Abstract
Over the 75-year lifetime of the British Pharmacological Society there has been an enormous expansion in our understanding of how opioid drugs act on the nervous system, with much of this effort aimed at developing powerful analgesic drugs devoid of the side effects associated with morphine – the Holy Grail of opioid research. At the molecular and cellular level multiple opioid receptors have been cloned and characterised, their potential for oligomerisation determined, a large family of endogenous opioid agonists has been discovered, multiple second messengers identified and our understanding of the adaptive changes to prolonged exposure to opioid drugs (tolerance and physical dependence) enhanced. In addition, we now have greater understanding of the processes by which opioids produce the euphoria that gives rise to the intense craving for these drugs in opioid addicts. In this article, we review the historical pathway of opioid research that has led to our current state of knowledge.
Keywords: Enkephalins, endorphins, morphine, opioid receptors, opioid analgesics
Introduction
Although the pain relieving and euphorigenic properties of extracts of the opium poppy have been known for centuries (Figure 1), it was only in the 20th century that there were substantial advances made in our understanding of how opiates, such as morphine produced their powerful and selective effects on the body. The major goals of opioid research are to understand the underlying biology of the endogenous opioid systems, the discovery of new analgesic drugs devoid of unwanted effects associated with morphine and the development of new therapies for the treatment of opioid addicts.
Figure 1.
Extracting the juice from the opium poppy. Taken from ‘Opiologia' a treatise concerning the nature, properties, true preparation and safe use and administration of opium. Sala (Angelus) 1618. Wellcome History of Medicine Library.
There have, over the years, been many exciting and influential developments such as the discovery of the endogenous opioid ligands, cloning of the multiple opioid receptors and a fuller appreciation of how opioids cause analgesia and euphoria. The effort expended has been richly rewarded with a more complete understanding of the opioid system but there remain several important questions to answer.
Opioid receptors
The proposal that morphine and related opioids cause analgesia by interacting with a specific receptor arose in the 1950s, based on the strict structural and stereochemical requirements essential for activity. With the benefit of hindsight, we can point to evidence from 40 years earlier of a specific (receptor-mediated) action with the observation that the N-allyl-derivative of codeine antagonised the respiratory depressant effect of morphine. The significance of this observation was only fully appreciated when the corresponding derivative of morphine (nalorphine) was shown to antagonise the analgesic effect of morphine in man. It took the recent development of a μ-opioid receptor knockout mouse, however, to provide the final proof that morphine-induced analgesia, reward and physical dependence result from activation of the μ-receptor.
In the 1960s and 1970s, Martin and co-workers (Martin, 1979) provided evidence for multiple opioid receptors when they demonstrated that a series of opioids displayed different profiles of pharmacological activity in vivo. They proposed that opioids activated three different types of receptor, called μ, κ and σ for which the prototypical agonists were morphine, ketocyclazocine, and N-allylnormetazocine (SKF 10047) respectively.
The identification of the δ-receptor followed the discovery of the first endogenous opioid receptor ligands, [Met]- and [Leu]-enkephalin (Hughes et al., 1975), when it was shown that their pattern of agonist activity in vitro differed from that of the prototypical opioid ligands (Lord et al., 1977). Importantly, the nonselective opioid antagonist, naloxone, was less effective in blocking enkephalin-induced inhibition of the nerve-evoked contractions of the mouse vas deferens, compared with its antagonism of the response to normorphine.
In the 1990s, genes encoding the μ-, δ- and κ-receptors were cloned (MOR-1, DOR-1, and KOR-1, respectively) and the cloned receptors displayed the topology characteristic of G-protein-coupled receptors. Later, cDNA encoding an ‘orphan' receptor was identified that had as high a degree of homology (>60%) towards the classical opioid receptors, as they shared among each other (see Henderson & McKnight, 1997). This receptor was originally referred to as opioid receptor-like (ORL1), and is accepted as a member of the ‘family' of opioid receptors on the basis of its structural homology towards the classical types. It should be noted that the terminology used for opioid receptors has in recent years been through several revisions. Originally the receptors were defined by those who first discovered them using the Greek letters, μ, δ and κ. Subsequently, on the advice of an IUPHAR nomenclature committee, this was changed to OP1 (δ), OP2 (κ) and OP3 (μ) and OP4 (opioid receptor-like (ORL1)). Fortunately this new terminology was short lived and on the recommendation of the International Narcotics Research Conference MOR, DOR, KOR, and NOR were accepted. Most recently the IUPHAR nomenclature committee has recommended that MOP receptor, DOP receptor, KOP receptor, and NOP receptor be used.
Based on pharmacological data, other types of novel but poorly characterised opioid receptors were proposed: these include ɛ-, ι-, λ- and ζ-receptors. Little attention has been paid to these entities in recent times.
The σ-receptor is no longer held to be an opioid receptor as it displays neither the stereoselectivity characteristic of opioid receptors nor antagonism by opioid antagonists. The term ‘σ-receptor' has remained in use, however, to describe the binding site for phencyclidine (PCP) in the ionotropic N-methyl-D-aspartate (NMDA) glutamate receptor complex. More recently the σ-receptor has become a unique nonopioid, non-PCP ‘receptor' (Quirion et al., 1992) with σ1 and σ2 subtypes proposed. The σ1 receptor has been characterised as a 223-amino-acid protein possessing only one transmembrane domain, which may function as part of a multisubunit complex. The identity of putative endogenous σ-receptor ligand(s) is still unclear but neuroactive steroids, particularly progesterone, are possible candidates.
Receptor oligomerisation
All four opioid receptors are thought to form homomeric as well as heteromeric receptor complexes (for review see Devi, 2001; Milligan, 2004). Each opioid receptor is now believed to be capable of forming a heterodimer with any other opioid receptor. Opioid receptors are, in fact, quite promiscuous and can form heterodimers with certain nonopioid receptors, for example, μ with α2a-adrenoceptors. Heterodimerisation between opioid receptors has been shown to result in changes in the pharmacology of the receptors (see below in Opioid receptor subtypes) as well as in changes in receptor coupling to second messengers and trafficking. Both μ- and δ-receptors internalise on exposure to agonists whereas κ-receptors do not. When δ/κ dimers are formed, the trafficking properties of the κ-receptor predominates and the heterodimer does not internalise on exposure to agonists of either receptor.
Opioid receptor subtypes
Subtypes of μ-, δ-, κ- and ORL1-receptors have been proposed largely on the basis of radioligand binding studies. As yet there is little or no evidence for different genes encoding these subtypes but in some cases receptor heterodimerisation of opioid receptors has been proposed as a possible explanation.
