Abstract
While there were early papers about the extracellular actions of purines, the role of ATP as a purinergic neurotransmitter in nonadrenergic, noncholinergic nerves in the gut and bladder in 1972 was a landmark discovery, although it met considerable resistance for the next 20 years. In the early 1990s, receptors for purines were cloned: four P1 receptor subtypes and seven P2X ionotropic and eight P2Y metabotropic receptor subtypes are currently recognized and characterized. The mechanisms underlying ATP release and breakdown are discussed. Purines and pyrimidines have major roles in the activities of non-neuronal cells as well as neurons. This includes fast signalling roles in exocrine and endocrine secretion, platelet aggregation, vascular endothelial cell-mediated vasodilation and nociceptive mechanosensory transduction, as well as acting as a cotransmitter and neuromodulator in most, if not all, nerve types in the peripheral and central nervous systems. More recently, slow (trophic) purinergic signalling has been implicated in cell proliferation, migration, differentiation and death in embryological development, wound healing, restenosis, atherosclerosis, ischaemia, cell turnover of epithelial cells in skin and visceral organs, inflammation, neuroprotection and cancer.
Keywords: ATP, cotransmitter, proliferation, purines, purinergic, pyrimidines, P2X receptors, P2Y receptors, secretion, trophic
The discovery of ATP as a neurotransmitter
A seminal paper by Drury & Szent-Györgyi (1929) described the potent actions of purine nucleotides and nucleosides, ATP and adenosine, on the heart and blood vessels. Green and Stoner, who were given the job of studying the role of ATP in wound shock during World War II, published a paper that reviewed cardiovascular studies in a book on the ‘Biological Actions of Adenine Nucleotides' in 1950. Purines were also shown to produce powerful responses of the intestine and the uterus (Mihich et al., 1954). Buchthal & Folkow (1948) found that acetylcholine (ACh)-evoked contraction of skeletal muscle fibres was potentiated by ATP. Emmelin & Feldberg (1948) found complex effects initiated by intravenous (i.v.) injection of ATP into cats affecting peripheral as well as central mechanisms. Injection of ATP into the lateral ventricle produced muscular weakness, ataxia and sedation. Application of ATP to various regions of the brain produced biochemical or electrophysiological changes (e.g. Galindo et al., 1967) and ATP was shown to have anti-anaesthetic actions. Pamela Holton (1959) presented the first hint of a transmitter role for ATP in the nervous system by demonstrating the release of ATP during antidromic stimulation of sensory nerves. For a more detailed historical review of the early papers describing extracellular actions of ATP, see Burnstock (1997a).
The classical view that autonomic control of smooth muscle consisted of antagonistic sympathetic noradrenergic and parasympathetic cholinergic nerves was challenged in the early 1960s by Burnstock and his colleagues Max Bennett, Graham Campbell, Mollie Holman and Mike Rand in Melbourne (Burnstock et al., 1963; 1964; 1966) (see Figure 1a, b and d), and also by Martinson & Muren (1963) in Goteborg. Stimulation of the guinea-pig taenia coli in the presence of adrenergic and cholinergic blocking agents produced fast inhibitory junction potentials, which were blocked by tetrodotoxin (see Figure 1c). The search for the transmitter responsible for the non-adrenergic, non-cholinergic (later termed NANC) nerve responses was pursued in the late 1960s with my colleagues Graham Campbell, Dave Satchell, Brian Dumsday and Anne Smythe, and it led to the surprising proposal that adenosine triphosphate or a related nucleotide might be the transmitter involved in both the gut and the bladder (Burnstock et al., 1970; 1972).
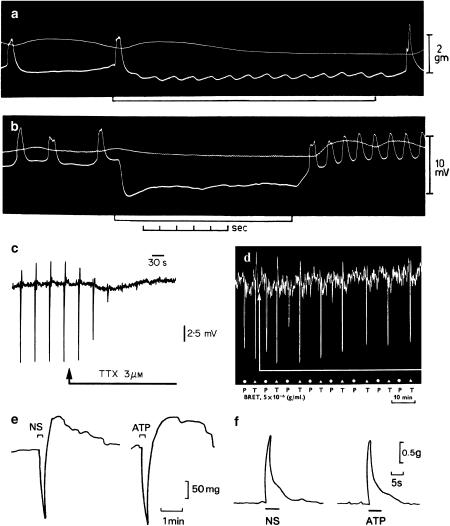
Figure 1.
(a) and (b) Sucrose gap records from smooth muscle of guinea-pig taenia coli showing inhibitory junction potentials in response to stimulation of intrinsic nerves. Frequencies of stimulation for (a) 1/s and (b) 30/s. Upper trace tension, and lower trace membrane potential. Note the phase of excitation in (b) which follows cessation of stimulation (From Burnstock et al. (1963). Reproduced with kind permission from Nature Publishing Group). (c) Sucrose gap recording of membrane potential changes in smooth muscle of guinea-pig taenia coli in the presence of atropine (0.3 μM) and guanethidine (4 μM). Transmural field stimulation (0.5 ms, 0.033 Hz, 8 V) evoked transient hyperpolarisations or inhibitory junction potentials, which were followed by rebound depolarisations. Tetrodotoxin (TTX, 3 μM) added to the superfusing Krebs solution (applied at arrow) rapidly abolished the response to transmural field stimulation. (From Burnstock (1986). Acta Physiol. Scand. 126, 67–91. Reproduced with kind permission from Blackwell Publishing). (d) Effects of bretylium (BRET, 5 × 10−6 g ml−1) on responses to stimulation of the guinea-pig perivascular nerve–taenia coli preparation after atropine. Bretylium abolishes responses to stimulation of the perivascular nerves at 30 pulses/s (P, at dots), but only reduced responses to field stimulation of the taenia with 10 pulses/s (T, at triangles) (From Burnstock et al. (1966). Reproduced with kind permission from Blackwell Publishing). (e) Responses of the guinea-pig taenia coli to intramural nerve stimulation (NS, 1 Hz, 0.5 ms pulse duration, for 10 s at supramaximal voltage) and ATP (2 × 10−6 M). The responses consist of a relaxation followed by a ‘rebound contraction'. Atropine (1.5 × 10−7 M), guanethidine (5 × 10−6 M) and sodium nitrite (7.2 × 10−4 M) were present. (From Burnstock & Wong, 1978, Br. J. Pharmacol. 62, 293–302. Reproduced with kind permission from the Nature Publishing Group). (f) A comparison of the contractile responses of the guinea pig bladder strip to intramural nerve stimulation (NS: 5 Hz, 0.2 ms pulse duration and supramaximal voltage) and exogenous ATP (8.5 μM). Atropine (1.4 μM) and guanethidine (3.4 μM) were present throughout (From Burnstock et al., 1978. Reproduced with kind permission from the Nature Publishing Group).
This ‘purinergic' hypothesis met considerable resistance, partly perhaps because ATP was recognised at that time as an intracellular molecule contained in all cells and of particular importance as an energy source, and it was considered that such a ubiquitous molecule was unlikely to act as a neurotransmitter, even though the presence of powerful ectoenzymes for the breakdown of ATP was already known. A colleague, Austin Doyle, Professor of Medicine in Melbourne, who was noted for his caustic wit, exclaimed to the audience during the farewell party for my move from Australia to England in 1975 that Burnstock was the inventor of the ‘pure-imagine' hypothesis!
