Abstract
γ-Aminobutyric acid (GABA) emerged as a potentially important brain chemical just over 50 years ago, but its significance as a neurotransmitter was not fully realized until over 16 years later. We now know that at least 40% of inhibitory synaptic processing in the mammalian brain uses GABA. Establishing its role as a transmitter was a lengthy process and it seems hard to believe with our current knowledge that there was ever any dispute about its role in the mammalian brain. The detailed information that we now have about the receptors for GABA together with the wealth of agents which facilitate or reduce GABA receptor mechanisms make the prospects for further research very exciting. The emergence of glycine as a transmitter seems relatively painless by comparison to GABA. Perhaps this is appropriate for the simplest of transmitter structures! Its discovery within the spinal cord and brainstem approximately 40 years ago was followed only 2 years later by the proposal that it be conferred with ‘neurotransmitter' status. It was another 16 years before the receptor was biochemically isolated. Now it is readily accepted as a vital spinal and supraspinal inhibitory transmitter and we know many details regarding its molecular structure and trafficking around neurones. The pharmacology of these receptors has lagged behind that of GABA. There is not the rich variety of allosteric modulators that we have come to readily associate with GABA receptors and which has provided us with a virtual treasure trove of important drugs used in anxiety, insomnia, epilepsy, anaesthesia, and spasticity, all stemming from the actions of the simple neutral amino acid GABA. Nevertheless, the realization that glycine receptors are involved in motor reflexes and nociceptive pathways together with the more recent advent of drugs that exhibit some subtype selectivity make the goal of designing selective therapeutic ligands for the glycine receptor that much closer.
Keywords: GABA, glycine, GABA receptors, glycine receptors, receptor structure
A brief history of GABA
While γ-aminobutyric acid (GABA) is now established as the most important inhibitory neurotransmitter in the mammalian CNS, its full acceptance in this role occurred only relatively recently (end of 1960s/early 1970s). Although it had been shown to be in biological tissues as early as 1910, its presence in the CNS was not described until some 40 years later. Only after 1950, when the free amino acid was positively identified in mammalian brain, did interest in its potential neurochemical significance arise. Nevertheless, during the ensuing decade, relatively few studies were reported which attempted to define the effects of GABA and related compounds on neuronal activity within the brain. Instead, more attention was drawn to the concurrent findings of Kuffler (1954), and Florey (1954) who described the presence of excitatory and inhibitory control mechanisms in crustacea. With little or no evidence, these authors suggested that crustacean preparations could provide ideal assay systems for detecting inhibitory and excitatory substances present in the mammalian brain. Hence, the crayfish stretch receptor preparation became an important assay in the search for mammalian neuroactive agents. Factor I (where ‘I' represented inhibitory action on neuronal activity) was extracted from mammalian brain by Florey & McLennan (1959) and shown to contain GABA which the authors suggested might well be the natural neurotransmitter. Of course, the establishment of any substance as a neurotransmitter requires the fulfillment of certain criteria. While these could be largely met in crustacea, where, for example, mimicry of the characteristics of the endogenous inhibitor at the lobster neuromuscular junction was achieved (Kravitz et al., 1963; Otsuka et al., 1966), the position in the mammalian CNS was largely negative until the late 1960s. Prior to this, many studies failed to obtain the required data and in some cases were even contrary to the expectations of an inhibitory action on neurones (Hayashi, 1958). So much so, that by 1964 a considerable amount of evidence against a CNS transmitter role had been accrued. Elliott & Van Gelder (1958) had concluded that GABA could not be a transmitter as there was no process for its rapid inactivation. However, they did note that brain tissue accumulated the amino acid, but said that this was inadequate for transmitter inactivation. We now know, of course, that rapid intracellular uptake does play a major part in the inactivation process (Iversen & Neal, 1968). Curtis (1959) and colleagues argued that the action of GABA in the CNS was probably of a general depressant nature rather than that of a neurotransmitter as the characteristics of its action were not consistent with a transmitter role. In addition, these authors noted that strychnine was unable to block the effects of GABA in the spinal cord, whereas it did block postsynaptic inhibition (Curtis, 1959). This was resolved when studies by Krnjevic & Schwartz (1967) on cerebral cortical neurones provided unequivocal evidence for GABA as an inhibitory transmitter (Figure 1). This was further supported by data obtained with the natural alkaloid, bicuculline, which showed that it not only blocked the action of GABA but also postsynaptic inhibition in the cerebral cortex. The apparent discrepancy in the spinal cord was later resolved in 1968 by Werman and colleagues (see later), who showed that glycine is the postsynaptic transmitter and its action could be blocked by strychnine (Curtis et al., 1968). After these major observations, there followed an exponential growth in research activities on GABA in an attempt to define all the necessary criteria and its overall role in the physiology of the brain. Its localization and synthetic pathway were defined and there were attempts to define its synaptic release and inactivation process(es). Information on pathway specific release was difficult to define biochemically, but GABAergic projections were identified and confirmed using electrophysiological techniques.
