Abstract
Antihormonal therapy targeted to the oestrogen receptor (OER) is recognized as a significant advance in the treatment and prevention of breast cancer. However, the research method used to achieve the current successes seen in the clinic was not linear but was based on the changing fashions in research and the application of appropriate testing models. The discovery and investigation of nonsteroidal antioestrogens by the pharmaceutical industry during the 1960s was initially an exciting prospect for clinical development. The drugs were superb antifertility agents in laboratory animals, so the prospect of marketing a ‘morning after' pill was a high priority. Unfortunately, the reproductive endocrinology of the rat was found to be completely different from that of the human. Antioestrogens, in fact, improved fertility by inducing ovulation in subfertile women so much of the drug development was discontinued. The successful reinvention of ICI46,474 from its origins as a failed contraceptive to a pioneering breast cancer treatment targeted to the OER presaged the development of the current menu of medicines targeted to a range of different survival mechanisms in cancer cells.
Keywords: Tamoxifen, raloxifene, aromatase inhibitors, oestrogen receptor
Introduction
The story of the development of tamoxifen as a pioneering medicine in cancer treatment has its origins in two separate areas of research that converged almost by accident, in the early 1970s. The first research path, followed between 1900 and 1974, sought to understand why only some of the women who developed breast cancer, responded to endocrine therapies. The second research path, which started in 1958, was unconnected to the first and started with the chance discovery of nonsteroidal antioestrogens, in the pharmaceutical industry. There was initial enthusiasm to develop the drugs as ‘morning after' pills because antioestrogens were postcoital contraceptives in laboratory animals. Unfortunately, these predictions did not translate to humans as antioestrogens actually induced ovulation in subfertile women. They guaranteed what they were designed to prevent! By the end of the 1960s, drug development of antioestrogens was of little or no interest, but the failure of one application became an opportunity for another.
Ovarian dependence for the growth of some breast cancers has been known since 1896 when Dr George Beatson (Beatson, 1896) reported that a woman with very advanced metastatic breast cancer responded dramatically to the removal of her ovaries. Unfortunately, not all breast cancers responded. A few years later, Mr Stanley Boyd of the Charing Cross Hospital accumulated all of the reported experience with oophorectomy for breast cancer in Great Britain (Boyd, 1900). Only one in three women responded objectively to oophorectomy and the duration of response was about a year. Despite this disappointing finding, endocrine ablation, that is, surgical or radiation induced oophorectomy, hypophysectomy or adrenalectomy, remained a palliative option for patients with advanced breast cancer for the first 60 years of the 20th century (Kennedy, 1965). However, there was no way of knowing which patients would respond. In other words, for every 100 surgical procedures performed, only 30 women would derive any benefit.
The pioneering studies by Dr Elwood Jensen would ultimately answer the question of which breast tumours would or would not respond to oestrogen withdrawal. Jensen synthesized high specific activity tritium labeled oestradiol and showed that unchanged steroid was bound to and retained by oestrogen target tissues (e.g. uterus and vagina) of the immature rat. In contrast, radio-labelled oestradiol initially bound to, but was not retained by nontarget tissues, for example, lung, heart and skeletal muscle (Jensen & Jacobson, 1962). Jensen reasoned that there must be an oestrogen receptor (OER) to mediate oestrogen action in a particular target tissue and applied this concept successfully to establish a predictive test for the treatment of breast cancer for ablative surgery (Jensen et al., 1971). Simply stated, the OER assay for breast cancer is based on the presence or absence of receptor protein extracted from a tumour biopsy. If the receptor is present, there is a high probability that the tumour will respond to ablative surgery, but if the receptor is absent, there is an extremely low probability of a tumour response.
The OER assay was also effective at predicting the outcome of breast cancer treatment with high-dose synthetic oestrogen or androgen therapy. However, the side effects of additive hormonal therapy were unacceptable for some patients (Kennedy, 1965).
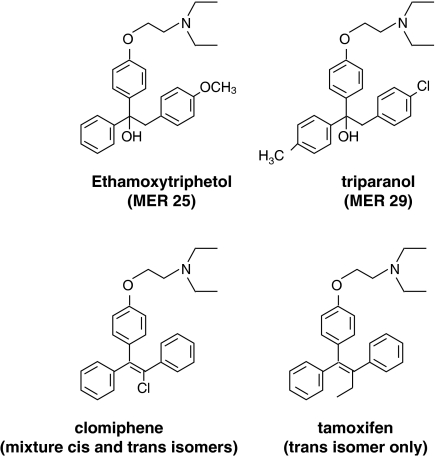
The story of the second research path started with serendipity. The first nonsteroidal antioestrogen MER25 (Figure 1) was discovered by accident at the Merrell laboratories in Cincinnati during the mid-1950s. Dr Leonard Lerner, a member of the hormone research program, was curious about the biological activity of a triphenylethanol, 1-(p-2 diethylaminoethoxyphenyl)-1-phenyl 2 p-methoxyphenyl ethanol, which was being tested as part of their cardiovascular program. Lerner had noted that the structure of the new triphenylethanol was similar to that of synthetic oestrogens, but Lerner found only antioestrogenic activity in all species tested and there was no other hormonal or antihormonal activity (Lerner et al., 1958). The compound was also a postcoital contraceptive so there was enormous enthusiasm for the development of a ‘morning after' pill. Unfortunately, the persistent investigation of numerous drugs of this class discovered and tested by several pharmaceutical companies (reviewed in Lunan & Klopper, 1975; Jordan, 1984) found no evidence of effectiveness in humans and, in fact, the opposite was true. Two of the compounds, clomiphene and tamoxifen (Figure 1), both induced ovulation in subfertile women (Lunan & Klopper, 1975) and both drugs were marketed for that indication.
