Abstract
Some 865 genes in man encode G-protein-coupled receptors (GPCRs). The heterotrimeric guanine nucleotide-binding proteins (G-proteins) function to transduce signals from this vast panoply of receptors to effector systems including ion channels and enzymes that alter the rate of production, release or degradation of intracellular second messengers. However, it was not until the 1970s that the existence of such transducing proteins was even seriously suggested. Combinations of bacterial toxins that mediate their effects via covalent modification of the α-subunit of certain G-proteins and mutant cell lines that fail to generate cyclic AMP in response to agonists because they either fail to express or express a malfunctional G-protein allowed their identification and purification. Subsequent to initial cloning efforts, cloning by homology has defined the human G-proteins to derive from 35 genes, 16 encoding α-subunits, five β and 14 γ. All function as guanine nucleotide exchange on–off switches and are mechanistically similar to other proteins that are enzymic GTPases. Although not readily accepted initially, it is now well established that β/γ complexes mediate as least as many functions as the α-subunits. The generation of chimeras between different α-subunits defined the role of different sections of the primary/secondary sequence and crystal structures and cocrystals with interacting proteins have given detailed understanding of their molecular structure and basis of function. Finally, further modifications of such chimeras have generated a range of G-protein α-subunits with greater promiscuity to interact across GPCR classes and initiated the use of such modified G-proteins in drug discovery programmes.
Keywords: G-protein, signal transduction, cyclic AMP, cholera toxin, pertussis toxin, drug discovery, protein acylation
Early days
As with the G-protein-coupled receptors (GPCRs), heterotrimeric guanine nucleotide-binding proteins (G-proteins) represent an ancient protein family that has been highly conserved over evolution. The capacity of a number of bacterial exotoxins to covalently modify the α-subunit of many of the heterotrimeric G-proteins, and hence alter their function, attests to this. Such toxins were key tools in the discovery and classification of the G-proteins and remain important reagents in many studies of G-protein function.
It was clear from the work of Sutherland and others in the late 1950s and early 1960s, which resulted in the award of the Nobel Prize for Physiology or Medicine in 1971, that a range of hormones was able to stimulate production of cyclic AMP (cAMP). It was not evident at that time, however, that this was a GTP-dependent process that required the intermediacy of a G-protein. This reflected the fact that ATP isolated from rabbit muscle and used as substrate for the generation of cAMP in such assays was contaminated with sufficient GTP to mask this requirement. It was not until enzymic synthesis of ATP became commonplace that the absolute requirement for GTP became apparent. Although the requirement for a GTP-dependent step was now evident, identification and characterisation of the putative GTP-dependent signal-transducing protein remained a tremendous challenge. However, as with many key steps in science, the confluence of information from apparently disparate strands of work underpinned this advance.
Studies on the action of an exotoxin produced by Vibrio cholerae, the bacterium responsible for the symptoms of the disease cholera, showed both that addition of the toxin to cells produced sustained generation and elevation of cAMP levels, and that it did so via its action as a mono-ADP-ribosyltransferase, that is, an enzyme able to catalyse the transfer of the ADP-ribose element of nicotinamide adenine dinucleotide (NAD+) to a protein substrate (Gill & Meren, 1978). In parallel, mutagenesis of the mouse lymphoma cell line S49 (Coffino et al., 1975) began to dissect and identify molecular components of the trans-plasma membrane signal transduction cascade by which β-adrenoceptor, agonists cause elevation of cAMP. Sustained elevation of cAMP in S49 cells results in their death. Thus, mutant lines that continued to prosper in the presence of β-adrenoceptor agonists were isolated. Although not the first mutant identified, the key initial mutant cell line was (with hindsight erroneously) named cyc− because, as it failed to generate cAMP in response to isoprenaline and other agonists but could be shown to still express a ligand-binding site with the characteristics of the β2-adrenoceptor, the most obvious conclusion was that it must lack expression of the cAMP-generating enzyme, adenylyl cyclase. However, a range of studies indicated that direct regulation of adenylyl cyclase/cAMP production could still occur in these cells. Furthermore, while treatment of membranes of wild-type S49 cells with activated cholera toxin and [32P]NAD+ resulted in incorporation of radioactivity into a polypeptide of some 45 kDa, this did not occur when membranes of S49 cyc− cells were used. Membranes of S49 cyc− cells thus lacked a key component of the cAMP generation cascade and hence provided an ideal background for reconstitution studies designed to purify the cholera toxin substrate.
Using rabbit liver as a source, this purification was a tour de force and identified a 45 kDa polypeptide corresponding to the cholera toxin substrate and transducing protein (Northup et al., 1980). However, despite efforts employing a range of chromatographic steps, the 45 kDa polypeptide copurified with a 35 kDa and (although originally overlooked because of its rapid mobility through SDS–PAGE) an 8–10 kDa polypeptide. The 45 kDa protein was thus defined as the α-subunit of the adenylyl cyclase stimulatory G-protein Gs and the corresponding 35 and 8–10 kDa polypeptides the β- and γ-subunits that make up the functional G-protein heterotrimer. For this, and a host of other key studies on the function and structure of hetero-trimeric G-proteins, Alfred G. Gilman (Gilman, 1995) was awarded the Nobel Prize for Physiology or Medicine in 1994 along with Martin Rodbell (Rodbell, 1995).
A number of developments in what are now viewed as key underpinning aspects of the basic pharmacology of receptor ligand-binding studies could now begin to be understood in molecular terms. For example, in competition binding studies using [3H]antagonist/agonists at the β2-adrenoceptor, the ‘low' Hill slope for full agonist ligands observed in membranes of wild-type S49 cells was converted into a single site that displayed only low affinity for the agonist in membranes of S49 cyc− cells. As this was equivalent to the effects of adding guanine nucleotides to assays performed on wild-type S49 cell membranes, such studies defined the receptor/G-protein complex as a high-affinity site for agonists, the isolated receptor as a low-affinity site and indicates that classical antagonists did not discriminate between the two. Such studies were therefore integral to the development of the ‘two-state' receptor model and subsequent adaptations of this concept (see also Rang, this issue).
