Abstract
This article traces the development of knowledge about the physiology and pharmacology of acetylcholine and its receptors between 1930 and 2005, with emphasis on contributions by members of the British Pharmacological Society, and by other British pharmacologists and physiologists.
Keywords: Nicotinic receptors, muscarinic receptors, acetylcholine release
The early years
Over the first half of the last century, the story of acetylcholine was substantially authored by British physiologists and pharmacologists – though of course with some very distinguished co-authors such as Otto Loewi. Thus, in 1953, at a symposium on chemical transmission held in Philadelphia (Symposium, 1954), 17 out of 29 papers (most on acetylcholine, but some on noradrenaline) were from the U.K. Indeed, in summing up, R.W. Gerard was led to say
It is not entirely clear to me why I was asked to summarize and close this program. As the lone American (not true, actually: one paper was by G.B. Koelle from Philadelphia, and another by A.S. Marrazzi from Maryland – but perhaps they had left!) in this distinguished international galaxy and as one of the few physiologists amidst the pharmacological cohorts….(and so on).
Of those talking about acetylcholine, the U.K. contingent contained the following members of the British Pharmacological Society: H.H. Dale (Figure 1a), W.S. Feldberg (Figure 1b), W.D.M. Paton (Figure 1c), W.L.M. Perry, A.S.V. Burgen, E.J. Zaimis, J.H. Burn and N. Ambache, plus the physiologist M. de B. Daly – though, of course, most of their principal work was not published in the BJP, since the latter was not started until 1946.
Figure 1.
Some U.K. cholinergic pharmacological luminaries. (a) H.H. Dale; (b) W. Feldberg; (c) W.D.M. Paton. Photographs © the Royal Society.
What was known in 1930?
The seminal paper on the actions of acetylcholine was that of Dale (1914). This arose from his work at the Wellcome Physiological (not Pharmacological!) Research Laboratories, on extracts of ergot. Eight years earlier, Hunt & Taveau had reported the strong vasodepressor action of acetylcholine, and the ergot extract produced a similar effect. Dale and his colleagues thought the extract might contain muscarine (at that time not identified chemically), but the active principle, isolated by A.J. Ewins in the same year, turned out to be acetylcholine. In this paper, Dale compared the effects of acetylcholine with choline and with various other esters and ethers of choline, and came to the key division into ‘muscarine' and ‘nicotine' effects. Thus, to quote from his first conclusion:
In the action of choline, and, with varying degrees of intensification, in the action of certain ethers and esters of choline, two distinct types of action can be detected – a ‘muscarine' action, paralysed by atropine, and a ‘nicotine' action, paralysed by excess of nicotine.
He also noted that
As numerous writers have pointed out, [the ‘muscarine' action] may be summarized…….as a reproduction of the effects of stimulating nerves belonging to the cranial and sacral divisions of the involuntary (autonomic) system…
Notwithstanding, he goes on to say
The question of a possible physiological significance [in this resemblance] …is one of great interest, but one for the discussion of which little evidence is available. Acetyl-choline is, of all the substances examined, the one whose action is most suggestive in this direction……….On the other hand, there is no known depot of choline derivatives, corresponding to the adrenine depot in the adrenal medulla nor, indeed, is there any evidence that a substance resembling acetyl-choline exists in the body at all.
This evidence came forth 15 years later, when Dale & Dudley (1929) isolated acetylcholine from ox and horse spleen. By then, of course, Otto Loewi had published his papers establishing the concept of chemical transmission in the heart. Dale & Dudley recognized this – and, indeed, noted an earlier, and rather comparable, experiment on the heart by W.E. Dixon more than 20 years previously. Dixon, a founder-member of the BPS (see also Cuthbert, this issue), had shown that an extract of dog heart under vagal inhibition slowed an isolated frog heart, and explicitly suggested that the vagus nerve released a chemical substance ‘inhibitin' that
combining with the heart muscle, results in cardiac standstill.
Dale & Dudley also raised the question whether the release of acetylcholine might account for the contractures of denervated skeletal muscle on parasympathetic nerve stimulation, a ‘nicotinic' effect, noted by many workers as far back as Sherrington in 1894, thereby implicitly extending the chemical transmission hypothesis to nicotinic receptors. Thus:
If we suppose, on the analogy of Loewi's experiments on the heart vagus, that parasympathetic nerves produce their effects by liberation of a chemical stimulant, we must also credit this substance with a stimulant action on denervated voluntary muscles; it must, therefore, be a substance having the two types of activity which are exhibited by the choline esters, and by acetylcholine with unique intensity. (Dale & Dudley, 1929)
Acetylcholine post-1930
Acetylcholine as a neurotransmitter
It is clear that the discovery of acetylcholine in the body was, for Dale, the ‘clincher' for its putative neurotransmitter function. Immediately thereafter, there appeared an avalanche of papers from Dale's laboratory at the National Institute for Medical Research, published (primarily in the Journal of Physiology) with a number of colleagues who dominated U.K. pharmacology over the subsequent years. Between 1934 and 1936, Dale, Feldberg and collaborators established a role for acetylcholine as a neurotransmitter from the vagus nerve to the stomach, from sympathetic nerves to sweat glands (which Dale had earlier suggested might be anomalously cholinergic), and at the skeletal neuromuscular junction. In the interim (and as a clear prelude to the work on skeletal muscle), W. Feldberg and his colleagues – initially in Berlin but subsequently at the National Institute – established the cholinergic nature of transmission from preganglionic sympathetic nerves to the adrenal medulla and to the sympathetic ganglion.