Figure 1.
The structures of the hypocholesteraemic drug, triparanol, which was withdrawn from the market in the late 1950s because it caused cataracts in patients, and the first nonsteroidal antioestrogen, ethamoxytriphetol or MER25. MER25 was too toxic and not particularly potent, so clinical studies were discontinued in favor of the triphenylethylene, clomiphene. This drug is a mixture of cis and trans geometric isomers and is used for the induction of ovulation in subfertile women. Tamoxifen is the pure trans isomer eventually developed for the induction of ovulation in subfertile women and for the treatment of breast cancer in elderly women.
The use of antioestrogens as agents to treat breast cancer was, at that time, a remote possibility in part because of concerns about long-term toxicities. The nonsteroidal antioestrogens had been noted to lower circulating cholesterol. Although this property could be viewed as a benefit, there was a link to a ‘cause celebre' within the pharmaceutical industry. MER25 was a structural derivative of the hypocholesteraemic drug triparanol (Figure 1) that, during the late 1950s, had been withdrawn from the market. Changes in cholesterol levels apparently increased desmosterol levels and this was believed to cause an increased incidence of cataract formation in young women. As a result, only agents that did not affect circulating desmosterol levels would be acceptable for long-term therapy (reviewed in Jordan, 2003).
An alternative solution at Alderley Park
Dr A.L. Walpole (Figure 2) led the team of reproductive endocrinologists at Imperial Clinical Industries (ICI) Pharmaceuticals Division (now AstraZeneca), who discovered the compound ICI46,474 (Harper & Walpole, 1967), which became tamoxifen. Walpole had broad interests in carcinogenesis, the endocrine treatment of cancer, cytotoxic chemotherapy as well as reproductive endocrinology (reviewed in Jordan, 1988). He was elected a member of the British Pharmacological Society in 1946 and published much of his screening work on cytotoxic chemotherapy in the British Journal of Pharmacology (reviewed in Jordan, 1988). At that time in the late 1940s, Walpole was a member of staff at ICI's Dyestuffs Division's Biological Laboratory in Wilmslow, Cheshire. This establishment was the predecessor of the Pharmaceuticals Division Research Laboratories built in 1956–57 at Alderley Park near Macclesfield, Cheshire.
Figure 2.

Dr Arthur L. Walpole, head of the reproduction and fertility control program at ICI Pharmaceuticals Division, discovered ICI46,474 that became tamoxifen. Although Walpole did not conduct any basic or clinical studies on the antitumour properties of ICI46,474, his faith in the compound ensured that financial and collegial support would be available for others. Tamoxifen was reinvented as an anticancer agent from its origins as a failed contraceptive. Walpole never saw the tremendous success of this discovery as he died suddenly on July 2, 1977.
However, throughout the whole of the 1960s and until his retirement in the early 1970s, the focus of Walpole's team was entirely on reproductive endocrinology (Walpole, 1968). Nevertheless, Harper & Walpole (1967) succeeded in identifying the trans isomer of a substituted triphenylethylene (ICI46,474), which was a partial oestrogen agonist/antagonist in the immature rat but remarkably a full oestrogen agonist in the mouse. Most importantly, ICI46,474 lowered circulating cholesterol without increasing desmosterol levels (Harper & Walpole, 1967).
The subsequent development of tamoxifen as a useful therapeutic agent was not a corporate priority in the late 1960s and early 1970s. ICI Pharmaceutical Division was not a ‘player' in the cancer therapy community but a series of chance events turned ICI46,474 from a laboratory curiosity into a pioneering medicine over the next 20 years (Jordan, 2003). It is unlikely that this drug development story will ever be repeated, but it now serves as an example of what can be achieved by having close personal relationships between scientists in academia and those in industry. Most importantly, it illustrates how consistent long-term research support for junior faculty can result in a strategy for successful drug development.
The situation in 1972
In April 1972, all the preliminary clinical data on ICI46,474 was reviewed by scientists at ICI Pharmaceuticals Division at Alderley Park, but there was reluctance to pursue the development of the drug as a short-term palliative treatment for breast cancer. Several factors were considered in the decision not to develop ICI46,474. The class of compounds had no patent protection in the United States (this would only be granted in 1985!), there was estimated to be no significant market for a palliative drug that would only be effective for a year in one in three breast cancer patients (at the time the incidence of metastatic breast cancer in the United Kingdom was only a few thousand patients per year), there was no infrastructure at Alderley Park to support a breast cancer program and finally there was no pipeline of compounds to replace ICI46,474 should subsequent studies reveal unacceptable toxicities. Most importantly, it was generally accepted that another endocrine therapy would add almost nothing to the medical armamentarium of breast cancer therapies. Overall, there was little initial enthusiasm for the use of a new antihormonal therapy that benefited a minority of patients for a short period. A review by Lunan & Klopper (1975) stated the situation at the time, but proclaimed the promise of antioestrogens as potential therapeutic agents.
Relatively little has been done to apply the results of animal experiments to humans, but there is now enough evidence to suggest that antioestrogens have a great potential application to human therapy.
A promising approach is to tailor or make particular antioestrogens for specific indications – for example, ovulation induction or antifertility or anticancer. In the antifertility field, it is particularly important that compounds of very low toxicity are sought since they may be inadvertently administered to pregnant women or given for very long periods to some women. As yet, antioestrogens have only been widely applied to the induction of ovulation and even in this field they were introduced on an empirical basis without fully understanding their mechanism of action. Now that we realize how they work, it is possible by animal experimentation, to determine what the effect of a particular modification of, for example, the triphenylethylene molecule is on a specific oestrogen activity such as nidation of the ovum. There are many applications for compounds capable of counteracting particular biological effects of oestrogen; seen in this light, antioestrogens carry a hopeful promise (Lunan & Klopper, 1975).