μ-receptor subtypes
Pasternak and co-workers suggested that the binding of [3H]-labelled μ- and δ-ligands was to two binding sites; a common high-affinity site was termed the μ1-site and the lower-affinity site was termed the μ2-site, with δ-ligands binding to their own lower-affinity δ-site (for review see Pasternak, 2005). Binding to the μ1-site was reported to be selectively blocked by naloxonazine. They also reported that naloxonazine blocked morphine-induced supraspinal analgesia but did not block morphine-induced respiratory depression or the induction of morphine dependence. Subsequent work in other laboratories has failed to confirm this classification. The pharmacology of the cloned MOR-1 corresponds to the μ2-site described above.
A further novel form of μ-receptor with which morphine itself does not interact but where morphine-6β-glucuronide and other 6-substituted analogues of morphine (e.g. heroin and 6-acetyl morphine) act to produce antinociception has been proposed (Pasternak, 2005). It has been suggested that this receptor is produced from an alternative transcript of the MOR-1 gene but the reliability of these observations awaits confirmation in other laboratories.
Splice variants of MOR-1 differing only in the C terminus region have been identified in a variety of species (Pasternak, 2005). Their significance remains to be elucidated but they may give rise to changes in receptor trafficking or agonist efficacy and potency.
δ-receptor subtypes
Two overlapping subdivisions of δ-receptors have been proposed: δ1/δ2 and δcx/δncx (for review see Traynor & Elliot, 1993). The δ1/δ2 subtypes arose from in vivo rodent experiments by Takemori and co-workers, in which δ-receptor agonists and antagonists displayed two patterns of supraspinal antinociceptive activity. There is little backing for this subdivision from radioligand-binding studies but some support has come from second messenger studies. The other subdivision proposed a δcx-subtype that was complexed with μ-receptors (and perhaps also κ-receptors) and a δncx-subtype not associated with an opioid receptor complex; the latter has been further resolved into δncx−1 and δncx−2. There is confusion about the relationship between the DOR-1 clone and the δ-receptor subtypes since the pharmacological properties of DOR-1 lie somewhere between those for δ1- and δ2-subtypes and the pharmacology of the δ/μ heterodimer does not correspond exactly to that described for either δ1/δ2 or δcx/δncx subtypes.
κ-receptor subtypes
The evidence for κ-receptor subtypes has come almost entirely from radioligand binding assays using nonselective ligands and the result is a much more complex and confusing literature than that for the other opioid receptors (Traynor, 1989). The demonstration of a κ-binding site analogous to the κ-receptor proposed by Martin initially proved difficult as the ketocyclazocine-like compounds, in addition to their κ-affinity, had affinity for μ- and δ-binding sites. To demonstrate specific κ-binding in the brain, it was necessary to quench (‘suppress') binding to μ- and δ-sites, by incubation with unlabelled ligands that bound selectively at these sites (Kosterlitz et al., 1981).
Several different groups have suggested κ1-, κ2- and κ3-subtypes but it is unclear how the putative subtypes relate to each other. Definitive functional pharmacological evidence supporting the existence of this confusing number of putative subtypes of the κ-receptor is lacking primarily because of the absence of subtype-specific ligands. The cloned KOR-1 appears to encode for the κ1 receptor. It has been suggested that the κ2 receptor results from the heterodimerisation of κ- and δ-receptors (Devi, 2001).
ORL1-receptor subtypes
There is as yet no convincing data to support the existence of ORL1-receptor subtypes although a C terminal splice variant has been described. As with κ-receptors the pharmacological delineation of ORL1-subtypes awaits the development of highly selective ligands, specifically antagonists.
Endogenous ligands
By the early 1970s the idea had arisen that the function of the opioid receptors in the brain was not to mediate the pharmacological effects of opium alkaloids and their congeners, but that there must exist endogenous agonists fulfilling a physiological function. Prime exponents of this concept were Hans Kosterlitz (Figure 2) and John Hughes. Their success in being the first to identify endogenous opioids was due to the use of a functional bioassay to measure morphine-like activity in extracts of brain and because they had no preconceived idea of the possible structure of the endogenous ligand, other than it was likely to be a small molecule with similarities to morphine and, as a putative neurotransmitter or neuromodulator, it would probably be labile (Kosterlitz, 1979). The isolation of sufficient material from pig brain for analysis took about 2 years and resulted in the identification of two closely related endogenous opioids, the pentapeptide enkephalins (Hughes et al., 1975; Table 1); the name is derived from the Greek en kephalos meaning ‘in the head'.
Figure 2.
Hans Walter Kosterlitz (1903–1996), the doyen of British opioid pharmacologists. Photograph courtesy of Alan North.
Table 1. Endogenous opioid peptides.
| Precursor | Opioid peptide product | Amino acid sequence |
|---|---|---|
| Pro-enkephalin | [Met]-enkephalin | YGGFM |
| [Leu]-enkephalin | YGGFL | |
| YGGFMRF | ||
| YGGFMRGL | ||
| Peptide E | YGGFMRRVGRPEWWMDYQKRYGGFM | |
| BAM 22P | YGGFMRRVGRPEWWMDYQKRYG | |
| Metorphamide | YGGFMRRVNH2 | |
| Pro-opiomelanocortin | β-Endorphin | YGGFMTSEKSQTPLVTLFKNAIIKNAYKKGE |
| Prodynorphin | Dynorphin A | YGGFLRRIRPKLKWDNQ |
| Dynorphin A(1–8) | YGGFLRRI | |
| Dynorphin B | YGGFLRRQFKVVT | |
| α-Neoendorphin | YGGFLRKYPK | |
| β-Neoendorphin | YGGFLRKYP | |
| Pronociceptin/orphanin-FQ | Nociceptin/orphanin-FQ | FGGFTGARKSARKLANQ |
| Endomorphin-1 | YPWF-NH2 | |
| Endomorphin-2 | YPFF-NH2 | |
| Prodermorphin and prodeltorphin* | Dermorphin | Y(D)AFGYPS-NH2 |
| Deltorphin | Y(D)MFHLMD-NH2 | |
| Deltorphin I | Y(D)AFDVVG-NH2 | |
| Deltorphin II | Y(D)AFEVVG-NH2 |
The pentapeptide sequences corresponding to [Met]-enkephalin and [Leu]-enkephalin contained in other opioid peptides are shown in bold. Note that β-endorphin and most of the opioid peptides derived from proenkephalin contain [Met]-enkephalin at their N-termini, whereas the sequence of [Leu]-enkephalin is present in those peptides derived from prodynorphin.