In our 1970 paper in the British Journal of Pharmacology (Burnstock et al., 1970), we set out to see what substances could satisfy the criteria set out by Eccles and others for establishing the identity of a neurotransmitter for the NANC inhibitory nerves in the guinea-pig taenia coli (a preparation I had learnt in Edith Bülbring's laboratory in Oxford before going to Melbourne) and for NANC nerves in the gastrointestinal tract of the guinea-pig, rabbit, rat and mouse, the toad stomach and turkey gizzard. Firstly, a putative transmitter must be synthesised and stored within the nerve terminals from which it is released. Once released, it must be mimicked by the exogenous application of the transmitter substance. Also, enzymes that inactivate the transmitter and/or uptake systems for the neurotransmitter or its derivatives must also be present, and, finally, drugs that affect the nerve-mediated response must be shown to modify the response to exogenous transmitter in a similar manner. Many substances were examined as putative transmitters in the NANC nerves of the gastrointestinal tract and bladder, but the substance that best satisfied the above criteria was the purine nucleotide, ATP (see Figures 1e, f and 2a). A tentative model of storage, release, receptor activation by and inactivation of ATP during purinergic transmission in the gut and urinary bladder was proposed in the 1972 Pharmacological Review (Burnstock, 1972) and is now generally accepted (see Burnstock, 1997b; 2001c, 2001d). A Volume of ‘Seminars in the Neurosciences' was devoted entirely to purinergic neurotransmission in 1996.
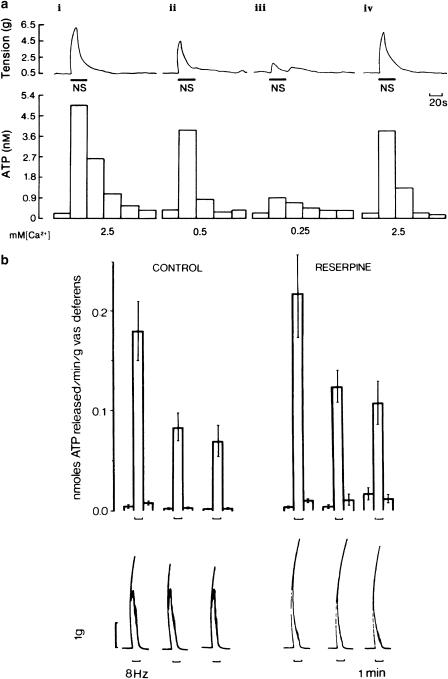
Figure 2.
(a) Effect of changing the calcium ion (Ca2+) concentration on the release of ATP from the guinea-pig isolated bladder strip during stimulation of intramural nerves. Upper trace: mechanical recording of changes in tension (g) during intramural nerve stimulation (NS: 20 Hz, 0.2 ms pulse duration, supramaximal voltage for 20 s). Lower trace: concentration of ATP in consecutive 20 s fractions of the superfusate. The Ca2+ concentration in the superfusate varied as follows: (i) 2.5 mM (normal Krebs); (ii) 0.5 mM; (iii) 0.25 mM; and (iv) 2.5 mM. The successive contractions were separated by 60 min intervals as indicated by the breaks in the mechanical trace. Atropine (1.4 μM) and guanethidine (3.4 μM) were present throughout. The temperature of the perfusate was between 22°C and 23°C (From Burnstock et al. (1978). Reproduced with kind permission from the Nature Publishing Group).(b) Release of endogenous ATP from control (n=32) and reserpine-treated (n=12) guinea-pig vasa deferentia during field stimulation at 8 Hz (pulse width 0.5 ms, 20 V). Upper panel: mean±s.e.m. nmol of ATP released min−1 g−1 of vas deferens. Lower panel: biphasic mechanical response to stimulation of the vas deferens for 1 min as denoted by the upward bracket. Note that the second slow phase of the mechanical response (mediated by NA) has gone in the reserpine-treated tissue (From Kirkpatrick & Burnstock (1987). Reproduced with kind permission from Elsevier).
During the 1970s and 1980s, it was not unusual for someone to approach me, after a talk, to say, emotionally, that they intended to devote their lives to destroying the purinergic hypothesis. I found this disquieting. On one such occasion I was talking together with Ulf von Euler, the discoverer of noradrenaline (NA), and he gave me some wonderful advice: ‘Look Geoff', he said, ‘I do not know whether your hypothesis is correct, but do not let these negative people upset you – they will come and go, but you must be careful to examine their arguments very carefully – if they are emotive, discard them, but if they are based on serious experimental data, you must try to repeat these experiments, interpret them objectively and, if true, you should be the first to question your own hypothesis'. This advice was very important to me and I did indeed follow it.
The original hypothesis based on the experiments carried out on the gut and bladder was that ATP was a neurotransmitter released by nerves distinct from those that release ACh or NA. So when we found that ATP was released from sympathetic nerves as well as ‘purinergic nerves' (Su et al., 1971), I thought that there was something seriously wrong with the hypothesis and I remember staying up all night writing a paper disclaiming and rejecting it. However, in the early hours of the morning, I thought of another possible interpretation – what if ATP was a cotransmitter with NA in sympathetic nerves? So, I put the disclaimer manuscript on hold and sought hints in the literature about the possibility of multiple transmitters being stored and released from single nerve fibres, leading to a commentary (Burnstock, 1976) entitled ‘Do some nerves release more than one neurotransmitter?', which included a paper on sympathetic cotransmission to the cat nictitating membrane (Langer & Pinto, 1976). Thinking back about the work that Mollie Holman and I carried out in the early 1960s on the electrophysiology of sympathetic neurotransmission in the vas deferens, we puzzled why the excitatory junction potentials (ejps) that we recorded in smooth muscle during nerve stimulation were not blocked by adrenoceptor antagonists. They were, however, blocked by guanethidine and bretylium, which were known to block transmitter release. Could it be that the ejps were due to the release of ATP as a cotransmitter? Years later, when ATP receptor antagonists became available, Peter Sneddon and I and other laboratories did indeed show this to be the case.
Sadly, some of the disbelievers in the purinergic hypothesis accused me at the time of being slippery by side-stepping the real issue and putting off rejecting the hypothesis by coming up with another hypothesis that contradicted another well-established principle in pharmacology – one nerve/one transmitter. Alternatively, it could be taken as an example of how reinterpretation of data can lead to an expansion of the significance of the original hypothesis.
Another interpretation of sympathetic nerve cotransmission, proposed by Neild & Hirst (1984), which was widely supported for a while, was that ejps were mediated in the vas deferens by their newly defined γ-adrenoceptors located at neuromuscular junctions. However, this was experimentally discounted when it was shown that NA failed to mimic the ejp, when ejps were shown to be blocked by desensitisation of the P2 receptor with a,β-methylene ATP (Sneddon & Burnstock, 1984b) and when reserpine, which depleted NA, did not change the ejps or the first component of contraction, while ATP was still released (Kirkpatrick & Burnstock, 1987) (Figure 2b).
