Abstract
Glutamatergic synaptic transmission in the mammalian central nervous system was slowly established over a period of some 20 years, dating from the 1950s. Realisation that glutamate and like amino acids (collectively known as excitatory amino acids (EAA)) mediated their excitatory actions via multiple receptors preceded establishment of these receptors as synaptic transmitter receptors. EAA receptors were initially classified as N-methyl-D-aspartate (NMDA) and non-NMDA receptors, the latter subdivided into quisqualate (later AMPA) and kainate receptors after agonists that appeared to activate these receptors preferentially, and by their sensitivity to a range of differentially acting antagonists developed progressively during the 1970s. NMDA receptors were definitively shown to be synaptic receptors on spinal neurones by the sensitivity of certain excitatory pathways in the spinal cord to a range of specific NMDA receptor antagonists. Importantly, specific NMDA receptor antagonists appeared to be less effective at synapses in higher centres. In contrast, antagonists that also blocked non-NMDA as well as NMDA receptors were almost universally effective at blocking synaptic excitation within the brain and spinal cord, establishing both the existence and ubiquity of non-NMDA synaptic receptor systems throughout the CNS. In the early 1980s, NMDA receptors were shown to be involved in several central synaptic pathways, acting in concert with non-NMDA receptors under conditions where a protracted excitatory postsynaptic potential was effected in response to intense stimulation of presynaptic fibres. Such activation of NMDA receptors together with non-NMDA receptors led to the phenomenon of long-term potentiation (LTP), associated with lasting changes in synaptic efficacy (synaptic plasticity) and considered to be an important process in memory and learning. During the 1980s, it was shown that certain glutamate receptors in the brain mediated biochemical changes that were not susceptible to NMDA or non-NMDA receptor antagonists. This dichotomy was resolved in the early 1990s by the techniques of molecular biology, which identified two families of glutamate-binding receptor proteins (ionotropic (iGlu) and metabotropic (mGlu) receptors). Development of antagonists binding to specific protein subunits is currently enabling precise identification of discrete iGlu or mGlu receptor subtypes that participate in a range of central synaptic processes, including synaptic plasticity.
Keywords: L-Glutamate, excitatory amino acids, ionotropic glutamate receptors, metabotropic glutamate receptors, synaptic transmission
Genesis (JCW)
It is something of a coincidence that the BPS and I both came into being at roughly the same time. It is another coincidence that until the late 1950s neither contributors to the Journal (as far as I am aware) nor I were remotely interested in glutamate as a substance worthy of pharmacological study. Much has emerged in this context over the past 50 years. An intriguing thought is that this article is not only glutamate-motivated, but also, in large part anyway, glutamate-mediated. I am tempted also to attribute any compositional shortcomings it may betray to suboptimal performance of the amino acid and its receptors within an ageing brain.
High concentrations of glutamate in brain, first recognised in the 1930s, engendered speculation of an important neurophysiological role of the amino acid, and this in turn led to a variety of trials in the 1940s of dietary glutamate and glutamine in the treatment of learning disorders and epilepsy. The role of glutamate in brain, aside from the obvious one as a protein constituent, was at that time considered more in terms of energy metabolism, given the close association of the amino acid with the Krebs cycle. An early indication of a special role of glutamate in electrophysiological processes was the observation by Hayashi (1954) that injection of glutamate into brain or carotid arteries produced convulsions. He speculated that glutamate was a transmitter in the mammalian CNS. However, many chemical agents can cause convulsions, including those that interfere with normal oxidative metabolism.
I myself became interested in pharmacology, and later glutamate, by a completely unrelated series of events. At 13, I began to study chemistry at High School and immediately manifested an interest in medical applications. Following instructions in my Boys Own Chemical Set, I added caustic soda to sulphuric acid, producing a substance called Glauber's Salt (sodium sulphate decahydrate). To my great alarm, my grandfather promptly ingested a considerable amount despite my protestations. I spent a couple of days in great anxiety, but he survived with no obvious ill effects. In retrospect this was probably the closest I ever came to preparing a therapeutically useful substance (albeit one already known since the 17th century) despite considerable later effort.
Later, with a PhD in chemistry, I faced a big problem – what actually to do for the rest of my life. The answer came out of the blue during one of many sleepless nights in 1956. Practically nothing was known about chemical mechanisms in the brain! It struck me with immense force that thought, feelings and ‘instructions for behaviour' were all generated within a mass of pinkish grey gelatinous substance inside our heads, made entirely of chemicals!! But what chemicals and how they interacted had been little studied to that time. In particular, what was the relationship between such chemical interactions and mental processes? Eureka! I would henceforth try to enter the field of neurochemistry, which coincidentally had just been recognised as a specific discipline in its own right, with the Journal of Neurochemistry having just published its first ever issue.
Living at Yale in a medical dormitory (but working in the Chemistry Department) I experienced medical influences of all kinds. One of my American friends was a neurophysiologist, who suggested that I get in touch with a renowned Australian compatriot of mine, J.C. Eccles (later Nobel Laureate). I did. Professor Eccles was most enthusiastic about the prospect of a chemist joining his physiological laboratory in Canberra and promptly offered me a job as a Research Fellow to work with David Curtis, on chemical transmitters in the brain. Interestingly, Eccles himself had only just begun to believe in them, but had now become a most enthusiastic convert from his long-held previous conviction that all central synaptic transmission was electrical. At the age of 26 my future direction in life was established.
On course
My involvement with glutamate followed almost immediately. Set the task (in early 1958) of isolating and identifying previously unknown chemical synaptic transmitters in the CNS, I drew up a list of the many chemical constituents of brain that had already been reported. Many were available from one source or another, some I synthesised. All were stockpiled ready to ascertain their individual effects on central nervous tissue in collaboration with David Curtis and John Phillis. Sodium glutamate was top of the list because of the high concentration of glutamic acid in brain, and because we had a 500 g bottle on the shelf! We did not know of Hayashi's work at this time. Our early discoveries have been detailed before (Curtis & Watkins, 1960; 1965; Curtis et al., 1960; 1961), and here I just present a few of the highlights.