Although it had been recognised from the early studies on cAMP production that certain receptor ligands were able to reduce, rather than increase, cAMP levels, and that ligand-binding studies on such receptors often produced data similar to the ‘guanine nucleotide shifts' in agonist affinity discussed above, serious efforts to identify an equivalent adenylyl cyclase inhibitory ‘Gi' G-protein again required both information from an apparently unrelated research area and the concretion of relevant information in a timely review from Martin Rodbell (Rodbell, 1980). Studies on the action of ‘islet-activating protein', an exotoxin produced by Bordetella pertussis, the causative agent of whooping cough, showed it to be a mono-ADP-ribosyltransferase able to modify covalently a 41 kDa polypeptide present in the membranes of essentially all cells (Katada & Ui, 1982). Furthermore, in a physiological context, ‘islet-activating protein' (now known generically as ‘pertussis toxin') attenuated α2-adrenoceptor regulation of insulin secretion from islet cells, suggesting that the molecular target for pertussis toxin might be ‘Gi'. Although a functional reconstitution assay akin to that used for the purification of Gs was not readily available, purification of the pertussis toxin substrate in fractions enriched in high-affinity GTPase activity resulted in the identification of the 41 kDa polypeptide as the α-subunit of ‘Gi', along with 35 and 8–10 kDa polypeptides that appeared identical to the β- and γ-subunits of Gs (Bokoch et al., 1984).
Extending the Gα-protein family
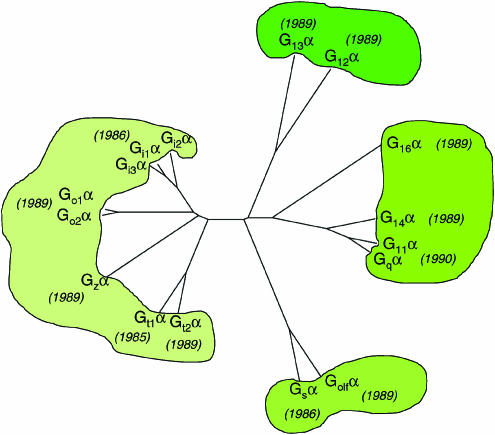
Although inhibition of a signal or a biological process by pretreatment with pertussis toxin rapidly became diagnostic of the involvement of ‘Gi', it was soon apparent that ‘Gi'α was not a single molecular species. A number of studies, particularly using brain as a rich source of polypeptides that were substrates for ADP-ribosylation by pertussis toxin, purified more than one polypeptide with apparent molecular mass close to 40 kDa (Huff et al., 1985). Although these could have represented nothing more than proteolytic cleavage products from a single ‘Gi'α-protein, they were recognised differentially by various antisera including those raised against the C-terminal region of rod transducin (Gt1) α (Pines et al., 1985). As with the G-protein-coupled photon receptor rhodopsin, high-level expression in the specialised architecture of mammalian rod outer segments had allowed the purification, detailed characterisation and cloning of Gt1α in the advance of other G-proteins (Figure 1). The major, ‘non-Gi', pertussis toxin substrate from the brain was thus designated Goα for G‘other' because its function was unclear.
Figure 1.
Homology of mammalian G-protein α-subunits. The relatedness of individual mammalian G-protein α-subunits is shown as an unrooted homology tree. The date of cloning of cDNAs corresponding to each family member is shown in parentheses.
Cholera toxin-catalysed [32P]ADP-ribosylation studies had indicated at least two forms of Gsα with amounts of the two forms varying between tissues. Cloning of cDNAs encoding Gsα now uncovered that these variants were derived from a single gene via alternative splicing of exon 3 (Bray et al., 1986). By contrast, cloning of cDNAs encoding the various pertussis toxin-sensitive ‘Gi' G-protein α-subunits indicated each of what became known as Gi1α, Gi2α and Gi3α to be the products of different genes. ‘Goα‘ was also the product of a separate gene that can be differentially spliced to generate at least two polypeptides, Go1α and Go2α (Figure 1). Although the Gi2α and Gi3α gene products are widely expressed, both Gi1α and the forms of ‘Goα' have more restricted distribution patterns that can generally be described as ‘neuroendocrine' (Table 1). Studies on specialised sensory systems, including olfactory, visual and lingual tissues, uncovered other G-protein α subunits, Golfactory, cone transducin (also called Gt2) and Ggustducin, respectively, with highly restricted distribution patterns that were highly related to, but distinct from, Gs or the previously identified Gi family members (Table 1).
Table 1. The family of mammalian heterotrimeric G-protein subunits: function and regulation.
| Family | Subtype | Effectors | Expression | Disease relevance | Pharmacological modulation |
|---|---|---|---|---|---|
| Gsα | Gs(S)α Gs(L)α Gs(XL)α Golfα | Adenylyl cyclases ↑(Gs,s(XL),olfα) Maxi K channel ↑(Gsα) Src tyrosine kinases (c-Src, Hck) ↑(Gsα) GTPase of tubulin ↑(Gsα) | Gsα: ubiquitous Golfα: olfactory neurons, certain CNS ganglia; digestive and urogenital tract | Gs(XL)α: brachydactyly, trauma-related bleeding tendency, neurological problems Gsα: McCune–Albright syndrome, cholera, pseudohypoparathyroidism type Ia/b, testotoxicosis, adenomas of pituitary and thyroid | Gsα: CTX Golfα: CTX |
| Gi/oα | Go1α Go2α Gi1–i3α Gzα Gt1/2α Ggustα | Adenylyl cyclase ↓ (Gi,o,zα) Rap1GAPII-dependent ERK/MAPkinase activation ↑ (Giα) Ca2+ channels ↓ (Gi,o,zα) K+ channels ↑ (Gi,o,zα) GTPase of tubulin ↑ (Giα) Src tyrosine kinases (c-Src, Hck) ↑ (Giα) Rap1GAP ↑ (Gzα) GRIN1-mediated activation of Cdc42 ↑ (Gi,o,zα) cGMP-PDE ↑ (G tα) Ggustα: ? | Go1–2α: neurons, neuroendocrine cells, astroglia, heart Gi1–i3α: neurons and many others Gzα: platelets, neurons, adrenal chromaffin cells, neurosecretory cells Gt1α: rod outer segments, taste buds Gt2α: cone outer segments Ggustα: sweet and/or bitter taste buds, chemoreceptor cells in the airways | Giα: whooping cough, adrenal and ovarian adenomas Gtα: congenital cone dysfunction, night blindness | Go(1/2)α: PTX Gi1-i3α: PTX Gzα: ? Gt1/2α: PTX, CTX Ggustα: PTX |
| Gq/11α | Gqα G11α G14α G15α G16α | Phospholipase Cβ isoforms ↑ p63-RhoGEF ↑ (Gq/11α) Bruton's tyrosine kinase ↑ (Gqα) K+ channels ↑ (Gqα) | Gq/11α: ubiquitous G15/16α: hematopoietic cells | Gq/11α: dermal hyperpigmentation and melanocytosis? | Gq/11α: YM-254890 G14α: ? G15α: ? G16α: ? |
| G12/13α | Gα12 Gα13 | Phospholipase D ↑ Phospholipase Cɛ ↑ NHE-1 ↑ iNOS ↑ E-cadherin-mediated cell adhesion: ↑ p115RhoGEF ↑ PDZ-RhoGEF ↑ Leukaemia-associated RhoGEF (LARG) ↑ Radixin ↑ Protein phosphatase 5 (PP5) ↑ AKAP110-mediated activation of PKA ↑ HSP90 ↑ | Ubiquitous | Recent SNPs identified but no disease correlation yet | G12α: ? G13α: ? |
| Gβ/γ | β1–5 γ1–12 | PLCβs ↑ Adenylyl cyclase I ↓ Adenylyl cyclases II, IV, VII ↑ PI-3 kinases ↑ K+ channels (GIRK1,2,4) ↑ Ca2+ (N-, P/Q-, R-type) channels ↓ P-Rex1 (guanine nucleotide exchange factor for the small GTPase Rac) ↑ c-Jun N-terminal kinase (JNK) ↑ Src kinases ↑ Tubulin GTPase activity ↑ G-protein-coupled receptor kinase recruitment to membrane ↑ Protein kinase D ↑ Bruton's tyrosine kinase ↑ p114-RhoGEF ↑ | β1γ1: retinal rod cells β3γ8: retinal cone cells β5: neurons and neuroendocrine organs β5(L): retina Most cell types express multiple β and γ subtypes | Gβ3: atherosclerosis, hypertension, metabolic syndrome | Gβγ: ? |
CTX=cholera toxin; PTX=pertussis toxin; ↑=enhances function; ↓=reduces function; YM-254890=a cyclic depsipeptide isolated from Chromobacterium sp QS3666.