Evidence followed for sympathetic cotransmission to various blood vessels (Sneddon & Burnstock, 1984a; Burnstock & Warland, 1987; see Burnstock, 1988) as well as to the vas deferens (Fedan et al., 1981; Sneddon & Burnstock, 1984b). Evidence for parasympathetic nerve cotransmission to the urinary bladder, involving ATP and ACh, was extended (see Burnstock, 2001c). It was originally assumed that NA and ATP were co-stored in small agranular vesicles as well as in large granular vesicles in sympathetic nerve terminal varicosities, but in a series of papers published with Jim Ellis in the British Journal of Pharmacology, in 1989, we showed that prejunctional modulation of the release of ATP and NA did not concur, suggesting that they may be stored in different populations of vesicles.
Gradually purinergic signalling was becoming accepted and during the meeting held by the New York Academy of Sciences in 1989 entitled the ‘Biological Actions of Extracellular ATP', Samuel C. Silverstein presented a poem:
Oh tell me Lord how could it be, That though our cells make ATP, It's not all used for energy, But sometimes is secreted free. It puzzles you, it puzzles me, While Geoffrey Burnstock smiles with glee At the many roles of ATP.
A strong boost to interest in purinergic mechanisms came in 1992 when purinergic synaptic transmission was demonstrated between neurones in coeliac ganglia by AnneMarie Surprenant and colleagues and by Eugene Silinsky and, in the brain, by Frances Edwards and colleagues at University College London (see Burnstock, 2003). Fortunately, cotransmission turned out to be the rule, rather than the exception, and in fact the significance of purinergic transmission was much extended by this concept. Evidence for ATP as a cotransmitter, albeit with variable levels of contribution, has been found for all peripheral or central nerves so far investigated (see Burnstock, 2004a).
The identity and roles of the cotransmitters in NANC nerves in the gastrointestinal tract have also been much debated (see Burnstock, 2001d). When the technique of immunohistochemistry for neuropeptides was used widely in the mid 1970s, the idea that vasoactive intestinal polypeptide (VIP) was the neurotransmitter, rather than ATP in the NANC nerves in the gut, gained ground and papers entitled ‘Peptidergic rather than purinergic' were published. However, the pharmacological experiments were not entirely supportive, partly because in most gut preparations the response to VIP was very slow and sustained after a long latency, in contrast to the fast relaxations and inhibitory junction potentials produced by nerve stimulation and ATP. After earlier hints from the laboratory of John Gillespie (particularly Anne Bowman and Billy Martin) in the early 1980s, a new contender for the NANC inhibitory transmitter emerged when nitric oxide (NO) was recognised in 1989/1990 by Rand and Snyder, Garthwaite, Boeckxystaens and others to be a neurotransmitter in the nervous system. Most laboratories now support the view that ATP, NO and VIP are cotransmitters in NANC inhibitory nerves, although the proportions vary markedly in different regions of the gut and in different species (see Burnstock, 2001d).
Purinoceptor subtypes
Purinergic receptors were first defined in 1976 and 2 years later a basis for distinguishing two types of purinoceptors, named P1 and P2 for adenosine and ATP/ADP, respectively, was proposed (Burnstock, 1978).
Four subtypes of P1 receptors have been cloned, namely A1, A2A, A2B and A3 (see Ralevic & Burnstock, 1998). All P1 adenosine receptors are typical G protein-coupled (metabotropic) receptors and specific agonists and antagonists are available for each subtype.
In 1985, P2 receptors were subdivided into P2X and P2Y subtypes on the basis of pharmacology (Burnstock & Kennedy, 1985). We now recognise that P2 purinoceptors belong to two major families, a P2Y family of G protein-coupled receptors (cloned in 1993 in collaboration with Eric Barnard; Webb et al., 1993) and by Kevin Lustig and a P2X family of ligand-gated ion channel receptors (cloned in 1994 by Valera, North and colleagues in Geneva and by Anthony Brake). Currently, seven subtypes of the P2X family and eight subtypes of the P2Y family have been cloned and functionally characterised (see Burnstock, 2004b). Members of the existing family of ionotropic P2X1–7 receptors show the following: intracellular N- and C-termini; two transmembrane spanning regions (TM1 and TM2), the first involved with channel gating and second lining the ion pore; large extracellular loop, with 10 conserved cysteine residues forming a series of disulphide bridges and an ATP-binding site, which may involve regions of the extracellular loop adjacent to TM1 and TM2. The P2X1–7 receptors show 30–50% sequence identity at the amino acid level. The stoichiometry of P2X1–7 receptors is thought to involve three subunits, which form a trimer. Heteromultimers as well as homomultimers are involved in forming the trimer ion pore. Heteromultimers are clearly established for P2X2/3 receptors in nodose ganglia, P2X4/6 receptors in central nervous system (CNS) neurones, P2X1/5 receptors in some blood vessels, P2X2/6 receptors in the brain stem and more recently P2X1/4 and P2X1/2 receptors. P2X7 receptors do not form heteromultimers, and P2X6 receptors will not form a functional homomultimer.
Metabotropic P2Y receptors are characterised by: an extracellular N-terminus and an intracellular C-terminus; seven transmembrane spanning regions; a high level of sequence homology between some transmembrane spanning regions; structural diversity of intracellular loops and C-terminus among P2Y subtypes, thus influencing the degree of coupling with Gq/11, Gs and Gi proteins. Each P2Y receptor binds to a single heterotrimeric G protein (typically Gq/11), although P2Y11 can couple to both Gq/11 and Gs, whereas P2Y12 couples to Gi. P2Y receptors may form homo- and heteromultimeric assemblies under some conditions, and many cells express multiple P2Y receptor subtypes. Some P2Y receptors are activated principally by nucleotide diphosphates (P2Y1,6,12,13), while others are activated mainly by nucleotide triphosphates (P2Y2,4). Some P2Y receptors are activated by both purine and pyrimidine nucleotides (P2Y2,4,6), and others by purine nucleotides alone (P2Y1,11,12,13). In response to nucleotide activation, recombinant P2Y receptors either activate phospholipase C and release intracellular calcium or affect adenylyl cyclase and alter cAMP levels.
2-MethylthioADP is a potent agonist of mammalian P2Y1 receptors, and MRS 2179 and 2500 are potent antagonists. At P2Y2 and P2Y4 receptors in the rat, ATP and UTP are equipotent. The P2Y6 receptor is UDP selective and MRS 2578 was identified recently as a selective antagonist. A P2Y12 receptor is found on platelets (Hollopeter et al., 2001) and selective antagonists are available. For P2Y13 and P2Y14 receptors, transduction is entirely through adenylate cyclase. An interesting question that has arisen by analogy with other G protein-coupled receptors is whether dimers can form between the P2Y subtypes.
ATP release and breakdown
Release of ATP from the perfused heart during coronary vasodilation in response to hypoxia was reported by Paddle & Burnstock (1974) and from exercising human forearm muscle by Tom Forrester and his colleagues in early papers. However, until recently, it was usually assumed that the only source of extracellular ATP acting on purinoceptors was damaged or dying cells, but it is now recognised that mechanically-induced ATP release from healthy cells is a physiological mechanism (see Bodin & Burnstock, 2001). There is an active debate, however, about the precise transport mechanism(s) involved. There is compelling evidence for exocytotic vesicular release of ATP from nerves, but for ATP release from non-neuronal cells, various transport mechanisms have been proposed, including ATP-binding cassette (ABC) transporters, connexin or pannexin hemichannels or possibly plasmalemmal voltage-dependent anion channels, as well as vesicular release.