Much early work on the interaction of antioestrogens with the mouse and rat OER was conducted by Dr Lars Terenius of the Department of Pharmacology in Lund, Sweden (Terenius, 1971a). He demonstrated that MER-25 and the antioestrogen U-11,100A (nafoxidine) had antitumour activity in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model (Terenius, 1971b, 1971c). However, nafoxidine was subsequently found to be far too toxic for general clinical use (Jordan, 2003). A preliminary study of ICI46,474 for the treatment of breast cancer in postmenopausal patients showed the same efficacy, but an improved side effect profile compared with standard high-dose oestrogens or androgens (Cole et al., 1971). The scene was set to reinvent ICI46,474 from its origins as an unsuccessful contraceptive to become a drug with ubiquitous targeted applications for the treatment of all stages of breast cancer and as a vanguard medicine for the prevention of the hormone responsive breast cancer.
ICI46,474 to tamoxifen
A full account of the use of tamoxifen for the treatment and prevention of breast cancer has been published in the British Journal of Pharmacology (Jordan, 1993); however, a brief account of the development of tamoxifen is appropriate in light of recent advances. Although tamoxifen was available for the untargeted treatment of breast cancer in postmenopausal women during the mid-1970s, a scientific strategy for the appropriate clinical application of tamoxifen was developed in the laboratory to target the drug to the tumours that would respond. Tamoxifen blocked the binding of oestradiol to human breast and rat mammary tumour OERs and prevented the induction and growth of OER positive DMBA-induced rat mammary carcinomas (Jordan, 1974; 1976; Jordan & Koerner, 1975; Jordan & Jaspan, 1976), However, the finding that long-term tamoxifen treatment in animals with early mammary cancer, that is, a low tumour burden (Jordan & Allen, 1980) was to prove remarkably effective for enhancing the survivorship of women with early node positive and node negative OER positive breast cancer. The original strategy for the evaluation of tamoxifen was to use it for 1 year of adjuvant treatment after surgery. The reason for this was that tamoxifen was only effective for the treatment of advanced breast cancer for about a year and there was a real concern that longer treatment duration would result in premature drug resistance. However, the subsequent evaluation of 1, 2 or 5 years of adjuvant tamoxifen treatment in OER positive premenopausal breast cancer patients produced survival advantages whereas 1 year of tamoxifen was completely ineffective. Similarly, patients with OER-negative tumours were unaffected by any duration of adjuvant tamoxifen treatment (EBCTCG, 1998).
Drug resistance does, however, develop with increasing duration of tamoxifen treatment. An evaluation of 5 vs 10 years of adjuvant tamoxifen shows that 10 years of adjuvant tamoxifen results in an increase in tumour recurrences and increased accumulation of serious side effects (endometrial cancer, blood clots) compared with 5 years of adjuvant therapy (Fisher et al., 2001). It is perhaps important to point out that drug resistance is not simply the loss of the OER or the changing pharmacokinetics of a drug given for long periods. Tumour cells are selected that become tamoxifen stimulated for growth in the breast and endometrium (Jordan, 2004).
Five years of tamoxifen therapy became the treatment strategy of choice throughout the 1990s. Remarkably, the application of 5 years of adjuvant tamoxifen therapy results in long-term benefit for patients that persists for at least a decade after stopping tamoxifen (EBCTCG, 2005). It is possible that a change in the biology of drug resistant breast cancer changes its sensitivity to oestradiol so that when tamoxifen stops low concentrations of the patient's own oestrogen kill the primed cancer cells (Jordan, 2004). Further work is necessary to understand this phenomenon.
Treatment to prevention
Thirty years ago, the prospects of developing preventives for breast cancer were remote. However, the expanded use of tamoxifen as a long-term treatment for breast cancer provided the appropriate level of clinical confidence to consider the evaluation of tamoxifen as the first preventive for any cancer. Several essential pieces of evidence provided a strong scientific basis for considering tamoxifen for testing as an agent to reduce the incidence of breast cancer in high-risk women.
Laboratory evidence, in a diverse range of animal models, demonstrated that tamoxifen could prevent carcinogen-induced rat mammary carcinogenesis (Jordan, 1974; 1976; Gottardis & Jordan, 1987) and mammary carcinogenesis in high incidence strains of virgin female mice (Jordan et al., 1991). However, the finding that contralateral breast cancer was reduced dramatically during adjuvant tamoxifen therapy was the most persuasive result in initiating clinical trials of tamoxifen as a preventative in women without breast cancer.
Although the clinical community had confidence that side effects with tamoxifen would be manageable in women at high risk for breast cancer, long-term toxicological concerns had to be resolved before staging full-scale randomized clinical trials. If tamoxifen acted as an antioestrogen to prevent oestrogen-stimulated breast cancer cell replication, would the antioestrogenic actions of tamoxifen on bone result in osteoporosis and would long-term antioestrogen action result in premature atherosclerosis? Paradoxically, tamoxifen was found to have selective oestrogenic and antioestrogenic actions in different oestrogen target tissues. Tamoxifen and keoxifene, the compound that later became known as raloxifene (Figure 3), were shown to be partial oestrogen agonists in ovariectomized rats (Jordan et al., 1987) and these data translated to enhanced bone density when tamoxifen was used to treat postmenopausal patients with breast cancer (Love et al., 1992). It has been known since the 1960s (Harper & Walpole, 1967) that tamoxifen lowered circulating cholesterol in rats and again these data translated to a significant reduction of circulating low-density lipoprotein cholesterol during the treatment of postmenopausal patients with tamoxifen (Love et al., 1991). Based on these preliminary studies and the huge clinical database, the evaluation of tamoxifen as a preventive went forward despite concerns about liver carcinogenesis based on rat studies (Jordan, 1995).