Dermorphin and deltorphins are derived from multiple precursors and all have a naturally occuring D-amino acid in position 2.
Soon after the enkephalins were discovered it was observed that a fragment of the pituitary hormone β-lipotropin(61–91) contained the sequence of [Met]-enkephalin at its amino-terminus; this 31 amino acid (‘C-fragment') was then shown to be a potent opioid agonist and was subsequently called β-endorphin. It was initially proposed that β-endorphin was a precursor of [Met]-enkephalin but the demonstration of their markedly different distribution eliminated the possibility of a precursor:product relationship, signifying that β-endorphin is an important opioid product in its own right.
Opioid precursors
Within 5 years of the discovery of the enkephalins, there were three families of opioid peptides, derived from different precursors: proenkephalin, pro-opiomelanocortin, prodynorphin, and all of the opioid peptide cleavage products contained the sequence of either [Met]-enkephalin or [Leu]-enkephalin as the first five amino acids (Table 1). These peptides vary in their affinity for μ-, δ- and κ-receptors, and have negligible affinity for ORL1-receptors, but none binds exclusively to one opioid receptor type (Corbett et al., 1993; Henderson & McKnight, 1997).
Pro-opiomelanocortin
Pro-opiomelanocortin is a multifunctional precursor giving rise to adrenocorticotropin (ACTH), α-, γ- and β-melanocyte stimulating hormone (MSH) but apparently only one opioid peptide, β-endorphin, although fragments of β-endorphin such as β-endorphin(1–27) may also have biological relevance. β-Endorphin appears to be an important mediator released from the brain and the pituitary, is equiactive at μ- and δ-receptors but with much lower affinity for κ-receptors (Corbett et al., 1993).
Proenkephalin
Proenkephalin contains four copies of [Met]-enkephalin, one copy of [Leu]-enkephalin and one copy each of the octapeptide [Met]-enkephalyl-Arg-Gly-Leu and the heptapetide [Met]-enkephalyl-Arg-Phe, which forms the C-terminus of the precursor (Table 1). [Met]- and [Leu]-enkephalins have high affinities for δ-receptors but C-terminal extensions lose this δ-preference (Corbett et al., 1993). Proenkephalin is widely expressed in neuronal and non-neuronal sites, and processing can differ markedly between tissues. A number of other opioid products are derived from proenkephalin (Table 1). These opioid peptides have been implicated in a wide variety of biological roles including analgesia but interest in BAM 22P, in particular, has recently been rekindled because of its high affinity for the sensory neuron-specific G-protein-coupled receptors (SNSRs). Interestingly it is the C-terminal region (BAM 8–22) that is important for interacting with SNSRs whereas its N-terminal region interacts with opioid receptors. The most potent agonist at SNSRs is γ-MSH, the nonopioid peptide derived from pro-opiomelanocortin.
Prodynorphin
In 1979, Goldstein and co-workers proposed the existence of ‘dynorphin', a potent endogenous opioid peptide with the sequence of [Leu]-enkephalin at its N-terminus, and this peptide was characterised as the full heptadecapeptide dynorphin A in 1981 (Goldstein et al., 1981). Soon afterwards the isolation of other endogenous [Leu]-enkephalin-containing peptides was reported; these peptides (Table 1) are derived from a common precursor, prodynorphin. The opioid fragments of prodynorphin have high affinity for κ-receptors but also have significant affinity for μ- and δ-receptors (Corbett et al., 1993).
Pronociceptin/orphanin FQ
The most recent addition to the endogenous opioid ligand ‘superfamily' is nociceptin/orphanin-FQ which is derived from a fourth opioid precursor, pronociceptin/orphanin-FQ (for review see Henderson & McKnight, 1997). Nociceptin/orphanin-FQ, the endogenous ligand for the ORL1-receptor, is a heptadecapeptide, which was isolated simultaneously in 1975 by the groups of Meunier and Civelli. Nociceptin/orphanin-FQ resembles the dynorphins (Table 1) in that it has a peptide backbone consisting of a number of basic amino acids important for binding to the receptor and contains the canonical Gly-Gly-Phe (GGF) sequence. The N-terminal amino acid of nociceptin/orphanin-FQ is phenylalanine rather than tyrosine, and this single change markedly influences the receptor selectivity of the peptides, with nociceptin/orphanin-FQ having virtually no affinity for μ-, δ- and κ-receptors.
Endomorphins
Over 25 years after the isolation and characterisation of the enkephalins, two C-terminally amidated tetrapeptides, endomorphin-1 and endomorphin-2, were extracted from the brain (Zadina et al., 1997). They have a tyrosine residue at the N-terminus but are otherwise structurally unrelated to the enkephalins (Table 1). The endomorphins have exceptionally high affinity and selectivity for the μ-receptor but as yet no precursor protein has been identified and thus there is still doubt about whether they are endogenous, or are peptides cleaved from larger proteins during the extraction procedure.
Endogenous morphine
In the original concept of endogenous opioids it was supposed that such a substance would be unlikely to be closely related to the alkaloid morphine. However, morphine itself has been shown to be present in tissues and body fluids although in much lower concentrations that in Papaver somniferum. Early reservations that ‘endogenous' morphine was of dietary origin have been discounted since precursors such as thebaine and codeine are also present in mammalian tissue. More importantly, SH-SY5Y human neuroblastoma cells are capable of synthesising morphine via a biosynthetic route similar to that of the opium poppy. The physiological role of endogenous morphine is still unclear but it is present in neurones and undergoes Ca2+-dependent release consistent with a neurotransmitter or neuromodulator role.
Atypical opioid peptides
There are many other peptides with opioid activity that are not derived from one of the four opioid precursors and have been grouped together as ‘atypical' opioid peptides (Teschemacher, 1993). These opioids are derived from a variety of parent proteins but almost all have a Tyr residue at their N-terminus. Atypical opioid peptides include the milk-derived β-casomorphins, haemorphins from haemoglobin and cytochrophins that are fragments of cytochrome b. They all have low affinity and selectivity for opioid receptors. In contrast, amphibia have proven to be an important source of selective and high affinity, atypical opioid peptides. Amphibian skin contains two families of D-amino-acid-containing peptides, the dermorphins and deltorphins (Table 1). Dermorphin is a μ-selective heptapetide without significant affinity at δ- and κ-receptors. The deltorphins on the other hand are highly selective for δ-receptors. The physiological significance of amphibian and other atypical opioid peptides remains unclear.