Although receptor-mediated production of inositol 1,4,5 trisphosphate, and hence elevation of intracellular [Ca2+], seemed conceptually similar to receptor regulation of cAMP production, an absolute requirement for a GTP-dependent step and therefore a G-protein was significantly more recalcitrant to demonstration. In part, this reflected that a membrane-based assay for ligand function was substantially more difficult to establish, that in most cell types neither cholera toxin nor pertussis toxin pretreatment modified this cascade and that ‘guanine nucleotide shifts' of agonist affinity in [3H]antagonist/agonist competition binding studies were generally small (and often negligible) for receptors that link predominantly to this pathway. However, determined purification efforts resulted in the identification and characterisation of Gqα and G11α as 42 kDa polypeptides that fulfilled the criteria for phosphoinositidase Cβ-linked G-proteins (Taylor et al., 1990). Essentially in parallel, and taking advantage of the high homology of other cloned Gα sequences, Mel Simon and colleagues cloned both Gqα and G11α (Strathmann & Simon, 1990) and showed these to be widely expressed. They also cloned the related G14α and G16α that have much more limited expression patterns (Figure 1, Table 1), although both can also link receptors to the elevation of intracellular [Ca2+]i. Finally, further efforts based on homology cloning identified two additional Gα-subunits, G12α and G13α, that form a separate subfamily (Figure 1) and are involved in communications between heterotrimeric G-protein-linked signalling pathways and cell responses regulated by monomeric GTP-binding proteins, including cellular shape and morphology and cell proliferation (Riobo & Manning, 2005).
The families of β- and γ-subunits
At least five different β- and 12 γ-subunits have been described (Table 1). Although a number of possible pairings have been indicated not to form, and tissue expression patterns may further limit the actual number of pairings in particular cells and tissues, there is still the potential for coexpression of a substantial number of pairs. Despite this, early studies suggested that β/γ complexes isolated along with different α-subunits or from different tissues were functionally interchangeable, except that β1/γ1 (the combination associated with Gt1α in rod outer segments) was generally less potent functionally than β/γ complexes isolated from the brain, for example. The β-subunits have a β-propeller structure, containing seven so-called WD (tryptophan-aspartate)-40 repeats, and the crystal structure of the β1/γ1 complex (Sondek et al., 1996) showed that the γ-subunit interacted with the β-subunit via an N-terminal coiled coil and a series of other extensive contacts along much of the length of the γ subunit sequence (Figure 2). Although β1−β4 are highly homologous, β5 is substantially less so, suggesting that it may play a different role(s). Although it can certainly interact with a number of the γ-subunits, unlike the other β-subunits, it dissociates from the γ and is also able to interact with a number of regulator of G-protein signalling (RGS) protein family members that contain a G-protein γ-subunit-like domain.
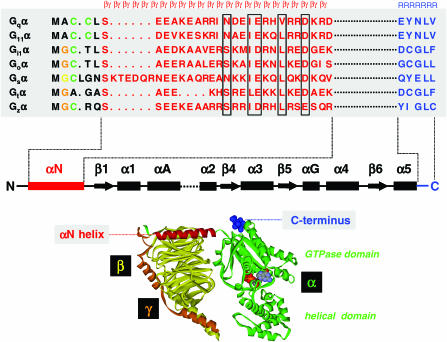
Figure 2.
β/γ and receptor contact sites on Gα. Top: Sequence alignment of the N- and C- terminal regions of selected Gα-subunits. Residues that are subject to N-linked myristoylation, thio-palmitoylation or N-linked palmitoylation are highlighted in orange, green and yellow, respectively. In each case, M (black) is the protein synthesis initiator, methionine, that is eliminated during protein synthesis. Residues comprising the N-terminal αN helix are highlighted in red and residues at the extreme C-terminus of Gα are shown in blue. The αN helix is required for binding β/γ-subunits, and particular β/γ contacts are boxed in black, the extreme C-terminus plays a key role in specific receptor recognition. In the secondary structure diagram below the aligned sequences, β/γ and receptor interaction sites are highlighted in red and blue, respectively. Only selected domains of Gα are shown, and for simplicity the domains between αA and the α2 helix have been omitted as indicated by the dotted line. Bottom: Illustration of the N-terminal αN helix (red) and the C-terminal receptor contact region (blue) in the context of the tertiary and quaternary structure of the resting state, inactive Gi1αβ1γ2 heterotrimer. The GDP molecule is buried between the GTPase and helical domain of Gα (green), the β-subunit is coloured yellow and the γ-subunit is shown in orange. The diagram was generated using the coordinates from the PDB file 1GP2 and visualised with WebLab ViewerPro.
Co- and post-translational modification of G-protein α-subunits: roles in membrane association
Unlike GPCRs, Gα-subunits are not transmembrane polypeptides and based on their cDNA sequences would appear to be essentially soluble proteins. It was anticipated, however, that they must be present at the plasma membrane to interact with receptors. Widely expressed members of the Giα subfamily have an N-terminal sequence corresponding to the consensus motif for N-myristoylation. Almost invariably, N-myristoyl CoA transferase cotranslationally adds the 14 carbon, saturated fatty acid (C14:0) myristic acid to the N-terminal glycine (Gly), following removal of the initiator methionine (Met) of polypeptides within the sequence (Met)-Gly-Xaa-Xaa-Xaa-Ser (see Figure 2). Attachment of this fatty acid chain increases the hydrophobicity of what is essentially a hydrophilic polypeptide, and key studies in this area in the early 1990s demonstrated that alteration of the glycine to alanine eliminated incorporation of radiolabelled myristate and resulted in the production of a protein that was present in the cytosol of cells.
