Abstract
Well over 80 years ago Philip Smith described the beneficial clinical effects of adrenocortical extracts in animal models of adrenal insufficiency. In the ensuing years, scientists across the globe have sought to understand the mechanisms by which adrenal hormones and their synthetic analogues produce their complex and varied actions. Particular attention has focused on the glucocorticoids, partly because they have a vital place in the treatment of inflammatory and autoimmune disorders but also because dysregulation of the secretion and/or activity of endogenous glucocorticoids is increasingly implicated in a number of common disorders that pose a growing clinical burden, such as obesity, type II diabetes, the metabolic syndrome, hypertension and depression. This review considers some of the key advances that have been made in our understanding of the physiology, pathology and pharmacology of the glucocorticoids. Emphasis is placed on the molecular mechanisms of glucocorticoid signalling and the complex mechanisms that regulate the access of steroids in the systemic circulation to their receptors in their various target cells and tissues. In addition, consideration is given to the irreversible ‘organisational' actions of glucocorticoids in perinatal life and to the potential role of the steroids in the aetiology of disease.
Keywords: Glucocorticoids, HPA axis, glucocorticoid receptors
Introduction
Glucocorticoids are extraordinary hormones. They influence the activity of almost every cell in the body, they modulate the expression of approximately 10% of our genes, they are essential for life but are also increasingly implicated in the pathogenesis of disease and they produce many unwanted effects when given therapeutically; yet over 50 years after their introduction to the clinic as anti-inflammatory drugs, they still have a major place in the treatment of disease. Perhaps, not surprisingly, glucocorticoids have intrigued and frustrated many researchers for decades. This short review will highlight some of the major advances in glucocorticoid biology, which have occurred in recent years and consider briefly the physiological functions and mechanisms that control the secretion of these steroid hormones.
Endogenous glucocorticoids
Physiological functions
The principal endogenous glucocorticoids are cortisol and corticosterone. Both steroids are produced by most mammalian species, but the ratio in which they are secreted varies from species to species. Cortisol is the predominant glucocorticoid in man; rodents produce mainly corticosterone, whereas the sheep, pig and dog each produce significant amounts of both. Cortisol/corticosterone exert widespread actions in the body which are essential for the maintenance of homeostasis and enable the organism to prepare for, respond to and cope with physical and emotional stress (Sapolsky et al., 2000). They promote the breakdown of carbohydrate and protein and exert complex effects on lipid deposition and breakdown. They are also important regulators of immune and inflammatory processes and are required for numerous processes associated with host defence. These properties underlie many of the stress-protective actions of the steroids as they quench the pathophysiological responses to tissue injury and inflammation and, thereby, prevent them proceeding to a point where they threaten the survival of the host (Munck & Naray-Fejes-Toth, 1992). They also form the basis of their use as anti-inflammatory and immunosuppressive drugs. Endogenous glucocorticoids also exert a spectrum of other actions. They raise blood pressure, in part, through their ability to alter the sensitivity of tissues to catecholamines and exert ‘aldosterone-like' actions which modulate electrolyte and water balance. They have complex effects on bone, exert both positive and negative effects on cell growth and are proapoptotic. Within the central nervous system (CNS), glucocorticoids target both neurones and glial cells. During development, these actions underpin important organisational events in the brain, while in adulthood, they contribute to neuronal plasticity and are implicated in the processes of neurodegeneration. Other central effects include complex changes in mood and behaviour and modulation of food intake, body temperature, pain perception and neuroendocrine function (Pearson Murphy, 2002).
In conditions in which sustained and pronounced elevations in circulating glucocorticoids occur, due either to hypersecretion of endogenous steroids (Cushing's syndrome/disease) or prolonged administration of exogenous steroids, the above effects become exaggerated and a plethora of unwanted pathologies emerge. These include a significant redistribution of fat, giving rise to centripetal obesity together with a characteristic ‘moon face' and ‘buffalo hump'; protein wasting and associated muscle weakness; hyperglycaemia and insulin-resistant diabetes mellitus (‘steroid diabetes'); hypertension, raised cholesterol, altered serum lipids, salt and water retention; immunodeficiency, poor wound healing and loss of connective tissue leading to easy bruising; impaired growth and development; osteoporosis; menstrual irregularities, infertility and other endocrine-related changes; and depression and, sometimes, impaired cognitive function. Conversely, insufficient glucocorticoid secretion, which may arise from Addison's disease (an autoimmune disorder causing degeneration of the adrenal cortex), the adrenogenital syndrome (an inherited disorder of glucocorticoid synthesis) or pituitary disease, is characterised by a vulnerability to stress, white blood cell excess, lymphoid tissue hypertrophy, hypotension, mood disturbances, weakness/lethargy, weight loss and hypoglycaemia. Adrenal insufficiency is often insidious in onset and may go undetected until stress or illness precipitates a crisis. By contrast, acute adrenal insufficiency usually manifests as cardiovascular shock in previously undiagnosed patients.
For many years glucocorticoid-associated pathologies were associated solely with substantive long-term changes in the glucocorticoid milieu. More recent findings, however, indicate that quite subtle but sustained changes in glucocorticoid secretion and/or activity are potentially hazardous and may contribute to the pathogenesis of a number of common diseases, including hypertension and other cardiovascular disease; insulin resistance, obesity and type II diabetes; depression; autoimmune inflammatory disease; and reproductive dysfunction (De Kloet et al., 1998; Gold et al., 2002; Seckl, 2004b).
Control of secretion
Cortisol, the principal glucocorticoid in humans, and its rodent counterpart, corticosterone, are synthesised from cholesterol in cells of the zona fasciculata of the adrenal cortex. Their release into the systemic circulation is pulsatile and pulse amplitude varies according to a distinct circadian pattern. Serum glucocorticoid concentrations thus show a 3–5-fold change over 24 h, being maximal just before waking and declining thereafter to reach a nadir early in the sleep phase. Glucocorticoids are also released in response to physical and/or emotional trauma. This ‘stress response' is superimposed upon the existing circadian tone and varies in magnitude according to the nature, intensity and duration of the stimulus and the individual's previous experience (Buckingham et al., 1996).
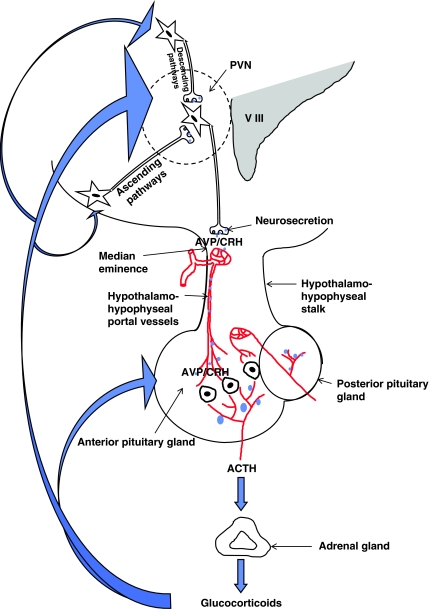
The circadian and stress-induced secretion of the glucocorticoids is governed by the hypothalamo-pituitary axis (Figure 1). The hypothalamus receives, monitors and integrates neural and humoral information from many sources. It thus acts as a sensor of changes in the external and internal environment. Using this information, the hypothalamus responds to adverse circumstances, be they physical or emotional, and circadian factors by activating the final common pathway to glucocorticoid synthesis. The first step is the release of two hypothalamic neurohormones, corticotrophin-releasing hormone (CRH) and arginine vasopressin (AVP), from parvocellular neurones that project from the paraventricular nucleus to the median eminence. CRH and AVP travel from the hypothalamus via the hypophyseal–portal blood vessels to the anterior pituitary gland where they act synergistically via specific receptors (type 1 corticotrophin-releasing hormone receptor and type 1b vasopressin receptor, respectively) to trigger the release of the adrenocorticotrophic hormone (corticotrophin, ACTH) from the corticotrophs (specific ACTH-producing cells) into the systemic circulation. ACTH in turn acts on the adrenal cortex via type 2 melanocortin receptors to initiate the synthesis of cortisol/corticosterone, which is released immediately into the systemic circulation by diffusion. The sensitivity of the hypothalamo-pituitary-adrenocortical (HPA) axis to incoming stimuli is modulated by a negative feedback (servo) system through which the sequential release of CRH/AVP and ACTH from the hypothalamus and anterior pituitary gland is suppressed by the glucocorticoids themselves. The magnitude of the HPA response to stress thus depends upon the pre-existing glucocorticoid tone (Buckingham et al., 1996).