Effector mechanisms
All of the opioid receptors are G-protein coupled receptors (GPCRs) and couple to their cellular effectors primarily through Gi/Go proteins and thus the majority of opioid responses are Pertussis toxin-sensitive (see also Milligan & Kostenis, this issue). The different behaviours mediated by each receptor type in the intact animal (e.g. μ – euphoria vs κ – dysphoria; μ – supraspinal analgesia vs ORL1 – supraspinal antagonism of opioid analgesia) result not from each type of receptor evoking different cellular responses but from the different anatomical distributions of each receptor.
Interaction with ion channels
Early iontophoretic studies in rat brain and spinal cord revealed that morphine could inhibit or excite single neurones. Opioid inhibition of neuronal excitability occurs largely by activation of potassium channels in the plasma membrane (Figure 3). Opioid receptors are now known to activate a variety of potassium channels, including G-protein-activated inwardly rectifying (GIRK), calcium-activated, inwardly rectifying, dendrotoxin-sensitive and M-type channels (Williams et al., 2001; see also Jenkinson, this issue). As with other members of the Gi/Go-coupled receptor family, each of the opioid receptors has been shown also to inhibit high threshold voltage-activated calcium channels. Paradoxically, in some cell types, opioid receptor activation can also cause an elevation of the free calcium concentration inside cells by releasing calcium from intracellular stores or enhancing calcium entry by a dihydropyridine-sensitive mechanism (Samways & Henderson, 2005).
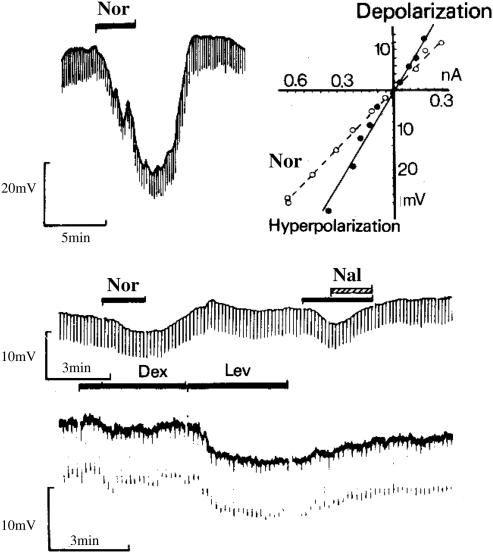
Figure 3.
First demonstration that the μ-receptor couples to activation of a potassium conductance. Membrane hyperpolarisations of guinea-pig myenteric neurones in response to normorphine (Nor) and levorphanol (Lev), but not to dextrorphan (Dex). The response to normorphine was reversed by coapplication of naloxone (Nal) (North & Tonini, 1979, with permission).
Inhibition of neurotransmitter release
The original observations that opioids inhibit neurotransmitter release were made in the peripheral nervous system. In 1917, Trendelenburg first reported that morphine inhibited the peristaltic reflex in the guinea-pig ileum but it took 40 years to demonstrate that this was due to inhibition of acetylcholine release. Later, morphine was also shown to inhibit the release of noradrenaline (and presumably also ATP) from postganglionic sympathetic nerve endings innervating the cat nictitating membrane and mouse vas deferens.
Early studies in the brain were impeded by the lack of understanding of neurotransmission within the CNS. Opiates were shown to inhibit the release of acetylcholine and noradrenaline from the cerebral cortex but these studies predated the general acceptance of amino acids as the major CNS neurotransmitters. Today there is ample evidence that the release of glutamate, GABA and glycine throughout the CNS can be inhibited by opioids acting at any one of the four opioid receptor types (Williams et al., 2001). In the late 1980s and early 1990s there was much debate about the relative importance of increased potassium conductance and inhibition of voltage-activated calcium channels in the inhibition of neurotransmitter release. Recently, it is has been demonstrated that opioid receptors may inhibit directly the neurotransmitter release machinery. Thus, opioids are able to inhibit neurotransmitter release by one or more mechanisms and the relative importance of each mechanism may vary from synapse to synapse.
Although the predominant action of opioids in the nervous system is inhibitory, in several brain regions important for either supraspinal analgesia (e.g. periaqueductal grey (PAG)) or euphoria/reward (e.g. ventral tegmental area (VTA)) opioids are excitatory. Initially there was controversy over the possible mechanisms (Henderson, 1983) but it is now accepted that opioid-induced excitations are due, not to a direct excitatory action of opioids, but to disinhibition; the apparent excitation of a neurone by opioids being the result of inhibition of the release of inhibitory neurotransmitters (e.g. GABA) from interneurones onto that cell. In the rostroventral medulla, μ-receptors are located presynaptically on GABAergic interneurones and give rise to disinhibition and enhanced neuronal output, whereas activation of the postsynaptic ORL1-receptors results in inhibition of neuronal output from this nucleus. Thus a simple difference in anatomical distribution explains why, supraspinally, μ agonists induce analgesia, whereas ORL1 agonists reduce opioid analgesia.
Inhibition of adenylyl cyclase
Opioid receptors, in common with other Gi/Go-coupled receptors, inhibit adenylyl cyclase resulting in a fall in intracellular cAMP. While this phenomenon has been extensively used in the study of opioid receptor-effector coupling, its physiological significance has been obscure. In primary afferent neurons, cAMP modulates the activation of hyperpolarisation-activated cation channels (Ih) (see Williams et al., 2001). By decreasing cAMP levels opioids effectively inhibit Ih, reducing pacemaker activity and lowering the rate of action potential firing thus reducing the flow of nociceptive information to the CNS. In opioid withdrawal, cAMP levels are elevated, and enhanced protein kinase A (PKA) activity increases neurotransmitter release. Under these circumstances, inhibition of adenylyl cyclase by opioids will lead to a reduction in the PKA-enhanced transmitter release.
Tolerance
Opioid receptor desensitisation and trafficking
Opioid receptors, like many other GPCRs, cycle to and from the plasma membrane from intracellular compartments. This cycling can be either constitutive or precipitated by agonist activation of the receptor. δ- and κ-receptors have been reported to be located predominantly in intracellular compartments and cycle to the plasma membrane in response to various stimuli including chronic morphine treatment.