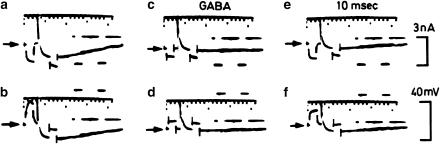
Figure 1.
Action of GABA on membrane potential and resistance of neocortical neurones. In each trace, two downward pulses were evoked by equal 20 ms pulses of current (hyperpolarizing in a, c and e and depolarizing in b, d and f) applied before and near the peak IPSP elicited by stimulating the cortical surface. Note the sharp drop in resistance during the IPSP (smaller responses in a and b); GABA produced a similar drop in resistance (smaller initial responses in c and d) as well as a hyperpolarization, indicated by the downward shift in the resting potential (arrows). Traces (e) and (f) show the recovery from the effects of GABA (taken from Dreifuss et al. (1969). Exp Brain Res 9: 137–154, with permission).
In the subsequent years of the 70s and 80s, considerable attention was given to defining the nature of the receptor through which GABA acts. This culminated with the emergence of the structure of the ionotropic receptor at the end of the 80s (Olsen & Tobin, 1990). However, many years before any molecular details were obtained it became apparent that the ionotropic receptor for GABA, which gates neuronal Cl− channels, comprises a protein complex on which a variety of compounds lacking any affinity for the GABA recognition site could act. Substances such as general anaesthetics and, later, neurosteroids were shown to potentiate the effect of GABA. But the action of the most important of these modulators, the benzodiazepines, was first described by Haefely et al. (1975). These important therapeutic agents act allosterically to increase the opening frequency of the GABA channel and in so doing provide a mechanism for inducing anxiolytic and sedative effects. Further studies led to the development of inverse agonists at the benzodiazepine binding site and this provided drugs with anxiogenic and convulsant properties.
Around this time in the mid-70s, Roberts and colleagues (Barber et al., 1978) were able to describe the distribution of the forming enzyme for GABA (GAD) in the mammalian spinal cord. They were able to show a high and discrete localization of this enzyme which acts as a marker for GABA, in terminals of interneurones which impinge on primary afferent terminals. The GABA released from these interneurones provides the basis for ‘presynaptic inhibition' within the spinal cord (see Barber et al., 1978). The concept was and still is that GABA released on to the primary afferent fibres produces a depolarization (rather than a hyperpolarization) of the terminals to decrease the evoked release of transmitter from the primary fibres due to shunting of the current in the nerve terminal. The depolarizing action would still be mediated via an increase in Cl− conductance if the reversal potential for Cl− in primary afferent neurones is more positive than the resting membrane potential. An increase in Cl− flow would depolarize the neuronal membrane with a decrease in transmitter release from the primary afferent terminal.
These definitive studies prompted us to try to develop a peripheral model for the presynaptic action of GABA in the spinal cord. At that time we had been focussing our studies on the action of GABA on peripheral sympathetic neurones and had shown that it depolarizes the cell bodies of these neurones. We argued that if this effect extended to the cell terminals which innervate peripheral tissues then a model for primary afferent inhibition could be developed (Figure 2). As it was not possible to measure the membrane potential of nerve terminals invading a peripheral organ, it was necessary to monitor the actual release of the sympathetic transmitter, noradrenaline, and we chose to do this in the isolated atrium of the rat heart. Much work had been performed by Iversen (1967) and colleagues during the 60s and 70s on the uptake and release of tritiated noradrenaline by and from rat isolated atria. Transmural stimulation of superfused atria which had previously been incubated in radiolabelled noradrenaline evoked a discrete release of the amine. As predicted when GABA (10 μM) was added to the superfusion solution, a reduction in the evoked release of labelled noradrenaline occurred. This was only really evident if the α2 adrenoceptor antagonist, yohimbine, was also present to increase the overflow of noradrenaline. Obtaining this effect with GABA was the goal we had hoped to achieve but, rather than the end, this turned out to be just the start of studies in this area.