ATP release by mechanical distortion of urothelial cells during distension of the bladder was first demonstrated by Ferguson and his colleagues in 1997 and later by us (Vlaskovska et al., 2001) and was also demonstrated in ureter (Knight et al., 2002), from mucosal epithelial cells during distension of the colorectum (Wynn et al., 2003). It is probably released from odontoblasts in tooth pulp, from epithelial cells in the tongue, epithelial cells in the lung, keratinocytes in the skin and glomus cells in the carotid body (Rong et al., 2003). Perhaps surprisingly, evidence was presented that the release of ATP from urothelial cells in the ureter (as well as from endothelial cells (Bodin & Burnstock, 2001)) is vesicular, since monensin and brefeldin A, which interfere with vesicular formation and trafficking, inhibited distension-evoked ATP release, but not gadolinium, an inhibitor of stretch-activated channels, or glibenclamide, an inhibitor of two members of the ABC protein family (Knight et al., 2002). There is increased release of ATP from endothelial cells during acute inflammation.
Ectonucleotidases that rapidly break down released ATP consist of nucleoside triphosphate diphosphohydrolases (NTPDases 1, 2, 3 and 8), nucleotide pyrophosphatases (NPP 1, 2 and 3), alkaline phosphatase and 5′-nucleotidase (Zimmermann, 2001).
Physiology and pathophysiology: fast and slow purinergic signalling
The concept of purinergic signalling has broadened through the years to include not only cotransmission in different nerve types in both peripheral and CNS, but also roles for purines acting on non-neuronal cells (see Abbracchio & Burnstock, 1998; Burnstock & Knight, 2004). Examples of fast purinergic signalling include cotransmission and neuromodulation (discussed earlier), exocrine and endocrine secretion, platelet aggregation, vascular endothelial cell-mediated vasodilatation and nociceptive mechanosensory transduction. Examples of slow (trophic) purinergic signalling include cell proliferation, differentiation and apoptosis in embryological development, wound healing (including bone resorption), atherosclerosis, restenosis and ischaemia, cell turnover of epithelial cells in skin and visceral organs, inflammation, neuroprotection and cancer.
Short-term signalling
P2 receptors have been shown to regulate ion transport in epithelial cells from a variety of different sources, where both ATP and UTP stimulate Cl− transport (see Abbracchio & Burnstock, 1998). These actions have very important implications for airways epithelia, which express P2Y receptors, mainly belonging to the P2Y2 or P2Y4 subtypes, and patients with disorders of airway electrolyte metabolism (e.g., cystic fibrosis) exhibit decreased mucociliary clearance and chronic airway infection (see Yerxa, 2001). ATP regulates gastric acid secretion and secretion from parotid and lachrymal acinar cells. P2Y2 receptor activation increases salt, water and mucus excretion, and thus represents a potential treatment for dry eye conditions (Yerxa, 2001). There is a substantial presence of purinoceptors in different regions of the nephron, the glomerulus and renal vascular system in the kidney, including P1 and P2 receptor subtypes involved in the regulation of renin secretion, glomerular filtration and the transport of water, ions, nutrients and toxins (Bailey et al., 2004) (see Figure 3).
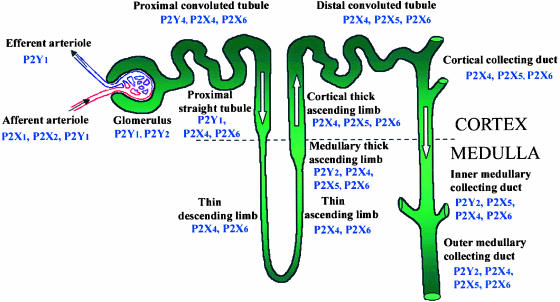
Figure 3.
Summary of the nephron segments immunopositive for P2 receptor subtypes (Modified from Turner, Vonend, Chan, Burnstock & Unwin, 2003. Cells, Tissues Organs 175, 105–117. Reproduced with kind permission from Karger AG, Basel).
Purinoceptors are widely expressed in endocrine glands (see Burnstock & Knight, 2004). For example, ATP modulates aldosterone production by adrenal cortex, and regulates prolactin release from anterior pituitary and vasopressin and oxytocin secretion from posterior pituitary. ATP and UTP inhibit estradiol and progesterone secretion from the ovary and mediate increases in intracellular calcium in Sertoli cells from testis. P2Y receptors, present on pancreatic β-cells, are involved in insulin secretion.
Platelets are known to express P2Y1, P2Y12 and P2X1 receptors (Hollopeter et al., 2001). Both P2Y1 and P2Y12 receptors inhibit platelet aggregation, but less is known about the role of P2X1 receptors. Clinical trials CAPRIE and CURE have provided clear evidence that the purinergic antithrombotic drugs clopidogrel and ticlopidine, which are antagonists to the platelet P2Y12 receptor, reduce the risks of recurrent strokes and heart attacks, especially when combined with aspirin. MRS 2500, a highly potent and selective antagonist for the P2Y1 receptor, has also been shown recently to have anti-aggregating activity on human platelets.
Regulation of vascular tone by purines and pyrimidines does not only involve P2X receptors on smooth muscle mediating vasoconstriction in response to perivascular nerve stimulation, but also P2 receptors on endothelial cells mediating vasodilation (see Burnstock, 2002). Activation of vascular endothelial P2Y1 and P2Y2 and probably P2Y4 and P2X4 receptors results in the release of endothelium-derived relaxing factor, mostly NO, and resultant vasodilatation. This important discovery challenged the influential earlier hypothesis by Berne (1963) that adenosine was the local regulator of blood flow following hypoxia in heart in many other vascular beds; it now seems likely that reactive hyperaemia is largely due to ATP, released from endothelial cells during hypoxia and acting on endothelial P2 receptors to release NO. Adenosine, produced following the breakdown of ATP, contributes to the later component of vasodilatation by the direct action of P1 receptors on vascular smooth muscle. The purinergic cotransmitter component in sympathetic nerves supplying vessels is increased in spontaneous hypertensive rats (see Ralevic & Burnstock, 1998).
The involvement of ATP in the initiation of pain was recognised early (Collier et al., 1966; Bleehen & Keele, 1977). A major advance was made when the P2X3 ionotropic receptor was cloned in 1995 and shown later to be predominantly localised in the subpopulation of small nociceptive sensory neurons that label with isolectin IB4 in dorsal root ganglia, whose central projections terminate in inner lamina II of the dorsal horn. Burnstock (1996) put forward a unifying purinergic hypothesis for the initiation of pain by ATP acting via P2X3 and P2X2/3 receptors associated with causalgia, reflex sympathetic dystrophy, angina, migraine, pelvic and cancer pain. This has been followed by an increasing number of papers expanding on this concept for acute, inflammatory, neuropathic and visceral pain (see reviews by Burnstock, 2001a; Jarvis, 2003). A hypothesis was proposed that purinergic mechanosensory transduction occurs in visceral tubes and sacs, including the ureter, bladder and gut, where ATP released from epithelial cells during distension acts on P2X3 homomultimeric and P2X2/3 heteromultimeric receptors on subepithelial sensory nerves to initiate impulses in sensory pathways to pain centres in the CNS (Burnstock, 1999). Subsequent studies of the bladder (Cockayne et al., 2000; Vlaskovska et al, 2001), ureter (Rong & Burnstock, 2004) and gut (Wynn et al., 2003) have produced evidence in support of this hypothesis, as well as in the tongue and tooth pulp. P2X3 knockout mice were used to show that ATP released from urothelial cells during distension of the bladder acting on P2X3 receptors on subepithelial sensory nerves also initiated bladder voiding reflex activities (Cockayne et al., 2000). ATP may also be released from the spinal cord terminals of primary afferent sensory nerves to act as a neurotransmitter in the dorsal horn (Chen & Gu, 2005). The search is on for selective P2X3 and P2X2/3 receptor antagonists that do not degrade in vivo. PPADS is a non-selective P2 antagonist, but has the advantage that it dissociates about 100–10,000 times more slowly than other known antagonists. The trinitrophenyl-substituted nucleotide TNP-ATP is selective and very potent at both P2X3 and P2X2/3 receptors. Tetramethylpyrazine, a traditional Chinese medicine, used as an analgesic for dysmenorrhoea, significantly inhibited the first phase of nociceptive behaviour induced by 5% formalin in the rat hindpaw pain model.