The generally accepted mechanism underlying μ- and δ-receptor desensitisation is that agonist-activated receptors on the plasma membrane are phosphorylated by G-protein-coupled receptor kinases (GRKs), which facilitates arrestin binding (Bailey & Connor, 2005) and prevents the receptor from coupling to G-proteins. Arrestin-bound receptors are rapidly concentrated in clathrin-coated pits and undergo dynamin-dependent internalisation into early endosomes. δ-receptors are then preferentially trafficked into lysosomes and are downregulated, whereas μ-receptors are trafficked into endosomes where they are dephosphorylated and recycled back to the plasma membrane in a resensitised state. Thus, for the μ-receptor internalisation can be considered to be involved in resensitisation, not in desensitisation. There is evidence that different C-terminus splice variants of the μ-receptor resensitise at different rates. Interestingly, κ-receptors do not appear to internalise in response to agonist activation.
Morphine tolerance
In the whole animal, tolerance to morphine has been shown to develop rapidly even after a single dose. Morphine tolerance is a complex phenomenon that may comprise adaptive changes at the level of the opioid receptors themselves (desensitisation), as well as a variety of more generalised changes, for example, altered gene expression or changes in the properties of neuronal circuits (Williams et al., 2001; Waldhoer et al., 2004). There is, however, still controversy concerning the role of μ-receptor desensitisation in mediating tolerance (Waldhoer et al., 2004; Bailey & Connor, 2005). Morphine induces less μ-receptor desensitisation than other opioid agonists and this led Jennifer Whistler and co-wokers to suggest that morphine may induce greater tolerance because it does not induce rapid receptor desensitisation. In β-arrestin 2 knockout mice, the analgesic effect of a single dose of morphine is enhanced and the development of chronic morphine tolerance attenuated implying an arrestin-dependent mechanism underlying the development of tolerance. However, β-arrestin may have functions unrelated to receptor desensitisation that influence the response to opioid agonists. The mechanism underlying morphine tolerance may in fact be more prosaic. Increasing protein kinase C (PKC) activity markedly enhances morphine-induced μ-receptor desensitisation (Bailey et al., 2004). Activation of PKC, for instance, by activation of Gq-coupled receptors or NMDA receptors, may be required for the development of morphine tolerance and may explain the reversal of morphine tolerance in vivo by PKC inhibitors.
Dependence
Physical dependence
Opiate withdrawal results in a rebound enhancement of cAMP production. How can a rise in cAMP production be translated into the characteristic opiate withdrawal syndrome observed in man? At many synapses, activation of PKA, a direct consequence of cAMP production, results in an enhancement of neurotransmitter release. Thus at opioid-sensitive synapses, withdrawal-induced adenylyl cyclase supersensitivity would result in increased neurotransmitter release (Williams et al., 2001). In addition, opioid withdrawal induces hyperexcitability in many brain regions. It has recently been demonstrated that opiate withdrawal activates a cation current in periaqueductal grey neurones that is mediated by the GABA transporter-1 (GAT-1) and requires activation of PKA for its expression (Bagley et al., 2005). Inhibition of GAT-1 or PKA prevented withdrawal-induced hyperexcitation and thus GAT-1 may be a potential target for therapeutic suppression of opiate withdrawal symptoms.
Psychological dependence
The importance of the VTA-nucleus accumbens projection in the rewarding effects of morphine was first suggested by the observation that rats will lever press to obtain morphine directly into the VTA. At a relatively simplistic level, μ-agonists can be thought to induce euphoria by indirectly enhancing dopamine release in the nucleus accumbens (see also Marsden, this issue). They do this in part by inhibiting GABA release from interneurones within the VTA, thus disinhibiting the dopaminergic VTA neurones that project to the accumbens. On the other hand, κ-agonists are dysphoric because they directly inhibit dopamine release from nerve terminals in the nucleus accumbens. Morphine, like cocaine, amphetamine and nicotine, promotes long-term potentiation (LTP) of glutamatergic transmission onto VTA dopaminergic neurones by mechanims that are not yet fully understood (Jones & Bonci, 2005). LTP is thought to be a synaptic correlate of learning and memory and its induction by drugs of abuse may explain the long lasting craving for these drugs that persists in addicts even when they are drug free.
Development of opioids as clinically useful analgesics
The driving force behind over 75 years of synthetic efforts in the opioid field has been the search for an alternative to morphine, which would induce powerful analgesia without the attendant effects of respiratory depression and, above all, liability for abuse. Most of the clinically available opioid drugs were obtained before the discovery of the endogenous peptide agonists and the characterisation of the opioid receptors and, indeed, many were obtained even before the receptors were postulated.
Morphine and related drugs
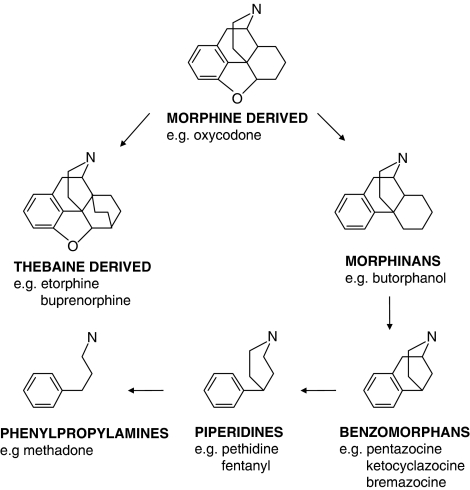
The majority of clinically available opioid analgesics are μ-agonists derived from chemical templates that relate to the natural opium alkaloids, with progressive simplification through the morphinans to the benzomorphans and the piperidines, to the phenylpropylamines such as methadone (Figure 4). It should not be supposed that the scheme shown indicates the temporal sequence for the derivation of the classes of opioids, or that this apparently elegant progression reveals a rational approach, for here, as in many drug-discovery and development programmes, serendipity also played a role.
Figure 4.
Chemical template of morphine-related drugs illustrating the progressive simplification of the structure.
With the introduction of the hypodermic syringe in the mid 19th century morphine itself became available for parenteral use as an improved analgesic, sedative and antitussive agent. The addictive properties of morphine soon became apparent, and one of the first ‘nonaddictive morphine substitutes' to be introduced was the 3,6-diacetylated derivative, diacetylmorphine (heroin). Heroin was an early example of a prodrug, with high analgesic potency attributable to rapid metabolism to 6-monoacetylmorphine and morphine (Casy & Parfitt, 1986), but the claims for reduced respiratory depression and dependence liability were soon shown to be ill-founded. Later, semisynthetic derivatives of morphine were introduced more tentatively, and several (e.g. hydrocodone and oxycodone) remain available today, in spite of little clear therapeutic advantage over morphine and considerable abuse liability.
