Abstract
The synaptic actions of most neurotransmitters are inactivated by reuptake into the nerve terminals from which they are released, or by uptake into adjacent cells. A family of more than 20 transporter proteins is involved. In addition to the plasma membrane transporters, vesicular transporters in the membranes of neurotransmitter storage vesicles are responsible for maintaining vesicle stores and facilitating exocytotic neurotransmitter release. The cell membrane monoamine transporters are important targets for CNS drugs. The transporters for noradrenaline and serotonin are key targets for antidepressant drugs. Both noradrenaline-selective and serotonin-selective reuptake inhibitors are effective against major depression and a range of other psychiatric illnesses. As the newer drugs are safer in overdose than the first-generation tricyclic antidepressants, their use has greatly expanded. The dopamine transporter (DAT) is a key target for amphetamine and methylphenidate, used in the treatment of attention deficit hyperactivity disorder. Psychostimulant drugs of abuse (amphetamines and cocaine) also target DAT. The amino-acid neurotransmitters are inactivated by other families of neurotransmitter transporters, mainly located on astrocytes and other non-neural cells. Although there are many different transporters involved (four for GABA; two for glycine/D-serine; five for L-glutamate), pharmacology is less well developed in this area. So far, only one new amino-acid transporter-related drug has become available: the GABA uptake inhibitor tiagabine as a novel antiepileptic agent.
Keywords: Neurotransmitter transporters, noradrenaline, serotonin, GABA, psychostimulants, antidepressants
Discovery
Most neurotransmitters are inactivated by uptake of the released chemical into the nerve terminal from which it had been released or into adjacent cells – a process mediated by a family of transporter molecules. This concept is only some 40 years old. Prior to this, it was generally assumed that the inactivation of neurotransmitters after their release from nerves was likely to involve rapid enzymatic breakdown, as seen with acetylcholinesterase. The degradation of monoamines by the enzyme monoamine oxidase was known early on, and in the 1950s, a second enzyme catechol-O-methyl transferase (COMT) was discovered and was thought to play a key role in inactivating noradrenaline and other catecholamines.
When tritium-labelled radioactive catecholamines of high specific activity became available in the late 1950s, experiments could be performed for the first time using quantities of monoamine small enough to mimic the very low concentrations of adrenaline or noradrenaline normally encountered in body fluids. The first experiments performed in the Axelrod laboratory at the National Institutes of Health with [3H]-adrenaline and later with [3H]-noradrenaline yielded an unexpected result. Although in laboratory animals most of the injected dose of labelled catecholamine was rapidly metabolized (mainly by COMT), a substantial proportion of the injected monoamine (30–40%) was removed from the circulation by a rapid uptake into tissues, where it remained for some time unchanged. A key observation was that the uptake of [3H]-noradrenaline into the heart was virtually eliminated in animals in which the sympathetic innervation had been destroyed by surgical removal of the superior cervical ganglion (Hertting et al., 1961). This led Hertting & Axelrod (1961) to propose that the reuptake of noradrenaline by the same nerves from which it had been released might represent a novel mechanism for inactivating this neurotransmitter (Figure 1).
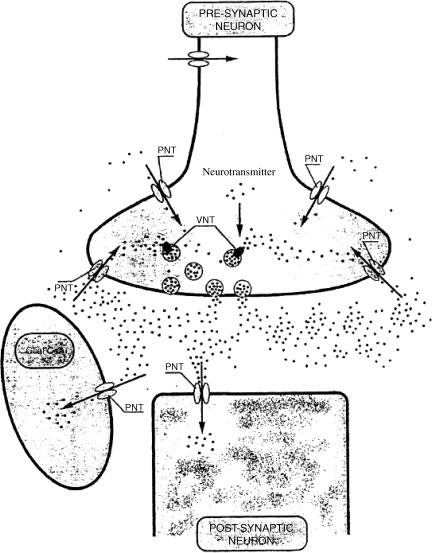
Figure 1.
The role of neurotransmitter transporters at the synapse. PNT=plasma membrane neurotransmitter transporter; VNT=vesicular neurotransmitter transporter. Redrawn from Masson et al. (1999).
The discovery of noradrenaline uptake was followed by the finding that similar but distinct transporters were involved in the inactivation of serotonin and dopamine, and that similar mechanisms existed for the inactivation of the amino-acid neurotransmitters GABA, glycine and L-glutamate (Iversen, 1971; Masson et al., 1999; Shigeri et al., 2004) (Figure 1). Research interest has focused on these mechanisms, including in recent years the identification and cloning of the genes encoding the transporter proteins involved and the development of knockout strains of genetically engineered mice lacking one or other of these gene products. The family of neurotransmitter transporters has turned out to be far more extensive than previously imagined, with more than 20 different members (Masson et al., 1999) (Table 1), and several have provided rich targets for CNS drug discovery.