Figure 2.
Diagrammatic representation of the rationale for determining whether the depolarizing action of GABA on sympathetic ganglion neurones would extend to the sympathetic terminals to provide a model for central presynaptic inhibition. While a Cl−-dependent action might exist on the terminals, what was detected by examining the influence of GABA on transmitter outflow was an action that was independent of Cl− and insensitive to recognized GABA antagonists.
In attempting to characterize the response to GABA, it soon became apparent that the effect was not Cl− dependent and was not mimicked by many accepted GABA receptor agonists. Moreover, the established GABA antagonists, such as bicuculline, failed to prevent the action of GABA. It was then that we suspected a novel receptor might be mediating the observed effect of GABA. Further support came with the subsequent observation that β-chlorophenyl GABA (baclofen) was a stereospecific agonist at the receptor. This compound had been launched some 8 years earlier as a GABA-related drug for the treatment of spasticity. However, it was never demonstrated to be a GABA mimetic despite numerous attempts to do so in recognized GABA receptor systems. By contrast, the action of baclofen was identical to that of GABA in the isolated atrium. So while we had failed to model the depolarizing action of GABA in the spinal cord, we appeared to have uncovered a novel action of GABA which was not associated with an increase in Cl− conductance.
It soon became apparent that this novel receptor was also present in other peripheral tissues, located primarily on autonomic nerve terminals, where its activation modulated the tissue response to nerve stimulation. But, of course, the role of GABA outside the brain is probably of limited significance and so it was essential that the presence and function of the receptor could be demonstrated within the CNS. We were able to demonstrate, in a manner similar to peripheral tissue, that GABA and baclofen could inhibit the K+-evoked release of neurotransmitter from brain slices in a bicuculline-insensitive manner (Bowery et al., 1980). The subsequent development of a binding assay for the native receptor using 3H-GABA or 3H-baclofen eventually provided unequivocal evidence for the presence of the receptor in the mammalian brain. It was at this point in 1981 that we decided to designate the term ‘GABAB' to describe this novel receptor, while referring to the classical ionotropic receptor as ‘GABAA' (Hill & Bowery, 1981; Bowery et al., 1983).
During the ensuing years, the significance of GABAB receptors in brain physiology became apparent, particularly when Roger Nicoll and colleagues obtained substantial evidence for a role in synaptic transmission within the rat hippocampus (Dutar & Nicoll, 1988). Characteristically, the activation of GABAB sites mediates slow hyperpolarization in contrast to fast GABAA-mediated hyperpolarization. While the latter is mediated by an increase in Cl− flux, the former is due to an increase in K+ conductance across the neuronal membrane. But GABAB receptors are coupled not only via second messengers to K+ channels but also to Ca2+ channels, predominantly of the N class, where receptor activation decreases ion conductance. This latter mechanism appears to predominate on axon terminals of neurones where a reduction in Ca2+ conductance, mediated by GABAB receptor activation, produces a decrease in transmitter release. This effect occurs in many regions of the mammalian brain although axo-axonic synaptic contacts have only been fully identified at primary afferent terminals in the spinal cord. Within the cord, small-diameter afferent fibre terminals appear to have a predominance of GABAB over GABAA receptors and the former may well contribute to the control of transmitter output from sensory fibres associated with nociception. While the phenomenon of primary afferent depolarization mediated via GABAA receptors is important in controlling transmitter release, it may be of greater importance on large-rather than small-diameter fibres.
GABA receptor structure
GABAA
Identification of the structure of GABAA receptors was achieved towards the end of the 1980s when it became clear that the receptor is a member of a superfamily to which nicotinic acetylcholine, glycine and 5HT3 receptors also belong. Their structures exhibit homology in the regions where ligand binding occurs and, of course, all of these receptors are ionotropic and associated with fast conductance events. The GABAA receptor complex has a pentameric structure comprising a variety of possible combinations of protein subunits. The receptor complex includes not only the binding site for GABA, which is accepted to be at the interface between the α and β subunits, but also binding sites for substances which modulate the actions of GABA. Notable among these is the benzodiazepine-binding site, which appears to be located at the interface of the α and γ subunits of the receptor complex. Thus, there exist certain forms of the GABAA receptor which lack the γ subunit or only specific forms of the α subunit which do not bind the benzodiazepines. However, most forms of the receptor complex have affinity for the benzodiazepines, which enables them to allosterically modulate the function of the GABAA receptor to modify the overall response to the transmitter. This functional potentiation provides the basis for the therapeutic action of this important group of anxiolytics and sedatives.