The introduction of a bulky substituent into the morphine structure gives the molecule antagonist activity. Nalorphine (N-allylnormorphine) was the first opioid agonist-antagonist to be subjected to extensive clinical testing. The early clinical work was with combinations of nalorphine and morphine in an attempt to find an ‘ideal ratio' where only the desired analgesic properties of morphine might be obtained (Houde, 1979; Martin, 1979). Nalorphine was subsequently found to be an analgesic in man with reduced respiratory depressant and abuse liability properties. These findings had the profoundest effect on the course of opioid drug discovery in the succeeding years, however, and pointed to the prospect that ‘narcotic agonist-antagonist drugs' might be the answer to the quest for nonaddictive analgesics. Nalorphine was subsequently observed to have dysphoric and psychotomimetic effects at high doses and is now accepted to be an antagonist at the μ-receptor and an agonist at the κ-receptor. Note: The term ‘narcotic agonist-antagonist' was introduced before the existence of multiple opioid receptors was accepted and was used to describe the activity of the drug in vivo. In this context, it encompasses both partial agonists and drugs that are agonists at one opioid receptor, while also being antagonists at another opioid receptor.
Subsequently antagonists with little or no agonist efficacy have been developed. Naloxone is a competitive antagonist with slightly higher affinity for μ-receptors over δ- and κ-receptors. Naltrexone has activity comparable to naloxone but has longer duration of action and higher oral availability (Gold et al., 1982). Several therapeutic indications for antagonists continue to be explored, beyond that of the simple antidote against opioid overdose. These include antagonists that do not cross the blood/brain barrier; such drugs may be of use in preventing opioid-induced nausea, vomiting and constipation.
Important advances in opioid chemistry were made in the 1960s by Bentley and Hardy (Casy & Parfitt, 1986). Their rationale had been to produce opioid ligands of increased rigidity, pursuing the notion that the flexibility of morphine permitted the interaction with different receptors, so producing the (undesirably) wide spectrum of effects. The result was to produce nonselective agonist and antagonist compounds of high affinity at all of the opioid receptor types, and, in the case of the agonists as exemplified by etorphine, agonist potency several thousand times that of morphine was achieved. Etorphine was a potent analgesic in man, but the therapeutic index was low on account of its respiratory depressant effect. Gratifyingly, some success was achieved from this innovative programme with the development of buprenorphine by Reckitt and Colman as an analgesic for intramuscular and sublingual use. Although it is a partial agonist at the μ-receptor (as well as being an antagonist at the κ-receptor) buprenorphine is a powerful analgesic in postoperative and cancer pain (Houde, 1979).
Building on the innovative work of May and Eddy and exploring the benzomorphan template, Sidney Archer and co-workers at Sterling-Winthrop developed pentazocine as a drug that had reduced potential for abuse and provided an adequate level of analgesia in various clinical settings (Houde, 1979; Harris, 1985). Subsequently reports of dysphoria and psychotomimesis were made (Houde, 1979) that are probably due to pentazocine being a κ agonist and μ antagonist.
The serendipitous nature of drug discovery is well psychoto-highlighted in the case of pethidine (meperidine in the U.S.A.), which was synthesised among a group of compounds being studied for spasmolytic activity and whose analgesic activity was identified in routine screening (Casy & Parfitt, 1986). It was introduced for clinical use in 1939 and has been used extensively in obstetrics. The importance of pethidine was that the 4-phenylpiperidine template (Figure 4) represented a novel chemical approach in the relentless search for the ideal opioid analgesic and thousands of analogues were synthesised over the following 25 years in many laboratories. It became clear, however, that just as the data from preclinical tests of μ-agonist activity were reliable in predicting analgesic potency in man, the correlation was just as good for dependence liability (Harris, 1985). On surveying such findings, Schaumann, who had developed pethidine, was driven to conclude pessimistically that it would ‘not be possible to find morphine-like analgesics without the undesirable addiction'.
A very important addition to the chemical classes of opioid analgesic drugs came with the introduction of 4-anilinopiperidines by Janssen around 1960 (Casy & Parfitt, 1986). This led not only to the powerful opioid analgesic fentanyl, but also to the major tranquilliser haloperidol. Janssen's approach had been to attempt to design compounds of high potency, as a possible approach to selectivity and low toxicity, and then to tailor the pharmacodynamic and pharmacokinetic properties to allow different therapeutic applications. Fentanyl and analogues such as sufentanil are among the most potent μ-agonists known and generally have been used as adjuncts for surgical anaesthesia. In this context, a short duration of action is preferred and another analogue, alfentanil, was useful in this respect. More recently the development of an ‘ultra-short-acting' analogue of fentanyl has been achieved by the substitution of an ester function that is required for biological activity (Feldman et al., 1991). Remifentanyl is rapidly metabolized by blood and tissue esterases, has a terminal elimination half-life of less than 10 min and does not accumulate in tissues. Consequently, it is ideal for use by continuous infusion in a perioperative setting.
Opioid agonists in the treatment of opioid withdrawal
Methadone produces analgesia comparable to that of morphine with a similar profile of side effects. It has good oral availability with a long duration of action and showed withdrawal effects that were slower to develop and less severe than for morphine (Isbell & Fraser, 1950). These properties were the basis of the now extensive use of methadone as maintenance therapy in opioid dependence. The related compound, L-alpha-acetyl-methadol (LAAM), has been introduced as a longer acting alternative to methadone.
The possible utility of sublingual buprenorphine as a treatment for opioid abuse was suggested over 25 years ago, with its long duration of action and relatively mild withdrawal syndrome, presumably due to slow dissociation from its receptors (Lewis et al., 1982) pointing to this utility. Approval was granted by the FDA for this use in 2002 either for the drug by itself, or with naloxone to prevent ‘diversion' and parenteral misuse.
κ-receptor agonists
Several benzomorphans deriving from the programmes that produced pentazocine, including ketocyclazine, ethylketocyclazocine and bremazocine became important compounds in defining the pharmacology of the κ-receptor, as has been explained. Although their clinical development was precluded because of psychotomimetic and dysphoric effects, the limited data from studies in man and the increasing evidence from preclinical studies showed that dependence liability was low. It was thought that the undesirable effects of this class was attributable to their lack of selectivity and, in spite of the evidence that the psychotomimetic effects were mediated by the κ-receptor (rather than the ‘sigma receptor'), there was some optimism that in a truly selective κ-agonist, the long-held hope for an opioid analgesic that lacked abuse potential would be realised. This hypothesis would not be put to the test until the Upjohn Company described a structurally novel class of selective κ-agonist (Vonvoightlander et al., 1983). The first compound, U-50,488, was an important structural lead that was developed further both by the Upjohn Company and by Parke-Davis. Both companies undertook clinical investigation with the related compounds spiradoline (Upjohn) and enadoline (Parke-Davis), although there are few reports with the former compound and none relating to analgesic activity. Enadoline was shown to have little efficacy against the pain of third molar extraction (Pande et al., 1996a) and, while there was a positive effect in postsurgical pain, comparable to that achieved with morphine, ‘neuropsychiatric adverse events' with enadoline were dose limiting (Pande et al., 1996b).