Table 1. Neurotransmitter transporters.
| Substrate | Subtypes | Name |
|---|---|---|
| Noradrenalline | NET | |
| Dopamine | DAT | |
| Serotonin | SERT | |
| GABA | Four | GAT1–GAT4 |
| GABA/betaine | BGT-1 | |
| Glycine | Two | GLYT1 and GLYT2 |
| Taurine | RB16a | |
| Proline | PROT | |
| L-Glutamate | Five | EAAT1–EAAT5 |
| Vesicular monoamine | Two | VMAT-1–VMAT-2 |
| Vesicular acetylcholine | VAChT | |
| Vesicular GABA/glycine | VGAT | |
| Vesicular glutamate | Three | VGLUT1–VGLUT3 |
Monoamine transporters
The noradrenaline transporter (NET) was cloned by Pacholczyk et al. (1991) and this soon lead to the discovery of other related members of the monoamine transporter gene family. Separate transporters exist for serotonin (SERT) and dopamine (DAT) (Masson et al., 1999). The monoamine transporters are dependent on sodium and chloride ions for their function. They use the electrochemical gradient of sodium between the outside and inside surfaces of the cell membrane to provide the thermodynamic energy required to pump neurotransmitters from low concentrations outside the cell to the much higher concentrations inside the cell. Chloride ions accompany the entry of neurotransmitter and sodium, and there is a net movement of positively charged ions into the cell, although not in sufficient amounts to appreciably alter the resting membrane potential of the cell.
The vesicular neurotransmitter transporters represent another family whose function is to maintain the very high concentrations of monoamine and amino-acid neurotransmitters in storage vesicles. They use the proton gradient that exists across the vesicular membrane as the motive force. The vesicular monoamine transporters (VMAT) recognize serotonin, dopamine, noradrenaline, adrenaline and histamine. VMAT-1 is present chiefly in amine-containing endocrine and paracrine cells in peripheral organs, while VMAT-2 is the predominant form found in monaminergic neurons in the CNS. It is also expressed in the histamine-containing cells of the stomach, and in the adrenal medulla and in blood cells. The Na+/Cl+-dependent transporters and the vesicular transporters are membrane proteins consisting of a single polypeptide chain of 500–600 amino acid residues, with 12 α-helical membrane-spanning domain (Kavanaugh, 1998). The molecular mechanisms underlying the function of the neurotransmitter transporters remain unclear. Unlike flux through an open ion channel, there must be a gating cycle every time solute is transported, but the exact molecular details of this are not understood.
Drugs as inhibitors of monoamine transporters
By far the most important CNS drugs that target the noradrenaline and serotonin neurotransmitter transporters (NET and SERT, respectively) are the tricyclic antidepressants and their modern counterparts. The discovery that imipramine potently inhibited the uptake of noradrenaline in sympathetic nerves (Axelrod et al., 1961), and the finding that this also applied in the brain (Glowinski & Axelrod, 1964) led to the first understanding of the mechanism of action of the tricyclic antidepressants. Following the discovery of the serotonin uptake system in brain, it soon became apparent that the classical tricyclic drugs imipramine and amitriptyline were potent as inhibitors of both noradrenaline and serotonin uptake (Table 2). This reinforced the monoamine hypothesis of depression as a monoamine deficiency state, and stimulated much further research in the pharmaceutical industry to discover new inhibitors of monoamine uptake. The debate as to whether inhibition of noradrenaline or serotonin was the most important in conferring antidepressant efficacy has swung one way and the other over the past 40 years, and there is no definitive answer to this question. An early effort to improve the selectivity of antidepressants was made in the 1970s by scientists at the CIBA-GEIGY Company in Switzerland (now Novartis), who developed the selective noradrenaline uptake inhibitor maprotiline (Table 2) (Waldmeier, 1996). This proved to be clinically effective as an antidepressant, but it was not a great success commercially and had few clear advantages over the classical TCAs. This idea was also swept away by the wave of enthusiasm for serotonin-selective reuptake inhibitors (SSRIs) in the 1990s. The first compound of this type was zimeledine, launched by Astra in Europe in the 1980s, but it had to be withdrawn because of serious adverse side effects (Carlsson, 2001). The real success of SSRIs started with fluoxetine (‘Prozac®'), although this compound had languished on the shelf for many years before being developed as an antidepressant (Wong et al., 1995). It was followed by several other SSRIs, several of which met with considerable commercial success. Although the SSRIs were no more efficacious than the first-generation tricyclic antidepressants, and did not act any faster, they were considerably safer in overdose and could be used more safely. Table 2 summarizes the affinities of currently used antidepressants on cloned human monoamine transporters expressed in tissue culture cell lines (Tatsumi et al., 1997). The availability of the human transporter proteins for screening represents a considerable advance. Although there are many published accounts of the effects of antidepressants on monoamine transporter mechanisms, most of these employed animal tissues and there are few reported studies in which a large number of drugs were tested under the same experimental protocols.