Long-term signalling
Purines and pyrimidines can both stimulate the progression of cells through the cell cycle and inhibit cell growth, depending upon their extracellular concentrations, the physiological state of target cells and/or the expression of specific P1 and P2 receptor subtypes (see Burnstock, 2002). Stimulation of DNA synthesis and cell proliferation by purines has been demonstrated in Swiss mouse 3T3 and 3T6 fibroblasts, thymocytes, haemopoietic cells, vascular smooth muscle cells, endothelial cells, primary astrocytes and astrocytoma cell lines (see Neary et al., 1996). The complex mechanisms responsible for purinergic signalling in cell proliferation, differentiation and cell death are reviewed by Abbracchio & Burnstock (1998). The mitogenic effects of ATP, via P2Y receptors linked to stimulation of phospholipase C and Ca2+ release from inositol-phosphate-sensitive intracellular stores, are synergistic with those induced by conventional polypeptide growth factors. Epithelial cell turnover of skin keratinocytes and of urothelial cells of the bladder and ureter involve P2Y1 and P2Y2 receptors mediating proliferation, P2X5 receptors mediating differentiation and P2X7 receptors mediating apoptotic cell death (Greig et al., 2003).
ATP and adenosine have been claimed to play roles in the cytological changes and morphogenetic movements occurring during early embryogenesis (see Burnstock, 2001b). In the past, evidence for a role of ATP in early development has been interpreted merely in terms of source of energy to support these activities. Now that the existence of specific extracellular receptors for nucleotides and nucleosides has been widely accepted, the results of a number of these studies can be reinterpreted in the light of the novel role of these compounds as extracellular messengers. A number of reports implicate ATP to play a critical role in differentiation and maturation, and in the acquisition of highly specialized functions. The expression of many of the receptor subtypes in embryos is transient and often lost in adults. However, these receptors may be expressed again under specific pathophysiological conditions, when regeneration and/or growth occurs following trauma or insults. A novel P2Y receptor (P2Y8), cloned and expressed in the neural plate of Xenopus embryos, may be involved in the early formation of the nervous system (Bogdanov et al., 1997). P2Y1 receptors are involved in the development of limb buds in chick embryos. Sequential expression of P2X5, P2X6 and P2X2 receptors during perinatal development of skeletal muscle has been reported (Ryten et al., 2001).
A role for purines and pyrimidines in promoting cell proliferation, migration, differentiation and death in wound healing has been proposed (see Abbracchio & Burnstock, 1998). There is recent evidence for a role for purinergic signalling in muscle regeneration. ATP plays a pivotal role in the control of vascular cell growth and neointima formation associated with hypertension, renal vascular injury and atherosclerosis. Several reports implicate purinergic signalling in bone development and remodelling (see Hoebertz et al., 2003; Figure 4). The multiple purinoceptors on bone and cartilage represent potential targets for the development of novel therapeutics to inhibit bone resorption in diseases such as rheumatoid arthritis, osteoporosis, tumour-induced osteolysis and periodontitis.
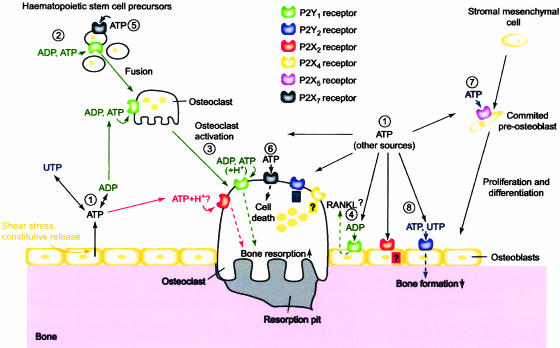
Figure 4.
Schematic diagram illustrating the potential roles played by extracellular nucleotides and P2 receptors in modulating bone cell function. Adenosine 5′-triphosphate (ATP), released from osteoblasts (e.g., through shear stress or constitutively) or from other sources, can be degraded to adenosine 5′-triphosphate (ADP) or converted into uridine 5′-triphosphate (UTP) via ecto-nucleotidases (1). All three nucleotides can act separately on specific P2 receptor subtypes, as indicated by the colour coding. ATP is a universal agonist, whereas UTP is only active at the P2Y2 receptor and ADP is only active at the P2Y1 receptor. ADP, via P2Y1 receptors, appears to stimulate both the formation (i.e., fusion) of osteoclasts from haematopoietic precursors (2) and the resorptive activity of mature osteoclasts (3). For the latter, a synergistic action of ATP and protons has also been proposed via the P2X2 receptor. ADP could also stimulate resorption indirectly through actions on osteoblasts, which in turn release pro-resorptive factors (e.g., receptor activator of nuclear factor kB ligand (RANKL)) (4). ATP at high concentrations might facilitate fusion of osteoclast progenitors through P2X7 receptor pore formation (5) or induce cell death of mature osteoclasts via P2X7 receptors (6). In osteoblasts, ATP, via P2X5 receptors, might enhance proliferation and/or differentiation (7). By contrast, UTP, via P2Y2 receptors, is a strong inhibitor of bone formation by osteoblasts (8). For some receptors (e.g., P2X4 and P2Y2 receptors on osteoclasts or P2X2 receptors on osteoblasts), evidence for expression has been found, but their role is still unclear (question marks). Dashed lines indicate signalling events in the cell. (From Hoebertz et al., 2003. Reproduced with kind permission from Elsevier).
Long-term (trophic) roles of purinergic signalling in vascular smooth muscle and endothelial cell proliferation and death have been implicated in atherosclerosis and restenosis (Burnstock, 2002; Di Virgilio & Solini, 2002). Trophic changes are also triggered by purines and pyrimidines, large amounts of which are released following trauma and ischaemia, that may play a role in limiting damage and in favouring repair mechanisms and restoration of physiological tissue homeostasis. Reperfusion of the ischaemic heart with either ATP or ATP synthase promoters results in significantly lower hypoxic damage and increased myocardial recovery.