The first such ‘highlight' turned out to be misleading, since we ‘confirmed' our preconceived idea that glutamate, a close structural relative of GABA, already a known inhibitor of neuronal activity at that time, would also be a depressant. Administered by David Curtis's newly adapted microelectrophoretic technique, L-glutamate resembled GABA in depressing neuronal field potentials (electrical voltages generated by groups of synaptically activated neurons in the vicinity of a recording electrode) in the spinal cord of the cat in vivo. This was a perfectly valid observation and paralleled the action of L-glutamate in producing spreading depression following topical application in the cerebral cortex. For a broader perspective of the actions of our stockpiled compounds, we used the isolated toad spinal cord preparation. Our results, published in this Journal (Curtis et al., 1961), clearly showed that, with concentrations of 10−4 to 10−2 M, L-glutamate first excited populations of neurones, then depressed them, the latter effect mirroring what we had seen in our microelectrophoretic experiments in vivo. More detailed studies on individual cat spinal cord neurones in vivo dramatically showed an initial depolarising effect of L-glutamate on central neurones, leading to repetitive spike discharge of the neurone, followed, in the case of sufficiently large depolarisation, by inactivation of spike generating mechanisms and complete suppression of cell activity (Curtis et al., 1960).
Following this first observation of the excitatory action of L-glutamate on single cells in vivo, we had excitedly written a Letter to Nature (mid-late 1958), very tentatively suggesting a possible transmitter function of the amino acid. Sent by surface mail from Canberra, it took 6–8 weeks to arrive, and during this period we tested the action of glutamate on a particular type of spinal interneurone, the inhibitory Renshaw cell, known to have a cholinergic input. L-Glutamate also excited the Renshaw cell, and this suggested to us that glutamate was ‘nonspecific', affecting different types of cells, and probably not via specific transmitter receptors. This could also explain why such a wide variety of acidic amino acids, including both D- and L-forms of glutamate, aspartate and structurally similar substances, also showed excitatory action, of near-equal potency. So, before hearing from Nature of the fate of our submitted Letter, we sent off an amendment – by airmail – withdrawing the transmitter speculation and, in fact, expressing the view that a specific transmitter function was unlikely. This overcautious view prevailed for the next 18 years.
The dark ages
Glutamate was an important intermediary metabolite in brain, but a transmitter? Hardly!. A transmitter function of L-glutamate did indeed seem quite unlikely in those early years. The hypothetical ‘receptor' would have to respond to many amino acids (either L- or D-, ‘natural' or ‘unnatural') with some general resemblance to glutamate. Furthermore, a range of glutamate enzyme inhibitors failed to affect the duration of action. Also, high concentrations were generally needed, 1000 times more than expected, say, for acetylcholine or noradrenaline at their peripheral neuroeffector sites. By the same token, we expected only very low concentrations of transmitters to be actually present in central nervous tissue. Instead, L-glutamate was among the most abundant of all small molecule constituents of brain. But the most serious objection to the transmitter possibility was that the reversal potential for L-glutamate-induced depolarisation of motoneurones was apparently different from that of the excitatory synaptic response. While the discrepancy could be explained in various ways, this result was clearly a set-back to our hypothesis, and many years were to elapse before a transmitter function for glutamate could be established.
It may well be asked how it came about that I continued to work on glutamate and like amino acids during the ensuing period of such general pessimism. The fact was simply that the effect of glutamate, whether or not mediated by transmitter receptors, was quite spectacular, and could not be ‘explained away'; also, it could well be implicated in brain dysfunction, particularly epilepsy. And, while seeming unlikely at this time (late 1950s, early 1960s), a transmitter function could not definitely be ruled out, especially as no antagonists were then known for the vast majority of synaptic excitations observed electrophysiologically in a wide variety of central neurones following stimulation of appropriate pathways. Specific antagonists for either the natural transmitters or glutamate-like excitatory substances were crucial in order to prove or disprove the involvement of such an amino acid in synaptic excitation at these synapses.
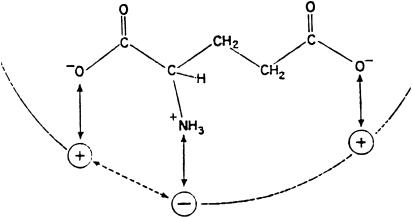
As a chemist, my interest lay in investigating structure–activity relations of glutamate analogues. The apparent nonstereoselectivity of the action of glutamate and aspartate demanded the study of analogues with more bulky substituents, where stereoselectivity might be more apparent. One of these, NMDA (Figure 1), turned out to be a particularly potent excitant, much more potent than the L form, and established beyond doubt that the site(s) of action of the amino acids could indeed exhibit a definite stereoselectivity, implying that a discrete membrane receptor site was actually involved in such action. We proposed a ‘three-point' receptor site (Curtis & Watkins, 1960; 1965) with which the charged groups of the anionic glutamate molecule interacted (Figure 2), this interaction causing a conformational change in the receptor or associated membrane molecules, which opened pores to allow extracellular sodium ions to flow down their electrochemical gradient and depolarise the cell.
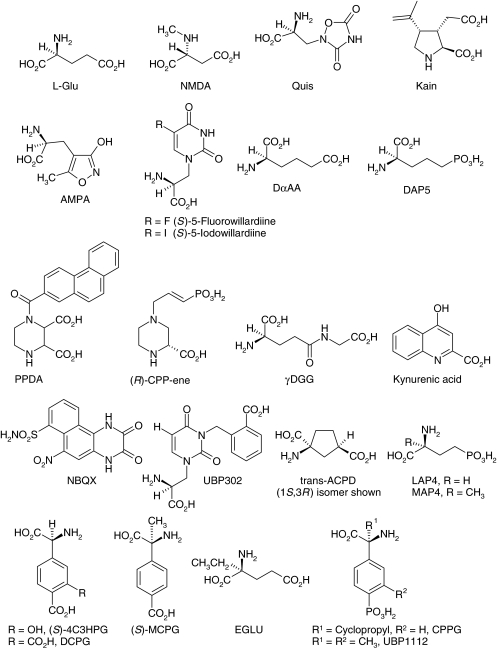
Figure 1.