Naturally, the concept of chemical transmission by acetylcholine was not universally accepted. It may seem quite plausible for the slow responses resulting from autonomic nerve stimulation, but how could it possibly explain the transmission of impulses across ganglionic synapses and at the neuromuscular junction with such short (ms) synaptic delays and with such short intervals between stimuli? This could hardly be answered satisfactorily with the technology available in the 1930s, so sides were drawn largely on the basis of the experimenter's own techniques (as is still frequently the case): pharmacologists, who like chemicals, were happy with chemical transmission, whereas electrophysiologists record electric currents or potentials so adhered to electrical transmission. This was not resolved for another 20 years or so, until the intracellular recordings of end-plate potentials by B. Katz and colleagues (Fatt & Katz, 1951), and, in particular, the demonstration by del Castillo & Katz (1957) that tubocurarine blocked the end-plate response to iontophoretically applied acetylcholine and to motor nerve stimulation with equal facility. Following the revolutionary discovery of single nicotinic receptor channels, the rapid time-course of the end-plate current could then be explained by the short life-time of the channel open state (Colquhoun, 1981), assuming a ‘one-hit' activation by released acetylcholine. The primary electrical events at autonomic synapses appear analogous (e.g., Rang, 1981; Mathie et al., 1987), though not yet studied in comparable detail and thus could pharmacology and electrophysiology be resolved.
But what about the central nervous system (CNS)? By the mid-50s, there was considerable circumstantial evidence for a role for acetylcholine in the CNS, such as the localized presence of choline acetyltransferase and acetylcholinesterase. This was summarized by W. Feldberg in the issue of Pharmacological Reviews referred to above (Symposium, 1954), and was soon to be supplemented by several demonstrations of the evoked release of acetylcholine from the cerebral cortex and into the cerebral ventricles (see Vogt, 1969). Add to this the well-known central effects of such drugs as atropine and hyoscine (scopolamine), and some function for acetylcholine seemed obligatory. The problem lay in the details. The first reasonably definitive evidence for a localized transmitter function came from Eccles et al. (1956), after his Pauline conversion from electrical to chemical transmission. Reasoning from ‘Dale's Principle' that the same chemical transmitter should be released from all terminals of the same neurone, he and his colleagues showed that the recurrent collaterals from cholinergic motor axons onto inhibitory interneurones (‘Renshaw cells') in the spinal cord were indeed cholinergic, in that excitation (recorded extracellularly) was enhanced by physostigmine and reduced by dihydro-β-erythroidine, with corresponding changes in the intracellularly recorded motoneurone recurrent ipsp (Eccles et al., 1956) (Intracellularly recorded Renshaw cell synaptic currents came much later and, interestingly, have revealed a minor component due to co-release of glutamate (e.g., Mentis et al., 2005)). This led to an orgy of experiments on the effects of acetylcholine and its congeners and antagonists on central neuronal electrical activity, assisted greatly by the introduction of iontophoretic methods for local drug application (e.g., McCance et al., 1968). However, though providing masses of valuable information, on the whole, this did not pinpoint any specific cholinergic synapses comparable to the Renshaw cell synapse. Retrospectively, one can suggest two main reasons for this.
First, one has the feeling that – faced with the example of fast nicotinic receptor-mediated transmission at the neuromuscular junction of striated muscle (NMJ) and an apparently similar receptor involvement at the Renshaw cell – investigators were expecting comparable events at other central synapses. In fact, only a few other clear instances of fast nicotinic synaptic transmission in the mammalian CNS have emerged (Jones et al., 1999). True, very many neurons have nicotinic receptors and exhibit inward currents in response to nicotinic agonists, but these receptors rarely seem to be involved in true cholinergic transmission. Some of these discrepancies are striking. Thus, the fasciculus retroflexus of Meynert (FRM), running from the medial habenula to the interpeduncular nucleus (IPN), contains the highest concentration of choline acetyltransferase of any fibre tract in the brain, and the interpeduncular neurones themselves have abundant nicotinic receptors. Hence, when we started experimenting on this system, we fully expected FRM–IPN transmission to be cholinergic, so we were quite astonished when it turned out to be glutamatergic! (Brown et al., 1983; McGehee et al., 1995).
Of course, nicotine itself has multiple effects on the brain. These (for smokers) are not all secondary to oral gratification, or to sensory stimulation, since characteristic effects can be seen on intracerebroventricular injection (e.g., Armitage et al., 1966). However, it now emerges that many, or most, of the effects of nicotine can be attributed to the activation of presynaptic receptors, with consequential enhancement or diminution in the release of other neurotransmitters (Wonnacott, 1997). These effects arise, not only from changes in excitability, but also from the entry of Ca2+ through Ca2+-permeable nicotinic channels (McGehee et al., 1995). Most important, perhaps, from a psychopharmacological viewpoint, is its effect on the release of dopamine and on the activation of dopaminergic neurones (Dani et al., 2001; see also Marsden, this issue), though it also increases the release of other transmitters, including acetylcholine itself. But nicotine is not a transmitter, and these receptors can hardly have been put in place to gratify a smoker's wishes: what remains a puzzle is when, or if, they actually see any acetylcholine (Sivilotti & Colquhoun, 1995).
