances insulin sensitivity in a GSH and NO dose-dependent manner.
24 h fasted Wistar rats were used. Hepatic GSH was supplemented by administration of glutathione monoethylester (GSH-E; 0.1/0.25/0.5/1/2 mmol kg−1) and 3-morpholinosidnonimine (SIN-1; 5/10 mg kg−1) was used as a NO donor. The drugs were administered either systemically (i.v.) or intraportally (i.p.v.). Insulin sensitivity was assessed using a transient euglycemic clamp.
Neither GSH-E nor SIN-1 increased insulin sensitivity when administered alone, both i.v. and i.p.v. Moreover, changes in insulin sensitivity were not observed when GSH-E was administered i.v. followed by either i.v. or i.p.v. SIN-1 at any of the doses tested. However, i.p.v. administration of GSH-E followed by i.p.v. SIN-1 10 mg kg−1 significantly increased insulin sensitivity in a GSH-E dose-dependent manner: 26.1±9.4% after 0.1 mmol kg−1 GSH-E; 44.6±7.9% after 0.25 mmol kg−1 GSH-E; 59.4±15.1% after 0.5 mmol kg−1 GSH-E; 138.9±12.7% after 1 mmol kg−1 GSH-E and 117.3±29.2% after a dose of 2 mmol kg−1 (n=23, P<0.005).
Our results confirm that insulin sensitivity is enhanced in a dose-dependent manner by co-administration of NO and GSH donors to the liver.
Keywords: Insulin sensitivity, glutathione ester, nitric oxide, liver, rapid insulin sensitivity test
Introduction
In the past decade, it has become increasingly clear that the liver plays a major role in the regulation of insulin action. Under hepatic parasympathetic neural control the liver secretes a humoral factor that acts selectively at the skeletal muscle to enhance peripheral glucose disposal by insulin (Petersen et al., 1994; Moore et al., 2002; Lautt, 2004). Inadequate hepatic signaling leads to a decreased insulin action and peripheral insulin resistance (Moore et al., 2002).
Our knowledge of the pathway that modulates insulin sensitivity through a humoral factor secreted by the liver (Lautt, 2004) has come a long way. It is now known that the sequential signaling requires cholinergic muscarinic activation of nitric oxide synthase (NOS) in the liver (Sadri et al., 1999; Guarino et al., 2004), followed by subsequent activation of hepatic guanylyl ciclase (Correia et al., 2002; Guarino et al., 2004). In agreement, it was observed that peripheral insulin resistance induced by hepatic parasympathetic denervation is reversed by intraportal (i.p.v.) administration of either cholinergic agonists or nitric oxide (NO) donors (Xie et al., 1996a; Guarino et al., 2001), which act downstream from the blocked site, while insulin resistance induced by NOS antagonism is reversed by administration of NO donors but not cholinergic agonists to the liver (Guarino et al., 2004).
Physiologically, the insulin-sensitizing effect of the liver is strictly related to the prandial status (Lautt et al., 2001). The hypoglycemic effect of an insulin bolus is maximal after a meal and decreases in about 55% after a 24 h fast (Lautt et al., 2001). We have recently proposed that the fine regulation of insulin action by the prandial status is dependent on hepatic glutathione (GSH) content, which is known to be strongly related to the nutritional status (Tateishi et al., 1977; Guarino et al., 2003). This hypothesis was highlighted by the observation that hepatic GSH depletion produced by administration of the γ-glutamylcysteine synthetase inhibitor, L-buthionine-[S,R]-sulfoximine (BSO), produced insulin resistance that was only partially inhibited by hepatic NOS blockade (Guarino et al., 2003). Therefore, GSH depletion and NOS blockade affect the same pathway at different steps, inhibiting the insulin-sensitizing signal in the liver. Moreover, exogenous NO was not able to restore insulin action in BSO-treated rats, which suggests that both GSH and NO are required in the liver to allow full peripheral insulin action (Guarino et al., 2003).
In the present study, we tested the hypothesis that physiological insulin resistance induced by fasting is reversed by co-administration of GSH and NO to the liver. Hepatic GSH was supplemented by administration of a GSH donor, glutathione monoethylester (GSH-E), which was previously shown to be effectively transported into hepatocytes and converted into GSH (Anderson et al., 1989). 3-Morpholinosidnonimine (SIN-1) was employed as the source of exogenous NO.
In this report, we describe for the first time that supply of GSH and NO to the liver of fasted rats enhances insulin sensitivity by restoring the hepatic insulin-sensitizing pathway.
Methods
Presurgical protocols
Male Wistar rats (8–9 weeks, Charles River, Spain) were housed one per cage and maintained in a temperature-controlled room, on a 12 h light/dark cycle. Rats had ad libitum access to standard rat chow (Panlab A04, Charles River, Spain) and tap water. The animals were fasted for a period of 24 h and experiments started between 9:00 and 10:00 a.m. Rats were anesthetized with an intraperitoneal injection of sodium pentobarbital (65 mg kg−1) and anesthesia was maintained throughout the experiment by continuous infusion into the internal jugular vein (1.0 mgml−1, 1.0 ml 100 g body wt−1 h−1). The temperature was maintained at 37.0±0.5°C using a heating pad (Homeothermic Blanket Control Unit 50–7061, Harvard Apparatus, U.S.A.) and monitored with a rectal probe thermometer.
All the animals were treated according to the European Union Directive for Protection of Vertebrates Used for Experimental and other Scientific Ends (86/609/CEE) and the US National Research Council Guide for the Care and Use of Laboratory Animals.