The benzodiazepines are not the only group of compounds that modulate GABA function; gaseous and intravenous anaesthetics as well as neurosteroids can all positively modulate the response to GABA by an action on the receptor complex at a site(s) distinct from either the GABA- or benzodiazepine-binding sites (see also Franks, this issue). Although the definitive structure of the GABAA receptor is unavailable, homology modelling based on the acetylcholine-binding protein has enabled us and others to provide insight into the location of allosteric binding sites. This is evidenced by the determination of the number of discrete binding sites for Zn2+ on GABAA receptors, which is quite a potent subtype-selective inhibitor of GABAA receptor function, particularly for receptors lacking the γ subunit or those incorporating the δ subunit (Hosie et al., 2003).
GABAB
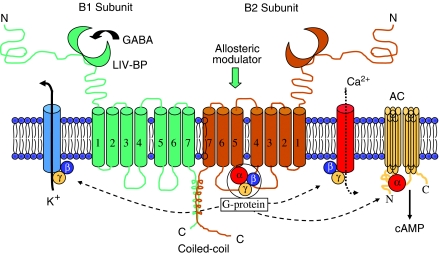
More than 10 years after the emergence of the structure of GABAA receptors, the composition of the GABAB receptor ‘slowly' began to be resolved. Why ‘slowly'? Because, the first structure reported by Kaupmann et al. (1997) was incomplete and although this group had made a major breakthrough there was still something missing. They determined that the receptor exists as a 7-transmembrane (7TM) spanning monomer with a long extracellular N-terminal sequence. In the light of previous knowledge obtained for other 7TM receptors, the possibility of a monomeric structure being responsible was not unexpected. Even a homodimeric structure such as exists for metabotropic glutamate receptors would not have been unreasonable. However, it soon became apparent that the structure reported by Kaupmann et al. (1997) when expressed in a cell line was incomplete. It did not function as predicted; in particular, the relative binding affinities of a series of receptor agonists were much weaker than observed at native receptors and the functional response to receptor activation was also weak. Within a year, the problem was resolved when it emerged from studies performed by three independent groups and published simultaneously in the same issue of Nature (1998), that the GABAB receptor exists as a heterodimer with two dissimilar 7TM subunits (GABAB1 and GABAB2) comprising the functional receptor (Figure 3). The receptor proteins GABAB1 and GABAB2 are produced independently within neurones and each acts as a chaperone to the other to transport it to the plasma membrane. Early studies suggested that GABAB2 might exist in a functional form within the plasma membrane, but evidence is lacking and it seems that the two receptor proteins couple via their C-terminal domains to be transported from the cellular endoplasmic reticulum as a heterodimer for insertion into the plasma membrane. GABAB1 provides the GABA-binding domain within its extracellular chain, while GABAB2 provides the G-protein coupling. In addition, data from more recent studies indicate that within the heptahelical domain of the second subunit, and not in the subunit containing the agonist-binding site, there is an allosteric site which can modulate the response to the agonist. The potential importance of allosteric modulation of GABAB receptors has yet to be fully realized, but it may well have therapeutic implications. Different isoforms of GABAB1 (1a–1f) have been reported although no supporting evidence to couple individual isoforms to functional receptor subtypes has been obtained. Moreover, only 1a, 1b and 1c appear to act as functional subunits. No substantial evidence exists for different functional forms of the GABAB2 subunit. Currently, there is also little or no firm pharmacological evidence for functional GABAB receptor subtypes although it seems hard to believe that only one receptor subtype exists as this would be unique among 7TM receptors. But then the heterodimeric structure of the native receptor may be unique.
Figure 3.
Heterodimeric structure of the GABAB receptor. The two 7TM receptor subunits (GABAB1 and GABAB2) are coupled via their intracellular C-termini. The binding domain for GABA is located in the extracellular domain of GABAB1. The heptahelical domain of GABAB2 contains an allosteric modulator site as well as the G-protein-coupling site. Neither of these appear to be present in the GABAB1 subunit. The receptor is coupled indirectly to K+ and Ca2+ channels, the former of which predominates postsynaptically while the latter is mainly presynaptic in origin. Note that the ligand binding domain is believed to be similar to the leucine/isoleucine/valine-binding protein (LIV-BP), one of the bacterial periplasmic binding proteins (modified from The GABA receptors, in Encyclopedia of Neuroscience, 3rd edn. Amsterdam: Elsevier, with permission).