A second problem was that most of the postsynaptic effects of synaptically released acetylcholine in the CNS are mediated not through nicotinic receptors, but through muscarinic receptors. These do not directly ‘gate' ion channels but produce effects through coupling to G-proteins (see below). In consequence, responses can be either excitatory or inhibitory and are very delayed, by several orders of magnitude, compared with the sub-millisecond delay in activating nicotinic channels. Excitatory synaptic responses usually, but not invariably, result from inhibition of one or more of the neuron's endogenous K+ currents; they take several hundreds of milliseconds to begin, can last for seconds, and are usually only manifest after repetitive afferent stimulation (e.g., Gahwiler & Brown, 1985). These are analogous to the slow muscarinic receptor-mediated responses previously recorded in peripheral tissues such as smooth muscle (Purves, 1974), sympathetic neurones (Adams & Brown, 1982) and myenteric neurones (North & Tokimasa, 1984). Incidentally, the presence of muscarinic receptors on nerve cells – in sympathetic ganglia – was, as usual, first shown by Dale in 1912, in experiments where he recorded the pupillodilator effect of pilocarpine applied to the cat's superior cervical ganglion. However, since this predates his 1914 paper, he called this effect ‘nicotine-like' rather than ‘muscarine-like'. In contrast, inhibitory responses result from the opening of K+ channels: these are rather faster and briefer (but still slow compared with, say, GABAA-mediated inhibition), and comparable to the response of cardiac cells to vagal stimulation (North, 1989; cf. Burgen & Terroux, 1953). These slow effects (excitatory or inhibitory) do not contribute directly to the ‘bit-by-bit' transmission required for the preservation of frequency coding in the CNS: this function is subsumed by glutamate and GABA or glycine. Hence, there is no necessity for ‘tight' synapses, and most cholinergic release sites (boutons or varicosities) do not show synaptic specializations (Descarries et al., 1997). Notwithstanding, the release process itself is still ‘fast', like that at the NMJ (Allen & Brown, 1996). A further complexity is that – like the nicotinic receptors – many of the muscarinic receptors are presynaptic, where their activation can either increase or reduce transmitter release. Functionally, the most important of these are likely to be those on cholinergic terminals, that is, autoreceptors. Thus, some time back, it was observed that atropine produced a very large increase in the evoked release of acetylcholine from the cerebral cortex (Mitchell, 1963). This is because atropine blocks the profound auto-inhibitory effect of acetylcholine on its own release from the processes of cholinergic basal forebrain neurones, from whence the cortical release stems (Allen & Brown, 1996).
Acetylcholine receptors
Although the pharmacological differences between nicotinic and muscarinic receptors are quite substantial, they are both stimulated by acetylcholine, and by carbachol. Hence, there was some astonishment when the two were first cloned and shown to be such totally different beasts. The nicotinic receptor is a pentameric subunit-assembled ion channel (e.g., Noda et al., 1982; Sumikawa et al., 1982; Karlin, 2002; Unwin, 2005; see also Bowman, this issue), whereas the muscarinic receptor, like the β-adrenergic receptor, is a homolog of the visual pigment, rhodopsin, and a member of the heptahelical, G-protein-coupled, receptor family (Fukuda et al., 1987; Peralta et al., 1987; see also Hill, this issue).
Muscarinic receptors
Prior to cloning, quite substantial evidence had accrued from pharmacological studies for at least two – possibly three – subtypes of the muscarinic receptor. This was suggested most directly by Burgen & Spero (1968) to explain the different agonist sensitivities and rank order for their effects on membrane K+ permeability and contraction in guinea-pig intestine – probably reflecting effects on M2 and M3 receptors (Zholos & Bolton, 1997). Further evidence came from receptor-binding studies that had shown the presence of at least two binding sites for agonists (high and low affinity) in rat brain membrane preparations. Subsequently, the antagonist pirenzepine was found to bind relatively selectively to the high-affinity site in rat brain, but with much lower affinity to smooth muscle and atria (Hammer et al., 1980), and this preferential affinity for ‘neural' muscarinic receptors over smooth muscle receptors was confirmed in functional studies (Brown et al., 1980). Further analysis revealed the presence of two types of receptor in the brain that bound pirenzepine with high and low affinity, respectively (Berrie et al., 1985). Thus, in a sense, it was not that surprising when, eventually, five subtypes (M1–M5) emerged from cloning experiments (Bonner et al., 1987), with differential coupling to G-proteins (M1, M3, M5 to the Gq/G11 family, and M2, M4 to the Gi/Go family), and, to some degree, differential sensitivities to some antagonists (Caulfield & Birdsall, 1998; Alexander et al., 2004).