A range of key ligands for glutamate receptors indicating the extensive variation of the simple glutamate structure that has led to selective agonists and antagonists for specific receptor subtypes (see Table 1) (Stereochemical nomenclature: In the interests of readability we have not always stated the enantiomer of glutamate applying to each mention of the amino acid. Where no enantiomer is stated, the L form is assumed. The modern terms R and S are used for more recently prepared synthetic compounds, although D and L are retained for others, for example, N-methyl-D-aspartate (NMDA) and L-2-amino-4-phosphonobutyrate (LAP4)).
Figure 2.
‘Three-point receptor' (Curtis & Watkins, 1960).
In addition to NMDA, other new substances synthesised in the early 1960s also showed potency apparently greater than L-glutamate (but without taking account of different rates of uptake, later shown to be important in potency assessment); for example D- and L-homocysteate. The naturally occurring glutamate relative, β-N-oxalyl-L-α,β-diaminopropionic acid (ODAP), isolated from the seeds of the poisonous pea Lathyrus sativus, and possibly a factor in the causation of lathyrism (a neurodegenerative disease) following ingestion (Curtis & Watkins, 1965), was also a potent excitant. Shinozaki and co-workers later reported a range of even more potent glutamate analogues, including kainic acid, from the marine alga Digenia simplex, and quisqualic acid, from plants of the genus Quisqualis (Figure 1). Later again, a new synthetic excitatory amino acid, α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA, Figure 1), was reported by Krogsgaard–Larsen and the Canberra team. This highly potent excitant was ultimately to become particularly important in the classification of glutamate-like actions.
During this period, attention was also focussed on the cellular uptake of glutamate and related substances (see also Iversen, this issue). Johnston and co-workers suggested that the observed excitatory potencies of glutamate analogues may reflect differences in rates of uptake. In particular, NMDA was a poor substrate for the uptake transporters. More than anything, however, these studies differentiated the two phenomena of uptake and excitatory action, which showed quite different structure–activity relationships, so it was clear that the excitatory effect could not be an indirect consequence of glutamate uptake, as had been proposed. Uptake was established as a highly credible mechanism for the clearance of an excitatory transmitter amino acid (Curtis & Johnston, 1974). This had already been generally accepted for GABA and glycine as inhibitory transmitters in the CNS, such transmitter action having been established, again after a long barren period, when specific antagonists became available. Such antagonists were still lacking in the excitatory amino acid field, but at least a mechanism for termination of the transmitter action had been identified.
Along with uptake studies, several laboratories were able to establish calcium-dependent release of L-glutamate and L-aspartate from synaptic terminals in response to electrical stimulation or potassium-induced depolarisation. A method of synthesis of L-glutamate in synaptic terminals was also established via the ‘glutamate–glutamine cycle'. In this model extracellular L-glutamate, after release into synaptic clefts from terminals, was taken up by glial cells, wherein it was rapidly converted into glutamine. The latter was able readily to pass back into terminals, thereafter to be re-converted into glutamate by the action of glutaminase, which is highly concentrated in synaptic endings. These studies together established reasonable ‘logistics' for a role of glutamate in synaptic transmission (Watkins, 1972).
A related field of research was also taking hold in this period, developed by Olney and associates on the toxic action of high extracellular concentrations of glutamate and analogues, which caused neuronal death (‘excitotoxicity'). A possible correlation with ischaemic and traumatic brain damage was suspected. Experimental destruction by excitatory amino acids (EAA), particularly by kainate, of discrete populations of cells has been widely used for neurochemical and neuroanatomical mapping studies.
Recognition of multiple receptors and the development of selective antagonists
The identification of multiple excitatory amino acid receptors preceded definitive establishment of synaptic function. In 1968, Hugh McLennan (Deceased, 2004) and colleagues (McLennan et al., 1968) compared DL-homocysteate (the racemic form of the ω-sulphono analogue of glutamate) with glutamate itself in different regions of the thalamus, and ascribed the regional differences observed in the relative potencies to the possibility of there being more than a single glutamate receptor. In the early 1970s, Arthur Duggan (1974) found that L-aspartate was somewhat more potent than L-glutamate on Renshaw cells whereas the reverse was true on other types of spinal interneurone, suggesting, among other possibilities, that the receptors on the cells differed. The Curtis/Johnston team then showed large differences in the relative potencies of kainate (a ‘glutamate analogue') and NMDA (an ‘aspartate analogue') on the same two groups of cells (McCulloch et al., 1974). These results were so clearcut that the multiple receptor idea now began to appear compelling.
About this time I moved to the University of Bristol and teamed up with Professor Tim Biscoe (Physiology) and Dr Dick Evans (Pharmacology). We immediately began to test new substances as possible antagonists of amino acid induced excitation, to see whether any such antagonism was specific for amino acid- relative to non-amino acid-induced responses, and, critically, to check for selective antagonism of one excitatory amino acid relative to another, with the hope that such substances could also be shown to block synaptic transmission, at least in some central synaptic pathways.
Our first success in differentiating excitatory amino acid-induced responses from one another was, unexpectedly, the mere inclusion of magnesium ions in the medium superfusing the isolated frog spinal cord, which we had adopted for our screening studies. Frog Ringer solution is traditionally magnesium-free, and we initially introduced it to try to abolish synaptic release of transmitter, as occurs at the neuromuscular junction. To our surprise, remarkably low concentrations of magnesium (0.1–2 mM) greatly reduced synaptic activity as evoked by stimulation of dorsal roots, and also depressed responses to EAA in the medium, but, crucially, only responses to some amino acids and not others. NMDA-induced responses were almost abolished, while responses to kainate and quisqualate were almost unaffected, and responses to other amino acids, including L-glutamate, were reduced to an intermediate degree. We initially classified depolarisations produced by glutamate analogues as magnesium-sensitive and magnesium-insensitive responses. Importantly, the depression of synaptic activity by magnesium ions was shown not to be associated with calcium antagonism (as at the neuromuscular junction), but with the ability of magnesium to block excitatory amino acid (particularly NMDA)-induced excitation.