Table 2. Antidepressants – inhibition of human serotonin (SERT), norepinephrine (NET) and dopamine (DAT) transporters.
| Generic name | Human SERT Kd (nM) | Human NET Kd (nM) | Human DAT Kd (nM) | Selectivity-SERT vs NET |
|---|---|---|---|---|
| Amitriptyline | 4.3 | 35 | 3250 | 8 |
| Amoxepine | 58 | 16 | 4310 | 0.3 |
| Bupropion | 9100 | 52,000 | 520 | 5.7 |
| Citalopram | 1.2 | 4070 | 28,100 | 3500 |
| Clomipramine | 0.3 | 38 | 2190 | 130 |
| Desipramine | 17.6 | 0.8 | 3190 | 0.05 |
| Dothiepin | 8.6 | 46 | 5310 | 5.3 |
| Doxepine | 68 | 29.5 | 12,100 | 0.4 |
| Fluoxetine | 0.8 | 240 | 3600 | 300 |
| Fluvoxamine | 2.2 | 1300 | 9200 | 580 |
| Imipramine | 1.4 | 37 | 8500 | 27 |
| Lofepramine | 70 | 5.4 | 18,000 | 0.08 |
| Maprotiline | 5800 | 11.1 | 1000 | 0.002 |
| Mirtazapine | >100,000 | 4600 | >100,000 | — |
| Nefazodone | 200 | 360 | 360 | 1.8 |
| Nortriptyline | 18 | 4.4 | 1140 | 0.24 |
| Paroxetine | .13 | 40 | 490 | 300 |
| Protriptyline | 19.6 | 1.4 | 2100 | 0.07 |
| Reboxetine* | 129 | 1.1 | — | 0.008 |
| Sertraline | 0.29 | 420 | 25 | 1400 |
| Trazodone | 160 | 8500 | 7400 | 53 |
| Trimipramine | 149 | 2450 | 780 | 16 |
| Venlafaxine | 8.9 | 1060 | 9300 | 120 |
Data from Tatsumi et al. (1997) and *Wong et al. (2000). The results are equilibrium dissociation constants (Kd) in nM, using [3H]-imipramine binding to human SERT, [3H]-nisoxetine binding to human noradrenaline transporter, and 3H-WIN35428 binding to human dopamine transporter (Tatsumi et al., 1997), or for reboxetine *(Wong et al., 2000) [3H]-citalopram binding to human SERT and [3H]-nisoxetine binding to the human NET. The most selective drugs are highlighted in bold in the right hand column.
Ironically, some of the most recently introduced antidepressants hark back to the less-selective compounds of the earlier era. Thus, duloxetine (Kirwin & Goren, 2004) and venlafaxine (Mendlewicz, 1995) are described as drugs that combine both noradrenaline and serotonin reuptake inhibition, although in vitro binding data show that venlafaxine binds with more than 100 times higher affinity to human SERT than to NET (Table 2). Reboxetine is the first antidepressant drug since maprotiline in a new class of NET-selective inhibitors (Hajos et al., 2004). Reboxetine is reported to be as effective as the SSRIs or older tricylics, but is not associated with sexual dysfunction, a common side effect of the SSRIs. It is claimed to be more effective than fluoxetine in improving the social adjustment of depressed patients.
The monoamine uptake inhibitors have proved very effective in the treatment not only of major depression but also for a series of other psychiatric illnesses. The SSRIs expanded the approved indications for the use of these drugs to include obsessive compulsive disorder, bulimia nervosa, panic disorder, post-traumatic stress syndrome, premenstrual tension and social anxiety syndrome (Iversen & Glennon, 2003). They have also proved hugely successful commercially, with worldwide sales in excess of $17 billion in 2003.