P2X7 and P2Y1 and P2Y2 receptors located on inflammatory and immune cells play a pivotal role in inflammation and immunomodulation. ATP and its breakdown product adenosine are released at sites of inflammation. ATP is involved in the development of inflammation through a combination of actions: release of histamine from mast cells, provoking production of prostaglandins, and the production and release of cytokines from immune cells. In contrast, adenosine exerts anti-inflammatory actions. Nucleoside triphosphate diphosphohydrolase ectoenzymes also affect immune reactions. ATP-induced apoptosis in macrophages via P2X7 receptors results in killing of the mycobacteria contained within them, in contrast to macrophage apoptosis produced by other agents (Lammas et al., 1997). While the cytotoxic actions of ATP on macrophages are via P2X7 receptors, the bacteriocidal effects of ATP (and UTP) are probably via P2Y2 receptors. P2X7 receptors may be a relevant target for therapeutic intervention in lung hypersensitivity reactions associated with chronic inflammatory responses. Extracellular nucleotides and their receptors have been implicated in the pathogenesis of inflammatory bowel disease. P2X7 receptors may play a role in the response of enteric glia to inflammation and P2X3 purinergic signalling enhancement in an animal model of colitis has been described. P2X3 receptor expression is increased in the enteric plexuses in human irritable bowel syndrome (IBS), suggesting a potential role in dysmotility and pain, and the possibility that P2X receptors are potential targets for the drug treatment of IBS has been raised (see Galligan, 2004). A-317491 is a potent and selective non-nucleotide antagonist of P2X3 and P2X2/3 receptors and it reduces chronic inflammatory and neuropathic pain in the rat (McGaraughty et al., 2003). In recent studies by Stone and Vulchanova, antisense oligonucleotides have been used to downregulate the P2X3 receptor in models of neuropathic and inflammatory pain. Further, Dorn and colleagues reported that P2X3 double-stranded short interfering RNA (siRNA) relieves chronic neuropathic pain.
In the brain, purinergic signalling is involved in nervous tissue remodelling following trauma, stroke, ischaemia or neurodegenerative disorders (see Burnstock, 2003). Neuronal injury releases fibroblast growth factor, epidermal growth factor and platelet-derived growth factor. In combination with these growth factors, ATP can stimulate astrocyte proliferation, contributing to the process of reactive astrogliosis and to hypertrophic/hyperplasic responses (Neary et al., 1996). P2Y receptor antagonists have been proposed as potential neuroprotective agents in the cortex, hippocampus and cerebellum. Adenosine, via A2A receptors, has a protective effect in a rat model of Parkinson's disease, perhaps by upregulating antioxidant states and reducing dopamine loss. ATP inhibits the release of the excitatory transmitter, glutamate, and stimulates the release of the inhibitory transmitter, GABA, from hippocampal nerves, thus serving a protective role. ATP is an extracellular signalling molecule between neurons and glial cells (see Fields & Stevens, 2000). Some of the responses to ATP released during brain injury are neuroprotective, but at higher concentrations ATP contributes to the pathophysiology initiated after trauma. Multiple P2X and P2Y receptor subtypes are expressed by astrocytes, oligodendrocytes and microglia. Microglia, immune cells of the CNS, are also activated by purines and pyrimidines to release inflammatory cytokines such as interleukins 1β and 6 and tumour necrosis factor α. Thus, while microglia may play an important role against infection in the CNS, overstimulation of this immune reaction may accelerate the neuronal damage. P2X7 receptors mediate superoxide production in primary microglia and are unregulated in a transgenic model of Alzheimer's disease, particularly around β-amyloid plaques. P2X4 receptors are induced in spinal microglia that appear to gate tactile allodynia after nerve injury (Tsuda et al., 2003).
The anticancer activity of adenine nucleotides was first described by Rapaport in 1983. Intraperitoneal injection of ATP into tumour-bearing mice results in significant anticancer activity against several fast-growing aggressive carcinomas. ATP inhibits the growth of murine colonic adenocarcinoma and human pancreatic carcinoma in mice as well as inhibiting the associated weight loss. In a comprehensive review about the use of ATP for the treatment of advanced cancer (Abraham et al., 2003), evidence was presented that (1) extracellular ATP inhibits the growth of a variety of human tumours, including prostate, breast, colon, liver, ovarian, colorectal, oesophageal and melanoma cancer cells, partly by mediating apoptotic cancer cell death, (2) ATP administration induces resistance of non-malignant tissue to chemo- and radiation therapy and (3) ATP has pronounced anticachexia effects, particularly in older patients, reducing weight loss, anorexia and hormonal aberrations, largely via its ability to expand blood plasma ATP pools. It was concluded that preclinical data support utilisation of ATP in the treatment of advanced cancers supported by Phase I and II human trials, which indicate that ATP has a future place as a useful anticancer agent. Recent studies from our laboratory have analysed the P2 receptor subtypes that contribute to ATP suppression of malignant melanomas, basal and squamous cell tumours and prostate and bladder cancers. In general, P2Y1 and P2Y2 receptors mediate proliferation or antiproliferation, P2X5 receptors mediate cell differentiation, which in effect is antiproliferative, and P2X7 receptors mediate apoptotic cell death. ATP administration is particularly effective in treating bladder tumours when combined with the more commonly used anticancer drug mitomycin.
Concluding comments
After a stormy start, purinergic signalling is now a well-established and rapidly expanding field. For example, two volumes devoted to ‘Purinergic and Pyrimidinergic Signalling' in the Handbook of Experimental Pharmacology were published recently (Abbracchio & Williams, 2001), as well as a Volume entitled ‘Purines and the autonomic nervous system: from controversy to clinic' (King & North, 2000). Following the conceptual advances about the molecular biology and the pharmacology and physiology of purinoceptors, the field has advanced and details have been established about purinergic receptors and their transduction mechanisms and about fast and slow (trophic) purinergic signalling in both neuronal and non-neuronal cells. An increasing range of tools have underlined these advances, including RT–PCR and immunohistochemistry of P2 receptor mRNA and protein expression, P2 receptor subtype characterisation in transfected cells, the development of transgenic mice deleting or over-expressing P2 receptor subtypes, medicinal chemistry methodology allowing the design of selective P2 receptor agonists and antagonists, use of antisense oligonucleotides and siRNA. It is hoped that selective antagonists for P2 receptor subtypes will soon be developed that can be applied in vivo and that studies can be undertaken to examine the behavioural roles of purinergic signalling in the brain.