Surgical preparation
The trachea was cannulated (polyethylene tubing, PE 240, Becton Dickinson, U.S.A.) to allow spontaneous respiration. A carotid artery-jugular vein arteriovenous shunt was set up as previously described (Lautt et al., 1998). Also, the portal vein was cannulated with a 24 g intravenous (i.v.) catheter (Optiva; Johnson & Johnson Medical, Italy) after laparotomy. At the end of the surgical procedure, the animals were heparinized with 100 IU kg−1 heparin.
Rats were allowed to stabilize from the surgical intervention for 50 min before any procedures were carried out. Mean arterial blood pressure (MAP) was monitored by briefly clamping the venous outlet of the shunt and the patency of flow was examined by recording pressure from the nonoccluded loop (Powerlab 8/s, AD Instruments; Chart/MacLab Software, U.S.A.). After stabilization arterial blood samples (25 μl) were collected every 5 min, and glucose concentration was immediately determined using a glucose analyser (1500 YSI Sport, Yellow Springs Instruments, U.S.A.) until three successive stable glucose concentrations were obtained. The mean of these three values is referred to as the basal glucose level. Drugs were administered i.v. by puncturing the shunt on the venous side (infusion line PE50, Becton Dickinson with a cut 23 g needle at the delivery end).
Rapid insulin sensitivity test
The methodology chosen to evaluate insulin sensitivity was the rapid insulin sensitivity test (RIST), since this transient euglycemic clamp can be carried out four consecutive times in the same animal with high reproducibility (Lautt et al., 1998). The RIST has been shown to be effective both in anesthetized and conscious animals, providing similar results independent of pentobarbital anesthesia (Latour et al., 2002).
The RIST starts with the administration of an insulin bolus (50 mU kg−1, i.v.), over 5 min, by means of an infusion pump (Perfusor fm, B-Braun). At 1 min after initiating the insulin infusion, arterial blood glucose was measured and glucose infusion (D-Glucose/saline, 100 mg ml−1, i.v.) was started at a rate of 5 mg kg−1 min−1. According to arterial glucose concentrations measured at 2 min intervals, the infusion rate of the glucose pump was readjusted to maintain euglycemia. When no further glucose infusion was required, usually within 35 min, the test was concluded. The amount of glucose necessary to maintain euglycemia along the test quantifies insulin sensitivity and is referred to as the RIST index (mg glucose kg−1) (Lautt et al., 1998).
Experimental protocols
1. Effect of i.p.v. administration of GSH-E on insulin sensitivity
The RIST index was determined in 24 h fasted rats. Afterward a dose of 0.5 or 1 mmol kg−1 GSH-E was administered i.p.v. as a 10 min bolus. These doses were chosen since they have been previously shown to enhance hepatic GSH levels (Grattagliano et al., 1995). After a 60 min period of stabilization, blood samples were collected to quantify arterial glucose levels. When a stable glucose baseline was reached, based on three successive blood samples taken 5 min apart, a new RIST was performed to evaluate the effect of GSH-E on insulin sensitivity.
2. Effect of i.p.v. administration of SIN-1 on insulin sensitivity
A fasted RIST was performed and afterward SIN-1 5 mg kg−1 or 10 mg kg−1 was infused i.p.v., as a 10 min bolus. The doses of SIN-1 were selected based on its ability to restore insulin sensitivity after hepatic NOS blockade or after muscarinic blockade (Guarino et al., 2004). 60 min after SIN-1 administration, the time required to achieve maximal effect after i.p.v. infusion (Guarino et al., 2003; 2004), blood samples were collected every 5 min to quantify arterial glucose levels. When a stable glycemia was reached a new RIST was performed.
3. Effect of combined administration of GSH-E and SIN-1 on insulin sensitivity
(a) Influence of the dosage and route of administration of SIN-1 on insulin sensitivity, when co-administered with i.p.v. GSH-E 1 mmol kg−1 In the first set of experiments, a RIST was performed in 24 h fasted animals followed by i.p.v. administration of GSH-E 1 mmol kg−1. After a 60 min period of stabilization, SIN-1 was administered i.p.v., either at a dose of 5 or 10 mg kg−1. A second RIST was carried out 90 min later.
In the second set of experiments, the protocol was very similar except that SIN-1 was administered i.v., either at a dose of 5 or 10 mg kg−1, after i.p.v. 1 mmol kg −1 GSH-E.
(b) Influence of the dosage and route of administration of GSH-E on insulin sensitivity, when co-administered with i.p.v. SIN-1 10 mg kg−1 In the first group of animals, a 24 h fasted RIST was performed followed by i.p.v. administration of GSH-E at different doses: 0.1, 0.25, 0.5, 1 and 2 mmol kg−1. After a 60 min period of stabilization SIN-1 was administered i.p.v. at a dose of 10 mg kg−1 and, 90 min after, a new RIST was executed.
In a second group, a 24 h fasted RIST was followed by i.v. administration of GSH-E at the same doses used before. After a 60 min period of stabilization, SIN-1 was provided at a dose of 10 mg kg−1, i.p.v. and, 90 min after, a new RIST was performed.
4. Hepatic GSH determination
At the end of the experiments, the liver was rapidly dissected out and immediately frozen on liquid nitrogen for storage at –80°C until further analysis. Liver GSH was determined using a modified peroxidase–reductase assay following a method described by Marinho et al. (1997). Briefly, the livers were powdered in liquid nitrogen and homogenized in HPO3 10% (wv−1). The suspension was centrifuged at 30,000 × g for 20 min and the supernatant was collected and neutralized. GSH peroxidase (15 U g of fresh liver−1) and 5 μl of H2O2 5 mM were added to the neutralized supernatant and the mixture was incubated for 30 min at 30°C. Reaction was stopped with 250 μl of HPO3 10% (wv−1), 0°C and GSSG formed was determined in the supernatant using GSSG reductase (47 U g of fresh liver−1) and NADPH. The livers of fed and 24 h fasted animals were used as controls.