Figure 1.
Diagram showing the hypothalamo-pituitary-adrenocortical (HPA) axis and principal loci of glucocorticoid feedback control. CRH=corticotrophin-releasing hormone; AVP=arginine vasopressin; and ACTH=adrenocorticotrophin hormone. Note that the intrahypothalamic parvocellular, CRH/AVP-secreting neurones originate in the paraventricular nucleus (PVN) and project to the median eminence where they terminate in close apposition to a capillary plexus (the primary plexus). CRH and AVP are secreted directly into these vessels and transported via the hypothalamo-hypophyseal portal system to the corticotrophs in the anterior pituitary gland. The CRH/AVP neurones are innervated by ascending nervous pathways from the brain-stem nuclei and by descending pathways from the limbic system and other centres (e.g. cortex). Circadian regulation is effected mainly via intrahypothalamic pathways from the suprachiasmatic nucleus (not shown). Local factors derived from glial cells (e.g. cytokines) and humoral factors (e.g. glucose) may also modulate the secretion of CRH/AVP. Similarly, locally produced substances also modulate the secretion of ACTH and glucocorticoids. Note (a) the concentration of AVP is considerably higher in hypothalamo-hypophyseal portal blood than in the systemic circulation; (b) vasopressin in the systemic circulation is derived from magnocellular neurones (not shown), which project from the PVN and supraoptic nuclei to the posterior pituitary gland.
Mechanisms of glucocorticoid action
Receptors
Glucocorticoids exert their actions principally via intracellular receptors which belong to the nuclear receptor superfamily and regulate the transcription of target genes. The biological actions of the steroids are thus generally slow in onset and persist for some time after the steroid has been cleared from the circulation. Although it was not recognised at the time, data from several early studies pointed to the existence of two receptors with differing affinity. For example, it was evident by the late 1970s that the dose regimens required to suppress basal ACTH secretion at the nadir of the circadian rhythm differed from those required to suppress the stress response (Buckingham & Hodges, 1974). Insight into these conundrums came with the characterisation of two distinct receptors, initially termed type 1 and type 2 corticosteroid receptors, but later renamed the mineralocorticoid receptor (MR) and glucocorticoid receptor (GR), respectively.
The MR has a high and approximately equal affinity (Kd approximately 0.5–2 nM) for cortisol, corticosterone and the mineralocorticoid, aldosterone. It has a restricted distribution, being localised mainly to the distal renal tubule and other cells/tissues concerned with Na+/K+ balance (e.g. sweat glands, parotid glands and colon), but is also found in specific brain regions, notably in neurones within the limbic system, entorhinal cortex and, to a lesser extent, the hypothalamus. The GR by contrast is of lower affinity (Kd cortisol/corticosterone approximately 10–20 nM), is glucocorticoid selective and does not bind aldosterone readily. GRs are widely distributed in the body; cell responsiveness depends not only on the presence of GRs but also on their concentration, which is known to fluctuate, for example, during development, during the cell cycle and following disturbances in endocrine status (De Kloet et al., 1998).
The free levels of endogenous glucocorticoids which diffuse from the circulation to bathe the tissues at the nadir of the circadian rhythm are in the region of 0.5–1 nM and considerably lower than those that occur at the peak of the circadian rhythm (20–30% of peak values), in stress or following administration of exogenous steroids. From this it was reasoned that the MR is responsible for mediating the effects of very low concentrations of glucocorticoids. However, when glucocorticoids levels are raised, for example in stress, when the free cortisol/corticosterone level may exceed 300 nM, the MRs are saturated and the GRs are responsible for mediating the observed biological effects. From these findings, it was easy to understand why hydrocortisone (cortisol) shows mineralocorticoid and glucocorticoid activity when used clinically in high doses and how small modifications of the cortisol molecule alter markedly the ratio of mineralocorticoid to glucocorticoid activity. The data did not, however, explain how MRs in tissues such as the kidney are preferentially regulated in vivo by aldosterone, the principal endogenous mineralocorticoid, despite their high affinity for cortisol/corticosterone. Answers to this problem emerged from studies that revealed tissue-specific mechanisms for the delivery of glucocorticoids to their receptors.
Delivery of endogenous glucocorticoids to their receptors
Access to target cells
While measurements of cortisol/corticosterone in the circulation provide a reasonable index of the activity of the HPA axis, they provide a surprisingly poor marker of delivery of the steroids to receptors in their target cells. Approximately 95% of cortisol/corticosterone in the circulation is bound to a carrier protein (corticosteroid-binding globulin, CBG). In principle, only the free steroid has ready access to target cells. However, in some cases (e.g. in inflamed tissues) local serine proteases facilitate delivery by liberating free steroid from its binding globulin, while in others (e.g. pituitary gland) locally expressed CBG may limit access by absorbing free steroid. The ability of glucocorticoids in the systemic circulation to reach target cells is also compromised by transporter proteins, which belong to the ATP-binding cassette (ABC) family and which lower intracellular glucocorticoid levels by actively extruding steroids from cells. These proteins, which are also called multidrug-resistant P-glycoproteins (MDR P-glycoproteins), are expressed in a tissue-specific manner and, like CBG, show substrate specificity. They thus provide a mechanism for tissue- and steroid-specific delivery of glucocorticoids to target cells, a phenomenon that may contribute to the subtle differences in pharmacological profile of various corticosteroids. In addition, dysregulation of these proteins may have a role in the development of glucocorticoid resistance. Particular interest has focused on the expression of MDRs in the blood–brain barrier as these appear to limit the access of steroids such as dexamethasone and, to a lesser extent, cortisol and corticosterone, to the brain (Meijer et al., 2003).
Pre-receptor metabolism
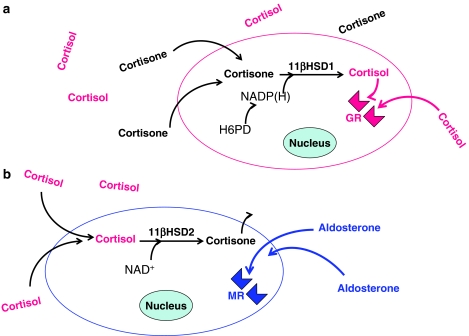
Probably the most important factor regulating the access of endogenous glucocorticoids to their receptors (GR or MR) is local metabolism of the steroids within the target cells themselves by 11β-hydroxysteroid dehydrogenase (11β-HSD) enzymes, a phenomenon sometimes termed pre-receptor metabolism. 11β-HSD catalyses the interconversion of cortisol and its inactive metabolite cortisone in humans (or corticosterone and 11-deoxycorticosterone in rodents; Figure 2). Its existence was first recognised in 1953, but it was not until the late 1980s, due largely to the seminal experiments of groups in Edinburgh and Melbourne, that the pivotal role of this enzyme family first became apparent.