Therapeutic significance of GABA receptor ligands
The contribution of GABAA receptors to clinical medicine has long been established and even commenced well before the role of GABA as a transmitter had been suggested. The barbiturates were first used clinically in the early part of the 19th century as anxiolytic hypnotics and anticonvulsants, and then later as intravenous anaesthetics. The association of their action with GABA receptors became clear during the 1970s when evidence for an action on the ionotropic GABA receptor complex was obtained. The barbiturates appear to produce their effects by potentiation of the response to GABA, and this is mediated by an increase in the Cl− channel mean open time, enabling more current to flow. The 1960s saw the introduction of the benzodiazepines which largely replaced the barbiturates as hypnotics and anxiolytics because of their improved safety, in particular with respect to respiratory depression, which was a major problem with the barbiturates. The advent of the benzodiazepines opened up a ‘Pandora's Box' of pharmacological activities, all of which are mediated by modulation of the GABAA receptor complex. The wide spectrum of activities led to the introduction of the term ‘inverse agonist' to describe those benzodiazepine receptor ligands which had opposite effects to the hypnotic/anxiolytic/anticonvulsant class, that is, anxiogenic and proconvulsant profiles. The importance of benzodiazepines to therapeutics cannot be underestimated as having been the primary treatment for anxiety and insomnia for nearly 40 years. Recognition of dependence and withdrawal symptoms produced by the benzodiazepines has now limited their usage, but there is no doubt that they still provide the ‘gold standard' by which other sedative drugs are compared.
An alternative approach to increasing GABA receptor activation is to inhibit GABA transport processes, primarily GAT-1, in neurones and glia to increase the extracellular concentration of GABA by decreasing its removal after synaptic release (see also Iversen, this issue). The anticonvulsant tiagabine appears to act in this way, but the increase in GABA levels would, presumably, make it available for action at both GABAA and GABAB sites. An increase in intracellular GABA making more available for release appears to be the mechanism responsible for vigabatrin, which acts as an anticonvulsant. It irreversibly blocks the enzyme GABA-T, which is responsible for the intracellular metabolism of GABA. While activation of GABAA receptors is clearly beneficial, there are no such clinical benefits to be obtained from GABAA receptor antagonism. Any impairment of GABAA-mediated inhibition provokes seizures and convulsions. By contrast, GABAB receptor antagonism has considerable potential as a therapeutic target even though there are currently no drugs that utilize this mechanism. The potential activities of such antagonists are as antidepressants, cognition enhancers and anti-absence epilepsy agents. Such effects have been demonstrated in animal models and the agent CGP36742 (now SG742) is in phase II clinical trials for treatment of mild cognitive impairment (Froestl et al., 2004). The mechanism underlying the cessation of absence seizures by GABAB antagonists in animal models surely implicates these receptors in seizure generation, but in which brain region is not clear as the application of an antagonist to the ventral lateral thalamus, reticular nucleus, sensory cortex or even parts of the motor cortex in a genetic rat model of absence can suppress the abnormal seizure activity. GABAB receptor agonists exacerbate absence seizures in the rat and drugs that increase GABA levels in humans are contraindicated in individuals with absence. This would seem to add further weight to the suggestion that GABAB mechanisms play an integral part in the seizure generation.
The GABAB agonist baclofen has, of course, been in clinical use for the treatment of spasticity for many years and the indications are that this therapeutic effect is mediated via presynaptic receptors on excitatory terminals within the spinal cord. A similar mechanism of action appears to explain the antinociceptive action of baclofen, presumably by suppressing the release of sensory transmitter from primary afferent terminals. However, the action on sensory terminals in man appears to be short-lived with rapid tolerance to baclofen occurring. This does not seem to occur so readily with the antispastic effect. An important observation made by Roberts and colleagues (Cousins et al., 2002) has noted that GABAB receptor agonists reduce the craving for drugs of addiction in man as well as rats. This exciting finding has prompted studies focused on allosteric modulators as possible anticraving agents. This could be an important step as allosteric modulation would be preferred to direct receptor activation as the degree of activation by a modulator is not only dependent on the presence of the natural receptor agonist but also, unlike an applied agonist, cannot overstimulate the receptor.