Drugs
GSH-E was purchased from Bachem, Switzerland. SIN-1, D-Glucose, GSH peroxidase, GSSG reductase, HPO3 and H2O2 were purchased from Sigma-Aldrich Chemical Co., Portugal. Human insulin (Humulin, Regular) was obtained from Lilly, Portugal. Pentobarbital (Eutasil) was obtained from Sanofi, Portugal. Heparin was purchased from B-Braun, Portugal. All chemicals were dissolved in saline.
Data analysis
The RIST data were analyzed using two-tailed paired Student's t-tests in the first three protocols and a hyperbola nonlinear fit in protocol (3b). Glutathione quantification data were compared using Student's t-tests or one-way ANOVA followed by a Tukey-Kramer multiple-comparison test as applicable. The data are expressed as mean±s.e.m. throughout. Differences were accepted as statistically significant at P<0.05. Whenever P-value is not indicated, differences are not statistically significant.
Results
Effect of i.p.v. administration of GSH-E on insulin sensitivity
None of the GSH-E doses used altered the MAP, which remained constant throughout the RISTs. The RIST index was unchanged by i.p.v. administration of GSH-E, either at a dose of 0.5 mmol kg−1 (n=5) or 1 mmol kg−1 (n=8) (Table 1). Intraportal GSH-E 0.5 mmol kg−1 did not significantly alter hepatic GSH (5.08±0.15 μmol g fresh liver−1) when compared to the control fasted animals (5.20±0.16 μmol g fresh liver−1). Intraportal administration of GSH-E 1 mmol kg−1 raised hepatic GSH levels to 7.24±0.39 μmol g fresh liver−1, which was not significantly different from control postprandial values: 7.10±0.29 μmol g fresh liver−1 (Table 1).
Table 1.
Effect of i.p.v. GSH-E on insulin sensitivity and hepatic GSH content in 24 h fasted rats
| RIST (mg glucose kg−1) | Hepatic GSH (μmol g fresh liver−1) | |||
|---|---|---|---|---|
| 24 h FAST | GSH-E IPV | |||
| GSH-E IPV | 0.5 (n=5) | 95.2±16.4 | 96.9±12.4 | 5.08±0.15 |
| GSH-E IPV | 1.0 (n=8) | 83.1±7.5 | 68.1±5.7 | 7.24±0.39*** |
P<0.001 vs GSH-E 0.5 IPV.
Effect of i.p.v. administration of SIN-1 on insulin sensitivity
The MAP decreased similarly after SIN-1 5 mg kg−1 (from 105.0±15.0 to 65.0±5.0 mmHg) and SIN-1 10 mg kg−1 (from 114.9±8.0 to 68.6±7.4 mmHg). Despite the initial drop induced by the drug, MAP remained constant throughout the RISTs.
Intraportal SIN-1 did not significantly change insulin sensitivity either at a dose of 5 mg kg−1 (n=5) or at a dose of 10 mg kg−1 (n=5) (Table 2). There was no change in hepatic GSH after administration of i.p.v. SIN-1 compared to control 24 h fasted animals (Table 2).
Table 2.
Effect of i.p.v. SIN-1 on insulin sensitivity and hepatic GSH content in 24 h fasted rats
| RIST (mg glucose kg−1) | Hepatic GSH (μmol g fresh liver−1) | |||
|---|---|---|---|---|
| 24 h FAST | SIN-1 IPV | |||
| SIN-1 IPV 5.0 (n=5) | 98.4±10.6 | 89.4±5.2 | 5.09±0.16 | |
| SIN-1 IPV 10.0 (n=5) | 93.5±10.4 | 88.7±6.9 | 5.24±0.08 | |
Effect of combined administration of GSH-E and SIN-1 on insulin sensitivity
(a) Influence of the dosage and route of administration of SIN-1 on insulin sensitivity, when co-administered with i.p.v. GSH-E 1 mmol kg−1
We tested the insulin-sensitizing effect of administration of GSH-E followed by two different doses of i.p.v. SIN-1. The dose of GSH-E used was 1 mmol kg−1, which we observed to be the dose required to replenish hepatic GSH to postprandial values (Table 1).
The MAP decreased after i.p.v. SIN-1 5 mg kg−1 from 120.0±4.7 to 61.5±4.3 mmHg (P<0.001). This was not significantly different from the drop in the MAP after administration of i.p.v. SIN-1 10 mg kg−1 (59.8±5.9 mmHg). Intravenous SIN-1 caused a decrease in the MAP of the same magnitude as i.p.v. SIN-1 (from 129.0±3.0 to 65.9±4.6 mmHg, after the dose of 5 mg kg−1, and to 63.1±3.8 mmHg after the dose of 10 mg kg−1).
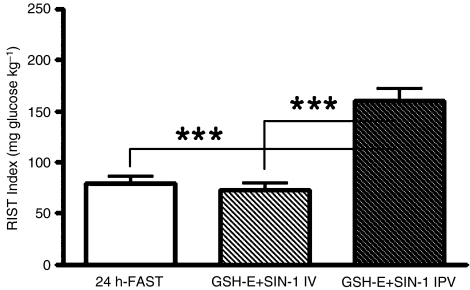
Combined administration of i.p.v. GSH-E 1 mmol kg−1 and i.p.v. SIN-1 5 mg kg−1 did not significantly increase insulin sensitivity (n=6), while combined administration of i.p.v. GSH-E 1 mmol kg−1 and i.p.v. SIN-1 10 mg kg−1 significantly improved insulin sensitivity (n=5, P<0.001) (Table 3 and Figure 1).