However, attachment of a single fatty acid is generally considered insufficient to anchor efficiently a soluble protein to the plasma membrane and a conserved cysteine residue at position 3 in all of the widely expressed Giα family members (Figure 2) proved to be the site for post-translational addition of the 16 carbon, saturated fatty acid (C16:0), palmitic acid. Interestingly, the two forms of Gtα do not have a cysteine at this position (Figure 2) and unlike the other pertussis toxin-sensitive G-proteins are easily removed from the membrane by the addition of guanine nucleotides, a feature that made purification of Gt1α a (relatively) trivial process. As thio-ester linkages are easily cleaved by dithiothreitol and other reducing agents, and because the linkage of palmitate is dynamic and can be regulated upon G-protein activation, the presence of this post-translational modification went unappreciated for a considerable period. However, it clearly plays a key role in G-protein targeting. Only the Gi-family G-protein α-subunits are targets for N-terminal myristoylation. All of the other Gα-subunits have one or more cysteine residues within the N-terminal 15 amino acids and in each case at least some of these are targets for post-translational palmitoylation.
The most actively studied have been Gsα and Gqα/G11α. For Gsα, Cys3 was rapidly identified as the site of thio-acylation (Mumby et al., 1994). However, although mutation of this amino acid did indeed prevent post-translational incorporation of [3H]palmitate that was sensitive to removal by treatments able to hydrolyse thio-ester bonds, a number of features of the mutated protein suggested the presence of a second, at that time unidentified, modification. Eventually, the application of mass spectrometry demonstrated that Gly2 (that is the de facto N-terminal amino acid following removal of the methionine initiator) was also palmitoylated. As this is an N-linked acylation, it is not susceptible to cleavage and dynamic regulation and thus was not detected in post-translational [3H]palmitate labelling studies. Both Gqα and G11α have a pair of adjacent cysteine residues at positions 9 and 10 that are targets for post-translational palmitoylation. These modifications are important for plasma membrane targeting and hence function. Indeed, the dual acylation of G-protein α-subunits appears key to the targeting and presence of a substantial fraction of the total cellular G-protein pool in specialised domains, often described as lipid rafts, that can be resolved from ‘bulk' membrane fractions by extraction with certain nonionic detergents and centrifugation through various density gradients (Ostrom & Insel, 2004).
Post-translational modification of γ-subunits
Although it was originally considered that the greater hydrophobicity of the β/γ complex might be sufficient to provide membrane association without further alterations, the presence in γ-subunits of a C-terminal Cys-A-A-X motif that had previously been shown to result in thio-ether-linked isoprenylation of other proteins including p21ras and the nuclear lamins suggested a similar set of post-translational modifications. This was shown to be the case, with the cysteine acting as the target for either farnesylation or geranylgeranylation depending on the identity of the C-terminal amino acid. As with the other proteins mentioned above, subsequent peptidase cleavage of the C-terminal three amino acids and carboxymethylation of the now C-terminal cysteine completes the maturation of the γ-subunit. Mutation to prevent γ-subunit isoprenylation limits effective interactions with the plasma membrane. Interactions with a β-subunit are important for effective modification and isoprenylation of the γ-subunit is important for effective interactions with both receptor and β/γ-regulated effectors.
Understanding the mechanism of G-protein action
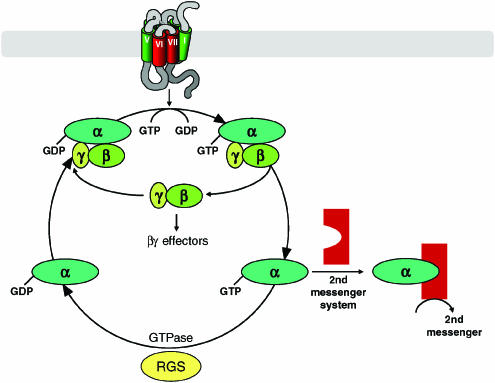
With clear understanding that a GTP-dependent step was integral to second messenger regulation, a range of studies was initiated to examine whether hydrolysis of GTP and hence a GTPase activity was essential for function. Initially for agonist stimulation of cAMP production in avian erythrocytes (Cassel & Selinger, 1976), and subsequently for the activation of a range of pertussis toxin-sensitive signals (Koski & Klee, 1981), a marked increase in high-affinity (or low Km) GTPase activity was observed in response to agonist ligands. Furthermore, purification schedules for the pertussis toxin-sensitive G-proteins incorporated high-affinity GTPase activity measures as functional assays. Such studies resulted in a basic model whereby, in the absence of stimulation, the Gα-subunit is GDP bound and associated with the β- and γ-subunits. The receptor, either via constitutive activity or in response to binding of an agonist, associates more effectively with the G-protein and acts as a guanine nucleotide exchange factor (Figure 3). This promotes release of GDP and its replacement with GTP, which is present in markedly higher cellular concentrations than GDP. Associated conformational rearrangements result in dissociation of the Gα-subunit from the β/γ complex and these components can then interact with and regulate effector systems including enzymes that generate second messengers and a variety of classes of ion channels (Figure 3, Table 1). The Gα-subunit is an intrinsic GTPase, hydrolysing the terminal phosphate of GTP to restore GDP to the nucleotide-binding pocket. This allows reassociation of the Gα with the β/γ complex and completion of the cycle (Figure 3).
Figure 3.
The nature of the G-protein α-subunit-bound nucleotide controls the extent and temporal kinetics of G-protein signaling. Conversion of a G-protein heterotrimer from the inactive, GDP-bound, to active GTP-bound state is promoted by interaction with a guanine nucleotide exchange factor (GEF), the most common of which are members of the GPCR family. Subsequent conformational changes promote separation of the GTP- bound α-subunit from the β/γ complex, whereupon both elements of the G-protein can regulate the activity of effector proteins that include second messenger generating enzymes and ion channels (see Table 1 for details). The intrinsic GTPase activity of the G-protein α-subunit hydrolyses the terminal phosphate of bound GTP and terminates function. This activity is accelerated by GTPase-activating proteins, the largest family of which are the regulators of G-protein signalling (RGS) proteins. Reassociation of Gα-GDP with the β/γ complex terminates effector regulation by the β/γ-subunits (Table 1) and completes the cycle. Further interaction with a GEF is now required to reinitiate the cycle.