Glossary
- ABC
ATP binding cassette
- ACh
acetylcholine
- ADP
adenosine diphosphate
- ATP
adenosine 5′-triphosphate
- CNS
central nervous system
- EDRF
endothelium-derived relaxing factor
- ejps
excitatory junction potentials
- GABA
γ-amino butyric acid
- IBS
irritable bowel syndrome
- NANC, non-adrenergic
non-cholinergic
- NA
noradrenaline
- NO
nitric oxide
- NTPD
nucleoside triphosphate diphosphohydrolase
- pl-VDAC
plasmalemmal voltage-dependent anion channel
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
- RT-PCR
reverse transcriptase polymerase chain reaction
- siRNA
short interfering RNA
- TM
transmembrane
- TNF-α
tumour necrosis factor-α
- UDP
uridine diphosphate
- UTP
uridine 5′-triphosphate
- VIP
vasoactive intestinal polypeptide
References
- ABBRACCHIO M.P., WILLIAMS M.(eds) (2001Handbook of Experimental Pharmacology: Purinergic and Pyrimidinergic Signalling, Vol. 151 (I and II) Berlin: Springer [Google Scholar]
- ABBRACCHIO M.P., BURNSTOCK G. Purinergic signalling: pathophysiological roles. Jpn. J. Pharmacol. 1998;78:113–145. doi: 10.1254/jjp.78.113. [DOI] [PubMed] [Google Scholar]
- ABRAHAM E.H., SALIKHOVA A.Y., RAPAPORT E. ATP in the treatment of advanced cancer. Curr. Top. Membr. 2003;54:415–452. [Google Scholar]
- BAILEY M.A., TURNER C., HUS-CITHAREL A., MARCHETTI J., IMBERT-TEBOUL M., MILNER P., BURNSTOCK G., UNWIN R. P2Y receptors present in the native and isolated rat glomerulus. Nephron. Physiol. 2004;96:P79–P90. doi: 10.1159/000076753. [DOI] [PubMed] [Google Scholar]
- BERNE R.M. Cardiac nucleotides in hypoxia: possible role in regulation of coronary blood flow. Am. J. Physiol. 1963;204:317–322. doi: 10.1152/ajplegacy.1963.204.2.317. [DOI] [PubMed] [Google Scholar]
- BLEEHEN T., KEELE C.A. Observations on the algogenic actions of adenosine compounds on human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- BODIN P., BURNSTOCK G. Purinergic signalling: ATP release. Neurochem. Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- BOGDANOV Y.D., DALE L., KING B.F., WHITTOCK N., BURNSTOCK G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J. Biol. Chem. 1997;272:12583–12590. doi: 10.1074/jbc.272.19.12583. [DOI] [PubMed] [Google Scholar]
- BUCHTHAL F., FOLKOW B. Interaction between acetylcholine and adenosine triphosphate in normal, curarised and denervated muscle. Acta Physiol. Scand. 1948;15:150–160. doi: 10.1111/j.1748-1716.1948.tb00492.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- BURNSTOCK G. Do some nerve cells release more than one transmitter. Neuroscience. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.1978A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones: A Multidisciplinary Approached. Straub, R.W. & Bolis, L. pp. 107–118.New York: Raven Press [Google Scholar]
- BURNSTOCK G. Sympathetic purinergic transmission in small blood vessels. Trends Pharmacol. Sci. 1988;9:116–117. doi: 10.1016/0165-6147(88)90185-x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.1997aHistory of extracellular nucleotides and their receptors The P2 Nucleotide Receptorsed. Turner, J.T., Weisman, G. & Fedan, J.S. pp. 3–40.Totowa, NJ: Humana Press Inc [Google Scholar]
- BURNSTOCK G. The past, present and future of purine nucleotides as signalling molecules. Neuropharmacology. 1997b;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J. Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol. Sci. 2001a;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.2001bPurinergic signalling in development Handbook of Experimental Pharmacology, Volume 151/I. Purinergic and Pyrimidinergic Signalling I – Molecular, Nervous and Urinogenitary System Functioned. Abbracchio, M.P. & Williams, M. pp. 89–127.Berlin: Springer-Verlag [Google Scholar]
- BURNSTOCK G.2001cPurinergic signalling in lower urinary tract Handbook of Experimental Pharmacology, Volume 151/I. Purinergic and Pyrimidinergic Signalling I – Molecular, Nervous and Urinogenitary System Functioned. Abbracchio, M.P. & Williams, M. pp. 423–515.Berlin: Springer-Verlag [Google Scholar]
- BURNSTOCK G.2001dPurinergic signalling in gut Handbook of Experimental Pharmacology, Volume 151/II. Purinergic and Pyrimidinergic Signalling II – Cardiovascular, Respiratory, Immune, Metabolic and Gastrointestinal Tract Functioned. Abbracchio, M.P. & Williams, M. pp. 141–238.Berlin: Springer-Verlag [Google Scholar]
- BURNSTOCK G. Purinergic signalling and vascular cell proliferation and death. Arterioscleros. Thromb. Vasc. Biol. 2002;22:364–373. doi: 10.1161/hq0302.105360. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.2003Purinergic receptors in the nervous system Curr. Top. in Membranes. Vol. 54. Purinergic Receptors and Signallinged. Schwiebert, E.M. pp. 307–368.San Diego: Academic Press [Google Scholar]
- BURNSTOCK G. Cotransmission. Curr. Opin. Pharmacol. 2004a;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Introduction: P2 receptors. Curr. Top. Med. Chem. 2004b;4:793–803. doi: 10.2174/1568026043451014. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G.E., KNIGHT G.E. Cellular distribution and functions of P2 receptor subtypes in different systems. Int. Rev. Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., WARLAND J.J.I. A pharmacological study of the rabbit saphenous artery in vitro: a vessel with a large purinergic contractile response to sympathetic nerve stimulation. Br. J. Pharmacol. 1987;90:111–120. doi: 10.1111/j.1476-5381.1987.tb16830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., RAND M.J. The inhibitory innervation of the taenia of the guinea-pig caecum. J. Physiol. 1966;182:504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., DUMSDAY B., SMYTHE A. Atropine resistant excitation of the urinary bladder: the possibility of transmission via nerves releasing a purine nucleotide. Br. J. Pharmacol. 1972;44:451–461. doi: 10.1111/j.1476-5381.1972.tb07283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M.E. Inhibition of the smooth muscle of the taenia coli. Nature. 1963;200:581–582. doi: 10.1038/200581a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., BENNETT M., HOLMAN M.E. Innervation of the guinea-pig taenia coli: are there intrinsic inhibitory nerves which are distinct from sympathetic nerves. Int. J. Neuropharmacol. 1964;3:163–166. doi: 10.1016/0028-3908(64)90003-6. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., SATCHELL D., SMYTHE A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., COCKS T., CROWE R., KASAKOV L. Purinergic innervation of the guinea-pig urinary bladder. Br. J. Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN M., GU J.G. A P2X receptor-mediated nociceptive afferent pathway to lamina I of the spinal cord. Mol. Pain. 2005;1:4. doi: 10.1186/1744-8069-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COCKAYNE D.A., HAMILTON S.G., ZHU Q.-M., DUNN P.M., ZHONG Y., NOVAKOVIC S., MALMBERG A.B., CAIN G., BERSON A., KASSOTAKIS L., HEDLEY L., LACHNIT W.G., BURNSTOCK G., MCMAHON S.B., FORD A.P.D.W. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O., JAMES G.W.L., SCHNEIDER C. Antagonism by aspirin and fenamates of bronchoconstriction and nociception induced by adenosine-5′-triphosphate. Nature. 1966;212:411–412. doi: 10.1038/212411a0. [DOI] [PubMed] [Google Scholar]