We then found that two totally different substances produced an almost identical pattern of antagonism of excitatory amino-acid-induced responses to that produced by magnesium ions. One of these was the substance HA-966, which some years earlier John Davies (a close friend and external collaborator for over 20 years, died suddenly 1991, aged 49) and I had shown to antagonise glutamate- and (especially) aspartate-induced excitation in rat brain in vivo and also some synaptically evoked responses in the brain stem. Another was diaminopimelic acid (DAP). Magnesium, HA-966 and DAP appeared to act at different sites to produce their almost identical actions (Evans et al., 1979). Taken together, these results constituted a breakthrough, enabling us unequivocally to confirm the Canberra suggestion of different receptors for NMDA and kainate. Quisqualate responses resembled those of kainate in being relatively unaffected by magnesium ions, HA-966, or diamino-substituted longer chain glutamate analogues. Other substances, for example, L-homocysteate, were more strongly antagonised, and in this respect resembled NMDA.
Further progress followed from an observation by McLennan and co-workers that DL-α-aminoadipate antagonised glutamate-induced responses whereas L-α-aminoadipate (LαAA) augmented them and was itself weakly excitatory. They concluded that D-α-aminoadipate (DαAA) (Figure 1) was a glutamate antagonist whereas LαAA was a glutamate agonist. This prompted us to study the effects of resolved stereoisomers of various mono- and diamino longer-chain analogues of glutamate, and it became clear that the D form of these substances were all NMDA-selective in their antagonist actions. Kainate- and quisqualate-induced responses were relatively unaffected, and other glutamate agonists were antagonised to varying intermediate degrees. These results were reported in the British Journal of Pharmacology, with considerable impact (Evans et al., 1979). We suggested the terms NMDA and non-NMDA receptors (Watkins & Evans, 1981) to replace our previous magnesium-based classification, and concluded that among our library of EAA we had some that acted mainly on NMDA receptors, others mainly on non-NMDA receptors, and many (including L-glutamate and L-aspartate) to varying degrees on both. Soon thereafter, Evans discovered a population of rapidly desensitising glutamate receptors on pain conducting C-fibres in dorsal roots that responded to kainate but not to NMDA or quisqualate. We therefore subdivided non-NMDA receptors into kainate and quisqualate types.
During the late 1970s and early 1980s, a range of antagonists was developed, most of which were highly specific for NMDA receptors, while others exhibited varying activity also on non-NMDA receptors, with little selectivity, however, being shown between kainate- and quisqualate-induced responses. The discovery of a series of phosphono compounds with greatly enhanced potency (Evans et al., 1982) resulted in a wealth of highly specific NMDA receptor antagonists of great value as research tools and (potentially) of future therapeutic application. These antagonists were shown in our laboratory to be of the competitive type, acting at the glutamate recognition site of the NMDA receptor. The phosphonic acid analogue of DαAA, D-2-amino-5-phosphonopentanoate (DAP5, Figure 1), is widely used as a pharmacological tool for identifying NMDA receptors. Other glutamate analogues, including γ-D-glutamylglycine (γDGG) (Figure 1) showed substantial activity at non-NMDA receptors in addition to their actions at NMDA receptors, and similar broad-spectrum antagonist activity was also shown by kynurenic acid (Figure 1). These antagonists were useful in the search for convincing evidence of the involvement of glutamate receptors, of different classes, in synaptic transmission throughout the 1980s. Later in the decade, a range of more potent and selective antagonists for non-NMDA receptors, particularly those of the quinoxalinedione type, such as CNQX and NBQX (Figure 1), was introduced by Honoré and co-workers, providing an enhanced armoury towards this objective.
With this progressive development of glutamate antagonists, new interest in the field was explosive.
‘Glutamatergic' synaptic transmission: yes or no?
From the very beginning, the BIG question following the discovery of the direct excitatory effect of L-glutamate on central neurones was whether the amino acid was indeed a synaptic transmitter in the brain and spinal cord and, if so, in what pathways.
The availability of highly selective NMDA receptor antagonists of the DαAA and DAP5 type allowed this question to be answered unequivocally and completely transformed the still-prevailing sceptical attitudes of the time. Our finding that these substances, along with magnesium ions and HA-966, all blocked gross electrical activity evoked by dorsal root stimulation in the frog and baby rat spinal cord in vivo, with potency paralleling that of their ability to block glutamate- and, particularly, NMDA-induced activity in the same preparations, was an extremely strong indication of excitatory amino-acid-mediated synaptic transmission, under the particular conditions used. It must be pointed out, however, that NMDA receptor sensitivity in these early experiments in vivo was greatly enhanced by the unphysiological absence of magnesium ions in our usual superfusion medium.
The crucial experiment, validating the conclusion that NMDA receptors were indeed synaptic receptors under more physiological conditions, was carried out by John Davies in 1977 on cat spinal Renshaw cells in vivo. Individual Renshaw cells were excited chemically either by acetylcholine or by L-glutamate, microelectrophoretically administered, or synaptically either cholinergically or noncholinergically by electrical stimulation of ventral or dorsal roots, respectively. DαAA blocked L-glutamate- (and L-aspartate-), as well as dorsal root-evoked excitation but did not block acetylcholine- or ventral root-evoked excitation. In contrast, the ganglionic acetylcholine antagonist DHβE blocked acetylcholine- and ventral root-evoked excitation, but neither L-glutamate- (or L-aspartate) nor dorsal root-evoked excitation. These experiments established, with a high degree of probability, that release of an L-glutamate-like transmitter was responsible for excitation of Renshaw cells via impulses in dorsal root afferent fibres (Biscoe et al., 1977). For various reasons, we actually plumped for L-aspartate as the transmitter, but our later observation that L-glutamate has a 10-fold higher affinity for 3H-DAP5 binding sites than L-aspartate (Olverman et al., 1984) suggests glutamate to be the more likely transmitter. Whatever the precise identity of the transmitter, this was indeed a seminal moment, the culmination of a 20-year search for definitive evidence, for or against, amino acids as excitatory transmitters in the CNS.