A major issue when purified G-protein subunits were initially studied biochemically and in isolation, was that the measured rate of GTP hydrolysis, and hence the rate of the cycle, appeared far too slow to account for the off-rate of physiological processes. This suggested that other proteins that accelerate this process must exist. Initially employing genetic screens in yeast, proteins able to accelerate Gα GTP hydrolysis were identified. Subsequently, members of the mammalian family of RGS proteins were isolated and shown to interact with and act as GTPase-activating proteins for various Gα-subunits (Figure 3).
Although conceptually simple and undoubtedly correct in essence, a number of elements of the GTPase cycle of activation and deactivation have been questioned. Perhaps the most discussed has been whether physical dissociation of the Gα and β/γ complex actually occurs in native systems. Fluorescence resonance energy transfer (FRET)-based studies have recently suggested that, at least for certain G-proteins, a conformational rearrangement might occur rather than separation (Bunemann et al., 2003). Furthermore, key studies on G-protein subunit dissociation utilised GTP analogues that are not (or only poorly) hydrolysed, rather than GTP, and a growing literature on protein scaffolding suggests that transmembrane signalling complexes may more resemble solid-state transistors than a series of independent and freely diffusing proteinaceous elements.
As cholera toxin produces persistent activation of adenylyl cyclase, it was inherently likely that the target amino acid for mono-ADP-ribosylation by the toxin would be central to the GTPase turn-off mechanism. Identification of the relevant arginine provided early clues to the mechanism of the GTPase activity that could be visualised and understood in chemical terms with the crystallisation of Gt1α (Noel et al., 1993). This advance also confirmed that the basic structure of the GTPase domain of the G-protein α-subunit was highly homologous to other GTPases including p21ras. Given the absolutely central role of this arginine, it is not surprising that it is conserved across the other Gα-subunits and that alteration of this residue by designed mutagenesis in the laboratory results in the production of constitutively active mutants that fail to turn off. Such mutant Gα-subunits have been used widely to understand the downstream signals that emanate from activation of specific G-proteins. Although rare, somatic mutation of this arginine (and other residues involved in the GTPase mechanism) in Gsα has been associated with the development of pituitary adenomas (Landis et al., 1989) (Table 1), because in these cells elevation of cAMP is a positive growth signal. As they can be performed as true enzyme kinetic studies by varying the concentration of GTP, direct measurements of the regulation of GTPase activity are perhaps the best means to examine effects of receptor ligands and G-protein-interacting proteins on the G-protein cycle. However, partially because the standard assay employs [γ-32P]GTP and requires a separation of substrate from product that is difficult to automate, such assays are not in widespread or routine use. Instead, measures of guanine nucleotide exchange monitor the regulation of binding of the GTP analogue [35S]GTPγS. Such assays are easy to perform and ‘bound' ligand can be separated from ‘free' by a simple filtration step or adapted to employ scintillation proximity. However, the high specific activity of [35S]GTPγS tends to ensure that assays are performed at very low nucleotide occupancy and because the nucleotide exchange step is not the point in the cycle regulated by RGS proteins for example, [35S]GTPγS binding studies are unable to provide direct information on the effects of such G-protein-interacting proteins.
Multitasking Gα-subunits: the relationship of structure to function
The G-protein α-subunits have to perform a range of functions and interact with a variety of other proteins to do so (Figure 2, Table 1). Clearly, they must have interfaces than interact selectively with receptor and effector subfamilies and they also must interact with the β/γ complex (Figure 2) as well as other guanine nucleotide exchange factors and GTPase-activating proteins. Although a number of distinct elements of the primary sequence of a Gα-subunit contribute to the selectivity of contacts with receptors (Kostenis et al., 2005), the extreme C-terminal tail (Figure 2) is both one of the most important and certainly has been the most extensively analysed.
Historically, two key studies particularly contributed to this assessment. Firstly, a second mutant S49 lymphoma cell line unable to generate cAMP in response to β2-adrenoceptor agonists was identified. In contrast to cyc− cells, this did express both the β2-adrenoceptor and the 45 kDa cholera toxin substrate. However, [3H]antagonist/agonist competition binding studies indicated only a single low-affinity agonist-binding site and that the affinity for agonist was unaffected by the addition of guanine nucleotides. Despite both the receptor and Gsα apparently being expressed, the lack of detectable interactions between them resulted in these cells being named unc (for uncoupled). Two-dimensional electrophoresis indicated that the form of Gsα in unc cells differed from wild type by a single ionic charge and cDNA cloning of these indicated the variation to be a single base substitution that resulted in replacement of arginine, six amino acids from the C-terminus in the wild type, by proline in the unc variant. The position of the alteration, near the extreme C-terminus, implied a key role for this region in interactions with the β2-adrenoceptor and, by extension, other Gsα-coupled receptors. Furthermore, the introduction of proline, the prototypic helix breaker amino acid, suggested a structural basis for the effect. Equally, as pertussis toxin was recognised to cause uncoupling of receptors from ‘Gi'-family G-proteins, the appreciation that the cysteine residue located four amino acids from the C-terminus of the α-subunit of each of the pertussis toxin-sensitive G-proteins were the target for pertussis toxin-catalysed ADP-ribosylation also implicated the extreme C-terminal region in such interactions.
By contrast, contacts between the G-protein α-subunit and β/γ complex were shown to involve the N-terminal domain of the α-subunit (Figure 2). Very limited tryptic digestion of G-protein α-subunits that targeted a site within the N-terminal 20–30 amino acids essentially eliminated interaction with β/γ. Far more details on the structure and interactions between G-protein subunits are provided by Cabrera-Vera et al. (2003).
Even the most diverse Gα-subunits share some 50% sequence identity. As members of protein families with such high homology generally share highly similar structures, it was therefore likely that chimaeric Gα-subunits could be generated that would fold appropriately and function. As a key receptor contact domain is provided by the extreme C-terminus, a chimaera consisting of the N-terminal half of Gsα and the C-terminal half of a ‘Gi'α would be expected to interact selectively with ‘Gi'-coupled receptors. The effector output would, however, be determined by whether key elements for such contacts resided in the N- or C-terminal domain. Using this basic strategy, the effector contact region was defined to be within the C-terminal half of the Gα-subunit but upstream from the receptor contact domain. The demonstration that the presence of only some three to five amino acids from the extreme C-terminus of a Gα was sufficient to determine the selectivity of receptor coupling (Conklin et al., 1993) has been integral to the development and use of such chimaeric G-proteins by academic researchers, but overwhelmingly by the drug discovery industry.