However, no antagonist shows more than a 10-fold selectivity for any one subtype; so subtype identification requires the use of two or more antagonists – a problem further exacerbated by the coexpression of multiple subtypes in many tissues and even in the same cell. An alternative approach to selectivity is through allosteric antagonism, stemming from the work of Clark & Mitchelson (1976) on the effects of gallamine on the heart (M2 receptors), and further developed by Lazareno & Birdsall (1995). Another approach to identifying individual subtype functions is to study subtype gene-deficient ('knock-out') mice. This has led to some interesting results – some expected, such as the absence of vagal bradycardia in M2 knock-out mice, and some surprising, such as disturbances of hypothalamic food-intake regulation in M3 knock-out mice, loss of acetylcholine-induced cerebral vasodilatation in M5 knock-out mice (Birdsall et al., 2001; Wess, 2004).
What emerges is the great variety of central functions collectively regulated by muscarinic receptors, such as basal ganglion motor activity, analgesia and hypothalamic function (temperature, feeding). The significance of this (and other functions) for future drug development have been extensively discussed (Birdsall et al., 2001; Wess, 2004). Knock-outs can also be helpful in sorting out contributions of different subtypes in systems showing multiple subtype expression – for example, in dissecting the receptors responsible for presynaptic inhibition of transmitter release in the peripheral nervous system (Trendelenburg et al., 2005).
Structurally and functionally, the muscarinic receptor appears to show a close homology to rhodopsin. Like other G-protein-coupled receptors, muscarinic receptors can form dimers or oligomers, but these are apparently subtype-specific, that is, homodimers, not heterodimers (Zeng & Wess, 2000); so dimerization is unlikely to have pharmacological consequences.
Nicotinic receptors
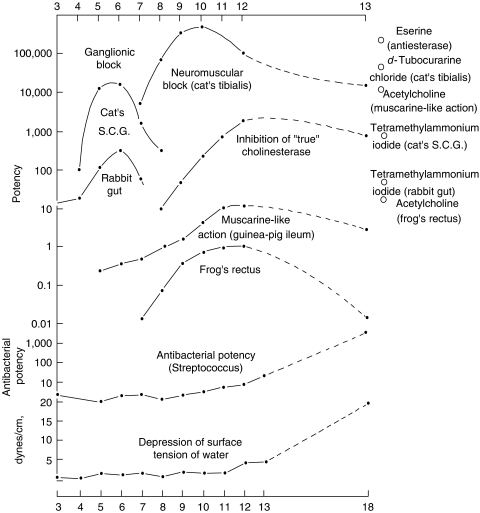
One of the most important post-1930 advances in nicotinic receptor pharmacology was the synthesis of the polymethylene bis-trimethylammonium (‘methonium') compounds, and the consequent demonstration of a clear difference between neural (ganglionic) and muscle (NMJ) receptors: the former were selectively blocked by hexamethonium and pentamethonium, whereas the NMJ receptor was first excited and then blocked by decamethonium (Paton & Zaimis, 1949) (Figure 2). This difference between nicotinic receptors was, apart from its scientific significance, also of immense clinical significance, since the fact that reducing the blood pressure with hexamethonium was actually life-saving in malignant hypertension was instrumental in persuading clinicians that the hypertension was indeed the cause of death, rather than an essential adaptation. This analysis provided the clinical impetus for all subsequent (and extensive) developments in antihypertensive pharmacotherapy. True, the selective ganglion-blocking action of tetraethylammonium (TEA) was already known, but TEA was too short-acting to be of much clinical use. Hexamethonium was a very imperfect drug from a practical viewpoint, with poor absorption and numerous side effects (amusingly summarized in Paton's caricature of ‘hexamethonium man' – see Text Box 1). These defects induced an avalanche of novel ganglion-blocking agents developed by U.K. and U.S. pharmaceutical companies, such as pentolinium (Mason & Wien, 1955), pempidine (Corne & Edge, 1958; Spinks et al., 1958), mecamylamine, trimetaphan and chlorisondamine, before alternative approaches to blood pressure control were developed (though some ganglion-blocking agents are still in use for selected purposes).
Figure 2.
Summary of the main pharmacological actions of the bistrimethylammonium series. Abscissa: number of carbon atoms in polymethylene chain. Ordinate: logarithmic scale of potency, with arbitrary origins (from Paton & Zaimis (1949), with permission).
Table 1. Hexamethonium Man.