A brief history of glycine
Amino acids that act as chemical neurotransmitters do not come any simpler in terms of structure than glycine. It is approximately 40 years since Aprison & Werman (1965) noted that the concentration of glycine in the spinal cord tissue is far higher than elsewhere in the brain and, because of this, it may have a role as a neurotransmitter. The levels of glycine were highest in the ventral horn, the location for spinal interneuronal terminals and the destruction of interneurones by anoxia correlated with a fall in tissue glycine levels, suggesting that the interneurones may be the repository of stored glycine. These seminal neurochemical investigations were later supported by early electrophysiological studies. These studies used the techniques pioneered by Curtis (1962) and Krnjevic & Phillis (1963), of extracellular recording and the microionophoretic application of drugs to in vivo preparations. In applying glycine to spinal neurones, both Curtis & Watkins (1960) and Werman et al. (1967) reported that the action of potential firing in such cells was reduced by glycine. To add to the growing evidence of glycine as a transmitter molecule, Shank & Aprison (1970) demonstrated that glycine could be synthesized by neurones and later on Hopkin & Neal (1970) were the first to demonstrate the release of this amino acid after applying stimulation. Once released, and having dissociated from the postsynaptic glycine receptors, glycine was sequestered and taken back into cells by Na+-dependent high-affinity transporters (usually referred to as uptake systems or carriers at that time).
Thus, glycine fulfilled many of the seminal criteria laid down by Werman (1966) before a substance could be accepted as a bone fide neurotransmitter molecule: namely, its concentration at the appropriate location in the nervous system, its ability to be released following stimulation and the presence of a mechanism to stop or limit transmission after release, that is, a transporter in the example of glycine. Werman noted that other criteria were also of importance, and this included the presence of receptors that are sensitive to glycine and the ability of other ligands to antagonize the action of glycine at its receptor (singular term, since in these early days receptor heterogeneity was not widely acknowledged, in part, because embryonic pharmacological studies had only a limited number of selective compounds to call upon). With regard to demonstrating the antagonism of glycine at its receptor, the naturally occurring convulsant alkaloid strychnine has proved very valuable. Studies by Young & Snyder (1973) and Curtis et al. (1968) revealed that strychnine was relatively selective for glycine, but at higher concentrations, Davidoff et al. (1969) revealed that it could also inhibit the action of GABA. Nevertheless, the one seminal finding that was to open up this field came from the realization that 3H-strychnine could bind irreversibly and photochemically label the glycine receptor if it was exposed to UV light (photoaffinity labelling: Graham et al., 1983). This discovery made it possible for the first time to consider biochemically isolating the glycine receptor (note still singular).
The glycine receptor: neurochemistry and molecular biology
It is now 32 years since the glycine receptor was identified, primarily by using radioligand binding with strychnine. The ability of this antagonist to bind irreversibly after UV irradiation enabled Betz and colleagues to start a seminal series of experiments, culminating in the isolation of the receptor on an affinity matrix containing 2-amino-strychnine-agarose (Pfeiffer et al., 1982). Three polypeptides were isolated as a result with masses, 48, 58 and 93 kDa. Strychnine seemed to bind irreversibly to the 48 kDa peptide, subsequently termed α, and this incorporation was blocked by glycine, which suggested that there should be some overlap in the respective binding sites for the agonist and antagonist (Graham et al., 1983). The 58 kDa polypeptide was less affected by strychnine (designated as β) (Graham et al., 1985) and both α and β peptides were considered necessary building blocks for forming the glycine receptor. In comparison, the larger 93 kDa polypeptide was regarded as a peripheral protein, probably cytoplasmic in origin, which we have now come to know as the structural protein, gephyrin (Triller et al., 1985; Schmitt et al., 1987). Gephyrin has also been associated quite closely with (but not directly bound to) the GABAA receptor in recent years. During this period in the early 1980s, we achieved the first functional isolation and expression of a glycine receptor outside the central nervous system by the injection of poly(A)-mRNA, prepared from brain tissue, into the Xenopus laevis oocyte heterologous expression system (Houamed et al., 1984; Smart et al., 1987). These heterologously expressed receptors exhibited all of the expected properties of neuronal glycine receptors, including their sensitivity to the antagonist strychnine.