Figure 2.
Diagram illustrating (a) the amplification of glucocorticoid action in GR-expressing cells by type 1 11β-hydroxysteroid dehydrogenase (11β-HSD1), which acts as a reductase and reactivates cortisone and (b) protection of mineralocorticoid receptors (MRs) by type 2 11β-hydroxysteroid dehydrogenase (11β-HSD2), which inactivates cortisol. Note that the reductase activity of 11β-HSD1 is dependent on the local generation of NADP(H) by hexose-6-phosphate dehydrogenase (H6PD). In rodents, 11β-HSD1 and 11β-HSD2 catalyse the interconversion of corticosterone and 11-deoxycorticosterone.
Stewart et al. (1988) described the first case of adult 11β-HSD deficiency as a condition of apparent mineralocorticoid excess characterised by hypertension, hypokalaemia, suppression of the renin–angiotensin system and impaired conversion of cortisol to cortisone. When established on a fixed Na+/K+ intake, the subject responded to dexamethasone with a reduction in urinary free cortisol (due to suppression of the HPA axis), natriuresis, K+ retention, increased plasma renin and a modest reduction in blood pressure. Addition of hydrocortisone to the treatment regimen reduced these beneficial effects by causing mineralocorticoid-like effects, which included Na+ retention, K+ loss, renin suppression and increased blood pressure. The authors concluded that the disease symptoms of 11β-HSD deficiency reflect activation of MR in the distal renal tubule by high intrarenal levels of cortisol. They further concluded that the kidney is a major site for the conversion of cortisol to cortisone, and that, in the normal state, intrarenal 11β-HSD inactivates cortisol and, thus, allows preferential binding of aldosterone to MR. Subsequent studies accorded with this view and revealed that MR-expressing tissues which are normally highly sensitive to aldosterone (kidney, parotid sweat glands and colon) show high levels of 11β-HSD activity. On the other hand, other tissues in which MRs are abundant (e.g. brain, heart) show little if any 11β-HSD activity, suggesting that that cortisol (or corticosterone) is the primary MR ligand at these sites (Edwards et al., 1996). These landmark studies opened a new chapter in the biology of glucocorticoids and, as described briefly below, it is now apparent that ‘pre-receptor' interconversion of active and inactive glucocorticoids plays a key role in determining not only the specificity of the MR but also the degree of GR activation in a number of tissues.
Two distinct isoforms of 11β-HSD have been cloned and characterised, type 1 and type 2 (11β-HSD1 and 11β-HSD2 (Edwards et al., 1996). Their properties are summarised in Table 1. Initially, attention focused on 11β-HSD2. This is a high-affinity, NAD+-dependent, constitutive enzyme that acts exclusively as a dehydrogenase. Results from in situ hybridisation and immunohistochemistry have confirmed data based on measures of enzyme activity that 11β-HSD2 is colocalised with the MR in tissues such as the kidney, parotid gland, sweat glands, colon and vascular smooth muscle cells. 11β-HSD2-null mice show a high degree of perinatal mortality; those that survive develop severe corticosterone-dependent hypertension and other features of apparent mineralocorticoid excess (Kotelevtsev et al., 1999) as also do patients with specific mutations of the 11β-HSD2 gene (Edwards et al., 1996) and animals or humans in which 11β-HSD2 activity is blocked with glycerrhetinic acid (the active component of liquorice) or its derivative carbenoxolone. 11β-HSD2 is also expressed in some tissues that lack MR, for example, placenta and developing brain, where it appears to provide protection from the potentially harmful effects of excess cortisol/corticosterone (Brown et al., 1996). Conversely, at loci in which 11β-HSD2 is not expressed (e.g. adult brain and cardiac myocytes) or is inactivated by a change in redox state (e.g. in damaged tissue), cortisol/corticosterone may act as the primary ligand of the MR (Funder, 2005).
Table 1. Comparison of type 1 and type 2 11β-HSD1 and 11β-HSD2.
| 11β-HSD1 | 11β-HSD2 | |
|---|---|---|
| Molecular mass | 34 kDa | 40 kDa |
| Principal function | Reductase | Dehydrogenase |
| Activity (Km) | Low affinity | High affinity |
| Cortisol: 17 μM | Cortisol:12 nM | |
| Corticosterone: 20 μM | Cortisosterone: 45 nM | |
| Cortisone: 200 μM | Dex: 140 nM | |
| Cortisone: NS | ||
| Aldosterone: NS | ||
| Cofactors | NADP(H) | NAD+ |
| Hexose-6-phosphate dehydrogenase | ||
| Distribution | Widespread | Discrete |
| Main loci | Liver | Renal tubule |
| Adipose tissue | Sweat glands | |
| Mature brain | Parotid glands | |
| Vasculature | Colon | |
| Developing brain | ||
| Placenta | ||
| Vasculature | ||
| Inhibitors | Glycerrhetinic acid | Glycerrhetinic acid |
| Carbenoxolone | Carbenoxolone | |
NS=not a substrate; Dex=dexamethasone.
The other isoform, 11β-HSD1, differs from 11β-HSD2 in many regards. It is expressed mainly in the liver, adipose tissue (particularly omental) and brain, but is also found in other tissues (see Table 1), and is subject to regulation by a variety of factors including glucocorticoids, stress, sex steroids, cytokines and perioxisome proliferator-activator receptor ligands. 11β-HSD1 is a low-affinity NADP(H)-dependent enzyme and in in vitro systems it shows bidirectional activity (i.e. both dehydrogenase and reductase). However, in vivo it appears to function solely as a reductase, relying on hexose-6-phosphate dehydrogenase (with which it is colocalised in the endoplasmic reticulum) to generate NADP(H) (Draper et al., 2003). 11β-HSD1 thus serves to regenerate biologically active cortisol/corticosterone from inert cortisone/11-dehydrocorticosterone.
As 11β-HSD1 is found mainly in tissues in which the high-affinity MR is sparse but the low-affinity GR is abundant, it has been argued that principal role of 11β-HSD1 is to amplify the local concentration of active glucocorticoids in those tissues in which the steroids have a key regulatory role, for example, the liver. Support for this hypothesis emerged from a number of studies, including phenotypic analysis of 11β-HSD1-null mice. These mice have raised corticosterone levels (due to impaired glucocorticoid feedback) but are resistant to the hyperglycaemia normally induced by stress or overfeeding; in addition, they show raised high-density lipoprotein cholesterol, lowered low-density lipoprotein cholesterol and reduced blood triglycerides. The metabolic responses appear to be driven by key changes which, by preventing amplification of the local corticosterone concentration, reduce gluconeogenesis and β-oxidation of lipids in the liver and possibly also attenuate glucocorticoid-dependent functions in visceral adipose tissue (Seckl, 2004a). Interestingly, when placed on a high fat diet, 11β-HSD1-null mice gain less weight than their wild-type counterparts and tend to deposit fat in subcutaneous rather than the visceral sites associated with metabolic disease. These and other results, including evidence that 11β-HSD1 blockade promotes insulin secretion, have led to the view that 11β-HSD1 may be an important factor in the development of insulin resistance, obesity and other metabolic disturbances. Consequently, drugs that block 11β-HSD1 selectively are now an important target for the pharmaceutical industry.