Particularly with overexpression of receptors, interactions with a range of G-proteins can be observed. The desire to have a single, generic assay suitable to detect agonist-activation of as many members of the G-protein-coupled receptor superfamily as possible, coupled with the widespread use of assays that monitor ligand regulation of [Ca2+]i, has resulted in the routine use of chimaeric G-proteins in which the C-terminal five amino acids of Gsα or forms of Giα are transplanted onto the backbone of Gqα. With the expression of a cocktail of authentic Gqα, and both Gq(i5)α and Gq(s5)α, receptors that interact with each of the three major G-protein classes should generate a common [Ca2+]i output in response to agonist activation. Extensions of this basic idea have taken advantage of the high promiscuity in receptor interactions of human G16α (and its murine orthologue G15α) as well as further mutagenesis to design even more receptor promiscuous variants of Gqα (Kostenis et al., 2005).
Uncovering roles of the β/γ complex
Initially, the β/γ complex was considered to act as little more than a binding partner for the Gα-subunit to suppress spontaneous signalling and potentially, because it was significantly more hydrophobic, to provide a membrane anchor for the Gα-subunit. However, the surprising finding that in a range of leucocytes, including neutrophils and related cell lines, treatment with pertussis toxin attenuated the ability of ligands such as formyl-Met-Leu-Phe (fMLP) to elevate [Ca2+]i appeared to be incompatible with the fact that the toxin was known to prevent productive interactions between receptors and Gi-family G-proteins and that such agonists did not inhibit adenylyl cyclase or reduce cAMP levels. With an assay for phospholipase C activity in membranes of such cells and the ability to purify significant levels of the β1/γ1 complex that associates with Gt1α in bovine rod outer segments, Peter Gierschik and colleagues demonstrated that β1/γ1 stimulated phospholipase C activity rather than inhibiting basal activity that might have reflected the presence of an activated Gα-subunit. Furthermore, addition of increasing amounts of GDP-bound, purified Gt1α reversed the effect of the β/γ complex. The obvious conclusion of such studies was that the inactive α-subunit bound to and inactivated the function of the β/γ complex.
Nowadays, blockade of an effect by expression of a cDNA encoding Gt1α (or another β/γ-interacting protein) is an essentially diagnostic means to implicate the β/γ complex in a functional response. However, in the late 1980s and early 1990s, the concept that only G-protein α-subunits were able to induce signals was so engrained that these conclusions were not readily accepted and other explanations, including the copurification with the β/γ of a small amount of active Gα, were actively promulgated. It required careful experimental design and high-profile reviews written by influential proponents of the β/γ signalling hypothesis, including David Clapham and Eva Neer (Clapham & Neer, 1993), before this hypothesis was generally accepted. However, even earlier, studies on muscarinic regulation of the atrial G-protein regulated inwardly rectifying K+ (GIRK) channel had shown a direct function of the β/γ complex (Logothetis et al., 1987). Furthermore, purification and application of bacterially expressed G-protein α- and β/γ-subunits resulted in similar conclusions in circumstances where there could be no possibility of copurification of the other G-protein element. The results from studies on leucocyte phospholipase Cβ activity still required explanation because, as noted earlier, Gq and G11 are not substrates for pertussis toxin catalysed ADP-ribosylation and treatment with this reagent does not inhibit receptor-mediated phospholipase Cβ activity in the majority of cells and tissues. Appreciation of the molecular diversity in species of phospholipase Cβ, their differential expression patterns, and responsivenes to Gα- and β/γ-subunits combined to provide the molecular explanation (Camps et al., 1992).
As noted earlier, initial studies suggested that β/γ-subunits purified along with a particular Gα might be essentially interchangeable. However, apart from β1/γ1 purified in association with Gt1α, it is likely that other β/γ preparations consisted of complex mixtures. The capacity to express and purify specific pairs of β+γ-subunits, particularly using insect Sf9 cells as expression host, allowed analysis of variation in function and potency of different pairings to interact with individual Gα-subunits, with different effectors (Wolfe et al., 2003) and, in conjunction with Gα-subunits, with different receptors (McIntire et al., 2002).
As with the α-subunits, a substantial number of β/γ-interacting proteins have since been identified. These are generally considered as β/γ effectors and include a variety of ion channel subunits (Table 1). As the regions of the β/γ complex that provide the interface for these interactions overlap, at least in part, with the regions involved in α-subunit interactions, it is probable that β/γ cannot bind both an effector and the α-subunit concurrently. Structural analysis of the complex between G-protein-coupled receptor kinase 2 and a β1/γ2 complex (Lodowski et al., 2003) has provided an elegant example of this concept.
G-proteins and disease
By far the most prevalent disease associated with alteration in G-protein activity and amount is cholera (Table 1). As described above, ADP-ribosylation of the α-subunit of Gs catalysed by the activated A-subunit of the exotoxin of V. cholerae, that is ingested via contaminated water, results in the persistent stimulation of adenylyl cyclase activity. This results in extrusion of water from cells of the intestinal epithelium and the watery diarrhoea and dehydration associated with the condition. A number of other bacterial exotoxins can produce similar effects via the same mechanism. As well as regulation of the activity of G-proteins by extraneous factors, alteration in levels of G-protein subunits has been reported to be associated, or at least correlated, with disease processes, including an upregulation of Giα-subunits in heart failure. Furthermore, a series of relatively rare endocrine conditions are linked to poor expression or mutation of a variety of Gα-subunits (Spiegel & Weinstein, 2004; Table 1). Although most reports have centred on the function of the α-subunits, a relatively common polymorphic variant of the β3-subunit has been associated with various cardiovascular phenotypes and aspects of the metabolic syndrome (Table 1), but as with many such studies, the contribution of this is likely to be modified by a series of other variations that are rarely examined in parallel.
Interfering with G-protein function
Cholera toxin and pertussis toxin have been invaluable tools in defining roles of Gs and the subfamily of pertussis toxin-sensitive G-proteins. Furthermore, the Pasteurella multocida toxin is able to cause activation of Gqα but not the highly related G11α (Zywietz et al., 2001). Despite the vast range of small molecule agonists and antagonists of GPCRs used as both therapeutic medicines and tool compounds, there is a remarkable dearth of small molecule reagents that interact directly with G-protein subunits and are useful to dissect and differentiate functions of closely related G-proteins. Despite a range of efforts to produce G-protein subtype selective inhibitors by alteration of the structure of suramin, at this time only YM-254890, a cyclic depsipeptide isolated from the culture broth of Chromobacterium sp, appears to be an inhibitor of the exchange of GDP for GTP upon Gqα/G11α activation and a useful and reasonably selective cell-permeable agent (Takasaki et al., 2004).