The isolation of the receptor polypeptides on the 2-amino-strychnine affinity column enabled sufficient peptide sequencing to be achieved on the isolated receptors, eventually allowing oligonucleotide probes to be constructed for the subsequent isolation of cDNA clones. These clones were found to encode for the entire 48 kDa polypeptide (Grenningloh et al., 1987). Interestingly, by comparing primary amino-acid sequences and presumed transmembrane (TM) topologies, the glycine receptor polypeptide shared many of the features of the nicotinic acetylcholine receptor subunits. On this basis, the ‘ligand-gated ion channel superfamily' was born. These features included the now classical TM signatures of these receptors: a large external N-terminus and much shorter external C-terminus; four TM domains, with the integral glycine ion channel being lined by hydrophilic residues associated with the TM2 domain; the prospect of at least two, and for glycine probably four, external cysteine residues engaging in disulphide bridge formation (which gives rise to the newer name for this receptor family of Cys-loop ligand-gated ion channels) and a large intracellular region between TM3 and TM4 that is a substrate for phosphorylation by numerous protein kinases such as cAMP-dependent protein kinase and protein kinase C, as well as being important for receptor anchoring at the glycinergic synapse and trafficking into and out of the cell surface membrane. A few years were to elapse before the cDNA for another part of the receptor was cloned and named the β polypeptide (Grenningloh et al., 1990a). This subunit, although larger, has a TM topology similar to the α subunit. A combined biochemical approach, using receptor subunit crosslinking, electrophoresis, antibody binding and sucrose sedimentation, was used to strongly suggest that the mature glycine receptor was a pentamer (Langosch et al., 1988), in accord with the structures of other members of the Cys-loop receptor family. This approach was also used to suggest that the stoichiometry of glycine receptors was 3α : 2β subunits (Kuhse et al., 1993), which held its position for some years until recently when studies using tandem glycine receptor cDNA constructs have placed greater emphasis on the β subunit, suggesting that the stoichiometry should now be revised to include 2α : 3β (Grudzinska et al., 2005) (Figure 4).
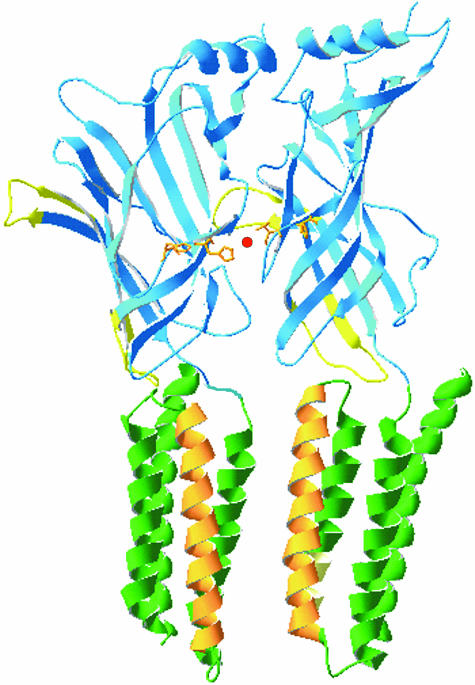
Figure 4.
The glycine receptor. This diagram shows two juxtaposed α1 subunits of the glycine receptor based on a homology model with the acetylcholine-binding protein and the TM domains taken from the nicotinic acetylcholine receptor. The N-terminal domains are shown in blue with the Cys loops depicted in yellow. The TM domains TM1, 3 and 4 are shown in green and the ion channel lining TM2 is shown in orange. The model illustrates the position of histidines 107 and 109 (orange) and their ability to coordinate a Zn2+ ion (red) at the interface between two adjacent α1 subunits. The alignments were generated using Deep View version 3.7.
Exactly as for the GABAA receptor, following the primary cloning of the receptor cDNAs, subsequent molecular cloning studies revealed a number of subunit isoforms. However, by contrast to GABA receptors, the level of subunit variation is rather modest. To date, there are four α subunits (α1–α4) (Grenningloh et al., 1990b; Kuhse et al., 1990; Matzenbach et al., 1994), but still just one β subunit. Further diversity in subunit structure is generated by alternative splicing of the receptor RNAs, yielding two forms of the α1 (denoted as α1 and α1ins), α2 (α2A and α2B) and α3 (α3S and α3L) subunits (Betz et al., 2000). Although potential diversity may exist for the β subunit, one of the β variants noted in human cells is lacking exon 8 and therefore probably not expressed. The glycine receptor-anchoring protein, gephyrin, has proved even more complicated with multiple gephyrin transcripts detected, which may have implications for the interaction of this molecule with many other structural proteins and biochemical processes (Prior et al., 1992; Rees et al., 2003). Moreover, gephyrin variants have also been noted outside the nervous system, for example, in the liver, heart and muscle.