What are we to make of these twists and turns in the history of the development of monoamine uptake inhibitors as antidepressants? How can drugs that are selective noadrenaline reuptake inhibitors be equally effective as those that selectively target serotonin reuptake? In practice, it is difficult to know how selective the monoamine uptake inhibitors are in vivo. None of the antidepressants is completely selective for NET or SERT. The SSRIs have some affinity for NET and some (e.g. paroxetine) are quite potent inhibitors of NET. In some cases, the formation of active metabolites alters the drug selectivity profile. Thus, the nonselective compound imipramine and the partially NET-selective compound lofepramine are extensively metabolized to desipramine, a highly potent and selective NE reuptake inhibitor. Similarly, whereas amitriptyline has little selectivity for NET or SERT, the metabolite nortriptyline is a selective NET inhibitor. It seems likely that both NET-selective agents and SSRIs exert their effects through some common final pathway in the brain. Perhaps the SSRIs act indirectly to modulate noradrenergic function (Gorman & Sullivan, 2000; Svensson, 2000). Experimental data from animal experiments using microdialysis probes showed increased levels of extracellular norepinephrine in rat hippocampus after chronic treatment with paroxetine (Svensson, 2000). The original monoamine hypothesis of depression as formulated by Schildkraut (1965) stated:
Some, if not all, depressions are associated with an absolute or relative deficiency of catecholamines, particularly norepinephrine, at functionally important adrenergic receptor sites in the brain. Elation conversely may be associated with an excess of such amines.
European opinion currently seems to be swinging back in support of the view that an upregulation of noradrenergic function may be the key element underlying the efficacy of antidepressant drugs (Gorman & Sullivan, 2000; Svensson, 2000), but most American psychiatrists continue to emphasise the importance of serotonin.
The molecular mechanisms in the brain that are triggered by the antidepressants, however, remain obscure (Iversen & Glennon, 2003). The fact that all drugs require a period of several weeks before they become fully effective suggest that they modify gene expression in the brain and that the resulting altered biochemical state takes a long time to become stabilized. Many theories have been proposed, including alterations in the expression of alpha and beta-adrenergic receptors, changes in transcription factors and/or neurotrophic factors, and even morphological alterations in the connectivity of monoaminergic nerves and the promotion of new nerve cell formation (Iversen & Glennon, 2003).
Inhibitors of monoamine uptake have found other medical uses. The amphetamines act by promoting the release of dopamine in the brain by virtue of their high affinity for the dopamine transporter. They enter dopaminergic neurones and displace endogenous dopamine by a combination of a depletion of vesicular stores and counter transport of dopamine outwards via the transporter (Rothman & Baumann, 2003). Amphetamine itself and the related drug methylphenidate (‘Ritalin®') have found increasing use in the treatment of children with attention deficit hyperactivity disorder (ADHD). A noradrenaline-selective NET inhibitor, atomoxetine, has also been introduced recently for the treatment of ADHD. The older antidepressant, bupropion, acts as a weak inhibitor of noradrenaline and dopamine uptake, with little effect on serotonin uptake, but it and some of its metabolites may indirectly activate noradrenergic mechanisms. The compound had little success as an antidepressant, but has been approved in the U.S.A. and Europe as an aid to smoking cessation (Hurt et al., 1999).
Apart from their medical uses monoamine transporters are also important targets for drugs of abuse. The dopamine transporter (DAT) is the key site of action for the psychostimulant amphetamines and for cocaine. Mice that are genetically engineered to knock out the expression of the DAT gene are profoundly hyperactive and fail to show any further stimulation of activity in response to cocaine or D-amphetamine (Giros et al., 1996). Such animals, nevertheless, will continue to self-administer cocaine (Rocha et al., 1998), suggesting that the rewarding properties of the drug cannot be explained entirely by its ability to inhibit DAT. Cocaine is also a potent inhibitor of both serotonin and noradrenaline reuptake. A corollary of the understanding that cocaine owes important parts of its overall CNS profile to mechanisms other than inhibition of DAT is that more selective inhibitors of dopamine reuptake might be useful and free of dependence liability. One such compound, brasofensine, has been proposed for the treatment of Parkinson's disease (Graul & Castaner, 1999). Other selective DAT inhibitors may be used for the treatment of the withdrawal phase of CNS drug abuse.
A different monoamine transporter, known originally as Uptake2 (Iversen, 1965; 1971) is present in several peripheral tissues and in the brain. It is not dependent on Na+ or Cl−, has a low affinity for substrates and a high capacity. It is sensitive to inhibition by O-methylated catecholamine metabolites and by steroids (Iversen, 1971). Uptake2 has been cloned in animals, where it is termed ‘organic cation transporter 3′ and in man where it is named ‘extraneuronal monoamine transporter' (Martel & Azevedo, 2003). This uptake system may represent a second line of defence that inactivates monoamines, which have escaped neuronal uptake, and thus prevents uncontrolled spread of the signal. Schildkraut & Mooney (2004) have suggested that inhibitors of this transporter might represent faster acting antidepressants.