As a result of the possibility of adverse CNS effects, several groups were proceeding in parallel with exploratory work on peripherally acting agents. The most advanced of these seems to be asimadoline, with development of the compound initially towards pain associated with osteoarthritis. Asimadoline was reported to increase the pain associated with knee surgery (Machelska et al., 1999), although recently there have been more promising early reports against abdominal pain (Delvaux et al., 2004). In support of this possible application, another peripherally acting κ-agonist, ADL 10-0101, is reported to have some utility in reducing the pain of pancreatitis (Eisnach et al., 2003).
δ-receptor agonists
The discovery of the enkephalins and of the δ-receptor led to the idea that the peptides themselves might be taken as ‘leads' for the development of a new class of opioid agonist. Progress towards the development of δ-selective agonists was slow, for the want of selective nonpeptide molecules. The first breakthrough was the discovery of a selective antagonist, naltrindole, an analogue of naltrexone, synthesised by Portoghese. The development of this compound was based on the ‘message-address' concept proposed for opioid peptides in which the molecule contains both a ‘message' and an ‘address' component; the message contributes to signal transduction, whereas the ‘address' confers receptor selectivity. Application of the message-address concept has been reported to be the basis of the successful identification of several nonpeptide δ-selective agonists such as TAN-67 and its close analogue SB 213698. However, the discovery of another series of piperazine derivatives, such as SNC-80 is less readily accounted for by this concept. The development status of these and other δ-selective agonists is not clear.
In addition to their antinociceptive activity δ-receptor agonists show antidepressant-like activity in animal behaviour models (Broom et al., 2002), suggesting that the δ-receptor could provide a therapeutic target for depression. However, δ-receptor agonists are also reported to have proconvulsant effects, which may limit their therapeutic usefulness.
Conclusion
Massive advances have been made in our understanding of the pharmacology of the opioid systems but despite this and the considerable effort expended in developing new and more selective molecules, there has been a disappointing lack of significant progress towards the Holy Grail of opioid research, that is, the development of a powerful analgesic drug, free from the undesirable effects of morphine. It may be that new drugs for the relief of pain will act through mechanisms unrelated to the opioid system, as exemplified by gabapentin, originally developed as an anticonvulsant.
Glossary
- ATP
adenosine triphosphate
- CNS
central nervous system
- cAMP
cyclic adenosine monophosphate
- GPCRs
G-protein coupled receptors
- GIRKs
G-protein-activated inwardly rectifying potassium channels
- GRKs
G-protein-coupled receptor kinases
- GAT-1
GABA transporter-1
- Ih
hyperpolarisation-activated cation channels
- LAAM
L-alpha-acetyl-methadol
- LTP
long-term potentiation
- ORL1
opioid receptor-like receptor 1
- PAG
periaqueductal grey
- PKA
protein kinase A
- PKC
protein kinase C
- SNSRs
sensory neuron-specific G-protein-coupled receptors
- VTA
ventral tegmental area
References
- BAGLEY E.E., GERKE M.B., VAUGHAN C.W., HACK S.P., CHRISTIE M.J. GABA transporter currents activated by protein kinase A excite midbrain neurons during opioid withdrawal. Neuron. 2005;45:433–445. doi: 10.1016/j.neuron.2004.12.049. [DOI] [PubMed] [Google Scholar]
- BAILEY C.P., CONNOR M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr. Opin. Pharmacol. 2005;5:60–68. doi: 10.1016/j.coph.2004.08.012. [DOI] [PubMed] [Google Scholar]
- BAILEY C.P., KELLY E., HENDERSON G. Protein kinase C converts morphine into a desensitizing agonist at μ-opioid receptors. Mol. Pharmacol. 2004;66:1592–1598. doi: 10.1124/mol.104.004747. [DOI] [PubMed] [Google Scholar]
- BROOM D.C., JUTKIEWICZ E.M., RICE K.C., TRAYNOR J.R., WOODS J.H. Behavioral effects of δ-opioid receptor agonists: potential antidepressants. Jpn. J. Pharmacol. 2002;90:1–6. doi: 10.1254/jjp.90.1. [DOI] [PubMed] [Google Scholar]
- CASY A.F., PARFITT R.T. Opioid Analgesics. New York: Plenum Press; 1986. [Google Scholar]
- CORBETT A.D., PATERSON S.J., KOSTERLITZ H.W.1993Selectivity of ligands for opioid receptors Handbook Exp. Pharmacol.104/1, ed. Herz, A. pp. 645–679.Berlin: Springer-Verlag [Google Scholar]
- DELVAUX M., BECK A., JACOB J., BOUZAMONDO H., WEBER F.T., FREXINOS J. Effect of asimadoline, a kappa opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment. Pharmacol. Ther. 2004;220:237–246. doi: 10.1111/j.1365-2036.2004.01922.x. [DOI] [PubMed] [Google Scholar]
- DEVI L.A. Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 2001;22:532–537. doi: 10.1016/s0165-6147(00)01799-5. [DOI] [PubMed] [Google Scholar]
- EISNACH J.C., CARPENTER R., CURRY R. Analgesia from a peripherally selective kappa-opioid receptor agonist in patients with chronic pancreatitis. Pain. 2003;101:89–95. doi: 10.1016/s0304-3959(02)00259-2. [DOI] [PubMed] [Google Scholar]
- FELDMAN P.L., JAMES M.K., BRACKEEN M.F., BILOTTA J.M., SCHISTER S.V., LAHEY A.P., LUTZ M.W., JOHNSON M.R., LEIGHTON H.J. Design, synthesis and pharmacological evaluation of ultrashort- to long acting opioid analgetics. J. Med. Chem. 1991;34:2202–2208. doi: 10.1021/jm00111a041. [DOI] [PubMed] [Google Scholar]