- DI VIRGILIO F., SOLINI A. P2 receptors: new potential players in atherosclerosis. Br. J. Pharmacol. 2002;135:831–842. doi: 10.1038/sj.bjp.0704524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRURY A.N., SZENT-GYÖRGYI A. The physiological activity of adenine compounds with special reference to their action upon the mammalian heart. J. Physiol. (London) 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMMELIN N., FELDBERG W. Systemic effects of adenosine triphosphate. Br. J. Pharmacol. Chemother. 1948;3:273–284. doi: 10.1111/j.1476-5381.1948.tb00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FEDAN J.S., HOGABOOM G.K., O'DONNELL J.P., COLBY J., WESTFALL D.P. Contributions by purines to the neurogenic response of the vas deferens of the guinea-pig. Eur. J. Pharmacol. 1981;69:41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- FIELDS R.D., STEVENS B. ATP: an extracellular signalling molecule between neurons and glia. Trends Neurosci. 2000;23:625–633. doi: 10.1016/s0166-2236(00)01674-x. [DOI] [PubMed] [Google Scholar]
- GALINDO A., KRNJEVIC K., SCHWARTZ S. Micro-iontophoretic studies on neurones in the cuneate nucleus. J. Physiol. 1967;192:359–377. doi: 10.1113/jphysiol.1967.sp008305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLIGAN J.J. Enteric P2X receptors as potential targets for drug treatment of the irritable bowel syndrome. Br. J. Pharmacol. 2004;141:1294–1302. doi: 10.1038/sj.bjp.0705761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREIG A.V.H., LINGE C., TERENGHI G., MCGROUTHER D.A., BURNSTOCK G. Purinergic receptors are part of a functional signalling system for proliferation and differentiation of human epidermal keratinocytes. J. Invest. Dermatol. 2003;120:1007–1015. doi: 10.1046/j.1523-1747.2003.12261.x. [DOI] [PubMed] [Google Scholar]
- HOEBERTZ A., ARNETT T.R., BURNSTOCK G. Regulation of bone resorption and formation by purines and pyrimidines. Trends Pharmacol. Sci. 2003;24:290–297. doi: 10.1016/S0165-6147(03)00123-8. [DOI] [PubMed] [Google Scholar]
- HOLLOPETER G., JANTZEN H.-M., VINCENT D., LI G., ENGLAND L., RAMAKRISHNAN V., YANG R.-B., NURDEN P., NURDEN A., JULIUS D., CONLEY P.B. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409:202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- HOLTON P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol. (London) 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JARVIS M.F. Contributions of P2X3 homomeric and heteromeric channels to acute and chronic pain. Expert Opin. Ther. Targets. 2003;7:513–522. doi: 10.1517/14728222.7.4.513. [DOI] [PubMed] [Google Scholar]
- KIRKPATRICK K., BURNSTOCK G. Sympathetic nerve-mediated release of ATP from the guinea-pig vas deferens is unaffected by reserpine. Eur. J. Pharmacol. 1987;138:207–214. doi: 10.1016/0014-2999(87)90434-1. [DOI] [PubMed] [Google Scholar]
- KING B.F., NORTH R.A.(eds) (2000Purines and the autonomic nervous system: from controversy to clinic J. Auton. Nerv. Syst. 811–299. [Google Scholar]
- KNIGHT G.E., BODIN P., DE GROAT W.C., BURNSTOCK G. ATP is released from guinea pig ureter epithelium on distension. Am. J. Physiol. Renal. Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- LAMMAS D.A., STOBER C., HARVEY C.J., KENDRICK N., PANCHALINGAM S., KUMARARATNE D.S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- LANGER S.Z., PINTO J.E.B. Possible involvement of a transmitter different from norepinephrine in residual responses to nerve stimulation of cat nicitating membrane after pretreatment with reserpine. J. Pharmacol. Exp. Ther. 1976;196:697–713. [PubMed] [Google Scholar]
- MARTINSON J., MUREN A. Excitatory and inhibitory effects of vagus stimulation on gastric motility in the cat. Acta Physiol. Scand. 1963;57:309–316. [Google Scholar]
- MCGARAUGHTY S., WISMER C.T., ZHU C.Z., MIKUSA J., HONORE P., CHU K.L., LEE C.H., FALTYNEK C.R., JARVIS M.F. Effects of A-317491, a novel and selective P2X3/P2X2/3 receptor antagonist, on neuropathic, inflammatory and chemogenic nociception following intrathecal and intraplantar administration. Br. J. Pharmacol. 2003;140:1381–1388. doi: 10.1038/sj.bjp.0705574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIHICH E., CLARKE D.A., PHILIOS F.S. Effect of adenosine analogs on isolated intestine and uterus. J. Pharmacol. Exp. Ther. 1954;111:335–342. [PubMed] [Google Scholar]
- NEARY J.T., RATHBONE M.P., CATTABENI F., ABBRACCHIO M.P., BURNSTOCK G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- NEILD T.O., HIRST G.D.S. The ‘γ-connection': a reply. Trends Pharmacol. Sci. 1984;5:56–57. [Google Scholar]
- PADDLE B.M., BURNSTOCK G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11:110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RONG W., GOURINE A., COCKAYNE D.A., XIANG Z., FORD A.P.D.W., SPYER K.M., BURNSTOCK G. Pivotal role of nucleotide P2X2 receptor subunit mediating ventilatory responses to hypoxia: knockout mouse studies. J. Neurosci. 2003;23:11315–11321. doi: 10.1523/JNEUROSCI.23-36-11315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RONG W., BURNSTOCK G. Activation of ureter nociceptors by exogenous and endogenous ATP in guinea pig. Neuropharmacology. 2004;47:1093–1101. doi: 10.1016/j.neuropharm.2004.08.003. [DOI] [PubMed] [Google Scholar]
- RYTEN M., HOEBERTZ A., BURNSTOCK G. Sequential expression of three receptor subtypes for extracellular ATP in developing rat skeletal muscle. Dev. Dyn. 2001;221:331–341. doi: 10.1002/dvdy.1147. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., BURNSTOCK G. ATP as a co-transmitter in rat tail artery. Eur. J. Pharmacol. 1984a;106:149–152. doi: 10.1016/0014-2999(84)90688-5. [DOI] [PubMed] [Google Scholar]
- SNEDDON P., BURNSTOCK G. Inhibition of excitatory junction potentials in guinea-pig vas deferens by α,β-methylene-ATP: further evidence for ATP and noradrenaline as cotransmitters. Eur. J. Pharmacol. 1984b;100:85–90. doi: 10.1016/0014-2999(84)90318-2. [DOI] [PubMed] [Google Scholar]
- SU C., BEVAN J.A., BURNSTOCK G. [3H]adenosine triphosphate: release during stimulation of enteric nerves. Science. 1971;173:337–339. doi: 10.1126/science.173.3994.336. [DOI] [PubMed] [Google Scholar]
- TSUDA M., SHIGEMOTO-MOGAMI Y., KOIZUMI S., MIZOKOSHI A., KOHSAKA S., SALTER M.W., INOUE K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- VLASKOVSKA M., KASAKOV L., RONG W., BODIN P., BARDINI M., COCKAYNE D.A., FORD A.P.D.W., BURNSTOCK G. P2X3 knockout mice reveal a major sensory role for urothelially released ATP. J. Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBB T.E., SIMON J., KRISHEK B.J., BATESON A.N., SMART T.G., KING B.F., BURNSTOCK G., BARNARD E.A. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 1993;324:219–225. doi: 10.1016/0014-5793(93)81397-i. [DOI] [PubMed] [Google Scholar]
- WYNN G., RONG W., XIANG Z., BURNSTOCK G. Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology. 2003;125:1398–1409. doi: 10.1016/j.gastro.2003.07.008. [DOI] [PubMed] [Google Scholar]
- YERXA B.R. Therapeutic use of nucleotides in respiratory and ophthalmic diseases. Drug Dev. Res. 2001;52:196–201. [Google Scholar]
- ZIMMERMANN H. Ectonucleotidases: some recent developments and a note on nomenclature. Drug Dev. Res. 2001;52:44–56. [Google Scholar]