| In his review of ‘Transmission and block in autonomic ganglia' at the 1953 Philadelphia meeting (Pharmacol. Rev., 6, 59–67), W.D.M. Paton wrote the following description of ‘hexamethonium man' as a description of the results of blocking autonomic ganglia: | |
| He is a pink complexioned person, except when he has stood for a long time, when he may get pale and faint. His handshake is warm and dry. He is a placid and relaxed companion; for instance, he may laugh, but he cannot cry because the tears cannot come. Your rudest story will not make him blush, and the most unpleasant circumstances will fail to make him turn pale. His collars and socks stay very clean and sweet. He wears corsets and may, if you meet him out, be rather fidgety (corsets to compress his splanchnic vascular pool, fidgety to keep the venous return going from the legs). He dislikes speaking much unless helped with something to moisten his dry mouth and throat. He is long-sighted and easily blinded by bright light. The redness of his eye-balls may suggest irregular habits and in fact his head is rather weak. But he always behaves like a gentleman and never belches or hiccups. He tends to get cold and keeps well wrapped up. But his health is good; he does not have chilblains and those diseases of modern civilization, hypertension and peptic ulcer, pass him by. He is thin because his appetite is modest; he never feels hunger pain and his stomach never rumbles. He gets rather constipated so that his intake of liquid paraffin is high. As old age comes on he will suffer from retention of urine and impotence, but frequency, precipitancy, and strangury will not worry him. One is uncertain how he will end, but perhaps if he is not careful, by eating less and less and getting colder and colder, he will sink into a symptomless, hypoglycaemic coma and die, as was proposed for the universe, a sort of entropy death. | |
| Inspired by this, in his summary of the meeting (Pharmac. Rev., 6, 123) R.W. Gerard came up with the following version in verse: | |
| When C-6 is about in excess, | |
| A man's organs yield under stress. | |
| In a corset they're tucked, | |
| His gut can't eruct, | |
| And he faces an entropic death. | |
Block of the nicotinic receptor at the NMJ by decamethonium was shown to result from local depolarization and consequent loss of excitability (Burns & Paton, 1951). This ‘depolarizing block' represented a new concept of drug action (see also Bowman, this issue). Subsequently, there were suggestions that block might arise instead from desensitization and an element of channel block emerged later, but it was clear that, in vivo, the principal cause was indeed end-plate depolarization (with consequent sodium channel inactivation), since block could be reversed by an opposing electric current (Burns & Paton, 1951). The ganglion block produced by hexamethonium, on the other hand, appeared to be a perfect example of ‘competitive' block, until Gurney & Rang (1984) showed that it actually blocked the open channels, rather than preventing them from opening. Tubocurarine – another archetypical competitive blocker at the NMJ – also produces some degree of channel block at the NMJ at hyperpolarized potentials (Colquhoun et al., 1979).
Notwithstanding the exact mechanism of block, the distinction between muscle and neural nicotinic receptors has been extended to the CNS, where virtually all of the nicotinic effects of acetylcholine are blocked by hexamethonium or, more conveniently, by the lipophilic mecamylamine. This distinction was emphasized by the cloning of the nicotinic receptor subunits. Thus, while the (adult) muscle receptor has a constant composition of two α1 subunits with one each of β1, ɛ and δ subunits, neural receptors are composed of quite distinct α and β subunits (or of homomeric α subunits), of which, collectively, there are 12 known types – α2–α10 and β2–β4. Since different subunit combinations show different agonist rank orders and some antagonist selectivity (at least, for toxins), much effort has been, and is being, devoted to finding out which subunit composition represents the physiologically relevant receptor in different neurons and at different synapses (McGehee & Role, 1995; Jones et al., 1999).
As with muscarinic receptors, information regarding the more global functions of different nicotinic receptor subunits has been derived from ‘knock-outs' (Cordero-Erausquin et al., 2000), and again with some surprises. Thus, though largely restricted to the autonomic nervous system, an α3 knock-out causes early postnatal death – possibly due to an intestinal disorder since it replicates a known human genetic intestinal defect with a short (3.6 months) survival time. At the other end of the spectrum, a β2 knock-out accelerates the reduction in cortical thickness with aging, as well as impairing passive avoidance learning. They also affect responses to nicotine – for example, both α4 and β2 knock-outs reduce the antinociceptive effect of nicotine, while β2 knock-outs also reduce nicotine self-administration.
Overall, more is now known about the nicotinic acetylcholine receptor than any other neurotransmitter receptor (Colquhoun et al., 2003). Single-channel analysis has revealed much about the likely conformational changes of a single receptor molecule on a sub-millisecond time-scale; the overall physical structure has been determined from cryo-electron microscopy at 4 Å resolution (Unwin, 2005), and the likely arrangement of atoms in the external domain of the receptor determined from X-ray crystallography of the homologous snail acetylcholine-binding protein. Much of this, plus the results of extensive mutational analysis concerning ligand-binding, channel-gating and channel conductance, are summarized by Karlin (2002). Thus, Dale's ‘nicotine action' of acetylcholine can now be explained in molecular terms.
Acetylcholine release
The cholinergic fibre, exemplified by its endings at the neuromuscular junction, also became the archetypical nerve terminal for studying transmitter release, especially through the work of Katz. It was here that quantal release was first demonstrated (Fatt & Katz, 1952) and the essential role of calcium elucidated (e.g., Jenkinson, 1957). These concepts proved generally applicable at other synapses, though with variations in the quantal content of the evoked synaptic response or release efficiency. It was also at the neuromuscular junction that the pharmacological concept of the ‘false transmitter' was first mooted, to explain the inhibitory action of triethylcholine (Bowman & Rand, 1961), even though this pharmacological modus operandi was later (and, more successfully, from a practical viewpoint) applied to aminergic fibres, with the introduction of α-methyldopa in antihypertensive therapy. More recent pharmacological approaches to modifying acetylcholine release have been directed at selective blockade of presynaptic muscarinic autoreceptors (see above).
The future?