There is also a growing interest in the role of 11β-HSD1 in the brain, particularly in the hypothalamus, hippocampus, cortex and cerebellum where the enzyme is expressed in abundance. Of particular note is a recent study that demonstrated improved cognitive function in elderly subjects treated with an inhibitor of 11β-HSD1 and evidence from a genetic study that demonstrated an association between haplotypes of the 11β-HSD1 gene and susceptibility to Alzheimer's disease (Seckl, 2004a).
Signal transduction at GRs
Genomic actions
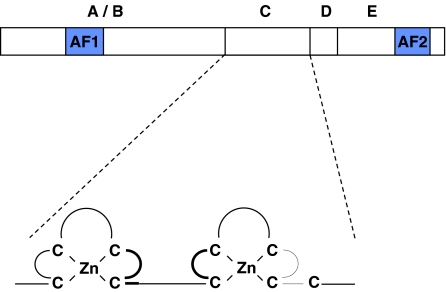
The GRs regulate, directly or indirectly, specific changes in DNA transcription and, hence, protein generation. They are located mainly in the cytoplasm as multiprotein complexes, which include various heat-shock proteins (e.g. hsp90). The GR shares a high degree of sequence homology with other members of the nuclear receptor family and consists of five distinct domains (Figure 3). The N-terminal A/B domains include activational function domain 1 (AF1), which facilitates transcriptional activity. The C-domain includes two cysteine-rich Zn2+ fingers and is responsible for receptor dimerisation and DNA binding. The D-domain or hinge region aids nuclear translocation as also does the C-terminal E-domain. The E-domain is also responsible for ligand binding, includes a second activational function domain 2 (AF-2) and may also have the ability to silence basal promoter activity.
Figure 3.
The five main domains of the GR, with some detail of the C-domain. The N-terminal A/B-domains include activational function domain 1 (AF-1), which facilitates transcriptional activity. The C-domain includes two cysteine-rich Zn2+ fingers and is responsible for receptor dimerisation and DNA binding. The D-domain or hinge region aids nuclear translocation as also does the C-terminal E-domain. The E-domain is also responsible for ligand binding, includes a second activational function domain 2 (AF-2) and may also have the ability to silence basal promoter activity. Mutations in the C-domain have given major insights into those facets of glucocorticoid action that are effected by receptor dimerisation and direct transcriptional regulation of the genome.
Early studies showed that activated GRs act as transcription factors, inducing or repressing the expression of target genes by direct interaction with specific glucocorticoid response elements (GREs) in the promoter region. Access of the ligand–receptor complex to the GREs is effected via a process involving dissociation of the chaperone proteins and sequential phosphorylation, dimerisation and nuclear translocation of the receptor. Access is also subject to regulation by intracellular proteins called co-activators and co-repressors. These proteins, which may be expressed in a cell- or tissue-specific manner, are recruited by the activated ligand–receptor complex (see also Jordan, this issue). They act, at least in part, as histone acetylases/deacetylases which modulate the structure of the core histone proteins that support the helical DNA structure, a process called chromatin remodelling. Co-activator proteins may thus facilitate access of RNA II polymerase and associated transcriptional complexes to target DNA sequences by acetylating key residues in the histone core and causing the DNA to unwind. Conversely, co-repressor modules limit the access of these regulatory molecules by histone deacetylation, which winds the DNA tightly around the histone core (Beato et al., 1995; Goulding, 2004).
In addition to acting as positive, or in some cases negative, transcription factors, activated GRs may also modulate gene transcription indirectly through protein–protein interactions. Initial evidence to this effect emerged from studies in cell lines, which revealed (a) that activated GRs repress activator protein 1 (AP-1, c-fos/c-jun) induced transcription of the collagenase gene, (b) that c-fos and c-jun effectively oppose the suppressive influence of GRs on collagenase expression and (c) that AP-1 and GR can be co-precipitated. Subsequent studies confirmed that GRs bind to AP-1 and thereby prevent its transcriptional activity. They also demonstrated that GR suppress transcription evoked by nuclear factor kappa B (NF-κB) but augment the responses to signal transduction and activator of transcription 5 (Stat-5) (Reichardt et al., 1998; Barnes & Adcock, 2003; Wintermantel et al., 2005). GRs may thus act as co-repressors or co-activators of other transcription factors as well as acting as transcription factors in their own right.
Further advances in our understanding of the significance of protein–protein interactions in effecting the actions of glucocorticoids came from analyses involving point mutations of the GR. Experiments in cultured cells focused on GR constructs with mutations in the DNA-binding and receptor dimerisation domains. The results demonstrated clearly that the transrepression effected through interactions with other transcription factors (e.g. AP-1 or NF-κB) does not require either dimerisation or DNA binding of the receptor. Further studies in vivo assessed the relative importance of GR signalling via DNA-dependent and -independent mechanisms. Mice with GRs deleted at birth as a result of lung actelectasis due to failure of surfactant production. In contrast, mice carrying a point mutation of the GR, which prevents receptor dimerisation (GRDim/Dim) are viable, indicating that transcriptional changes dependent on the binding of GR to DNA are not essential for survival (Reichardt et al., 1998). More detailed analyses of mice bearing this construct have given considerable insight into those facets of GR signalling that are mediated by direct transcriptional activity and those that involve interactions with other transcription factors. In particular, they have pointed to the importance of interactions with transcription factors, most notably NF-κB, in repressing the expression of pro-inflammatory cytokines (Barnes & Adcock, 2003). In addition, they have revealed a role for GR in promoting postnatal growth by serving as a co-activator of Stat-5 in the signalling pathway used by growth hormone. However, the majority of endocrine and metabolic actions of the steroids appear to be mediated by direct binding of GR dimers to DNA (i.e. transactivation).
These findings have led to a search for compounds that possess strong transrepressional activity via NF-κB or AP-1 (and hence ability to suppress the expression of proinflammatory genes), but have little direct transactivational activity. Such compounds should reduce the risk of systemic side effects when used as anti-inflammatory drugs. Several such compounds (termed dissociated steroids) have been shown to be promising in vitro (e.g. RU24858, RU40066), but the separation of anti-inflammatory and transactivational properties in vivo is disappointing, although a nonsteroidal GR agonist (ZK 216348) shows an improved therapeutic index relative to prednisolone (Barnes & Adcock, 2003). An important advance is the elucidation of the ligand-binding domain of GRs, as this has paved the way for improved design of dissociated steroids.
Nongenomic actions
Although glucocorticoid actions are effected mainly by changes in gene transcription, some occur too rapidly to be explained in this way. For example, cortisol hyperpolarises hippocampal neurones within 1–2 min contact and depresses the release of ACTH from the anterior pituitary gland over a similar time frame. The mechanisms responsible for these rapid effects are unknown, although there is evidence that they may reflect membrane perturbations, which compromise energy-dependent functions, alterations in Ca2+ flux or interactions with specific membrane bound G-protein-coupled receptors (Maier et al., 2005). Other data indicate that the nongenomic actions of glucocorticoids are effected via the intracellular GRs and that postreceptor signalling involves a complex kinase cascade. A role for the intracellular receptors is further supported by evidence that the activated GR complex may regulate post-transcriptional events including mRNA transcript stability, mRNA translation and post-translational processing. Such actions may be particularly relevant to steroid effects manifest before the full transcriptional effects of the genomic actions are apparent (Buckingham et al., 1996).