Evolution (JCW and DEJ)
Indications of differing synaptic roles of glutamate receptors
Interest in the field burgeoned in the 1980s, propelled by the availability of potent and selective NMDA receptor antagonists, as well as a range of less selective glutamate receptor antagonists. Early findings indicated that the vast majority of central synapses used a glutamate-like transmitter, acting at receptors that were mostly of the non-NMDA type. Collingridge & colleagues (1983) showed, however, that although the primary response of CA1 cells in the hippocampus to low-intensity stimulation of the Schaffer collateral-commissural pathway was mediated by non-NMDA receptors, high-intensity stimulation of this pathway led to the additional activation of NMDA receptors and to the generation of long-term potentiation (LTP). Indeed, the generation of LTP, considered to be an important process in memory and learning, was actually dependent on the activation of NMDA receptors. This was a major advance in our understanding of synaptic plasticity, and also pointed to the possibility of a special role of NMDA receptors in central synaptic transmission. Other examples of synaptic plasticity, for example in the visual cortex, were also soon shown to involve both types of glutamate receptors.
It was becoming evident that most synaptic pathways in the CNS used non-NMDA glutamate receptors for the primary activation of a postsynaptic cell, with NMDA receptors being involved in subsequent manifestations of the response to more intense or protracted afferent stimulation. With Renshaw cells, for example, the initial (fastest) response to dorsal root stimulation was mediated by non-NMDA receptors, the later spikes in the train being generated by NMDA receptors. In general, low-intensity stimulation, particularly that whereby only a single spike was generated in the postsynaptic cell, caused activation only of non-NMDA receptors in a variety of brain and spinal cord synaptic pathways.
Two special features of the NMDA receptor discovered about the same time are fundamental to the role of NMDA receptors in synaptic transmission and plasticity. One was the voltage dependency of the Mg2+-block of these receptors, which explained why activation of these receptors was enhanced with more intense stimulatory synaptic input (Nowak et al., 1984). A greater synaptic drive increases the postsynaptic depolarisation, relieving the magnesium block of NMDA receptors and allowing the synaptically released glutamate to activate the latter to an extent dependent on the initial synaptic drive. The second special feature of the NMDA receptor is the increased influx of calcium ions mediated by this receptor type on activation relative to that effected by non-NMDA receptors (Macdermott et al., 1986). The level of free intracellular calcium ions is considered to be an important factor in the biochemical processes associated with LTP.
An additional feature of the NMDA receptor would appear to be of fundamental importance, but its full significance remains to be clarified. This is the action of glycine as a co-agonist with glutamate in activating the receptor (Johnson & Ascher, 1987). Likewise, the physiological significance of the allosteric actions of certain polyamines such as spermine and spermidine in potentiating responses of the receptor requires further elucidation.
Clinical interest
Throughout the 1980s, the therapeutic potential of glutamate receptor agonists and antagonists excited mounting interest, which was increased by the discovery that known clinical drugs were able to block glutamate receptors. Thus, the dissociative anaesthetic ketamine and like compounds, as well as some opioids, were shown by Lodge and co-workers to block NMDA receptor ion channels (see also Franks, this issue). With initial targets of epilepsy, spasticity, and neuroprotection, the latter in cases of ischaemic neuronal damage such as occurs in stroke, and head and spinal injury, several glutamate receptor agonists and antagonists entered clinical trials. One of these (CPP-ene, Figure 1) reached Phase III in trials for neuroprotection in patients with head injury but was not shown to improve the neurological outcome, possibly because of too long a delay between the acute episode and administration of the drug.
No greater success was achieved with other agents that acted at different sites from the transmitter recognition sites first targeted, such as the ‘glycine-site' on the NMDA receptor. Some known NMDA receptor and broad spectrum EAA antagonists, such as HA-966, kynurenic acid and CNQX, were shown to act at this site of the NMDA receptor. A range of more potent and specific glycine-site antagonists was subsequently developed (Monaghan et al., 2004), but none has achieved clinical success. The same would seem to apply in the case of agents that act at the polyamine-activated allosteric site identified on the NMDA receptor. All agents that block the activation of NMDA receptors, irrespective of the site of action, have anticonvulsant effects, but have failed in clinical trials for epilepsy and other neurological disorders, mainly because of psychotomimetic side effects. Side effects were not unexpected in view of the ubiquitous involvement of glutamatergic synapses in the CNS, and some optimism remains for success in the future as the roles of specific glutamate receptor subtypes in neuronal function and dysfunction become better understood. It is encouraging in this respect that lamotrigine, which depresses glutamate release from synaptic terminals, has proved clinically useful as an adjunct to other anticonvulsant drugs for the treatment of partial seizures.
The ‘big bang': ionotropic and metabotropic glutamate receptors
The sufficiency of the three-receptor concept began to be questioned during the 1980s when the actions of two particular compounds with ‘glutamate-like' structures did not seem to conform with those of known agonists or antagonists at NMDA, kainate or quisqualate receptors. One of these was ‘trans'-ACPD and the other LAP4 (Figure 1) (reviewed by Monaghan et al., 1989). The former caused excitation, but was not susceptible to antagonism by NMDA or non-NMDA receptor antagonists, while the latter depressed synaptic excitation but was without glutamate receptor antagonist activity. Further, curious reports began to emerge about receptor-mediated EAA-induced biochemical effects that were not antagonised by known glutamate receptor antagonists (Monaghan et al., 1989).
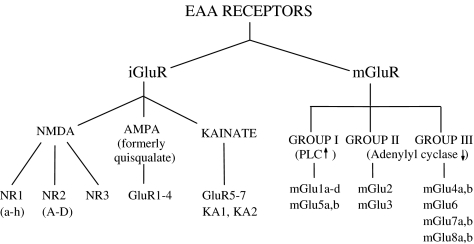
Enter molecular biologists, to greatly clarify the classification of glutamate receptors (Nakanishi, 1992; Hollmann & Heinemann, 1994). Two major families were recognised, those that mediate fast synaptic responses by opening ion channels, called ionotropic glutamate (iGlu) receptors, and those that cause slower synaptic effects, associated with biochemical changes, called metabotropic glutamate (mGlu) receptors (reviewed in Schoepp et al., 1999) (Scheme 1). The main classes of iGlu receptors are now accepted as comprising NMDA, AMPA (replacing quisqualate as the prototype, in view of its greater selectivity) and kainate receptors. The other major class, mGlu receptors, are linked to G proteins and comprise three groups: group I, consisting of mGlu1 and mGlu5, linked to phospholipase C activation and causing an increase in intracellular inositol trisphosphate concentration and calcium mobilisation, group II (mGlu2 and mGlu3) and group III (mGlu4, 6, 7 and 8), which inhibit adenylyl cyclase activity, resulting in a fall in intracellular cAMP concentration. Group I mGlu receptors are generally associated with excitatory synaptic responses and groups II and III with depression of synaptic responses, via inhibition of glutamate release. Groups of specific protein subunits were identified, assemblies of which constitute different iGlu and mGlu receptor subtypes, as shown in Scheme 1.