What of the future? At the molecular level, we await the crystal structures of the complete nicotinic and muscarinic receptors, but these will surely come soon. At the functional level, one anticipates an increasingly detailed description of the precise role of individual nicotinic receptor subunits in defined pathways and systems from targeted and timed knockouts and from siRNA knockdowns (which are not restricted to mice!), and, hopefully, the circumstances under which they are activated. This will also take us outside the nervous system, since nicotinic receptors (especially α7 receptors) are present and functional in epithelial cells, various tumour cells and in elements of the immune system, such as B-lymphocytes (Skok et al., 2003). From these two approaches, and others, we might optimistically look forward to some potentially beneficial advances in pharmacotherapy of disorders such as schizophrenia, neuropathic pain, Alzheimer's disease, and some forms of cancer and immune disorders – or at least the replacement of nicotine with less addictive substitutes.
As for muscarinic actions, some advances have already emerged from the identification of muscarinic receptor subtypes in the treatment of bladder incontinence (Michel & de la Rosette, 2005) and bronchoconstriction (Hansel & Barnes, 2002; see also Barnes, this issue), and there may be scope for some additional treatment of Parkinson's disease via an M4 receptor antagonist (Birdsall et al., 2001; Wess, 2004). However, the initial optimism that M1-receptor agonists might be beneficial in Alzheimer's disease has not yet been fully rewarded.
Overall, given the limited number, and overlapping distributions and functions, of muscarinic receptor subtypes, further ‘quantum leaps' in therapeutic drug development based on subtype-specific agonists, antagonists or inverse agonists seem somewhat remote; perhaps allosteric enhancers and inhibitors offer more scope, since they modify ongoing functions, rather than imitating or suppressing them. Of course, this is also what anticholinesterase drugs do, and they certainly have some valuable uses, but their limitations in the treatment of Alzheimer's disease are equally apparent – though one gets the feeling that this may be as much dependent on patient selection as on drug activity. Let us, therefore, stay optimistic.
A final point. Not only is 2006 the 75th anniversary of the founding of the British Pharmacological Society, it is also the hundredth anniversary of Hunt & Taveau's first report on the hypotensive effect of acetylcholine. So it is our oldest transmitter, yet is still generating new ideas, new research and new drugs.
Apologies
I apologise to all my non-U.K. friends and colleagues for this ‘Britocentric' essay. I hope they understand the reason for it, and will forgive me! Also, the references quoted above are frequently only one example of many relevant papers, a consequence of editorial restrictions and not a scientific value judgement.
References
- ADAMS P.R., BROWN D.A. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurones. J. Physiol. 1982;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., MATHIE A., PETERS J.A. Acetylcholine (muscarinic) receptors: guide to receptors and channels. Br. J. Pharmacol. 2004;141:S6. doi: 10.1038/sj.bjp.0705672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALLEN T.G., BROWN D.A. Detection and modulation of acetylcholine release from neurites of rat basal forebrain cells in culture. J. Physiol. 1996;492:453–466. doi: 10.1113/jphysiol.1996.sp021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARMITAGE A.K., MILTON A.S., MORRISON C.F. Effects of nicotine and some nicotine-like compounds injected into the cerebral ventricles of the cat. Br. J. Pharmacol. Chemother. 1966;27:33–45. doi: 10.1111/j.1476-5381.1966.tb01639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRIE C.P., BIRDSALL N.J., HULME E.C., KEEN M., STOCKTON J.M. Solubilization and characterization of high and low affinity pirenzepine binding sites from rat cerebral cortex. Br. J. Pharmacol. 1985;85:697–703. doi: 10.1111/j.1476-5381.1985.tb10566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRDSALL N.J.M., NATHANSON N.M., SCHWARZ R.D. Muscarinic receptors: it's a knockout. Trends Pharmacol. Sci. 2001;22:215–219. [Google Scholar]
- BONNER T.I., BUCKLEY N.J., YOUNG A.C., BRANN M.R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- BOWMAN W.C., RAND M.J. Actions of triethylcholine on neuromuscular transmission. Br. J. Pharmacol. 1961;17:176–195. doi: 10.1111/j.1476-5381.1961.tb01278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN D.A., DOCHERTY R.J., HALLIWELL J.V. Chemical transmission in the rat interpeduncular nucleus in vitro. J. Physiol. 1983;341:655–670. doi: 10.1113/jphysiol.1983.sp014831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN D.A., FORWARD A., MARSH S.J. Antagonist discrimination between ganglionic and ileal muscarinic receptors. Br. J. Pharmacol. 1980;71:362–364. doi: 10.1111/j.1476-5381.1980.tb10948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A.S.V, SPERO L. The action of acetylcholine and other drugs on the efflux of potassium and rubidium from smooth muscle of the guinea-pig intestine. Br. J. Pharmacol. 