Splice variants of the GR
The responses to glucocorticoids often vary quite considerably between individuals. This may be explained, in part, by GR polymorphisms or haplotypes. However, increasing evidence suggests that differential expression of splice variants of the GR gene is important in this regard. Two splice variants, GR-β and GR-γ, are well characterised. GR-β is a C-terminally truncated GR variant which is unable either to bind ligand or to induce gene transcription. Its expression is augmented by proinflammatory cytokines (e.g. tumour necrosis factor α) and molecular studies suggest that it may act as a dominant-negative inhibitor of GR and, thereby, contribute to the phenomenon of glucocorticoid resistance (Goulding, 2004). GR-γ is the most widely expressed of the variants identified to date. Its amino-acid sequence includes an additional residue (arginine) between the two Zn2+ fingers of the DNA-binding domain. This renders the receptor less transcriptionally active than the native GR and its relative expression may thus affect tissue sensitivity to glucocorticoids (Stevens et al., 2004).
Glucocorticoid-regulated genes
The advent of microarray technology has facilitated the identification of numerous glucocorticoid-regulated genes. However, the picture is far from clear. Predictably, these studies have identified genes concerned with: inflammation and immunity, cell growth, differentiation and apoptosis; endocrine function and metabolism; signal transduction and membrane transport; neurotransmission; and bone turnover. However, they have also identified a large number of genes whose functions are either unknown or fall outside these categories. In addition, they have revealed a highly complex scenario in which the profile of differentially expressed genes differs according to the cell type(s) studied, the steroid contact time and the nature of the steroid itself. Transferred to the situation in vivo, the expression profile will inevitably be modified further by the local milieu, which will also include the many other factors that contribute to the regulation of these target genes.
While difficult to interpret, the data from microarrays illustrate admirably the amazing complexity of glucocorticoid action and the resultant capacity of the steroids to exert very fine control over a broad range of physiological and pathophysiological functions. This is amply illustrated by consideration of the plethora of glucocorticoid-regulated genes concerned with the processes of inflammation (reviewed in Barnes & Adcock, 2003; Goulding, 2004). The gene encoding the anti-inflammatory protein annexin 1 (ANXA1, also termed lipocortin 1) was one of the first of these to be identified.
ANXA1 was originally identified by Flower & Blackwell (1979) as a glucocorticoid-inducible protein, which inhibits the activity of phospholipase A2, and hence the generation of proinflammatory eicosanoids. Subsequent studies using immunoneutralisation strategies, ANXA1 peptides and, most recently, ANXA1-null mice have confirmed and extended our understanding of the role of this protein in the control of inflammatory responses (Figure 4; Hannon et al., 2003). In addition, they have identified other roles of ANXA1, most notably in the regulation of neuroendocrine function, cell growth, differentiation and apoptosis (Croxtall et al., 2003; John et al., 2004). However, the mode of action of ANXA1 remains poorly understood. In some instances, it appears to act as an intracellular signalling molecule. In others, it serves as a mediator of cell–cell communication. This is perhaps best exemplified in the neuroendocrine system. In the anterior pituitary gland, ANXA1 is expressed by the nonsecretory folliculostellate cells where it is found mainly in the projections that make contact with the endocrine cells (Figure 5). Glucocorticoids cause rapid serine phosphorylation and translocation of the protein to the cell surface where it appears to suppress the secretion of ACTH by acting on membrane-bound receptors. The nature of these receptors awaits definition, but data from studies on both the host defence and neuroendocrine systems suggest that members of the formyl peptide receptor family may be important in this regard (Perretti, 2003; John et al., 2004). ANXA1 may thus target receptors recognised by bacterial peptides and also by the anti-inflammatory lipoxin molecules.
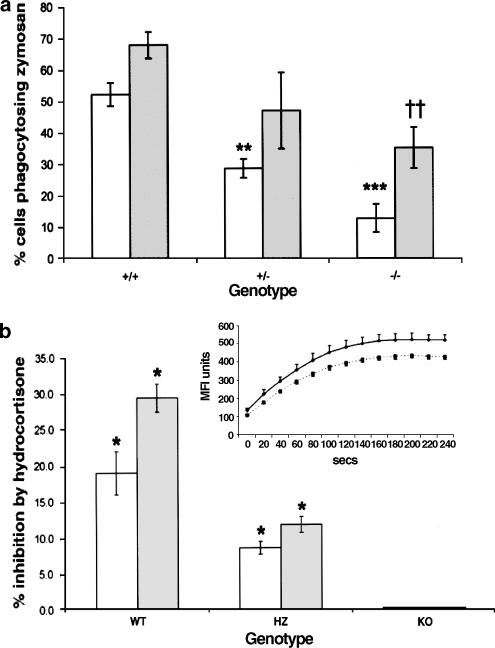
Figure 4.
Phagocytic behaviour of leucocytes from ANXA1−/− mice. (a) Phagocytosis of untreated (light shading) or opsonised (dark shading), zymosan by peritoneal macrophages from ANXA1+/+, ANXA1+/− and ANXA1−/− mice. Pooled data from two experiments were analysed relative to ANXA1+/+ controls, using two-tailed Student's t-test; n=6 per group. **P<0.01; ***P<0.001, significantly different from Anx-1+/+ opsonised values. ††P<0.01 different from ANXA1+/+ unopsonised values. (b) Inhibition by 10 μM hydrocortisone of IgG phagocytosis in peritoneal macrophages from different genotypes, using the RedOxyburst technique. Each experiment is typical of four similar experiments. White columns, % inhibition of reaction maximum; shaded columns, % inhibition of reaction rate. *P<0.05; relative to vehicle-treated controls. Inset: Typical trace showing inhibition of reaction by 10 μM hydrocortisone. Each point on the kinetic reaction plot above is the mean of three readings and the error bars represent standard errors of the mean. Reprinted from Hannon et al. (2003) with permission.
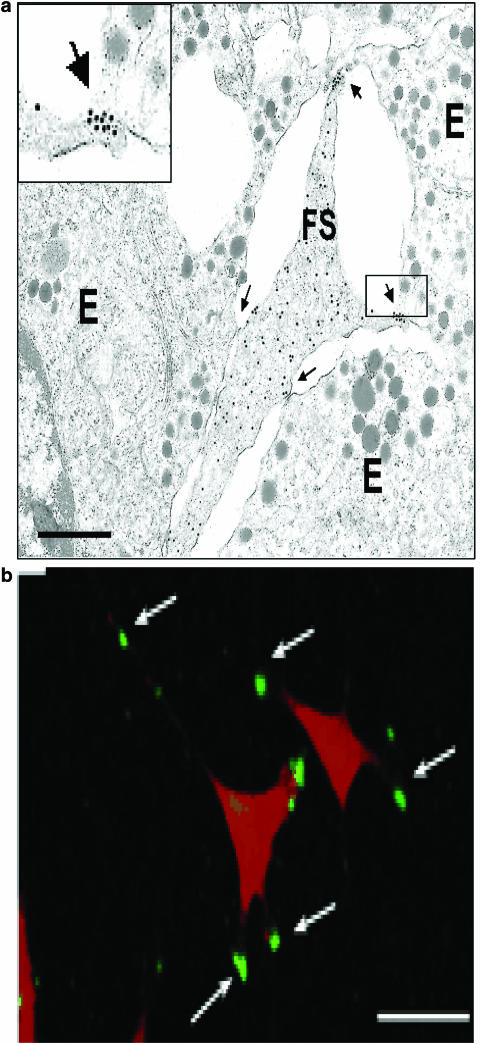
Figure 5.