Scheme 1.
Classification of glutamate receptors.
The recognition of the two distinct families of ionotropic and metabotropic glutamate receptors immediately explained glutamate receptor-mediated biochemical events that did not show the same pharmacological profile as glutamate-induced electrophysiolgical responses. Furthermore, the two agents, trans-ACPD and LAP4, which had caused much scratching of heads in the early 1980s, were soon shown to mediate their ‘anomalous' effects via metabotropic rather than ionotropic glutamate receptors. Trans-ACPD activates both group I and group II mGlu receptors, and LAP4 is a specific agonist (indeed, almost a diagnostic one) of group III mGlu receptors (reviewed by Schoepp et al., 1999).
Now the field really boomed! Selective agonists and antagonists for specific iGlu and mGlu receptor subtypes were progressively developed (Table 1), leading to the identification of discrete receptor subtypes that were involved in particular neuronal pathways and processes. These can only be touched upon here, and the reader is referred to recent reviews for more detailed accounts (Schoepp et al., 1999; Schoepp, 2001; Lerma, 2003; Monaghan et al., 2004; Kew & Kemp, 2005). Examples of specific iGlu and mGlu receptor subtypes that have been shown to participate in various synaptic processes include:
Group I mGlu receptors in LTP and LTD in the CA3–CA1 hippocampal pathway, in association with the AMPA and NMDA iGlu receptors previously identified; also mGlu5 as the particular subtype involved in tripping a proposed molecular switch required for the induction of this form of LTP (Bortolotto et al., 1994).
Group II mGlu receptors (mGlu2 and 3) in the control of transmitter release (including glutamate, GABA and 5HT). Agonists and positive allosteric modulators of these receptors have potential application in anxiety-related disorders and perhaps schizophrenia.
Group III mGlu receptors, including mGlu8, in synaptic depression in the spinal cord. Certain evidence suggests that anxiety and stress-related disorders might also be a useful therapeutic target for mGlu8 agonists. The mGlu7 subtype has been shown to be involved in the control of glutamate release in cerebellar granule cells via PICK1 coupled inhibition of P/Q Ca2+ channels.
GluR5 (kainate) iGlu receptors in the NMDA receptor-independent form of LTP in the mossy fibre/CA3 synapse in the hippocampus, and the involvement of this glutamate receptor protein also in the modulation of both excitatory and inhibitory transmission in the hippocampus (Bortolotto et al., 1999). Antagonism of this receptor subtype with LY382884 has shown potential value in the treatment of chronic pain, epilepsy, cerebral ischaemia and migraine.
Coassembly of NR2B and NR2D NMDA receptor subunits in cerebellar Golgi cells to form a subtype that is restricted to extrasynaptic sites. In addition, distinct subpopulations of NMDA receptor subtypes may be involved in hippocampal LTP and LTD.
Table 1. Selective iGlu and mGlu receptor ligandsa.
| iGlu receptors | |||
|---|---|---|---|
| NMDA | AMPA | Kainate | |
| Agonists | NMDA Tetrazolylglycine | AMPA; LY404187b (S)-5-fluorowillardiine | Kainate; ATPA (S)-5-iodowillardiine LY339434; SYM 2081 |
| Antagonists | CPP (selective NR2A/NR2B vs NR2C/NR2D) PEAQX (selective NR2A vs NR2B) PPDA (selective NR2C/NR2D vs NR2A/NR2B) Ro 25-6981c (NR2B selective) | NBQX; LY293558 (S)-ATPO GYKI53655c | LY382884; UBP302 NS 3763c |
| mGlu receptors | |||
| Group I | Group II | Group III | |
| Agonists | (S)-DHPG; CHPG (mGlu5) (1S,3R)-ACPD; Ro 01-6128b (mGlu1); DFBb (mGlu5) | LY354740 LY487379b | (S)-AP4; DCPG (mGlu8); PHCCb (mGlu4) |
| Antagonists | LY367385 (mGlu1); (S)-MCPG; MPEPc (mGlu5); MTEPc (mGlu5); CPCCOEtc (mGlu1); BAY36-7620c (mGlu1) | LY341495; EGLU | CPPG; UBP1112 |
For structures, see Figure 1 and supplementary information online.
Positive allosteric modulator.
Negative allosteric modulator.
The study of protein–protein interactions, phosphorylation–dephosphorylation reactions and receptor trafficking is a rapidly developing area of growing importance with respect to glutamate receptor function. These phenomena are considered particularly relevant in the synaptic plasticity field and have been recently reviewed (Collingridge et al., 2004).
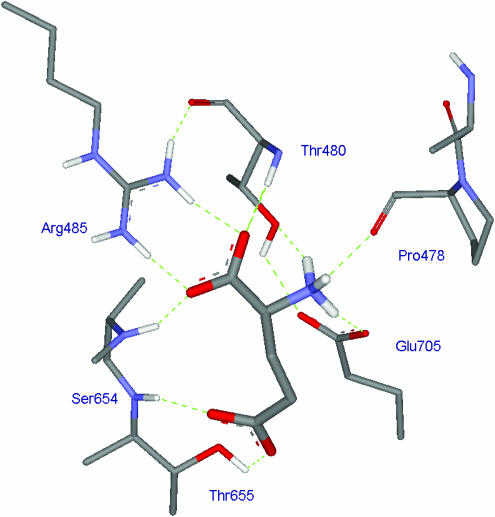
One of the most recent, and, to a chemist, dramatic and exciting advances in the field has been the ability to isolate and crystallise the so-called S1S2 ligand binding domain of a range of specific glutamate receptor protein subunits (the ligand binding domains of GluR2, GluR5, GluR6, NR1 and NR2A have been crystallised to date), with and without bound ligand (for a review, see Mayer, 2005). X-ray analysis of these proteins and protein–ligand complexes has elucidated fine molecular detail of the binding site, and the conformational changes different agonists and antagonists induce to open or block the ion channels or modulate the manifestation of their activity. Figure 3 shows the molecular binding of glutamate to the S1S2 construct of GluR2. One could only dream of such molecular detail half a century ago (cf Figure 2). The phenomenon of desensitisation is particularly relevant to AMPA receptor function, and has been studied at the molecular level by Mayer and co-workers (Jin et al., 2003). Evolving from our work in the 1990s, Mayer and co-workers have obtained X-ray crystal structures of a range of 5-substituted willardiines bound to the S1S2 GluR2 construct (Jin et al., 2003).