1968;34:99–115. doi: 10.1111/j.1476-5381.1968.tb07954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A.S.V, TERROUX K.G. On the negative inotropic effect in the cat's auricle. J. Physiol. 1953;120:449–464. doi: 10.1113/jphysiol.1953.sp004910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNS B.D., PATON W.D.M. Depolarization of the motor end-plate by decamethonium and acetylcholine. J. Physiol. 1951;115:41–73. doi: 10.1113/jphysiol.1951.sp004652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAULFIELD M.P., BIRDSALL N.J. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol. Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- CLARK A.L., MITCHELSON F. The inhibitory effect of gallamine on muscarinic receptors. Br. J. Pharmacol. 1976;58:323–331. doi: 10.1111/j.1476-5381.1976.tb07708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D. How fast do drugs work. Trends Pharmacol. Sci. 1981;2:212–217. [Google Scholar]
- COLQUHOUN D., DREYER F., SHERIDAN R.E. The actions of tubocurarine at the frog neuromuscular junction. J. Physiol. 1979;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLQUHOUN D., UNWIN N., SHELLEY C., BATTON C., SIVILOTTI L.G.2003Nicotinic acetylcholine receptors Drug Discovery and Drug Developmented. Abrahams, D, pp. 357–405.New York: John Wiley; (Burger's Medicinal Chemistry). [Google Scholar]
- CORDERO-ERAUSQUIN M., MARUBIO LM., KLINK R., CHANGEUX J.-P. Nicotinic receptor function: new perspectives from knockout mice. Trends Pharmacol. Sci. 2000;21:211–217. doi: 10.1016/s0165-6147(00)01489-9. [DOI] [PubMed] [Google Scholar]
- CORNE S.J., EDGE N.D. Pharmacological properties of pempidine (1:2:2:6:6-pentamethylenepiperidine), a new ganglion-blocking compound. Br. J. Pharmacol. 1958;13:339–349. doi: 10.1111/j.1476-5381.1958.tb00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALE H.H. The action of certain esters and ethers of choline, and their relation to muscarine. J. Pharmacol. Exp. Ther. 1914;6:147–190. [Google Scholar]
- DALE H.H., DUDLEY H.W. The presence of histamine and acetylcholine in the spleen of the ox and horse. J. Physiol. 1929;68:97–123. doi: 10.1113/jphysiol.1929.sp002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANI J.A., JI D., ZHOU F.M. Synaptic plasticity and nicotine addiction. Neuron. 2001;31:349–352. doi: 10.1016/s0896-6273(01)00379-8. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. The identity of intrinsic and extrinsic acetylcholine receptors in the motor end-plate. Proc. R. Soc. London B. 1957;146:357–361. doi: 10.1098/rspb.1957.0016. [DOI] [PubMed] [Google Scholar]
- DESCARRIES L., GISIGER V., STERIADE M. Diffuse transmission by acetylcholine in the CNS. Prog. Neurobiol. 1997;53:603–625. doi: 10.1016/s0301-0082(97)00050-6. [DOI] [PubMed] [Google Scholar]
- ECCLES J.C., ECCLES D.M., FATT P. Pharmacological investigations on a central synapse operated by acetylcholine. J. Physiol. 1956;131:154–169. doi: 10.1113/jphysiol.1956.sp005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J. Physiol. 1951;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. Spontaneous subthreshold activity at motor nerve endings. J. Physiol. 1952;117:109–128. [PMC free article] [PubMed] [Google Scholar]
- FUKUDA K., KUBO T., AKIBA I., MAEDA A., MISHINA M., NUMA S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987;327:623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- GAHWILER B.H., BROWN D.A. Functional innervation of cultured hippocampal neurones by cholinergic afferents from co-cultured septal explants. Nature. 1985;313:577–579. doi: 10.1038/313577a0. [DOI] [PubMed] [Google Scholar]
- GURNEY A.M, RANG H.P. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br. J. Pharmacol. 1984;82:623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMMER R, BERRIE C.P., BIRDSALL N.J., BURGEN A.S., HULME E.C. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature. 1980;283:90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- HANSEL T.T., BARNES P.J. Tiotropium bromide: a novel once-daily anticholinergic bronchodilator for the treatment of COPD. Drugs Today (Barc.) 2002;38:585–600. doi: 10.1358/dot.2002.38.9.696535. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H. The nature of the antagonism between calcium and magnesium ions at the neuromuscular junction. J. Physiol. 1957;138:434–444. doi: 10.1113/jphysiol.1957.sp005860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES S., SUDWEEKS S., YAKEL J.L. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- KARLIN A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- LAZARENO S., BIRDSALL N.J. Detection, quantitation, and verification of allosteric interactions of agents with labeled and unlabeled ligands at G protein-coupled receptors: interactions of strychnine and acetylcholine at muscarinic receptors. Mol. Pharmacol. 1995;48:362–378. [PubMed] [Google Scholar]
- MASON D.F.J., WIEN R. The actions of heterocyclic bisquaternary compounds, especially of a pyrrolidinium series. Br. J. Pharmacol. 1955;10:124–132. doi: 10.1111/j.1476-5381.1955.tb00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIE A., CULL-CANDY S.G., COLQUHOUN D. Single-channel and whole-cell currents evoked by acetylcholine in dissociated sympathetic neurons of the rat. Proc. R. Soc. London B. 1987;232:239–248. doi: 10.1098/rspb.1987.0072. [DOI] [PubMed] [Google Scholar]