Expression of ANXA1 (annexin 1) in folliculostellate (FS) cells. (a) Electron micrograph from a freeze substituted mouse anterior pituitary section showing immunogold detection of ANXA1 in an FS cell adjacent to three endocrine (E) cells. Gold particles (15 nm) are scattered over the cytoplasm and adjacent to the plasma membrane of the cell; they are also localised on the FS cell surface at the ends of processes contacting endocrine cells (see enlarged inset). Arrows indicate intercellular junctions. Scale bar: 1 μm. (b) Immunofluorescence staining of ANXA1 in a murine follicuostellate (TtT/GF) cell line. Surface ANXA1 fluorescence (green) is evident on processes of TtT/GF cell (nucleic acids stained red with propidium iodide). Arrows indicate bright patches of cell surface ANXA1. Scale bars: 20 μm. Reprinted from Chapman et al. (2002) with permission.
Organisational actions of glucocorticoids
Glucocorticoids and development
Glucocorticoids play an important role in the regulation of growth and development. For example, they are required for the normal maturation of the lung and the CNS and play a critical role in the control of postnatal growth. Many of these actions are ‘activational', that is, they are reversed in the absence of steroid. However, some actions of the steroids in development are irreversible or ‘organisational' and effectively ‘programme' adult physiology. Disturbances in the glucocorticoid milieu at critical stages of development may thus have long-term and potentially harmful effects on physiology, thereby influencing disease susceptibility. Perhaps not surprisingly, glucocorticoid levels are normally maintained within very narrow levels during development. Furthermore, in rodents at least, the neonate is refractory to stress and is thus protected from the potentially harmful effects of raised corticosteroid levels (Buckingham et al., 1997).
The foetal origin of disease
Evidence that adverse events in early life may increase disease susceptibility in adulthood first emerged from the work of Barker's group which noted the correlation between low birth weight and increased risk of cardiovascular and metabolic disorders in later life (Barker et al., 1989). Subsequent studies in rodents, pigs, sheep and humans linked malnutrition and stress in early life to an array of common adult pathologies, including hypertension, coronary heart disease, impaired glucose tolerance, hyperlipidaemia, type II diabetes mellitus and CNS disturbances, including anxiety and locomotor dysfunction. The molecular mechanisms underpinning these events are a focus of much current research with evidence supporting roles for genetic factors, nutrients and mediators such as growth factors, cytokines and hormones. Support for a role for glucocorticoids emerged from several sources. Firstly, malnutrition and early life stress produce permanent tissue-specific changes in GR expression, in particular causing downregulation of GR within the HPA axis, and thereby impairing the negative feedback regulation of glucocorticoid secretion in adulthood. Secondly, many of the pathological consequences of early life stress or malnutrition are similar to those of Cushing's syndrome, that is, hypertension, insulin resistance, osteoporosis and behavioural changes. Thirdly, the developing foetus is protected from fluctuations in maternal cortisol by expression of high levels of 11β-HSD2 in the placenta, which effectively captures and inactivates cortisol before it reaches the foetus. Correlations between low birth weight and placental 11β-HSD2 have been described. Moreover, rats exposed in utero to the nonselective 11β-HSD2 inhibitor, carbenoxolone, develop hypertension in adult life (Seckl, 2004b).
More direct evidence of a role for glucocorticoids has emerged from studies in experimental animals exposed to dexamethasone during perinatal life. Unlike cortisol and corticosterone, dexamethasone is a poor substrate for 11β-HSD2 and, thus, passes readily from the mother to the developing foetus (Edwards et al., 1996). Furthermore, in the neonate, dexamethasone has ready access to brain tissues due to the immaturity of the ABC transporter, P-glycoprotein, in the blood–brain barrier.
Consequences of perinatal glucocorticoid treatment
Early studies indicated that rats treated with glucocorticoids in the first 7 days of postnatal life show significant changes in CNS function in adulthood including loss of GRs, altered monoamine turnover, deficits in motor coordination, hyper-reactivity with stereotypy, impaired conditioned avoidance and disturbances in reproductive function (Olton et al., 1975; Benesova & Pavlik, 1989); in addition, basal and stress-induced HPA function is attenuated. Subsequent studies have focused mainly on the impact of glucocorticoid treatment during gestation.
In rats, prenatal dexamethasone treatment, given either throughout gestation or during the week before parturition, produces tissue-specific changes in GR expression together with an array of metabolic, cardiovascular, neuroendocrine and behavioural pathologies in adulthood (Kofman, 2002; Seckl, 2004b). The exact profile is dependent on the time and duration of glucocorticoid exposure and the sex of the individual. Similar but less extensive findings have been reported in the sheep and the guinea-pig. In the rat, prenatal dexamethasone treatment augments the basal and stress-induced activity of the adult HPA axis. These changes have been attributed, in part, to downregulation of the GRs within the HPA axis, in particular the hippocampus, and consequent diminution of the negative feedback effects of glucocorticoids on the secretion of ACTH, although other adaptive responses may contribute to the overall effect. The hyperactivity of the HPA axis is accompanied by hypertension in the adult rat. This may be partly explained by the raised corticosteroid levels, but the ‘programmed' rats also exhibit altered responses to vasoactive substances such as endothelin and increased expression of type 1 and type 2 angiotensin receptors. Moreover, in the sheep, baroreceptor reflexes are impaired by antenatal glucocorticoid treatment (O'Regan et al., 2004; Seckl, 2004b).
Prenatal dexamethasone treatment in both the rat and the sheep induces hyperglycaemia and hyperinsulinaemia in adulthood. These changes are accompanied not only by raised serum glucocorticoids but also by increased GR expression in the liver and visceral fat pads and, hence, increased tissue sensitivity to the diabetogenic properties of the steroid. A consequence of this is the upregulation of hepatic phosphoenolpyruvate carboxykinase (PEPCK), an enzyme which is rate limiting in gluconeogenesis and which, when overexpressed, impairs glucose tolerance. The consequences of raised GR expression in adipose tissue require further study, but may contribute to the manifestation of insulin resistance (Seckl, 2004b).
Diverse behavioural changes occur in adult rats subjected to antenatal glucocorticoid treatment, with increased fear and anxiety-type behaviours being reported by several groups (Kofman, 2002). These have been associated with changes in the amygdala where expression of the anxiogenic peptide CRH is increased. As glucocorticoids increase CRH expression in the central nucleus of the amygdala and basal corticosterone levels, and GR expression in the amygdala are both augmented by antenatal glucocorticoid treatment, it is plausible that the glucocorticoids have a causal role. In addition, there is evidence that prenatal glucocorticoid treatment produces long-term changes in central dopaminergic systems in rodents (Diaz et al., 1997) and impairments of myelination in sheep.
Clinical implications
To what extent the observations made in experimental animals can be translated to humans is unclear. However, there is cause for concern as synthetic glucocorticoids, for example, dexamethasone, are used in perinatal medicine to mature the lung in conditions of threatened or actual preterm birth. A limited number of cohort studies have provided evidence of impairments in cognitive function and a higher incidence of cerebral palsy in children aged 4 years and of raised blood pressure and increased frequency of infectious disease. However, such data need to be verified by randomised controlled trials, which take into account differences in the dosing regimen and type of corticosteroid employed (Kofman, 2002; Seckl, 2004b).