Table 3.
Effect of combined administration of i.p.v. GSH-E 1 mmol kg−1 and SIN-1 on insulin sensitivity
| RIST 24 h FAST | RIST GSH-E IPV 1 mmol kg−1+SIN-1 IPV | ||
|---|---|---|---|
| SIN-1 IPV 5.0 (n=6) | 73.3±8.9 | 90.1±7.6 | |
| SIN-1 IPV 10.0 (n=5) | 72.5±6.9 | 159.9±11.4*** | |
| SIN-1 IV 5.0 (n=3) | 55.4±14.4 | 71.3±11.0 | |
| SIN-1 IV 10.0 (n=5) | 92.4±14.4 | 73.4±6.2 |
P<0.001 vs RIST 24 h fast.
Figure 1.
RIST index after a 24 h-fast, followed by a RIST after co-administration of i.p.v. GSH-E 1 mmol kg−1 and i.v. (n=5) or i.p.v. (n=5) SIN-1 10 mg kg−1. I.v. SIN-1 after i.p.v. GSH-E did not change insulin sensitivity while i.p.v. SIN-1 after i.p.v. GSH-E significantly increased insulin sensitivity. Values are means±s.e.m. ***P<0.001.
Intravenous SIN-1 following i.p.v. GSH-E did not increase insulin sensitivity either at a dose of 5 mg kg−1 (n=3) or 10 mg kg−1 (n=5) (Table 3 and Figure 1).
There was an increase in hepatic GSH levels to postprandial values in all groups of animals tested (data not shown).
(b) Influence of the dosage and route of administration of GSH-E on insulin sensitivity, when co-administered with i.p.v. SIN-1 10 mg kg−1
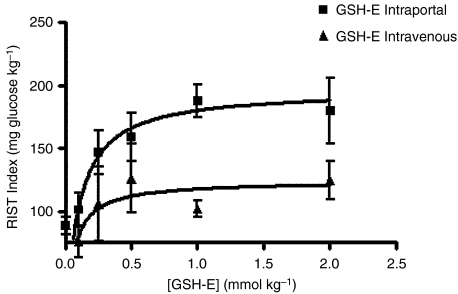
In the first group of 24 h fasted rats, different doses of GSH-E were administered in the portal vein followed by i.p.v. SIN-1 10 mg kg−1. Insulin sensitivity increased after administration of i.p.v. GSH-E followed by i.p.v. SIN-1. This increase was dependent of the dose of GSH-E administered: from 82.4±6.6 to 101.1±13.4 mg glucose kg−1 for a GSH-E dose of 0.1 mmol kg−1, corresponding to an increase of 26.1±9.4% (n=4); from 89.1±18.5 to 146.8±17.2 mg glucose kg−1 for a dose of 0.25 mmol kg−1, corresponding to an increase of 44.6±7.9% (n=4); from 95.2±16.4 to 158.8±19.2 mg glucose kg−1 for a dose of 0.5 mmol kg−1, corresponding to an increase of 59.4±15.1% (n=5); from 83.1±7.5 to 187.3±13.0 mg glucose kg−1 for a dose of 1 mmol kg−1, corresponding to an increase of 138.9±12.7% (n=8) and from 76.4±15.6 to 179.9±26.0 mg glucose kg−1 for a dose of 2 mmol kg−1, corresponding to an increase of 117.3±29.2% (n=4), P<0.005.
In the second group of animals, GSH-E was administered i.v. followed by i.p.v. SIN-1 10 mg kg−1. No changes in insulin sensitivity were observed, even at the highest dose of GSH-E tested (2 mmol kg−1): from 74.9±3.0 to 75.6±12.8 mg glucose kg−1 for a GSH-E dose of 0.1 mmol kg−1 (n=3); from 86.8±14.9 to 105.7±29.1 mg glucose kg−1 for a dose of 0.25 mmol kg−1 (n=3); from 94.9±9.3 to 99.2±11.8 mg glucose kg−1 for a dose of 0.5 mmol kg−1 (n=3); from 93.8±3.3 to 105.6±9.0 mg glucose kg−1 for a dose of 1 mmol kg−1 (n=3) and from 105.4±6.6 to 124.8±15.1 mg glucose kg−1 for a dose of 2 mmol kg−1 (n=3). Figure 2 represents the GSH-E dose-RIST index after SIN-1 response curve, both for i.p.v. and i.v. administration of GSH-E.
Figure 2.
RIST after SIN-1 as a function of the dose of GSH-E administered intravenously, n=12 or intraportally, n=23. Insulin sensitivity is dependent on the dose of GSH-E. GSH-E is more potent and more efficient when administered intraportally than systemically.
The MAP decreased after SIN-1, despite the dose of GSH-E administered and the route of administration of the drug. We observed that the MAP was not significantly different in all groups of animals tested (data not shown).
As expected, there was an increase in hepatic GSH values dependent on the dose of i.p.v. GSH-E administered (Table 4).
Table 4.
Effect of GSH-E and SIN-1 i.p.v. administration in the hepatic GSH content
| GSH-E IPV (mmol kg−1) | |||||
|---|---|---|---|---|---|
| 0.1 (n=4) | 0.25 (n=4) | 0.5 (n=5) | 1.0 (n=8) | 2.0 (n=4) | |
| Hepatic GSH (μmol g fresh liver−1) | 3.80±1.11** | 4.20±0.91* | 4.30±0.42* | 7.20±0.40 | 6.13±0.36 |
P<0.05,
P<0.01 compared to control postprandial values (7.10±0.29 μmol g fresh liver−1).