The neurotransmitter transporter family has provided many valuable targets for psychopharmacology. There is every prospect that this will continue. It might seem that the monoamine transporters had already been fully exploited, but the re-emergence of NET-specific antidepressants and the possible applications of selective inhibitors of DAT suggest that there may still be room for innovation even in such a crowded field.
Transporters for amino-acid neurotransmitters GABA
The existence of a high-affinity uptake of exogenous GABA by slices of rat cerebral cortex was demonstrated more than 30 years ago (Iversen & Neal, 1968). Iversen & Snyder (1968) subsequently showed that radiolabelled GABA was taken up into a distinct subpopulation of synaptosomes that could be separated physically from those accumulating catecholamines by density-gradient centrifugation, and Bloom & Iversen (1971) used electron microscopic autoradiography to show that exogenous GABA was taken up by a distinct subpopulation of nerve endings in slices of rat cerebral cortex.
It was immediately apparent that GABA uptake represented an interesting drug-discovery target, as the catecholamine and serotonin reuptake systems were already known to be key targets for antidepressants. However, this idea was not to see fruition for another 30 years. The medicinal chemistry of inhibitors of GABA uptake was pioneered by Krogsgaard Larsen et al. (1987), and tiagabine was developed by Lundbeck as the first GABA uptake inhibitor to be marketed as a novel antiepileptic (Schachter, 1999). It is interesting to see that tiagabine, launched initially for use in epilepsy, is now being investigated for other possible indications, in the treatment of psychosis, generalized anxiety, sleep in the elderly and drug addiction (Iversen, 2004).
There are at least three different GABA transporters, located in both neurons and glial cells (Masson et al., 1999). The one targeted by tiagabine (GAT1) is neuronal, while the others are on glia and other non-neural cells. There must be a rich scope for the future development of selective inhibitors of these other sites of GABA uptake – yielding new pharmacological tools of as yet unknown profiles or utility (Iversen, 2004).
Glycine/D-serine
Glycine plays a dual role in CNS neurotransmission. In the spinal cord and brainstem, it is released as an inhibitory neurotransmitter, but in all regions of the CNS, it also acts as a potent coagonist with L-glutamate at glutamate receptors of the NMDA subtype. Two specific transporters for glycine exist, GLYT1 and GLYT2, and in addition glycine is transported by the System A-family of ‘small neutral amino acid' (SNAT) transporters, which are expressed in both neurons and glial cells and transport a range of other amino acids (Schousboe et al., 2004). GLYT1 and SNAT predominate in the forebrain, and are thus likely to be associated with the role of glycine as a coagonist at NMDA receptors, whereas GLYT2 sites are colocalized with strychnine-sensitive glycinergic mechanisms in the spinal cord and hindbrain. D-Serine is also a potent coagonist with L-glutamate at NMDA receptors, and some reports have suggested that a specific transporter may also exist to regulate its function in CNS (Javitt et al., 2002).
Boosting the availability of glycine or D-serine at the NMDA receptor is of pharmacological interest mainly because of the ‘glutamate hypothesis' of schizophrenia, which states that the psychotic symptoms of the illness may be associated with glutamate underactivity, particularly at NMDA receptors (Javitt, 2004). Sarcosine is a naturally occurring inhibitor of GLYT1, and preliminary clinical trial results suggest that it may benefit schizophrenic patients when added to existing antipsychotic drug treatment (Tsai et al., 2004). Selective GLYT1 and GLY2 inhibitors are also under development (Bradaia et al., 2004).
L-Glutamate
L-Glutamate is the most widely used fast excitatory neurotransmitter in mammalian CNS. In view of its ubiquitous function, it is not surprising that many different categories of receptors and receptor subtypes are targets for the amino-acid, and that many different transporter mechanisms are involved in glutamatergic neurotransmission. There are five transporters in the excitatory amino acid family (Shigeri et al., 2004). They differ from the monoamine transporters in requiring both sodium and potassium ions for their function, but are not chloride-dependent. Like the monoamine transporters they are membrane proteins of 500–600 amino-acid residues with six to 10 membrane spanning domains. The transporters have an uneven distribution in CNS. EAAT1 and EAAT2 together account for the majority of the glutamate transport capacity in the brain, and they are almost exclusively expressed in astroglial cells. EAAT1 is preferentially expressed in the cerebellum, whereas EAAT2 is more prevalent in the forebrain. EAAT3 is expressed mainly in neurons, as are EAAT4 (in cerebellar Purkinje cells), while EAAT5 is found in non-neural retinal Müller cells (Schousboe et al., 2004).