Glucocorticoids and disease
Glucocorticoids were first introduced for the treatment of chronic inflammatory disease (e.g. rheumatoid arthritis) well over 50 years ago and, despite their multiple unwanted effects, they remain the most effective means of controlling this debilitating disease (see also Barnes, this issue). For many years, the clinically useful anti-inflammatory and immunosuppressive properties of the steroids were considered to be ‘pharmacological' and unrelated to the homeostatic physiological actions of endogenous glucocorticoids. However, the pioneering work of Munck and colleagues (reviewed in Munck & Naray-Fejes-Toth, 1992) challenged this dogma and a large body of data now supports the premise that endogenous glucocorticoids have a fundamental role in the regulation of host defence processes and that dysregulation of glucocorticoid secretion and/or action may contribute to the pathogenesis of immune or inflammatory disease.
Increasingly, glucocorticoids are also implicated in the aetiology of other common diseases, for example, hypertension, obesity, type II diabetes and depression. In many cases, their pathological effects appear to be primarily related to dysregulation of the processes regulating the delivery of the steroids to their receptors, receptor dysfunction or the potential consequences of inappropriate early life programming rather than alterations in cortisol secretion itself. However, abnormalities in HPA function have been described in a number of psychiatric conditions. For example, increased HPA activity is common in major depression, anorexia nervosa, obsessive-compulsive disorders and panic states, whereas HPA function is generally reduced in post-traumatic stress and seasonal affective disorders. Particular interest has centred on the role of cortisol in severe depression (Pariante et al., 2004).
Hyperactivity of the HPA axis in major depression is one of the most consistent findings in biological psychiatry. A substantial proportion of patients show raised concentrations of cortisol in the plasma, urine and cerebrospinal fluid, exaggerated adrenocortical responses to exogenous ACTH and enlarged pituitary and adrenal glands. In addition, CRH levels in the hypothalamus and cerebrospinal fluid are increased and CRH is now strongly implicated in the manifestation of the disease symptoms (e.g. decreased appetite, psychomotor disturbances and altered sleep patterns). These changes have been attributed, in part, to failure of the negative feedback effects of cortisol in the brain and pituitary gland, a hypothesis that is supported by evidence that depressed patients are relatively unresponsive to the suppressive effects of dexamethasone on basal and CRH-stimulated ACTH release and on CRH levels in the cerebrospinal fluid. These and other data have led to suggestions that cortisol resistance may have a causal role in the pathogenesis of depression (Pariante et al., 2004; Ridder et al., 2005). In support of this premise, a number of structurally unrelated antidepressant drugs augment brain GR expression and enhance the sensitivity of the HPA axis to glucocorticoid feedback inhibition when given long-term in both normal animals and in animal models of disease (Pariante et al., 2004). These data are consistent with clinical reports that the beneficial effects of antidepressant drugs are associated with normalisation of HPA function as also do data suggesting that antidepressants augment GR signalling by inhibiting the membrane steroid transporters and, thereby, increasing the intracellular concentrations of the steroid (Muller et al., 2004; Pariante et al., 2004). These and other data support the premise that defective cortisol signalling plays an important part in the pathogenesis of depression and that characterisation of the underlying molecular defects may identify new targets for therapeutic intervention.
Conclusions
A short review cannot possibly do justice to the literature surrounding the glucocorticoids. Suffice it to say that, in recent years, our understanding of the biology of the glucocorticoids has increased exponentially as increasing layers of complexity have been uncovered and explored. These findings have given significant insight into the critical role of these steroid hormones in the maintenance of homeostasis and, when dysregulated, the pathogenesis of disease. They have also made major contributions to our understanding of the molecular basis of the advantageous and unwanted effects of the steroids as drugs and paved the way for the development of more selective compounds.
Acknowledgments
I am grateful to the Wellcome Trust, BBSRC, Pfizer and GSK for their support of my group's work on the HPA axis.
Glossary
- ACTH
adrenocorticotrophic hormone
- AF domains
activational function domains
- ANXA1
annexin 1
- AP-1
activator protein 1
- AVP
arginine vasopressin
- 11β-HSD
11 beta-hydroxysteroid dehydrogenase
- CBG
corticosteroid-binding globulin
- CRH
corticotrophin-releasing hormone
- Dim
dimerisation
- GR
glucocorticoid receptor
- GRE
glucocorticoid response element
- HPA
hypothalamo-pituitary-adrenocortical
- MR
mineralocorticoid receptor
- MDR P-glycoprotein
multidrug-resistant P-glycoprotein
- NF-κB
nuclear transcription factor kappa B
- Stat-5
signal transduction and activator of transcription 5
References
- BARKER D.J., OSMOND C., GOLDING J., KUH D., WADSWORTH M.E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ. 1989;298:564–567. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNES P.J., ADCOCK I.M. How do corticosteroids work in asthma. Ann. Intern. Med. 2003;139:359–370. doi: 10.7326/0003-4819-139-5_part_1-200309020-00012. [DOI] [PubMed] [Google Scholar]
- BEATO M., HERRLICH P., SCHUTZ G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- BENESOVA O., PAVLIK A. Perinatal treatment with glucocorticoids and the risk of maldevelopment of the brain. Neuropharmacology. 1989;28:89–97. doi: 10.1016/0028-3908(89)90073-7. [DOI] [PubMed] [Google Scholar]
- BROWN R.W., DIAZ R., ROBSON A.C., KOTELEVTSEV Y.V., MULLINS J.J., KAUFMAN M.H., SECKL J.R. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM J.C., HODGES J.R. Interrelationships of pituitary and plasma corticotrophin and plasma corticosterone in adrenalectomized and stressed, adrenalectomized rats. J. Endocrinol. 1974;63:213–222. doi: 10.1677/joe.0.0630213. [DOI] [PubMed] [Google Scholar]
- BUCKINGHAM J.C., CHRISTIAN H.C., GILLIES G.E., PHILIP J.G., TAYLOR A.D.1996The hypothalamo-pituitary-adrenocortical immune axis The Physiology of Immunityeds. Kendall, M.D. & Marsh, J.A. pp. 331–354.Boca Raton, FL: CRC Press Inc [Google Scholar]
- BUCKINGHAM J.C., FLOWER R.J., KIKKERT R., LOXLEY H.D. Secretion of corticotrophin-releasing hormone and arginine vasopressin by the neonatal rat hypothalamus in vitro: age-dependent maturation of the responses to cytokines and modulation by glucocorticoids and lipocortin 1. Dev. Dysfunct. 1997;10:482–502. [Google Scholar]