Discussion
The hypoglycaemic effect of insulin is enhanced by food intake, increasing approximately 55% from the fasted to the fed state (Lautt et al., 2001; Lautt, 2004). Previous studies suggest that hepatic GSH and hepatic NO play a crucial role in the insulin-sensitizing effect induced by a meal (Guarino et al., 2003). This hypothesis is supported by the observation that both GSH and NO synthesis are decreased in the fasted state (Tateishi et al., 1977; Grongnet et al., 2003). Moreover, blockade of GSH synthesis (Guarino et al., 2003) or of hepatic NOS (Sadri et al., 1999; Guarino et al., 2004) in fed rats mimics the insulin resistance observed after a 24 h fast.
We now report that co-administration of GSH and NO to the liver of fasted animals restores the insulin-sensitizing effect induced by feeding. Furthermore, the enhancement of insulin sensitivity is dependent on the dose of GSH and NO administered to the liver.
The RIST methodology
The majority of the studies that focus on insulin resistance use classical methodological approaches to evaluate insulin sensitivity like the hyperinsulinemic-euglycemic clamp (HIEC) or the insulin tolerance test. Although the HIEC is the ‘gold-standard' technique used in scientific research, it is nonphysiological since high insulin levels are not usually sustained for long periods after a meal (Clark et al., 2003). We chose to use the RIST as it avoids the vagal withdrawal and sympathetic activation induced by sustained hyperinsulinemia observed during the HIEC (Van De Borne et al., 1999). Also, the RIST is reproducible for four consecutive times in the same anesthetized animal allowing paired experimental design (Lautt et al., 1998), provides results that are not altered by pentobarbital anaesthesia (Latour et al., 2002) and avoids the interference of counter-regulatory hormones (Xie et al., 1996b). In order to evaluate the site of action of the pharmacological manipulations, we performed RISTs after both i.v. and i.p.v. perfusion of GSH-E and SIN-1, which allowed us to discriminate between hepatic and systemic effects of the drugs. However, we were not able to determinate the tissues that experienced changes in insulin sensitivity after the pharmacological treatment, since the RIST evaluates whole-body glucose disposal by insulin. The basal fasted RISTs showed some discrepancy, which may be explained by interindividual variability in the animals response to insulin.
NO is not enough
The effect of NO on insulin sensitivity has been thoroughly studied by several groups (Baron, 1996; Scherrer et al., 2000; Steinberg et al., 2000; Guarino et al., 2004; Lautt, 2004; Mather et al., 2004). A current working hypothesis is that NO enhances insulin sensitivity due to its vasodilatory properties, increasing the delivery of insulin and glucose to insulin-target tissues (Clark et al., 2003). Our results counteract this hypothesis, indicating that the effect of NO on peripheral insulin sensitivity is not simply hemodynamic. We observed that despite its notorious vasodilator effects, administration of SIN-1 into the portal vein required the presence of elevated hepatic GSH levels in order to improve insulin sensitivity. Moreover, insulin sensitivity increased only when the SIN-1 was administered in the portal vein together with a GSH donor although i.v. SIN-1 decreased MAP to the extent that i.p.v. SIN-1 did. This indicates that the site of action for SIN-1 is the liver and not the vasculature (Sadri et al., 1998). Our results further indicate that the effect of hepatic NO on insulin sensitivity was dose-dependent given that a significant increase in insulin sensitivity was observed only at the highest dose of i.p.v. SIN-1 tested (10 mg kg−1).
Whereas evidence favors an hepatic NO-dependent mechanism that controls insulin action, there is still some controversy regarding the source of NO. Porszasz et al. proposed that NO is of sensory neural origin (Porszasz et al., 2002), based on the observation that sensory denervation of the anterior hepatic plexus leads to insulin resistance of the same magnitude as observed by ours and Lautt's group after hepatic NOS antagonism (Guarino et al., 2003; Sadri et al., 1998). However, Porszasz et al. used Wistar rats fasted for 24 h, which corresponds to a state of full blockade of the hepatic insulin-sensitizing mechanism. Thus, the deleterious effect that selective sensory denervation of the anterior hepatic plexus has on insulin sensitivity is most likely independent of the postprandially activated pathway that we are studying. Both ours and Lautt's group have shown that this pathway is triggered by activation of hepatic parasympathetic nerves that act through muscarinic cholinergic receptors leading to NO production (Xie et al., 1995; Sadri et al., 1998; Guarino et al., 2004).
The role of GSH in insulin sensitivity
Reports from other investigators suggest that administration of GSH to insulin resistant individuals decreases oxidative stress, leading to enhanced insulin sensitivity (Paolisso et al., 1992a, 1992b; De Mattia et al., 1998). Increased oxidative stress is known to play a role in the pathogenesis of insulin resistance (Ceriello et al., 2004; Da Ros et al., 2004). Several mechanism of action have been proposed to explain the deterioration of insulin signaling by oxidative stress like an inhibitory effect on tyrosine kinase activity of the insulin receptor (Hansen et al., 1999) or alterations in the expression and translocation capacity of the glucose transporter GLUT-4 (Khamaisi et al., 2000; Tirosh et al., 2000) among others. Supporting the idea of increased oxidative stress in diabetes, decreased GSH levels were found in blood and tissues of diabetic rats (Khamaisi et al., 2000; Seven et al., 2004) and humans (De Mattia et al., 1998). Paolisso et al. (1992a, 1992b) and De Mattia et al. (1998) observed that GSH administration increases insulin sensitivity in diabetic patients due to its antioxidant properties since, in these individuals, GSH infusion scavenges free radicals ameliorating insulin action at the receptor level and partially restoring insulin sensitivity. In contrast, control healthy subjects appear to benefit less from GSH administration (Paolisso et al., 1992a; De Mattia et al., 1998), which indicates that GSH administration improves insulin resistance significantly only when oxidative stress is enhanced. This is in agreement with our data in healthy Wistar rats, where administration of GSH-E per se did not increase insulin sensitivity. These animals had neither alteration at the insulin receptor level nor increased oxidative stress, which may have rendered the anti-oxidant effects of GSH on insulin action minimal.