Recent molecular biological analysis has revealed the unexpected finding that several of the genes associated with the function of L-glutamate as a neurotransmitter in the CNS are also expressed in a variety of peripheral tissues, in which it is thought that L-glutamate may play a role as an autocrine or paracrine signal molecule. These include bone, testis, pancreas and the adrenal, pituitary and pineal glands (Hinoi et al., 2004). Among the genes expressed in these tissues are some of the members of the glutamate transporter family, especially EAAT1 (Hinoi et al., 2004).
After release at excitatory synapses, the actions of L-glutamate are terminated by the concerted action of receptor desensitization and removal by the extremely active astroglial glutamate transporters. An efficient and rapid removal of L-glutamate from the synapse is essential not only to prevent the spread of excitation but also to limit the potential excitotoxic effects that excess L-glutamate can have when glutamate receptors of the NMDA subtype are overactivated (Schousboe et al., 2004). Some forms of neurodegenerative disease may be associated with a dysfunction of astroglial glutamate transport (Gegelashvili et al., 2001).
Three subtypes of vesicular glutamate transporters (VGLUT1–3) have been identified. They are membrane proteins of around 600 amino-acid residues, with eight to 10 transmembrane domains; like the monoamine vesicular transporters, they rely on a proton gradient to capture and retain L-glutamate (Shigeri et al., 2004). The presence of the vesicular transporters is essential for the rapid exocytotic release of glutamate from nerve terminals. The vesicular transporters are differentially expressed in different brain regions, thus helping to define subsets of excitatory neurons. VGLUT1 and VGLUT2 are expressed only in excitatory neurons, whereas VGLUT3 is also expressed neurons releasing other neurotransmitters – suggesting a different role of glutamate in such cells (Fremeau et al., 2004).
So far, there has been little pharmacological development around the glutamate transporters. Since understanding of the relative roles played by the various transporters is still incomplete, it is difficult to predict which might form attractive targets for drug discovery (Javitt, 2004). There is also the potential hazard that blocking the removal of L-glutamate from the synaptic space could encourage seizure activity or facilitate excitotoxic damage. Nevertheless, some progress has been made in exploring various medicinal chemical approaches to the design of both cell membrane and vesicular glutamate transporters (Shigeri et al., 2004). There has been some progress in identifying subtype selective inhibitors. The compound threo-3-methylglutamic acid was reported to inhibit the EAAT2 but not the EAAT1 transporter in vitro (Eliasof et al., 2001), and the compound WAY-855 was also reported to be a selective inhibitor of EAAT2 (Dunlop et al., 2003). Selective nonsubstrate inhibitors capable of penetrating the blood–brain barrier are needed, however, to determine the pharmacological potential of inhibitors of the excitatory amino-acid transports family.
Glossary
- GABA
gamma aminobutyric acid
References
- AXELROD J., WHITBY L., HERTTING G. Effect of psychotropic drugs on the uptake of 3H-norepinephrine by tissues. Science. 1961;133:383. doi: 10.1126/science.133.3450.383. [DOI] [PubMed] [Google Scholar]
- BLOOM F.E., IVERSEN L.L. Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography. Nature. 1971;229:628–630. doi: 10.1038/229628a0. [DOI] [PubMed] [Google Scholar]
- BRADAIA A., SCHLICHTER R., TROUSLARD J. Role of glial and neuronal glycine transporters in the control of glycinergic and glutamatergic synaptic transmission in lamina X of the rat spinal cord. J. Physiol. 2004;559:169–186. doi: 10.1113/jphysiol.2004.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A. A paradigm shift in brain research. Science. 2001;294:1021–1024. doi: 10.1126/science.1066969. [DOI] [PubMed] [Google Scholar]