Although the approach has not been used as avidly in recent times, the application of antisense technologies to knockdown levels of Gα-, β- and γ-subunits provided some of the most compelling early evidence for specificity of G-protein subunits in transducing the function of receptors to particular effector end points (Kleuss et al., 1992). However, despite the elegance of these experiments, both a resistance to the concept of β- and γ-subunit selectivity and a general unwillingness or inability to reproduce these studies held back acceptance. To date, application of siRNA-based approaches to reduce expression of G-protein subunits and hence explore their function has been limited (Barnes et al., 2005) but it is clearly an approach likely to be employed more widely. Mice, and cells and tissues derived from them, lacking the expression of particular G-protein subunits have, however, been of considerable use in understanding the role of these polypeptides in physiological processes (Wettschureck et al., 2004) and the development of tissue-specific and conditional knockouts of particular use in understanding the roles in postnatal life of G-proteins that are required for embryonic development.
As pertussis toxin modifies a conserved cysteine residue located in a key receptor interface of the Gi G-proteins, mutation to any other amino acid abolishes pertussis toxin sensitivity. However, the physicochemical nature of the amino acid used to replace this cysteine can determine the effectiveness of receptor interaction (Bahia et al., 1998) and such mutants were used to demonstrate how relative agonist-efficacy varies with the effectiveness of this interface (Jackson et al., 1999). Given the overall similarity of the pertussis-toxin-sensitive G-proteins and their routine coexpression, the ability to express pertussis toxin-resistant forms of these G-proteins individually in pertussis toxin-treated cells also provided a means to individually assess the activity of each isoform against a specific receptor or effector.
Perhaps the greatest impact in attempts to interfere with receptor-G-protein-mediated signalling has been based on minigene vectors encoding short peptides corresponding to the C-terminal regions of G-protein α-subunits that can selectively interfere with this process (Gilchrist et al., 2002), while conceptually similar ideas that employ membrane-targeted peptides corresponding to G-protein-interacting regions of the intracellular surfaces of receptors have also been employed in model systems to treat systemic inflammatory responses (Kaneider et al., 2005) and thrombosis (Covic et al., 2002).
Future perspectives
The function and structure of heterotrimeric G-proteins has been studied intensively for more than 20 years and the full panoply of genes encoding their subunits in man is now known. The importance of G-proteins in transducing signals from GPCRs will ensure they continue to be investigated actively to understand their selectivity in receptor recognition and a major advance in this respect will be the cocrystallisation of a receptor (or a receptor dimer) with a heterotrimeric G-protein. The application of FRET-based techniques is likely to increase in order to understand more fully the molecular movements associated with activation and to explore whether the α- and β/γ-subunits do indeed physically separate upon activation in native systems. The role of GPCR-interacting proteins other than those that regulate the efficiency of the GTPase cycle are also likely to be the subject of investigation as will recent suggestions that G-proteins may mediate a range of effects without receptor-mediated signal transduction. This will lead to a final understanding of the mode of action and regulation of this protein family.
Glossary
- cAMP
3′5′ cyclic adenosine monophosphate
- GEF
guanine nucleotide exchange factor
- G-protein
guanine nucleotide binding protein
- GTPγS
guanosine 5′-γ thiotriphosphate
- NAD+
nicotinamide adenine dinucleotide
- RGS
regulator of G-protein signalling
References
- BAHIA D.S., WISE A., FANELLI F., LEE M., REES S., MILLIGAN G. Hydrophobicity of residue351 of the G-protein Gi1 alpha determines the extent of activation by the alpha2A-adrenoceptor. Biochemistry. 1998;37:11555–11562. doi: 10.1021/bi980284o. [DOI] [PubMed] [Google Scholar]
- BARNES W.G., REITER E., VIOLIN J.D., REN X.-R., MILLIGAN G., LEFKOWITZ R.J. β-Arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- BOKOCH G.M., KATADA T., NORTHUP J.K., UI M., GILMAN A.G. Purification and properties of the inhibitory guanine nucleotide-binding regulatory component of adenylate cyclase. J. Biol. Chem. 1984;259:3560–3567. [PubMed] [Google Scholar]
- BRAY P., CARTER A., SIMONS C., GUO V., PUCKETT C., KAMHOLZ J., SPIEGEL A., NIRENBERG M. Human cDNA clones for four species of G alpha s signal transduction protein. Proc. Natl. Acad. Sci. U.S.A. 1986;83:8893–8897. doi: 10.1073/pnas.83.23.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUNEMANN M., FRANK M., LOHSE M.J. Gi protein activation in intact cells involves subunit rearrangement rather than dissociation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:16077–16082. doi: 10.1073/pnas.2536719100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABRERA-VERA T.M., VANHAUWE J., THOMAS T.O., MEDKOVA M., PREININGER A., MAZZONI M.R., HAMM H.E. Insights into G-protein structure, function, and regulation. Endocr. Rev. 2003;24:765–781. doi: 10.1210/er.2000-0026. [DOI] [PubMed] [Google Scholar]
- CAMPS M., CAROZZI A., SCHNABEL P., SCHEER A., PARKER P., GIERSCHIK P. Isozyme-selective stimulation of phospholipase C-beta 2 by G-protein beta gamma-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- CASSEL D., SELINGER Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim. Biophys. Acta. 1976;452:538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E., NEER E.J. New roles for G-protein beta gamma-dimers in transmembrane signalling. Nature. 1993;365:403–406. doi: 10.1038/365403a0. [DOI] [PubMed] [Google Scholar]
- COFFINO P., BOURNE H.R., TOMKINS G.M. Somatic genetic analysis of cyclic AMP action: selection of unresponsive mutants. J. Cell. Physiol. 1975;85:603–610. doi: 10.1002/jcp.1040850312. [DOI] [PubMed] [Google Scholar]