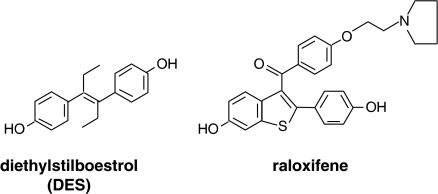
Figure 3.
The structure of the potent synthetic oestrogen, diethylstilboestrol, which was used extensively in clinical practice from the 1940s. High doses were used for the treatment of breast and prostate cancer before the development of antioestrogens and antiandrogens. For comparison, also shown is the structure of the SERM raloxifene that is used for the treatment and prevention of osteoporosis and is currently being tested as a preventive for breast cancer. The structure builds on a stilboestrol-like backbone with an appropriately positioned basic side chain that is the hallmark of antioestrogen action in the breast and the uterus (Jordan, 1984).
A vanguard toxicology study of over 2000 high-risk women started in the mid-1980s (Powles et al., 1989) but a full randomized placebo controlled clinical trial designed to test the worth of tamoxifen as a preventive was started in the United States in 1992. A total of 13,800 high-risk pre- and postmenopausal women volunteered for the trial. Breast cancer incidence was reduced by 50% in tamoxifen-treated women compared to placebo treated controls. The anticipated side effects of increases in blood clots and endometrial cancer were noted only in postmenopausal women (Fisher et al., 1998). Tamoxifen was approved by the Food and Drug Administration in the United States for the reduction of breast cancer risk in pre- and postmenopausal women in 1998.
Overall, tamoxifen has improved the prognosis of millions of women with a diagnosis of breast cancer. This antioestrogen has a favorable therapeutic index and has clearly improved survival of women with breast cancer (EBCTCG, 2005). Tamoxifen was the first targeted therapy for breast cancer (Jensen & Jordan, 2003) and the first to be used as a chemopreventive. Most importantly, tamoxifen opened the door for the development of a whole range of new and safer medicines that hold the promise of further improvements in healthcare.
Wider aspects of tamoxifen's success
Other treatments for breast cancer
The successful development of tamoxifen as a breast cancer drug (Lerner & Jordan, 1990) from its origins in the 1960s as a prospective postcoital contraceptive (Harper & Walpole, 1967) illustrates that the prudent selection of appropriate and relevant laboratory models and an examination of mechanisms of action can, under the appropriate scientific circumstances, lead to better medicines. Improvements in the survival of patients with breast cancer acted as an incentive to seek other agents that improved disease control and reduced side effects.
The aromatase inhibitors block the synthesis of oestrogen from androgens in the peripheral tissues, such as adipose tissue, and muscle in postmenopausal women (Miller, 2003). Although aromatase inhibitors can block the conversion of androgens to oestrogens in the ovary of premenopausal women, the drop in oestrogen levels induces a compensatory increase in gonadotrophins from the hypothalamopituitary axis, which reverses the blockade of the ovarian aromatase enzyme. As a result, aromatase inhibitors can only be used in postmenopausal women without ovarian function. There are both suicide inhibitors (formestane, exemestane) and competitive inhibitors (anastrozole, letrozole) of the aromatase enzyme system (Miller, 2003). Overall, aromatase inhibitors are improving patient prognosis if used instead of tamoxifen (Howell et al., 2005), after a couple of years of tamoxifen (Coombes et al., 2004) or after 5 years of tamoxifen (Goss et al., 2003). Indeed, the reduced incidence of side effects and the findings from adjuvant treatment studies of a greater reduction in contralateral breast cancer compared to tamoxifen have already acted as an incentive to initiate chemoprevention trials with aromatase inhibitors. However, the fact that aromatase inhibitors can only be used in postmenopausal women leaves the premenopausal population who develop breast cancer (about 30% of breast cancers annually) vulnerable unless tamoxifen is used as a chemopreventive. Fortunately, the risk benefit ratio for high-risk women who use tamoxifen as a chemopreventive is the most favorable during the premenopausal period. Thus, tamoxifen does not increase incidence of blood clots, stroke or endometrial cancer in premenopausal women (Fisher et al., 1998).
Aromatase inhibitors reduce oestrogen synthesis everywhere in the postmenopausal woman's body, unlike tamoxifen which maintains or improves bone density in this group (Love et al., 1992). These pharmacological actions on bone translate to more fractures in women treated with aromatase inhibitors (ATAC Trialists' Group, 2002). Aromatase inhibitors cause a modest increase in bone loss (Lonning et al., 2005) so the long-term application of aromatase inhibitors as chemopreventive agents requires a periodic evaluation of bone density and appropriate therapeutic intervention with bisphosphonates to avoid premature osteoporosis.
Another approach to treatment of breast cancer targeting the breast tumour OER with pharmacological agents to block the growth signal transduction pathway has proved extremely successful. Overexpression (gene amplification) of the human epidermal growth factor-2 (HER2) oncogene in human breast cancer is associated with a type of disease with a poor prognosis, that is independent of OER status (Slamon et al., 1987). The targeting of the HER-2 cell surface survival signaling pathway with the humanized antibody trastuzumab is currently improving survival of patients with this poor prognosis disease (Slamon et al., 2001). Just as tamoxifen first successfully targeted the OER in breast cancer, the use of a range of antibodies and synthetic small molecules to target different survival pathways for cancers in general is a current fashion in cancer therapeutics.
Unfortunately, at this stage, it is not always possible to identify the precise target in a tumor for intervention. Nevertheless, the technological advances in overall gene profiling with gene arrays for the whole human genome have already creating advances in identifying portraits of good and bad prognosis breast cancer. This approach is improving the prediction of responses and marrying the correct treatment to the correct tumour. Although there is no single answer to cancer, the combined incremental advances in target identification and the development of new agents will together improve healthcare delivery enormously within the next 30 years.