- DUNLOP J., ELIASOF S., STACK G., MCILVAIN H.B., GREENFIELD A., KOWAL D., PETROSKI R., CARRICK T. WAY-855 (3-amino-tricyclo[2.2.1.02.6]heptane-1,3-dicarboxylic acid): a novel, EAAT2-preferring, nonsubstrate inhibitor of high-affinity glutamate uptake. Br. J. Pharmacol. 2003;140:839–846. doi: 10.1038/sj.bjp.0705509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELIASOF S., MCILVAIN H.B., PETROSKI R.E., FOSTER A.C., DUNLOP J. Pharmacological characterization of threo-3-methylglutamic acid with excitatory amino acid transporters in native and recombinant systems. J. Neurochem. 2001;77:550–557. doi: 10.1046/j.1471-4159.2001.00253.x. [DOI] [PubMed] [Google Scholar]
- FREMEAU R.T., JR, VOGLMAIER S., SEAL R.P., EDWARDS R.H. VGLUT's define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- GEGELASHVILI G., ROBINSON M.B., TROTTI D., RAUEN T. regulation of glutamate transporters in health and disease. Prog. Brain Res. 2001;132:267–286. doi: 10.1016/S0079-6123(01)32082-4. [DOI] [PubMed] [Google Scholar]
- GIROS B., JABER M., JONES S.R., WIGHTMAN R.M., CARON M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- GLOWINSKI J., AXELROD J. Inhibition of uptake of tritiated-noradrenaline in the intact rat brain by imipramine and structurally related compounds. Nature. 1964;204:1318–1319. doi: 10.1038/2041318a0. [DOI] [PubMed] [Google Scholar]
- GORMAN J.M., SULLIVAN G. Noradrenergic approaches to antidepressant therapy. J. Clin. Psychiatry. 2000;61 (Suppl 1):13–16. [PubMed] [Google Scholar]
- GRAUL A., CASTANER J. Brasofensine sulfate; antiparkinsonian dopamine reuptake inhibitor. Drugs Future. 1999;24:128–132. [Google Scholar]
- HAJOS M., FLEISHAKER J.C., FILIPIAK-REISNER J.K., BROWN M.T., WONG E.H. The selective norepinephrine reuptake inhibitor antidepressant reboxetine: pharmacological and clinical profile. CNS Drug Rev. 2004;10:23–44. doi: 10.1111/j.1527-3458.2004.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERTTING G., AXELROD J. Fate of tritiated noradrenaline at the sympathetic nerve endings. Nature. 1961;192:172–173. doi: 10.1038/192172a0. [DOI] [PubMed] [Google Scholar]
- HERTTING G., AXELROD J., KOPIN I.J., WHITBY L. Lack of uptake of catecholamines after chronic denervation of sympathetic nerves. Nature. 1961;189:66. doi: 10.1038/189066a0. [DOI] [PubMed] [Google Scholar]
- HINOI E., TAKARADA T., UESHIMA T., TSUCHIHASHI Y., YONEDA Y. Glutamate signaling in peripheral tissues. Eur. J. Biochem. 2004;271:1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- HURT J.R., GOLDSTEIN M.G., HURT R.D., SHIFFMAN S. Recent advances in the pharmacotherapy of smoking. JAMA. 1999;281:72–76. doi: 10.1001/jama.281.1.72. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L. The uptake of catecholamines at high perfusion concentrations in the isolated rat heart: a novel catecholamine uptake process. Br. J. Pharmacol. 1965;25:18–33. doi: 10.1111/j.1476-5381.1965.tb01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVERSEN L.L. Role of transmitter uptake mechanisms in synaptic neurotransmission. Br. J. Pharmacol. 1971;41:571–591. doi: 10.1111/j.1476-5381.1971.tb07066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVERSEN L.L. GABA pharmacology – what prospects for the future. Biochem. Pharmacol. 2004;68:1537–1540. doi: 10.1016/j.bcp.2004.06.039. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L., GLENNON R.A.2003Antidepressants Burger's Medicinal ChemistryVol. 6: Nervous System Agents, ed. Abraham, D.J. Chapter 8, pp. 483–524.Wiley: New York [Google Scholar]
- IVERSEN L.L., NEAL M.J. The uptake of 3H-GABA by slices of rat cerebral cortex. J. Neurochem. 1968;15:1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L., SNYDER S.H. Synaptosomes: different populations storing catecholamines and gamma-aminobutyric acid in homogenates of rat brain. Nature. 1968;220:795–798. doi: 10.1038/220796a0. [DOI] [PubMed] [Google Scholar]
- JAVITT D.C. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry. 2004;9:984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- JAVITT D.C., BALLA A., SERSHEN H. A novel alanine-insensitive D-serine transporter in rat brain synaptosomal membranes. Brain Res. 2002;941:146–149. doi: 10.1016/s0006-8993(02)02557-x. [DOI] [PubMed] [Google Scholar]