- CONKLIN B.R., FARFEL Z., LUSTIG K.D., JULIUS D., BOURNE H.R. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- COVIC L., MISRA M., BADAR J., SINGH C., KULIOPULOS A. Pepducin-based intervention of thrombin-receptor signaling and systemic platelet activation. Nat. Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- GILL D.M., MEREN R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILCHRIST A., LI A., HAMM H.E. G alpha COOH-terminal minigene vectors dissect heterotrimeric G-protein signalling. Sci. STKE. 2002;118:PL1. doi: 10.1126/stke.2002.118.pl1. [DOI] [PubMed] [Google Scholar]
- GILMAN A.G. G-proteins and regulation of adenylyl cyclase. Biosci. Rep. 1995;15:65–97. doi: 10.1007/BF01200143. [DOI] [PubMed] [Google Scholar]
- HUFF R.M., AXTON J.M., NEER E.J. Physical and immunological characterization of a guanine nucleotide-binding protein purified from bovine cerebral cortex. J. Biol. Chem. 1985;260:10864–10871. [PubMed] [Google Scholar]
- JACKSON V.N., BAHIA D.S., MILLIGAN G. Modulation of relative intrinsic activity of agonists at the alpha2A adrenoceptor by mutation of residue351 of G-protein Gi1alpha. Mol. Pharmacol. 1999;55:195–201. doi: 10.1124/mol.55.2.195. [DOI] [PubMed] [Google Scholar]
- KANEIDER N.C., AGARWAL A., LEGER A.J., KULIOPULOS A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat. Med. 2005;11:661–6655. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- KATADA T., UI M. Direct modification of the membrane adenylate cyclase system by islet-activating protein due to ADP-ribosylation of a membrane protein. Proc. Natl. Acad. Sci. U.S.A. 1982;79:3129–3133. doi: 10.1073/pnas.79.10.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEUSS C., SCHERUBL H., HESCHELER J., SCHULTZ G., WITTIG B. Different beta-subunits determine G-protein interaction with transmembrane receptors. Nature. 1992;358:424–426. doi: 10.1038/358424a0. [DOI] [PubMed] [Google Scholar]
- KOSKI G., KLEE W.A. Opiates inhibit adenylate cyclase by stimulating GTP hydrolysis. Proc. Natl. Acad. Sci. U.S.A. 1981;78:4185–4189. doi: 10.1073/pnas.78.7.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSTENIS E., WAELBROECK M., MILLIGAN G. Techniques: promiscuous Gα proteins in basic research and drug discovery. Trends Pharmacol. Sci. 2005;26:595–602. doi: 10.1016/j.tips.2005.09.007. [DOI] [PubMed] [Google Scholar]
- LANDIS C.A., MASTERS S.B., SPADA A., PACE A.M., BOURNE H.R., VALLAR L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- LODOWSKI D.T., PITCHER J.A., CAPEL W.D., LEFKOWITZ R.J., TESMER J.J. Keeping G-proteins at bay: a complex between G-protein-coupled receptor kinase 2 and Gbetagamma. Science. 2003;300:1256–1262. doi: 10.1126/science.1082348. [DOI] [PubMed] [Google Scholar]
- LOGOTHETIS D.E., KURACHI Y., GALPER J., NEER E.J., CLAPHAM D.E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- MCINTIRE W.E., MYUNG C.S., MACCLEERY G., WANG Q., GARRISON J.C. Reconstitution of G-protein-coupled receptors with recombinant G-protein alpha and beta gamma subunits. Methods Enzymol. 2002;343:372–393. doi: 10.1016/s0076-6879(02)43146-1. [DOI] [PubMed] [Google Scholar]
- MUMBY S.M., KLEUSS C., GILMAN A.G. Receptor regulation of G-protein palmitoylation. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOEL J.P., HAMM H.E., SIGLER P.B. The 2.2 A crystal structure of transducin-alpha complexed with GTP gamma S. Nature. 1993;366:654–663. doi: 10.1038/366654a0. [DOI] [PubMed] [Google Scholar]
- NORTHUP J.K., STERNWEIS P.C., SMIGEL M.D., SCHLEIFER L.S., ROSS E.M., GILMAN A.G. Purification of the regulatory component of adenylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 1980;77:6516–6520. doi: 10.1073/pnas.77.11.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTROM R.S., INSEL P.A. The evolving role of lipid rafts and caveolae in G-protein-coupled receptor signaling: implications for molecular pharmacology. Br. J. Pharmacol. 2004;143:235–245. doi: 10.1038/sj.bjp.0705930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINES M., GIERSCHIK P., MILLIGAN G., KLEE W., SPIEGEL A. Antibodies against the carboxyl-terminal 5-kDa peptide of the alpha subunit of transducin crossreact with the 40-kDa but not the 39-kDa guanine nucleotide binding protein from brain. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4095–4099. doi: 10.1073/pnas.82.12.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIOBO N.A., MANNING D.R. Receptors coupled to heterotrimeric G-proteins of the G12 family. Trends Pharmacol. Sci. 2005;26:146–154. doi: 10.1016/j.tips.2005.01.007. [DOI] [PubMed] [Google Scholar]
- RODBELL M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980;284:17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- RODBELL M. Nobel Lecture. Signal transduction: evolution of an idea. Biosci. Rep. 1995;15:117–133. doi: 10.1007/BF01207453. [DOI] [PubMed] [Google Scholar]
- SONDEK J., BOHM A., LAMBRIGHT D.G., HAMM H.E., SIGLER P.B. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- SPIEGEL A.M., WEINSTEIN L.S. Inherited diseases involving G-proteins and G-protein-coupled receptors. Annu. Rev. Med. 2004;55:27–39. doi: 10.1146/annurev.med.55.091902.103843. [DOI] [PubMed] [Google Scholar]
- STRATHMANN M., SIMON M.I. G-protein diversity: a distinct class of alpha subunits is present in vertebrates and invertebrates. Proc. Natl. Acad. Sci. U.S.A. 1990;87:9113–9117. doi: 10.1073/pnas.87.23.9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKASAKI J., SAITO T., TANIGUSHI M., KAWASAKI T., MORITANI Y., HAYASHI K., KOBORI M. A novel Galphaq/11-selective inhibitor. J. Biol. Chem. 2004;279:47438–47445. doi: 10.1074/jbc.M408846200. [DOI] [PubMed] [Google Scholar]
- TAYLOR S.J., SMITH J.A., EXTON J.H. Purification from bovine liver membranes of a guanine nucleotide-dependent activator of phosphoinositide-specific phospholipase C. Immunologic identification as a novel G-protein alpha subunit. J. Biol. Chem. 1990;265:17150–17156. [PubMed] [Google Scholar]
- WETTSCHURECK N., MOERS A., OFFERMANNS S. Mouse models to study G-protein-mediated signaling. Pharmacol. Ther. 2004;101:75–89. doi: 10.1016/j.pharmthera.2003.10.005. [DOI] [PubMed] [Google Scholar]
- WOLFE J.T., WANG H., HOWARD J., GARRISON J.C., BARRETT P.Q. T-type calcium channel regulation by specific G-protein betagamma subunits. Nature. 2003;424:209–213. doi: 10.1038/nature01772. [DOI] [PubMed] [Google Scholar]
- ZYWIETZ A., GOHLA A., SCHMELZ M., SCHULTZ G., OFFERMANS S. Pleiotropic effects of Pasteurella multocida toxin are mediated by Gq-dependent and -independent mechanisms. Involvement of Gq but not G11. J. Biol. Chem. 2001;276:3840–3845. doi: 10.1074/jbc.M007819200. [DOI] [PubMed] [Google Scholar]