While GSH by itself did not increase insulin sensitivity, co-administration of a GSH donor with NO to the liver of fasted rats enhanced insulin action. The increment in insulin sensitivity reached a maximum after i.p.v. administration of GSH-E 1 mmol kg−1 followed by i.p.v. SIN-1 10 mg kg−1. This shows that only when hepatic GSH reaches postprandial values will administration of NO to the liver enhance insulin sensitivity, since 1 mmol kg−1 GSH-E was the lowest dose required to raise hepatic GSH to fed levels (Table 1). The improvement of insulin sensitivity is also dependent on the dose of NO, since SIN-1 10 mg kg−1 after GSH-E enhanced insulin action while SIN-1 5 mg kg−1 did not.
It has been described by other investigators that feeding increases both hepatic GSH (Tateishi et al., 1977) and NO synthesis (Sadri et al., 1999; Grongnet et al., 2003). The increase in both NO and GSH that occurs after a meal may be the feeding signal that triggers the hepatic insulin sensitising pathway. According to this hypothesis, fasting, increased oxidative stress, decreased activity of NO synthase or any other process that leads to depletion of GSH and/or NO will result in insulin resistance (Khamaisi et al., 2000; Latour et al., 2002; Guarino et al., 2003; 2004; Ceriello et al., 2004; Lautt, 2004). Our results show that restoring GSH and NO levels to postprandial values brings insulin sensitivity back to normal levels.
The importance of the liver
For the first time we have demonstrated that, in fasted animals, insulin sensitivity is enhanced after co-administration of i.p.v., but not systemic, NO and GSH donors to the liver, as long as postprandial hepatic GSH levels are reached. Our results support the previously suggested hypothesis that the liver plays a central role in the control of insulin sensitivity (Takayama et al., 2000; Petersen et al., 1994). Owing to technical limitations of the RIST, we did not identify the target in which insulin sensitivity was improved. Despite this, because our experiments were conducted in 24 h fasted animals and in the presence of euglycemia, when the role of the liver in glucose disposal is minimal (Moore et al., 2003), the hypothesis that the drugs are acting directly in the liver to reduce hepatic glucose output seems unlikely.
We propose that hepatic GSH and NO mimic the feeding signal that has been described by Lautt as the trigger for the synthesis of the hepatic insulin-sensitizing substance (HISS) (Lautt, 2003; 2004). Lack of HISS release by the liver causes insulin resistance in the skeletal muscle (Lautt et al., 2001; 2004). Defects in insulin action due to impairment in the HISS pathway are detected only in the postprandial period, long before any alteration can be perceived in the fasted state. This may correspond to the early stages of insulin resistance and highlights the importance of evaluating postprandial glycemia: instead of just the fasting glycemia: in the early diagnosis of insulin resistance. We propose that decreased hepatic NO and/or GSH levels are involved in the etiology of postprandial insulin resistance through impaired HISS secretion by the liver. Additional studies are required to evaluate the insulin-sensitising effect of GSH/NO administration to pathological animal models that show HISS-dependent insulin resistance, like the obese Zucker rat, the spontaneously hypertensive rat, sucrose fed rat, liver disease induced by chronic bile-duct ligation and offspring of fetal alcohol exposure and aging (Lautt, 2004).
The enhancement of insulin action by administration of a GSH donor together with a NO donor to the liver brings about a new perspective on alternative therapeutic approaches to early-stage insulin resistance.
Acknowledgments
We acknowledge Professor Dr M. Graça Morais and the Department of Biochemistry/FCM-UNL for the valuable help with the glutathione quantification technique. This study was supported by Fundação para a Ciência e Tecnologia grant POCTI/SAU/14009/1998 and by the Portuguese Diabetes Association (APDP). MPG was supported by a Fundação para a Ciência e Tecnologia fellowship BD/4916/2001.
Abbreviations
- GSH
glutathione
- GSH-E
glutathione monoethylester
- i.p.v.