Treatment of osteoporosis
The selective antioestrogenic and oestrogenic actions of tamoxifen in breast and bones was also noted with keoxifene (Gottardis & Jordan, 1987; Jordan et al., 1987), a failed breast cancer drug that was reinvented as the first selective oestrogen receptor modulator (SERM) raloxifene (Figure 3) for the treatment and prevention of osteoporosis (Ettinger et al., 1999) with breast and endometrial safety (Cummings et al., 1999).
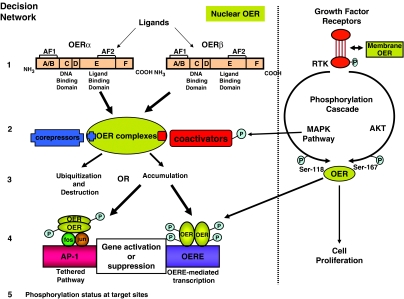
The SERMs appear to modulate the OER signal transduction pathway in different OER target tissues through a complex, and not completely understood, subcellular decision network (Figure 4). The shape of the ligand is important to program the OER complex as an agonist or antagonist at the target site (Jordan, 1984). The subsequent shape of the receptor complex determines whether coactivator proteins will interact to promote agonist actions or corepressor proteins will bind to block the signal transduction pathway. Additionally, the complex can now decide whether to activate genes directly through an interaction with DNA or indirectly via a tethered site (AP-1) for gene transcription. Finally, the whole signal transduction pathway can be modulated by cell surface receptors that create a phosphorylation cascade to change protein–protein interactions and half-lives through site-specific phosphorylation. Based on the decision pathway, it is likely that different target sites could have higher proportions of genes regulated through tethered pathways or that there could be subtle differences based on phosphorylation cascades.
Figure 4.
The potential decision network in oestrogen target tissues that could program a ligand receptor complex to activate oestrogenic or antioestrogenic responses. There are two distinct oestrogen receptors (OERs) (alpha and beta) that are differentially distributed throughout the body. The shape of the ligand can change the shape of the receptor complex. This is turn preprograms the complex to bind either a co-activator or co-repressor protein to enhance the intrinsic activity of the complex for oestrogenic responses or reduces intrinsic activity for antioestrogenic responses, respectively. The final decision point is to activate or suppress genes directly at DNA oestrogen response elements (OERE) or tether the AP-1 sites to increase gene transcription. Overall, a tissue can modify the decision network through cell surface receptor tyrosine kinases (RTK) enhancing the phosphorylation cascade. This in turn can increase phosphorylation of coactivators or the OER. The balance of decision outcomes modulates the response of a particular tissue.
Raloxifene is currently being evaluated as a chemopreventive in breast cancer based on the fact that raloxifene reduces tumour incidence as a secondary end point in an osteoporosis trial (Cummings et al., 1999). The reason for the apparent anomaly that raloxifene is poorly effective as a therapy for late breast cancer but appears to prevent breast cancer, is the finding that the bioavailability of raloxifene is only 2%. The low levels of drug are rapidly (48 h) cleared by phase II metabolism, so only low concentrations are available to penetrate the formed breast tumour. These pharmacokinetics contrast with those of tamoxifen, which has an extremely long half-life (14 days) and is a prodrug metabolized to active anticancer agents (Jordan, 1984). Nevertheless, the continuous low levels of raloxifene are clearly effective at preventing development of microscopic precancerous lesions.
Raloxifene is also being evaluated as a means of combating coronary heart disease. The SERMs were known to lower circulating cholesterol in laboratory animals (Harper & Walpole, 1967) and, latterly, in postmenopausal women (Love et al., 1991; Walsh et al., 1998). Clearly, clinical studies should continue in this important area of therapeutics.
What is particularly interesting is the current interest in developing selective agonist/antagonists for all members of the steroid hormone receptor super-family. Although no new agent has yet been tested successfully, the promise of a glucocorticoid without bone thinning actions or tissue specific androgens or antiandrogens is extremely appealing.
Personal postscript
The story of the early momentum for the development of tamoxifen owes much to members of the British Pharmacological Society. The Society provided an intellectual home for Arthur Walpole with his interest in anticancer drugs during the early years of his career (reviewed in Jordan, 1988). Although he was to be redirected at ICI's Pharmaceutical Division to focus on the control of reproduction (Walpole, 1968), his desire to see the introduction of an effective anticancer drug would be pivotal to the story. Regrettably, he would not live to see the success of tamoxifen. Nevertheless, he provided support to others to ensure that tamoxifen would be successful.
Edward (Ted) R. Clark, Senior Lecturer at the Department of Pharmacology, University of Leeds, was particularly interested in the structure–function relationships of nonsteroidal oestrogens and antioestrogens. In 1969, supported by a research scholarship from the Medical Research Council, I decided to study the OER in Clark's laboratory. An exciting series of reports had recently been published showing that the OER could easily be extracted from the rat uterus and isolated by sucrose density gradient analysis. My project was going to be simple: I was to establish the new technique of sucrose gradient analysis, isolate the receptor and crystallize the protein with an oestrogen and an antioestrogen. Through X-ray crystallography in the Astbury Department of Biophysics at the University of Leeds, we would then establish the three dimensional shape of the complexes to explain antioestrogen action. The goal was to solve a fundamental question in pharmacology: what is the molecular mechanism of action for a drug?