- CHAPMAN L., NISHIMURA A., BUCKINGHAM J.C., MORRIS J.F., CHRISTIAN H.C. Externalization of annexin I from a folliculo-stellate-like cell line. Endocrinology. 2002;143:4330–4338. doi: 10.1210/en.2002-220529. [DOI] [PubMed] [Google Scholar]
- CROXTALL J.D., GILROY D.W., SOLITO E., CHOUDHURY Q., WARD B.J., BUCKINGHAM J.C., FLOWER R.J. Attenuation of glucocorticoid functions in an Anx-A1−/− cell line. Biochem. J. 2003;371:927–935. doi: 10.1042/BJ20021856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE KLOET E.R., VREUGDENHIL E., OITZL M.S., JOELS M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- DIAZ R., FUXE K., OGREN S.O. Prenatal corticosterone treatment induces long-term changes in spontaneous and apomorphine-mediated motor activity in male and female rats. Neuroscience. 1997;81:129–140. doi: 10.1016/s0306-4522(97)00141-3. [DOI] [PubMed] [Google Scholar]
- DRAPER N., WALKER E.A., BUJALSKA I.J., TOMLINSON J.W., CHALDER S.M., ARLT W., LAVERY G.G., BEDENDO O., RAY D.W., LAING I., MALUNOWICZ E., WHITE P.C., HEWISON M., MASON P.J., CONNELL J.M., SHACKLETON C.H., STEWART P.M. Mutations in the genes encoding 11beta-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat. Genet. 2003;34:434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- EDWARDS C.R., BENEDIKTSSON R., LINDSAY R.S., SECKL J.R. 11 Beta-hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids. 1996;61:263–269. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- FLOWER R.J., BLACKWELL G.J. Anti-inflammatory steroids induce biosynthesis of a phospholipase A2 inhibitor which prevents prostaglandin generation. Nature. 1979;278:456–459. doi: 10.1038/278456a0. [DOI] [PubMed] [Google Scholar]
- FUNDER J.W. Mineralocorticoid receptors: distribution and activation. Heart Fail. Rev. 2005;10:15–22. doi: 10.1007/s10741-005-2344-2. [DOI] [PubMed] [Google Scholar]
- GOLD P.W., DREVETS W.C., CHARNEY D.S. New insights into the role of cortisol and the glucocorticoid receptor in severe depression. Biol. Psychiatry. 2002;52:381–385. doi: 10.1016/s0006-3223(02)01480-4. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J. The molecular complexity of glucocorticoid actions in inflammation – a four-ring circus. Curr. Opin. Pharmacol. 2004;4:629–636. doi: 10.1016/j.coph.2004.06.009. [DOI] [PubMed] [Google Scholar]
- HANNON R., CROXTALL J.D., GETTING S.J., ROVIEZZO F., YONA S., PAUL-CLARK M.J., GAVINS F.N., PERRETTI M., MORRIS J.F., BUCKINGHAM J.C., FLOWER R.J. Aberrant inflammation and resistance to glucocorticoids in annexin 1−/− mouse. FASEB J. 2003;17:253–255. doi: 10.1096/fj.02-0239fje. [DOI] [PubMed] [Google Scholar]
- JOHN C.D., CHRISTIAN H.C., MORRIS J.F., FLOWER R.J., SOLITO E., BUCKINGHAM J.C. Annexin 1 and the regulation of endocrine function. Trends Endocrinol. Metab. 2004;15:103–109. doi: 10.1016/j.tem.2004.02.001. [DOI] [PubMed] [Google Scholar]
- KOFMAN O. The role of prenatal stress in the etiology of developmental behavioural disorders. Neurosci. Biobehav. Rev. 2002;26:457–470. doi: 10.1016/s0149-7634(02)00015-5. [DOI] [PubMed] [Google Scholar]
- KOTELEVTSEV Y., BROWN R.W., FLEMING S., KENYON C., EDWARDS C.R., SECKL J.R., MULLINS J.J. Hypertension in mice lacking 11beta-hydroxysteroid dehydrogenase type 2. J. Clin. Invest. 1999;103:683–689. doi: 10.1172/JCI4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIER C., RUNZLER D., SCHLINDLER J., GRABNER G., WALDHAUS W., KOHLER K., LUGER A. G-protein-coupled glucocorticoid receptors on the pituitary cell membrane. J. Cell Sci. 2005;118:3353–3361. doi: 10.1242/jcs.02462. [DOI] [PubMed] [Google Scholar]
- MEIJER O.C., KARSSEN A.M., DE KLOET E.R. Cell- and tissue-specific effects of corticosteroids in relation to glucocorticoid resistance: examples from the brain. J. Endocrinol. 2003;178:13–18. doi: 10.1677/joe.0.1780013. [DOI] [PubMed] [Google Scholar]
- MULLER M.B., UHR M., HOLSBOER F., KECK M.E. Hypothalamic-pituitary-adrenocortical system and mood disorders: highlights from mutant mice. Neuroendocrinology. 2004;79:1–12. doi: 10.1159/000076041. [DOI] [PubMed] [Google Scholar]
- MUNCK A., NARAY-FEJES-TOTH A. The ups and downs of glucocorticoid physiology. Permissive and suppressive effects revisited. Mol. Cell Endocrinol. 1992;90:C1–C4. doi: 10.1016/0303-7207(92)90091-j. [DOI] [PubMed] [Google Scholar]
- OLTON D.S., JOHNSON C.T., HOWARD E. Impairment of conditioned active avoidance in adult rats given corticosterone in infancy. Dev. Psychobiol. 1975;8:55–61. doi: 10.1002/dev.420080108. [DOI] [PubMed] [Google Scholar]
- O'REGAN D., KENYON C.J., SECKL J.R., HOLMES M.C. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am. J. Physiol. Endocrinol. Metab. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- PARIANTE C.M., THOMAS S.A., LOVESTONE S., MAKOFF A., KERWIN R.W. Do antidepressants regulate how cortisol affects the brain. Psychoneuroendocrinology. 2004;29:423–447. doi: 10.1016/j.psyneuen.2003.10.009. [DOI] [PubMed] [Google Scholar]
- PEARSON MURPHY B.E.2002Glucocorticoids, overview Encyclopaedia of Stressed. Kink, G. pp. 244–260.San Diego: Academic Press [Google Scholar]
- PERRETTI M. The annexin 1 receptor(s): is the plot unravelling. Trends Pharmacol. Sci. 2003;24:574–579. doi: 10.1016/j.tips.2003.09.010. [DOI] [PubMed] [Google Scholar]
- REICHARDT H.M., KAESTNER K.H., TUCKERMANN J., KRETZ O., WESSELY O., BOCK R., GASS P., SCHMID W., HERRLICH P., ANGEL P., SCHUTZ G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- RIDDER S., CHOURBAJI S., HELLWEG R., URANI A., ZACHER C., SCHMID W., ZINK M., HORTNAGL H., FLOR H., HENN F.A., SCHUTZ G., GASS P. Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 2005;25:6243–6250. doi: 10.1523/JNEUROSCI.0736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAPOLSKY R.M., ROMERO L.M., MUNCK A.U. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- SECKL J.R. 11beta-hydroxysteroid dehydrogenases: changing glucocorticoid action. Curr. Opin. Pharmacol. 2004a;4:597–602. doi: 10.1016/j.coph.2004.09.001. [DOI] [PubMed] [Google Scholar]
- SECKL J.R. Prenatal glucocorticoids and long-term programming. Eur. J. Endocrinol. 2004b;151 (Suppl 3):U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- STEVENS A., DONN R., RAY D. Regulation of glucocorticoid receptor gamma (GRgamma) by glucocorticoid receptor haplotype and glucocorticoid. Clin. Endocrinol. (Oxford) 2004;61:327–331. doi: 10.1111/j.1365-2265.2004.02097.x. [DOI] [PubMed] [Google Scholar]
- STEWART P.M., CORRIE J.E., SHACKLETON C.H., EDWARDS C.R. Syndrome of apparent mineralocorticoid excess. A defect in the cortisol–cortisone shuttle. J. Clin. Invest. 1988;82:340–349. doi: 10.1172/JCI113592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERMANTEL T.M., BERGER S., GREINER E.F., SCHUTZ G. Evaluation of steroid receptor function by gene targeting in mice. J. Steroid Biochem. Mol. Biol. 2005;93:107–112. doi: 10.1016/j.jsbmb.2004.12.033. [DOI] [PubMed] [Google Scholar]