Figure 3.
Crystal structure of L-glutamate bound to the S1S2 construct of GluR2. Note the α-carboxyl group of glutamate forms a salt bridge with Arg485 and a hydrogen bond with Thr480 while the ω-carboxyl group forms hydrogen bonds with Ser654 and Thr655. The α-amino group of glutamate forms a salt bridge with Glu705 and hydrogen bonds with Thr480 and Pro478.
Advances in computer-aided molecular design have facilitated the construction of homology models of glutamate receptor subunits that have not yet been crystallised. Homology models of NR2 subunits based on either the X-ray crystal structures of the S1S2 domains of GluR2 or NR1 have been used to predict the binding modes of agonists such as glutamate and NMDA and antagonists such as DAP5 and PPDA (Kinarsky et al., 2005 and references therein).
X-ray crystal structures of the extracellular ligand-binding domain of the mGlu1 receptor with and without bound ligand have also been reported, allowing conformational changes in the presence of the agonist glutamate and the antagonist MCPG to be elucidated (Tsuchiya et al., 2002).
The future
With the emergence of such sophisticated techniques as those described above, one can look forward to the development of the field with great optimism and excitement. The complexity of glutamatergic transmission would seem to offer almost limitless scope for research in the future. Initial study is likely to be ‘more of the same', that is, defining what glutamate receptor proteins are involved in central synaptic pathways of all kinds, and the inter-relation of different EAA receptor systems. Whereas, 50 years ago, the search began for what was expected to be a considerable range of centrally active transmitters, subserving different functions, now it is recognised that such diverse functions are probably fulfilled by subtypes of glutamate receptors acting in conjunction with each other, and possibly just a limited number of other transmitter systems. On the ‘applied' front, we are bound to witness multiple clinical advances with increasing knowledge of specific glutamate receptor roles in a variety of neurological, mental and affective disorders. The further development of specific agonists and antagonists for these receptors will almost certainly lead to new drugs for such disorders. A particular avenue being actively pursued by drug companies is the possible application of positive and negative allosteric modulators of glutamate receptor subtypes rather than competitive agonists or antagonists. This could offer greater ‘fine control' of central synaptic processes.
The stage is well and truly set for the next half century of glutamatergic research and development. One can expect spectacular advances.
Acknowledgments
We are greatly indebted to Professor Graham Collingridge for his critical comments and helpful suggestions in the preparation of this manuscript. We also thank Professor Humphrey Rang for his immense help with the condensation of our original draft. JCW is most grateful also for his wife Beatrice's help in typing sections of the text and her advice on the vagaries of word processing in the case of his own laboured efforts in this respect.
Glossary
- AMPA
(S)-α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid
- BJP
British Journal of Pharmacology
- BPS
British Pharmacological Society
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- CNS
central nervous system
- CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
- γDGG
γ-D-glutamylglycine
- DαAA
D-α-aminoadipic acid
- DAP
α,ɛ-diaminopimelic acid
- DAP5
D-2-amino-5-phosphonopentanoic acid
- DCPG
(S)-3,4-dicarboxyphenylglycine
- DHβE
dihydro-β-erythroidine
- EAA
excitatory amino acid
- EGLU
(S)-α-ethylglutamic acid
- GABA
γ-aminobutyric acid
- 5HT
5-hydroxytryptamine
- HA-966
3-amino-1-hydroxypyrrolidin-2-one
- iGlu
ionotropic glutamate
- Kain
kainic acid
- LαAA
L-α-aminoadipic acid
- LAP4
(S)-2-amino-4-phosphonobutanoic acid
- L-Glu
L-glutamic acid
- LTD
long-term depression
- LTP
long-term potentiation
- MAP4
(S)-α-methyl-2-amino-4-phosphonobutanoic acid
- mGlu
metabotropic glutamate
- NBQX
2,3-dihydroxy-6-nitro-7-sulphamoyl-benzo[f]quinoxaline
- NMDA
N-methyl-D-aspartic acid
- ODAP
β-N-oxalyl-α,β-diaminopropionic acid
- PICK1
protein interacting with C kinase-1
- PLC
phospholipase C
- PPDA
cis-1-(phenanthrene-2-carbonyl)piperazine-2,3-dicarboxylic acid
- Quis
quisqualic acid
- (R)-CPP-ene
(R)-(E)-4-(3-phosphonoprop-2-enyl)piperazine-2-carboxylic acid
- (S)-4C3HPG
(S)-4-carboxy-3-hydroxyphenylglycine
- (S)-MCPG
(S)-α-methyl-4-carboxyphenylglycine
- trans-ACPD
trans-1-aminocyclopentane-1,3-dicarboxylic acid
- UBP1112
(RS)-α-methyl-3-methyl-4-phosphonophenylglycine
- UBP302
(S)-1-(2-amino-2-carboxyethyl)-3-(2-carboxybenzyl)pyrimidine-2,4-dione
Footnotes
Supplementary information is available at the British Journal of Pharmacology website (http://www.nature.com/bjp)
Dedicated to the memory of John Davies and Hugh McLennan
Supplementary Material
References
- BISCOE T.J., EVANS R.H., FRANCIS A.A., MARTIN M.R., WATKINS J.C., DAVIES J., DRAY A. D-α-aminoadipateas a selective antagonist of amino acid-induced and synaptic excitation of mammalian spinal neurones. Nature (London) 1977;270:743–745. doi: 10.1038/270743a0. [DOI] [PubMed] [Google Scholar]
- BORTOLOTTO Z.A., BASHIR Z.I., DAVIES C.H., COLLINGRIDGE G.L. A molecular switch activated by metabotropic glutamate receptors regulates induction of long-term potentiation. Nature. 1994;368:740–743. doi: 10.1038/368740a0. [DOI] [PubMed] [Google Scholar]