Progress was slow in establishing the technique of receptor purification by sucrose gradient analysis, and I rapidly switched my thesis topic to study the structure–activity relationships of nonsteroidal antioestrogens. As it turned out, this was a good strategic decision, as it has taken nearly 30 years to achieve partial success, with the structure of the OER ligand binding domain complexed with oestradiol and raloxifene being solved in 1997 (Brzozowski et al., 1997).
In 1972, however, there was little academic interest in the pharmacology of antioestrogens. I had completed my thesis entitled ‘Structure Activity Relationships of Some Substituted Triphenylethylenes and Triphenylethanols', but it was clear that no one was recommending antioestrogen research as a sound career choice; it was perceived as a dead end. I also wanted to develop antioestrogens as anticancer agents but targeted to the OER. The question was how. To make matters worse, the University of Leeds encountered difficulty in securing a qualified examiner for my thesis. Sir Charles Dodds, the discoverer of the synthetic oestrogen diethylstilboestrol (Figure 3), declined with regrets because he had not kept up with the literature during the 20 years since his retirement!
In 1971, the Department of Pharmacology appointed a new professor, Michael (Mike) Barrett. Barrett made two important decisions that would affect the course of my life. Firstly, he proposed Arthur Walpole from ICI Pharmaceuticals Division as my external examiner. After some initial protests from the University administration that Walpole was ‘from industry', my examination went ahead in August 1972. I had met Walpole in 1967 when I was a summer scholar at Alderley Park, but the meeting in 1972 started a collaboration to turn ICI46,474 into tamoxifen that would end only with his sudden death on July 2, 1977. Secondly, Barrett took the unusual step of supporting me, then a PhD student, for a faculty position in his department. However, in 1972, I was first required to obtain additional research experience elsewhere. Barrett solved this problem by arranging for me to work with his friend, Mike Harper, who had moved from ICI to the Worcester Foundation for Experimental Biology in Massachusetts. This was the home of the oral contraceptive and Harper who evaluated the reproductive endocrinology of ICI46,474 with Walpole at ICI (Harper & Walpole, 1967) was now heading up a team to develop prostaglandins as a ‘once a month' contraceptive.
I remember my telephone interview with Harper: ‘Can you come in September?' ‘Will $12,00,000 be a suitable salary?' and, ‘Do you mind working on prostaglandins?' The salary was three times my proposed salary as a lecturer at Leeds. Yes, yes, yes, I replied and headed to the library to find out what prostaglandins were.
When I arrived at the Worcester Foundation in September 1972, events took a bizarre turn. Harper had left to head a program at the World Health Organization and I was told sit down and write up the research plan that I would complete over the next 2 years. I could do anything I liked but some of the work must involve prostaglandins.
A phone call to Walpole secured his support to conduct laboratory studies with ICI46,474. He ensured I had the support of the newly acquired ICI Americas (Stuart Pharmaceuticals), in Delaware, and I was tasked to convince clinical trials group in America to use the new antioestrogen in their treatment protocols. ICI Americas provided financial resources and valuable human breast tumour specimens to establish that tamoxifen blocked the binding of oestrogen to the soluble receptor (Jordan & Koerner, 1975). As luck would have it President Nixon had ‘declared war on cancer' in 1971 and the Worcester Foundation had just appointed Jensen, then Director of the Ben May Laboratory for Cancer Research in Chicago, as a member of their scientific advisory board. Jensen generously agreed to arrange for me to visit his laboratory in Chicago to learn techniques in OER assay and the DMBA-induced rat mammary carcinogen model. I felt no urgency to publish my results as no one really seemed to care about a new hormone therapy for breast cancer. I soon realized my error when I returned to the Department of Pharmacology at the University of Leeds in 1974 and started writing.
The clinical and scientific research staff at ICI Pharmaceuticals Division (Roy Cotton, Barry Furr, Brian Newbould and Arthur Walpole) were extremely helpful to me in my research plans. With their financial support (and unlimited quantities of rats chauffeured over to Leeds each week from Alderley Park!) we established a successful ICI/Leeds University joint research scheme and my ‘Tamoxifen Team' in Leeds was able to create a scientific strategy that would serve as a foundation for the appropriate clinical application of tamoxifen as a treatment and preventive for breast cancer.
Acknowledgments
The results described in this paper were only made possible through the efforts of the 14 doctoral students and 30 postdoctoral fellows who turned ideas into action. Current studies are supported by the Specialized Program of Research Excellence in Breast Cancer 5P50CA89018-4, the Avon Foundation and the Weg gift to the Fox Chase Cancer Center.