- GOLD M.S., DACKIS C.A., POTTASH A.L.C., STERNBACH H.H., ANITTO W.J. Naltrexone, opiate addiction and endorphins. Med. Res. Rev. 1982;2:211–246. doi: 10.1002/med.2610020302. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN A., FISCHLI W., LOWNEY L.I., HUNKAPILLER M., HOOD L. Porcine pituitary dynorphin: complete amino acid sequence of the biologically active heptadecapeptide. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7219–7223. doi: 10.1073/pnas.78.11.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRIS L.S. Nathan B. Eddy memorial award lecture. NIDA Res. Monographs. 1985;67:4–13. [PubMed] [Google Scholar]
- HENDERSON G. Electrophysiological analysis of opioid action in the central nervous system. Br. Med. Bull. 1983;39:59–64. doi: 10.1093/oxfordjournals.bmb.a071792. [DOI] [PubMed] [Google Scholar]
- HENDERSON G., MCKNIGHT A.T. The orphan opioid receptor and its endogenous ligand – nociceptin/orphanin FQ. Trends Pharmacol. Sci. 1997;18:293–300. [PubMed] [Google Scholar]
- HOUDE R.W. Analgesic effectiveness of the narcotic agonist-antagonists. Br. J. Clin. Pharmacol. 1979;7:297S–308S. doi: 10.1111/j.1365-2125.1979.tb04704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES J., SMITH T.W., KOSTERLITZ H.W., FOTHERGILL L.A., MORGAN B.A., MORRIS H.R. Identification of two related peptides from the brain with potent opiate agonist activity. Nature. 1975;258:577–579. doi: 10.1038/258577a0. [DOI] [PubMed] [Google Scholar]
- ISBELL H., FRASER H.F. Addiction to analgesics and barbiturates. Pharmacol. Rev. 1950;2:355–397. [PubMed] [Google Scholar]
- JONES S., BONCI A. Synaptic plasticity and drug addiction. Curr. Opin. Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- KOSTERLITZ H.W. The best laid schemes o' mice an' men gang aft agley. Ann. Rev. Pharmacol. Toxicol. 1979;19:1–12. doi: 10.1146/annurev.pa.19.040179.000245. [DOI] [PubMed] [Google Scholar]
- KOSTERLITZ H.W., PATERSON S.J., ROBSON L.E. Characterization of the κ-subtype of the opiate receptor in the guinea-pig brain. Br. J. Pharmacol. 1981;73:939–949. doi: 10.1111/j.1476-5381.1981.tb08749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS J.W., RANCE M.J., SANGER D.J.1982The pharmacology and abuse potential of buprenorphine: a new antagonist analgesic Advances in Substance Abuse: Behavioral and Biological Researched. Mello, M.K., pp. 103–154.Greenwich, CT: JAI Press [Google Scholar]
- LORD J.A.H., WATERFIELD A.A., HUGHES J., KOSTERLITZ H.W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977;267:495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- MACHELSKA H., PFLUGER M., WEBER W., PIRANVISSEH-VOLK M., DAUNERT J.D., DEHAVEN R., STEIN C. Peripheral effects of the κ-opioid agonist EMD 61753 on pain and inflammation in rats and humans. J. Pharmacol. Exp. Ther. 1999;290:354–361. [PubMed] [Google Scholar]
- MARTIN W.R. History and development of mixed opioid agonists, partial agonists and antagonists. Br. J. Clin. Pharmacol. 1979;7:273S–279S. doi: 10.1111/j.1365-2125.1979.tb04700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLIGAN G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol. Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- NORTH R.A., TONINI M. The mechanism of action of narcotic analgesics in the guinea-pig ileum. Br. J. Pharmacol. 1979;61:541–550. doi: 10.1111/j.1476-5381.1977.tb07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PANDE A.C., PYKE R.E., GREINER M., COOPER S.A., BENJAMIN R., PIERCE M.W. Analgesic efficacy of the kappa-receptor agonist, enadoline, in dental surgery pain. Clin. Neuropharmacol. 1996a;19:92–97. doi: 10.1097/00002826-199619010-00009. [DOI] [PubMed] [Google Scholar]
- PANDE A.C., PYKE R.E., GREINER M., WIDEMAN G.L., BENJAMIN R., PIERCE M.W. Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clin. Neuropharmacol. 1996b;19:451–456. doi: 10.1097/00002826-199619050-00009. [DOI] [PubMed] [Google Scholar]
- PASTERNAK G.W. Molecular biology of opioid analgesia. J. Pain Symptom Manage. 2005;29:S2–S9. doi: 10.1016/j.jpainsymman.2005.01.011. [DOI] [PubMed] [Google Scholar]
- QUIRION R., BOWEN W.D., ITZHAKM Y., JUNIENM J.L., MUSACCHIOM J.M., ROTHMAN R.B., SU T.P., TAM S.W., TAYLOR D.P. A proposal for the classification of sigma binding sites. Trends Pharmacol. Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- SAMWAYS D.S.K., HENDERSON G.2005Opioid elevation of intracellular free calcium: possible mechanisms and physiological relevance Cellular Signalling[epub ahead of print]. [DOI] [PubMed]
- TESCHEMACHER H.1993Atypical opioid peptides Handbook of Exp. Pharmacol.104/1, ed. Herz, A. pp. 499–528.Berlin: Springer-Verlag [Google Scholar]
- TRAYNOR J.R. Subtypes of the kappa-opioid receptor: fact or fiction. Trends Pharmacol. Sci. 1989;10:52–53. doi: 10.1016/0165-6147(89)90074-6. [DOI] [PubMed] [Google Scholar]
- TRAYNOR J.R., ELLIOT J. δ-Opioid receptor subtypes and cross-talk with μ-receptors. Trends Pharmacol. Sci. 1993;14:84–86. doi: 10.1016/0165-6147(93)90068-u. [DOI] [PubMed] [Google Scholar]
- VONVOIGHTLANDER P.F., LAHTI R.A., LUDENS J.H. U-50,488: a selective and structurally novel non-μ (κ) opioid agonist. J. Pharmacol. Exp. Ther. 1983;224:7–12. [PubMed] [Google Scholar]
- WALDHOER M., BARTLETT S.E., WHISTLER J.L. Opioid receptors. Annu. Rev. Biochem. 2004;73:953–990. doi: 10.1146/annurev.biochem.73.011303.073940. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.T., CHRISTIE M.J., MANZONI O. Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 2001;81:299–343. doi: 10.1152/physrev.2001.81.1.299. [DOI] [PubMed] [Google Scholar]
- ZADINA J.E., HACKLER L., GE L.J., KASTIN A.J. A potent and selective endogenous agonist for the μ-opiate receptor. Nature. 1997;386:499–502. doi: 10.1038/386499a0. [DOI] [PubMed] [Google Scholar]