intraportal
- MAP
mean arterial pressure
- NO
nitric oxide
- NOS
nitric oxide synthase
- RIST
rapid insulin sensitivity test
- SIN-1
3-morpholinosidnonimine
References
- ANDERSON M.E., MEISTER A. Glutathione monoesters. Anal. Biochem. 1989;183:16–20. doi: 10.1016/0003-2697(89)90164-4. [DOI] [PubMed] [Google Scholar]
- BARON A.D. The coupling of glucose metabolism and perfusion in human skeletal muscle. The potential role of endothelium-derived nitric oxide. Diabetes. 1996;45 (Suppl 1):S105–S109. doi: 10.2337/diab.45.1.s105. [DOI] [PubMed] [Google Scholar]
- CERIELLO A., MOTZ E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 2004;24:816–823. doi: 10.1161/01.ATV.0000122852.22604.78. [DOI] [PubMed] [Google Scholar]
- CLARK M.G., WALLIS M.G., BARRETT E.J., VINCENT M.A., RICHARDS S.M., CLERK L.H., RATTIGAN S. Blood flow and muscle metabolism: a focus on insulin action. Am. J. Physiol. Endocrinol. Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- CORREIA N.C., GUARINO M.P., RAPOSO J., MACEDO M.P. Hepatic guanylyl cyclase inhibition induces HISS-dependent insulin resistance. Proc. West Pharmacol. Soc. 2002;45:57–58. [PubMed] [Google Scholar]
- DA ROS R., ASSALONI R., CERIELLO A. Antioxidant therapy in diabetic complications: what is new. Curr. Vasc. Pharmacol. 2004;2:335–341. doi: 10.2174/1570161043385538. [DOI] [PubMed] [Google Scholar]
- DE MATTIA G., BRAVI M.C., LAURENTI O., CASSONE-FALDETTA M., ARMIENTO A., FERRI C., BALSANO F. Influence of reduced glutathione infusion on glucose metabolism in patients with non-insulin-dependent diabetes mellitus. Metabolism. 1998;47:993–997. doi: 10.1016/s0026-0495(98)90357-2. [DOI] [PubMed] [Google Scholar]
- GRATTAGLIANO I., WIELAND P., SCHRANZ C., LAUTERBURG B.H. Disposition of glutathione monoethyl ester in the rat: glutathione ester is a slow release form of extracellular glutathione. J. Pharmacol. Exp. Ther. 1995;272:484–488. [PubMed] [Google Scholar]
- GRONGNET J.F., DAVID J.C. Reciprocal variations of nNOS and HSP90 are associated with fasting in gastrointestinal tract of the piglet. Dig. Dis. Sci. 2003;48:365–372. doi: 10.1023/a:1021948031333. [DOI] [PubMed] [Google Scholar]
- GUARINO M.P., AFONSO R.A., RAIMUNDO N., RAPOSO J.F., MACEDO M.P. Hepatic glutathione and nitric oxide are critical for hepatic insulin-sensitizing substance action. Am. J. Physiol. Gastrointest. Liver Physiol. 2003;284:G588–G594. doi: 10.1152/ajpgi.00423.2002. [DOI] [PubMed] [Google Scholar]
- GUARINO M.P., CORREIA N.C., LAUTT W.W., MACEDO M.P. Insulin sensitivity is mediated by the activation of the ACh/NO/cGMP pathway in rat liver. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G527–G532. doi: 10.1152/ajpgi.00085.2004. [DOI] [PubMed] [Google Scholar]
- GUARINO M.P., CORREIA N.C., RAPOSO J., MACEDO M.P. Nitric oxide synthase inhibition decreases output of hepatic insulin sensitizing substance (HISS), which is reversed by SIN-1 but not by nitroprusside. Proc. West Pharmacol. Soc. 2001;44:25–26. [PubMed] [Google Scholar]
- HANSEN L.L., IKEDA Y., OLSEN G.S., BUSCH A.K., MOSTHAF L. Insulin signaling is inhibited by micromolar concentrations of H(2)O(2). Evidence for a role of H(2)O(2) in tumor necrosis factor alpha-mediated insulin resistance. J. Biol. Chem. 1999;274:25078–25084. doi: 10.1074/jbc.274.35.25078. [DOI] [PubMed] [Google Scholar]
- KHAMAISI M., KAVEL O., ROSENSTOCK M., PORAT M., YULI M., KAISER N., RUDICH A. Effect of inhibition of glutathione synthesis on insulin action: in vivo and in vitro studies using buthionine sulfoximine. Biochem. J. 2000;349:579–586. doi: 10.1042/0264-6021:3490579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LATOUR M.G., LAUTT W.W. Insulin sensitivity regulated by feeding in the conscious unrestrained rat. Can. J. Physiol. Pharmacol. 2002;80:8–12. doi: 10.1139/y01-094. [DOI] [PubMed] [Google Scholar]
- LAUTT W.W. Practice and principles of pharmacodynamic determination of HISS-dependent and HISS-independent insulin action: methods to quantitate mechanisms of insulin resistance. Med. Res. Rev. 2003;23:1–14. doi: 10.1002/med.10022. [DOI] [PubMed] [Google Scholar]
- LAUTT W.W. A new paradigm for diabetes and obesity: the hepatic insulin sensitizing substance (HISS) hypothesis. J. Pharmacol. Sci. 2004;95:9–17. doi: 10.1254/jphs.95.9. [DOI] [PubMed] [Google Scholar]
- LAUTT W.W., MACEDO M.P., SADRI P., TAKAYAMA S., DUARTE RAMOS F., LEGARE D.J. Hepatic parasympathetic (HISS) control of insulin sensitivity determined by feeding and fasting. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;281:G29–G36. doi: 10.1152/ajpgi.2001.281.1.G29. [DOI] [PubMed] [Google Scholar]
- LAUTT W.W., WANG X., SADRI P., LEGARE D.J., MACEDO M.P. Rapid insulin sensitivity test (RIST) Can. J. Physiol. Pharmacol. 1998;76:1080–1086. doi: 10.1139/cjpp-76-12-1080. [DOI] [PubMed] [Google Scholar]