Glossary
- AP-1
activating protein 1
- DMBA
dimethylbenz(a)anthracene
- OER
oestrogen receptor
- SERM
selective oestrogen receptor modulator
References
- ATAC TRIALISTS' GROUP Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet. 2002;359:2134–3139. doi: 10.1016/s0140-6736(02)09088-8. [DOI] [PubMed] [Google Scholar]
- BEATSON G.T. On the treatment of inoperable cases of carcinoma of the mamma: suggestions for a new method of treatment with illustrative cases. Lancet. 1896;2:104–107. [PMC free article] [PubMed] [Google Scholar]
- BOYD S. On oophorectomy in cancer of the breast. BMJ. 1900;ii:1161–1167. [Google Scholar]
- BRZOZOWSKI A.M., PIKE A.C., DAUTER Z., HUBBARD R.E., BONN T., ENGSTROM O., OHMAN L., GREENE G.L., GUSTAFSSON J.A., CARLQUIST M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- COLE M.P., JONES C.T., TODD I.D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI 46474. Br. J. Cancer. 1971;25:270–275. doi: 10.1038/bjc.1971.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOMBES R.C., HALL E., GIBSON L.J., PARIDAENS R., JASSEM J., DELOZIER T., JONES S.E., ALVAREZ I., BERTELLI G., ORTMANN O., COATES A.S., BAJETTA E., DODWELL D., COLEMAN R.E., FALLOWFIELD L.J., MICKIEWICZ E., ANDERSEN J., LONNING P.E., COCCONI G., STEWART A., STUART N., SNOWDON C.F., CARPENTIERI M., MASSIMINI G., BLISS J.M. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N. Engl. J. Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- CUMMINGS S.R., ECKERT S., KRUEGER K.A., GRADY D., POWLES T.J., CAULEY J.A., NORTON L., NICKELSEN T., BJARNASON N.H., MORROW M., LIPPMAN M.E., BLACK D., GLUSMAN J.E., COSTA A., JORDAN V.C. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- EBCTCG Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;354:1451–1467. [PubMed] [Google Scholar]
- EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- ETTINGER B., BLACK D.M., MITLAK B.H., KNICKERBOCKER R.K., NICKELSEN T., GENANT H.K., CHRISTIANSEN C., DELMAS P.D., ZANCHETTA J.R., STAKKESTAD J., GLUER C.C., KRUEGER K., COHEN F.J., ECKERT S., ENSRUD K.E., AVIOLI L.V., LIPS P., CUMMINGS S.R. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators [see comments] [published erratum appears in JAMA 1999 Dec 8; 282 (22): 2124] JAMA. 1999;282:637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- FISHER B., COSTANTINO J.P., WICKERHAM D.L., REDMOND C.K., KAVANAH M., CRONIN W.M., VOGEL V., ROBIDOUX A., DIMITROV N., ATKINS J., DALY M., WIEAND S., TAN-CHIU E., FORD L., WOLMARK N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- FISHER B., DIGNAM J., BRYANT J., WOLMARK N. Five versus more than five years of tamoxifen for lymph node-negative breast cancer: updated findings from the National Surgical Adjuvant Breast and Bowel Project B-14 randomized trial. J. Natl. Cancer Inst. 2001;93:684–690. doi: 10.1093/jnci/93.9.684. [DOI] [PubMed] [Google Scholar]
- GOSS P.E., INGLE J.N., MARINO S., ROBERT N.J., MUSS H.B., PICCART M.J., CASTIGLIONE M., TU D., SHEPHERD L.E., PRITCHARD K.I., LIVINGSTON R.B., DAVIDSON N.E., NORTON L., PEREZ E.A., ABRAMS J.S., THERASSE P., PALMER M.J., PATER J.L. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy for early-stage breast cancer. N. Engl. J. Med. 2003;349:1–10. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- GOTTARDIS M.M., JORDAN V.C. Antitumor actions of keoxifene and tamoxifen in the N-nitrosomethylurea-induced rat mammary carcinoma model. Cancer Res. 1987;47:4020–4024. [PubMed] [Google Scholar]
- HARPER M.J., WALPOLE A.L. A new derivative of triphenylethylene: effect on implantation and mode of action in rats. J. Reprod. Fertil. 1967;13:101–119. doi: 10.1530/jrf.0.0130101. [DOI] [PubMed] [Google Scholar]
- HOWELL A., CUZICK J., BAUM M., BUZDAR A., DOWSETT M., FORBES J.F., HOCTIN-BOES G., HOUGHTON J., LOCKER G.Y., TOBIAS J.S., ATAC TRIALISTS' GROUP Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- JENSEN E.V., BLOCK G.E., SMITH S., KYSER K., DESOMBRE E.R. Estrogen receptors and breast cancer response to adrenalectomy. Natl. Cancer Inst. Monogr. 1971;34:55–70. [PubMed] [Google Scholar]
- JENSEN E.V., JACOBSON H.I. Basic guides to the mechanism of estrogen action. Recent Progr. Hormone Res. 1962;18:387–414. [Google Scholar]
- JENSEN E.V., JORDAN V.C. The estrogen receptor: a model for molecular medicine. The Dorothy P. Landon AACR Prize for Translational Research. Clin. Cancer Res. 2003;9:1980–1989. [PubMed] [Google Scholar]
- JORDAN V.C. Antitumour activity of the antioestrogen ICI 46,474 (tamoxifen) in the dimethylbenzanthracene (DMBA)-induced rat mammary carcinoma model. J. Steroid. Biochem. 1974;5:354. [Google Scholar]
- JORDAN V.C. Effect of tamoxifen (ICI 46,474) on initiation and growth of DMBA-induced rat mammary carcinomata. Eur. J. Cancer. 1976;12:419–424. doi: 10.1016/0014-2964(76)90030-x. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C. Biochemical pharmacology of antiestrogen action. Pharmacol. Rev. 1984;36:245–276. [PubMed] [Google Scholar]