- MCCANCE I., PHILLIS J.W., WESTERMAN R.A. Acetylcholine-sensitivity of thalamic neurones: its relationship to synaptic transmission. Br. J. Pharmacol. 1968;32:635–651. doi: 10.1111/j.1476-5381.1968.tb00463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGEHEE D.S., HEATH M.J., GELBER S., DEVAY P., ROLE L.W. Nicotine enhancement of fast excitatory synaptic transmission in CNS by presynaptic receptors. Science. 1995;269:1692–1696. doi: 10.1126/science.7569895. [DOI] [PubMed] [Google Scholar]
- MCGEHEE D.S., ROLE L.W. Physiological diversity of nicotinic acetylcholine receptors expressed by vertebrate neurons. Annu. Rev. Physiol. 1995;57:521–546. doi: 10.1146/annurev.ph.57.030195.002513. [DOI] [PubMed] [Google Scholar]
- MENTIS G.Z., ALVAREZ F.J., BONNOT A., RICHARDS D.S., GONZALEZ-FORERO D., ZERDA R., O'DONOVAN M.J. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc. Natl. Acad. Sci. U.S.A. 2005;102:7344–7349. doi: 10.1073/pnas.0502788102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL M.C., DE LA ROSETTE J.J. Role of muscarinic receptor antagonists in urgency and nocturia. BJU Int. 2005;96 (Suppl 1):37–42. doi: 10.1111/j.1464-410X.2005.05651.x. [DOI] [PubMed] [Google Scholar]
- MITCHELL J.F. The spontaneous and evoked release of acetylcholine from the cerebral cortex. J. Physiol. 1963;165:98–116. doi: 10.1113/jphysiol.1963.sp007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NODA M., TAKAHASHI H., TANABE T., TOYOSATO M., FURUTANI Y., HIROSE T., ASAI M., INAYAMA S., MIYATA T., NUMA S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982;299:793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Drug receptors and the inhibition of nerve cells. Br. J. Pharmacol. 1989;19:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTH R.A., TOKIMASA T. The time course of muscarinic depolarization of guinea-pig myenteric neurones. Br. J. Pharmacol. 1984;82:85–91. doi: 10.1111/j.1476-5381.1984.tb16444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATON W.D.M., ZAIMIS E.J. The pharmacological actions of polymethylene bistrimethyl-ammonium salts. Br. J. Pharmacol. Chemother. 1949;4:381–400. doi: 10.1111/j.1476-5381.1949.tb00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERALTA E.G., WINSLOW J.W., PETERSON G.L., SMITH D.H., ASHKENAZI A., RAMACHANDRAN J., SCHIMERLIK M.I., CAPON D.J. Primary structure and biochemical properties of an M2 muscarinic receptor. Science. 1987;236:600–605. doi: 10.1126/science.3107123. [DOI] [PubMed] [Google Scholar]
- PURVES R.D. Muscarinic excitation: a microelectrophoretic study on cultured smooth muscle cells. Br. J. Pharmacol. 1974;52:77–86. doi: 10.1111/j.1476-5381.1974.tb09689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANG H.P. The characteristics of synaptic currents and responses to acetylcholine of rat submandibular ganglion cells. J. Physiol. 1981;311:23–55. doi: 10.1113/jphysiol.1981.sp013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIVILOTTI L., COLQUHOUN D. Acetylcholine receptors: too many channels, too few functions. Science. 1995;269:1681–1682. doi: 10.1126/science.7569892. [DOI] [PubMed] [Google Scholar]
- SKOK M.V., KALASHNIK E.N., KOVAL L.N., TSETLIN V.I., UTKIN Y.N., CHANGEUX J.-P., GRAILHE R. Functional nicotinic acetylcholine receptors are expressed in B lymphocyte-derived cell lines. Mol. Pharmacol. 2003;64:885–889. doi: 10.1124/mol.64.4.885. [DOI] [PubMed] [Google Scholar]
- SPINKS A., YOUNG E.H.P., FARRINGTON J.A., DUNLOP D. The pharmacological actions of pempidine. Br. J. Pharmacol. 1958;13:501–520. doi: 10.1111/j.1476-5381.1958.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUMIKAWA K., HOUGHTON M., SMITH J.C., BELL L., RICHARDS B.M., BARNARD E.A. The molecular cloning and characterisation of cDNA coding for the alpha subunit of the acetylcholine receptor. Nucleic Acids Res. 1982;10:5809–5822. doi: 10.1093/nar/10.19.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYMPOSIUM Symposium on neurohumoral transmission. Pharmacol. Rev. 1954;6:1–131. [PubMed] [Google Scholar]
- TRENDELENBURG A.U., MEYER A., WESS J., STARKE K.2005Distinct mixtures of muscarinic receptor subtypes mediate inhibition of noradrenaline release in different mouse peripheral tissues, as studied with receptor knockout mice Br. J. Pharmacol.June 20 (Epub ahead of print). [DOI] [PMC free article] [PubMed]
- UNWIN N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- VOGT M. Release from brain tissue of compounds with possible transmitter function: interaction of drugs with these substances. Br. J. Pharmacol. 1969;37:325–337. doi: 10.1111/j.1476-5381.1969.tb10570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WESS J. Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu. Rev. Pharmacol. Toxicol. 2004;44:423–450. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- WONNACOTT S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- ZENG F., WESS J. Molecular aspects of muscarinic receptor dimerization. Neuropsychopharmacology. 2000;23 (4 Suppl):S19–S31. doi: 10.1016/S0893-133X(00)00146-9. [DOI] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Muscarinic receptor subtypes controlling the cationic current in guinea-pig ileal smooth muscle. Br. J. Pharmacol. 1997;122:885–893. doi: 10.1038/sj.bjp.0701438. [DOI] [PMC free article] [PubMed] [Google Scholar]