- KAVANAUGH M.P. Neurotransmitter transport: models in flux. Proc. Natl. Acad. Sci. U.S.A. 1998;95:12737–12738. doi: 10.1073/pnas.95.22.12737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRWIN J.L., GOREN J.L. Duloxetine: a dual serotonin-norepinephrine reuptake inhibitor for tr for the treatment of major depressive disorder. Pharmacotherapy. 2004;25:396–401. doi: 10.1592/phco.25.3.396.61600. [DOI] [PubMed] [Google Scholar]
- KROGSGAARD LARSEN P., FALCH E., LARRSON O.M., SCHOUSBOE A. GABA uptake inhibitors: relevance to a relevance to antiepileptic drug research. Epilepsy Res. 1987;1:77–93. doi: 10.1016/0920-1211(87)90012-x. [DOI] [PubMed] [Google Scholar]
- MASSON J., SAGNÉ C., HAMON M., EL MESTIKAWY S. Neurotransmitter transporters in the central nervous system. Pharmacol. Rev. 1999;51:439–464. [PubMed] [Google Scholar]
- MARTEL F., AZEVEDO I. An update on the extraneuronal monoamine transporter (EMT): characteristics, distribution and regulation. Curr. Drug Metab. 2003;4:313–318. doi: 10.2174/1389200033489433. [DOI] [PubMed] [Google Scholar]
- MENDLEWICZ J. Pharmacologic profile and efficacy of venlafaxine. Int. Clin. Psychopharmacol. 1995;10 (Suppl 2):5–13. doi: 10.1097/00004850-199503002-00003. [DOI] [PubMed] [Google Scholar]
- PACHOLCZYK T., BLAKELEY R.D., AMARA S.G. Expression cloning of an Antidepressant antidepressant-sensitive noradrenaline transporter. Nature. 1991;350:350–353. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- ROCHA B.A., FUMAGALLI F., GAINETDINOV R.R., JONES S.R., ATOR R., GIROS B., MILLER G.W., CARON M.G. Cocaine self-administration in dopamine transporter knockout mice. Nature Neurosci. 1998;1:132–137. doi: 10.1038/381. [DOI] [PubMed] [Google Scholar]
- ROTHMAN R.B., BAUMANN M.H. Monoamine transporters and psychostimulant drugs. Eur. J. Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- SCHACHTER S.C. Tiagabine. Epilepsia. 1999;40 (Suppl 5):S17–S22. doi: 10.1111/j.1528-1157.1999.tb00915.x. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT J.J. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT J.J., MOONEY J.J. Toward a rapidly acting antidepressant: the normetanephrine and extraneuronal monoamine transporter (uptake 2) hypothesis. Am. J. Psychiatry. 2004;161:909–911. doi: 10.1176/appi.ajp.161.5.909. [DOI] [PubMed] [Google Scholar]
- SCHOUSBOE A., SARUP A., BAK L.K., WAAGEPETERSEN H.S., LARSSON O.M. Role of astrocytic transport processes in glutamatergic and GABAergic neurotransmission. Neurochem. Int. 2004;45:521–527. doi: 10.1016/j.neuint.2003.11.001. [DOI] [PubMed] [Google Scholar]
- SHIGERI Y., SEAL R.P., SHIMAMOTO K. Molecular pharmacology of glutamate transporters, EAATs and VGLUTs. Brain Res. Rev. 2004;45:250–265. doi: 10.1016/j.brainresrev.2004.04.004. [DOI] [PubMed] [Google Scholar]
- SVENSSON T.H. Brain noradrenaline and the mechanisms of action of antidepressant drugs. Acta Psychiatr. Scand. 2000;101 (Suppl 402):18–27. doi: 10.1034/j.1600-0447.2000.02604.x. [DOI] [PubMed] [Google Scholar]
- TATSUMI M., GROSHAN K., BLAKELEY R.D., RICHELSON E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur. J. Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- TSAI G., LANE H.Y., YANG P., CHONG M.Y., LANGE N. Glycine transporter I inhibitor, N-methylglycine (sarcosine), added to antipsychotics for the treatment of schizophrenia. Biol. Psychiatry. 2004;55:452–456. doi: 10.1016/j.biopsych.2003.09.012. [DOI] [PubMed] [Google Scholar]
- WALDMEIER P.1996From mental illness to neurodegeneration The Psychopharmacologistsed D. Healy, pp. 565–586.London: Chapman & Hall [Google Scholar]
- WONG D.T., BYMASTER F.P., ENGELMAN E.A. Prozac (fluoxetine, Lilly 110140), the first serotonin uptake inhibitor and an antidepressant drug. Twenty years since its first publication. Life Sci. 1995;57:411–441. doi: 10.1016/0024-3205(95)00209-o. [DOI] [PubMed] [Google Scholar]
- WONG E.H.F., SONDERS M.S., AMARA S.G., TINHOLT P.M., PIERCEY M.F.P., HOFFMANN W.P., HYSLOP D.K., FRANKLIN S., PORSOLT R.D., BONSIGNORI A., CARFAGNA N., MCARTHUR R.A. Reboxetine: a pharmacologically potent, selective, and specific norepinephrine reuptake inhibitor. Biol. Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]