- BORTOLOTTO Z.A., CLARKE V.R.J., DELANY C.M., PARRY M.C., SMOLDERS I., VIGNES M., HOO K.H., BRINTON B., FANTASKE R., OGDEN A., GATES M., ORNSTEIN P.L., LODGE D., BLEAKMAN D., COLLINGRIDGE G.L. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- COLLINGRIDGE G.L., ISAAC J.T., WANG Y.T. Receptor trafficking and synaptic plasticity. Nat. Rev. Neurosci. 2004;5:952–962. doi: 10.1038/nrn1556. [DOI] [PubMed] [Google Scholar]
- COLLINGRIDGE G.L., KEHL S.J., MCLENNAN H. Excitatory amino acids and synaptic transmission in the Schaffer-commissural pathway of the rat hippocampus. J. Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D.R., JOHNSTON G.A.R. Amino acid transmitters in the mammalian central nervous system. Ergebn. Physiol. 1974;69:97–188. doi: 10.1007/3-540-06498-2_3. [DOI] [PubMed] [Google Scholar]
- CURTIS D.R., PHILLIS J.W., WATKINS J.C. The chemical excitation of spinal neurones by certain acidic amino acids. J. Physiol. 1960;150:656–682. doi: 10.1113/jphysiol.1960.sp006410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D.R., PHILLIS J.W., WATKINS J.C. Actions of amino acids on the isolated hemisected spinal cord of the toad. Br. J. Pharmacol. 1961;16:262–283. doi: 10.1111/j.1476-5381.1997.tb06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D.R., WATKINS J.C. The excitation and depression of spinal neurones by structurally related amino acids. J. Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- CURTIS D.R., WATKINS J.C. The pharmacology of amino acids related to gamma-aminobutyric acid. Pharm. Rev. 1965;17:347–391. [PubMed] [Google Scholar]
- DUGGAN A.W. The differential sensitivity to L-glutamate and L-aspartate of spinal interneurones and Renshaw cells. Exp. Brain Res. 1974;19:522–528. doi: 10.1007/BF00236115. [DOI] [PubMed] [Google Scholar]
- EVANS R.H., FRANCIS A.A., HUNT K., OAKES D.J., WATKINS J.C. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br. J. Pharmacol. 1979;67:591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS R.H., FRANCIS A.A., JONES A.W., SMITH D.A.S., WATKINS J.C. The effects of a series of ω-phosphonic α-carboxylic amino acids on electrically evoked and amino acid induced responses in isolated spinal cord preparations. Br. J. Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI T. Effects of sodium glutamate on the nervous system. Keio J. Med. 1954;3:192–193. [Google Scholar]
- HOLLMANN M., HEINEMANN S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- JIN R., BANKE T.G., MAYER M.L., TRAYNELLIS S.F., GOUAUX E. Structural basis for partial agonist action at ionotropic glutamate receptors. Nat. Neurosci. 2003;6:803–810. doi: 10.1038/nn1091. [DOI] [PubMed] [Google Scholar]
- JOHNSON J.W., ASCHER P. Glycine potentiates the NMDA response in cultured brain neurones. Nature (London) 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- KINARSKY L., FENG B., SKIFTER D., MORLEY R.M., SHERMAN S., JANE D.E., MONAGHAN D.T. Identification of subunit-specific and antagonist specific amino acids in the NMDA receptor glutamate binding pocket. J. Pharm. Exp. Ther. 2005;313:1066–1074. doi: 10.1124/jpet.104.082990. [DOI] [PubMed] [Google Scholar]
- KEW J.N.C., KEMP J.A. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology. 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- LERMA J. Roles and rules of kainate receptors in synaptic transmission. Nat. Rev. Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- MAYER M.L. Glutamate receptor ion channels. Curr. Opin. Neurobiol. 2005;15:282–288. doi: 10.1016/j.conb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- McCULLOCH R.M., JOHNSTON G.A.R., GAME C.J.A., CURTIS D.R. The differential sensitivity of spinal interneurones and Renshaw cells to kainate and N-methyl-D-aspartate. Exp. Brain Res. 1974;21:515–518. doi: 10.1007/BF00237169. [DOI] [PubMed] [Google Scholar]
- MACDERMOTT A.B., MAYER M.L., WESTBROOK G.L., SMITH S.J., BARKER J.L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. Nature (London) 1986;321:519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- MCLENNAN H., HUFFMAN R.D., MARSHALL K.C. Patterns of excitation of thalamic neurones by amino acids and by acetylcholine. Nature (London) 1968;219:387–388. doi: 10.1038/219387a0. [DOI] [PubMed] [Google Scholar]
- MONAGHAN D.T., BRIDGES R.J., COTMAN C.W. The excitatory amino acid receptors: their classes, pharmacology and distinct properties in the function of the central nervous system. Ann. Rev. Pharmacol. Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- MONAGHAN D.T., MORE J.C.A., FENG B., JANE D.2004Glutamate receptors Dopamine and Glutamate in Psychiatric Disordersed. Smith W.J. & Smith, M.E.A. pp. 79–116.Totawa, NJ: Humana Press Inc [Google Scholar]
- NAKANISHI S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- NOWAK L., BREGESTOVSKI P., ASCHER P., HERBET A., PROCHIANTZ A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature (London) 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- OLVERMAN H., JONES A.W., WATKINS J.C. L-Glutamate has higher affinity than other amino acids for [3H]-D-AP5 binding sites in rat brain membranes. Nature (London) 1984;307:460–462. doi: 10.1038/307460a0. [DOI] [PubMed] [Google Scholar]
- SCHOEPP D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharm. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- SCHOEPP D.D., JANE D.E., MONN J.A. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- TSUCHIYA D., KUNISHIMA N., KAMIYA N., JINGAMI H., MORIKAWA K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+ PNAS. 2002;99:2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS J.C. Metabolic regulation in the release and action of excitatory amino acids. Biochem. Soc. Symp. 1972;36:33–47. [PubMed] [Google Scholar]
- WATKINS J.C., EVANS R.H. Excitatory amino acid transmitters. Ann. Rev. Pharmacol. Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.