- JORDAN V.C. The development of tamoxifen for breast cancer therapy: a tribute to the late Arthur L. Walpole. Breast Cancer Res. Treat. 1988;11:197–209. doi: 10.1007/BF01807278. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C. Current view of the use of tamoxifen for the treatment and prevention of breast cancer. Gaddum Memorial Lecture. Br. J. Pharmacol. 1993;110:507–517. doi: 10.1111/j.1476-5381.1993.tb13840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JORDAN V.C. What if tamoxifen (ICI 46,474) had been found to produce rat liver tumors in 1973? A personal perspective. Ann. Oncol. 1995;6:29–34. doi: 10.1093/oxfordjournals.annonc.a059035. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C. Tamoxifen: a most unlikely pioneering medicine. Nat. Rev. Drug Disc. 2003;2:205–213. doi: 10.1038/nrd1031. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell. 2004;5:207–213. doi: 10.1016/s1535-6108(04)00059-5. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C., ALLEN K.E. Evaluation of the antitumour activity of the non-steroidal antioestrogen monohydroxytamoxifen in the DMBA-induced rat mammary carcinoma model. Eur. J. Cancer. 1980;16:239–251. doi: 10.1016/0014-2964(80)90156-5. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C., JASPAN T. Tamoxifen as an antitumour agent: oestrogen binding as a predictive test for tumour response. J. Endocrinol. 1976;68:453–460. doi: 10.1677/joe.0.0680453. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C., KOERNER S. Tamoxifen (ICI 46,474) and the human carcinoma 8S oestrogen receptor. Eur. J. Cancer. 1975;11:205–206. doi: 10.1016/0014-2964(75)90119-x. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C., LABABIDI M.K., LANGAN-FAHEY S. Suppression of mouse mammary tumorigenesis by long-term tamoxifen therapy. J. Natl. Cancer Inst. 1991;83:492–496. doi: 10.1093/jnci/83.7.492. [DOI] [PubMed] [Google Scholar]
- JORDAN V.C., PHELPS E., LINDGREN J.U. Effects of anti-estrogens on bone in castrated and intact female rats. Breast Cancer Res. Treat. 1987;10:31–35. doi: 10.1007/BF01806132. [DOI] [PubMed] [Google Scholar]
- KENNEDY B.J. Hormone therapy for advanced breast cancer. Cancer. 1965;18:1551–1557. doi: 10.1002/1097-0142(196512)18:12<1551::aid-cncr2820181206>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- LERNER L.J., HOLTHAUS J.F., THOMPSON C.R. A non-steroidal estrogen antagonist 1-(p-2-diethylaminoethoxyphenyl)-1-phenyl-2-p-methoxyphenylethanol. Endocrinology. 1958;63:295–318. doi: 10.1210/endo-63-3-295. [DOI] [PubMed] [Google Scholar]
- LERNER L.J., JORDAN V.C. The development of antiestrogens for the treatment of breast cancer: Eighth Cain Memorial Award Lecture. Cancer Res. 1990;50:4177–4189. [PubMed] [Google Scholar]
- LONNING P.E., GEISLER J., KRAG L.E., ERIKSTEIN B., BREMNES Y., HAGEN A.I., SCHLICHTING E., LIEN E.A., OFJORD E.S., PAOLINI J., POLLI A., MASSIMINI G. Effects of exemestane administered for 2 years versus placebo on bone mineral density, bone biomarkers, and plasma lipids in patients with surgically resected early breast cancer. J. Clin. Oncol. 2005;23:1–12. doi: 10.1200/JCO.2005.07.097. [DOI] [PubMed] [Google Scholar]
- LOVE R.R., MAZESS R.B., BARDEN H.S., EPSTEIN S., NEWCOMB P.A., JORDAN V.C., CARBONE P.P., DEMETS D.L. Effects of tamoxifen on bone mineral density in postmenopausal women with breast cancer. N. Engl. J. Med. 1992;326:852–856. doi: 10.1056/NEJM199203263261302. [DOI] [PubMed] [Google Scholar]
- LOVE R.R., WIEBE D.A., NEWCOMB P.A., CAMERON L., LEVENTHAL H., JORDAN V.C., FEYZI J., DEMETS D.L. Effects of tamoxifen on cardiovascular risk factors in postmenopausal women. Ann. Intern. Med. 1991;115:860–864. doi: 10.7326/0003-4819-115-11-860. [DOI] [PubMed] [Google Scholar]
- LUNAN C.B., KLOPPER A. Antioestrogens: a review. Clin. Endocrinol. 1975;4:551–572. doi: 10.1111/j.1365-2265.1975.tb01568.x. [DOI] [PubMed] [Google Scholar]
- MILLER W.R. Aromatase inhibitors: mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 2003;30:3–11. doi: 10.1016/s0093-7754(03)00302-6. [DOI] [PubMed] [Google Scholar]
- POWLES T.J., HARDY J.R., ASHLEY S.E., FARRINGTON G.M., COSGROVE D., DAVEY J.B., DOWSETT M., MCKINNA J.A., NASH A.G., SINNETT H.D. A pilot trial to evaluate the acute toxicity and feasibility of tamoxifen for prevention of breast cancer. Br. J. Cancer. 1989;60:126–131. doi: 10.1038/bjc.1989.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAMON D.J., CLARK G.M., WONG S.G., LEVIN W.J., ULLRICH A., MCGUIRE W.L. Human breast cancer:correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- SLAMON D.J., LEYLAND-JONES B., SHAK S., FUCHS H., PATON V., PHARM D., BAJAMONDE A., FLEMING T., EIERMANN W., WOLTER J., PEGRAM M., BASELGA J., NORTON L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- TERENIUS L. Structure–activity relationships of anti-oestrogens with regard to interaction with 17-beta-oestradiol in the mouse uterus and vagina. Acta Endocrinol. (Copenhagen) 1971a;66:431–447. doi: 10.1530/acta.0.0660431. [DOI] [PubMed] [Google Scholar]
- TERENIUS L. Anti-oestrogens and breast cancer. Eur. J. Cancer. 1971b;7:57–64. doi: 10.1016/0014-2964(71)90095-8. [DOI] [PubMed] [Google Scholar]
- TERENIUS L. Effect of anti-oestrogens on initiation of mammary cancer in the female rat. Eur. J. Cancer. 1971c;7:65–70. doi: 10.1016/0014-2964(71)90096-x. [DOI] [PubMed] [Google Scholar]
- WALPOLE A.L. Non steroidal drugs in relation to ovulation and implantation. J. Reprod. Fertil. 1968;14 Suppl:3–14. [Google Scholar]
- WALSH B.W., KULLER L.H., WILD R.A., PAUL S., FARMER M., LAWRENCE J.B., SHAH A.S., ANDERSON P.W. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women [see comments] JAMA. 1998;279:1445–1451. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]