- MARINHO H.S., BAPTISTA M., PINTO R.E. Glutathione metabolism in hepatomous liver of rats treated with diethylnitrosamine. Biochim. Biophys. Acta. 1997;1360:157–168. doi: 10.1016/s0925-4439(96)00075-0. [DOI] [PubMed] [Google Scholar]
- MATHER K.J., LTEIF A., STEINBERG H.O., BARON A.D. Interactions between endothelin and nitric oxide in the regulation of vascular tone in obesity and diabetes. Diabetes. 2004;53:2060–2066. doi: 10.2337/diabetes.53.8.2060. [DOI] [PubMed] [Google Scholar]
- MOORE M.C., CHERRINGTON A.D., WASSERMAN D.H. Regulation of hepatic and peripheral glucose disposal. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:343–364. doi: 10.1016/s1521-690x(03)00036-8. [DOI] [PubMed] [Google Scholar]
- MOORE M.C., SATAKE S., BARANOWSKI B., HSIEH P.S., NEAL D.W., CHERRINGTON A.D. Effect of hepatic denervation on peripheral insulin sensitivity in conscious dogs. Am. J. Physiol. Endocrinol. Metab. 2002;282:E286–E296. doi: 10.1152/ajpendo.00201.2001. [DOI] [PubMed] [Google Scholar]
- PAOLISSO G., DI MARO G., PIZZA G., D'AMORE A., SGAMBATO S., TESAURO P., VARRICCHIO M., D'ONOFRIO F. Plasma GSH/GSSG affects glucose homeostasis in healthy subjects and non-insulin-dependent diabetics. Am. J. Physiol. 1992a;263:E435–E440. doi: 10.1152/ajpendo.1992.263.3.E435. [DOI] [PubMed] [Google Scholar]
- PAOLISSO G., GIUGLIANO D., PIZZA G., GAMBARDELLA A., TESAURO P., VARRICCHIO M., D'ONOFRIO F. Glutathione infusion potentiates glucose-induced insulin secretion in aged patients with impaired glucose tolerance. Diabetes Care. 1992b;15:1–7. doi: 10.2337/diacare.15.1.1. [DOI] [PubMed] [Google Scholar]
- PETERSEN K.F., TYGSTRUP N. A liver factor increasing glucose uptake in rat hindquarters. J. Hepatol. 1994;20:461–465. doi: 10.1016/s0168-8278(05)80490-8. [DOI] [PubMed] [Google Scholar]
- PORSZASZ R., LEGVARI G., NEMETH J., LITERATI P.N., SZOLCSANYI J., SZILVASSY Z. The sensory nitrergic nature of the hepatic insulin sensitizing substance mechanism in conscious rabbits. Eur. J. Pharmacol. 2002;443:211–212. doi: 10.1016/s0014-2999(02)01603-5. [DOI] [PubMed] [Google Scholar]
- SADRI P., LAUTT W.W. Blockade of nitric oxide production in the liver causes insulin resistance. Proc. West Pharmacol. Soc. 1998;41:37–38. [PubMed] [Google Scholar]
- SADRI P., LAUTT W.W. Blockade of hepatic nitric oxide synthase causes insulin resistance. Am. J. Physiol. 1999;277:G101–G108. doi: 10.1152/ajpgi.1999.277.1.G101. [DOI] [PubMed] [Google Scholar]
- SCHERRER U., SARTORI C. Defective nitric oxide synthesis: a link between metabolic insulin resistance, sympathetic overactivity and cardiovascular morbidity. Eur. J. Endocrinol. 2000;142:315–323. doi: 10.1530/eje.0.1420315. [DOI] [PubMed] [Google Scholar]
- SEVEN A., GUZEL S., SEYMEN O., CIVELEK S., BOLAYIRLI M., UNCU M., BURCAK G. Effects of vitamin E supplementation on oxidative stress in streptozotocin induced diabetic rats: investigation of liver and plasma. Yonsei Med. J. 2004;45:703–710. doi: 10.3349/ymj.2004.45.4.703. [DOI] [PubMed] [Google Scholar]
- STEINBERG H.O., PARADISI G., HOOK G., CROWDER K., CRONIN J., BARON A.D. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- TAKAYAMA S., LEGARE D.J., LAUTT W.W. Dose-related atropine-induced insulin resistance: comparing intraportal versus intravenous administration. Proc. West Pharmacol. Soc. 2000;43:33–34. [PubMed] [Google Scholar]
- TATEISHI N., HIGASHI T., NARUSE A., NAKASHIMA K., SHIOZAKI H. Rat liver glutathione: possible role as a reservoir of cysteine. J. Nutr. 1977;107:51–60. doi: 10.1093/jn/107.1.51. [DOI] [PubMed] [Google Scholar]
- TIROSH A., RUDICH A., BASHAN N. Regulation of glucose transporters–implications for insulin resistance states. J. Pediatr. Endocrinol. Metab. 2000;13:115–133. doi: 10.1515/jpem.2000.13.2.115. [DOI] [PubMed] [Google Scholar]
- VAN DE BORNE P., HAUSBERG M., HOFFMAN R.P., MARK A.L., ANDERSON E.A. Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am. J. Physiol. 1999;276:R178–R183. doi: 10.1152/ajpregu.1999.276.1.R178. [DOI] [PubMed] [Google Scholar]
- XIE H., LAUTT W.W. M1 muscarinic receptor blockade causes insulin resistance in the cat. Proc. West Pharmacol. Soc. 1995;38:83–84. [PubMed] [Google Scholar]
- XIE H., LAUTT W.W. Insulin resistance caused by hepatic cholinergic interruption and reversed by acetylcholine administration. Am. J. Physiol. 1996a;271:E587–E592. doi: 10.1152/ajpendo.1996.271.3.E587. [DOI] [PubMed] [Google Scholar]
- XIE H., LAUTT W.W. Insulin resistance of skeletal muscle produced by hepatic parasympathetic interruption. Am. J. Physiol. 1996b;270:E858–E863. doi: 10.1152/ajpendo.1996.270.5.E858. [DOI] [PubMed] [Google Scholar]