In respect of the potential glycine receptor heterogeneity that can arise from four α subunits and a single β subunit, can anything be said about where and at what time do these receptor subunits appear in the nervous system? A long-held consensus view is that, with regard to immature (embryonic and neonatal) spinal neurones, the α2 subunit is the abundant isoform (transcripts are found across the central nervous system), and is most likely expressed as a homomeric receptor, while α1 subunits appear to dominate in older more mature neurones (particularly evident in the spinal cord and brainstem), but now expressed as a heteromer in association with the β subunit (Becker et al., 1988). This developmental switch is operative during early postnatal life, being completed at around day 20. Inevitably, such a changeover will cause the coexistence of various glycine receptor isoforms and, in more recent work, there is evidence that the α2 subunit may not simply disappear as originally thought, but be expressed long into adult life, particularly in the auditory brainstem and retina (Lynch, 2004). By contrast, both the α3 and α4 glycine receptor subunits are considered as relatively minor subunits compared to α1 and α2. The α3 subunit is expressed in mature neurones with an expression pattern that mirrors that observed for α1 glycine subunit, but only weaker. Significantly, in trying to determine the physiological role of selected glycine receptor subunits, ablation of α3 in the spinal cord indicated that it played a pivotal role in prostaglandin-mediated inflammatory pain transmission in the spinal cord dorsal horn (Harvey et al., 2004). The α4 subunit is quite rare and can be found in and also outside the nervous system. Curiously, the β subunits are quite widely expressed across the nervous system and this pattern of expression does not precisely correlate with α subunit expression, suggesting that the β subunit may have other roles since by itself it is incapable of forming homomeric glycine receptors.
There are many parallels between the development of the glycine receptor field and that for the GABA receptors. However, one point of divergence is that to date, the glycine receptor is an unusual member of the Cys loop ligand-gated ion channel family in not having any glycine receptor counterpart in the metabotropic receptor families. Thus, all signalling via glycine proceeds via the ionotropic receptor, and, of course, via its other main activity as a coagonist at the NMDA receptor. Curiously, however, given the lack of any direct link to G-protein-coupled signal pathways, glycine receptors can be modulated by G protein βγ subunits, which increase glycine potency and increase the duration of glycinergic synaptic currents (Yevenes et al., 2003). This is thought to proceed via a direct interaction with the glycine receptor α1 subunit, but this modulation, via G protein βγ subunits, does not occur at the GABAA receptor.
Inhibitory synapses
Given the diversity in glycine receptor structure, what subunits are we likely to find expressed next to glycine-releasing presynaptic nerve terminals in the spinal cord and brainstem? If α2 subunits predominate in embryonic neurones, can they participate in inhibitory transmission? To be effective, neurotransmitter receptors need to be anchored in the postsynaptic membrane opposite nerve terminals releasing the appropriate transmitter. For α subunit homomeric glycine receptors, it seems unlikely that they will be sequestered at synapses unless they coassemble with β subunits which are vital for linking to gephyrin. In addition, the activation of these homomeric α2 receptors is rather slow (Mangin et al., 2003), not in keeping with rapid transmission at these synapses. So for the α2 homomers, it seems likely that they are probably extrasynaptic, being activated by basal levels of glycine, and may be instrumental in neuronal development, whereas those α2 subunits that are assembled with β subunits could be sequestered by gephyrin and have a synaptic location (Lynch, 2004). At later stages of development, glycine receptor subunits exhibiting faster kinetics (e.g., α1) are probably dominant at the glycinergic inhibitory synapse and there is also the possibility of mixed α subunit receptors which could affect the onset and duration of mIPSCs (Legendre, 2001) (Figure 5). The diversity of glycinergic synapses is further demonstrated by the fact that some synapses can corelease both glycine and GABA from the same axon terminals, causing the activation of the appropriate glycine and GABAA receptors.
Figure 5.
The glycinergic synapse. Schematic representation of a typical glycinergic synapse. The glycine receptors are shown as pentamers of stoichiometry 3α : 2β and also the more recent preferred stoichiometry of 2α : 3β. The receptors are anchored via the β subunits to gephyrin and thus to the microfilaments and microtubules. Presynaptic glycine is packaged into vesicles via the vesicular inhibitory amino-acid transporter (VIAAT) before release. After dissociation from the receptor, either of two discretely localized glycine transporters (GlyT1 or 2) sequester the glycine, which can then be re-packaged into synaptic vesicles or hydrolysed via the glycine cleavage system (GCS).
Molecular pharmacology
The glycine receptor has a very modest pharmacological profile compared to its main comparator, the GABAA receptor. This profile remains largely unaffected whether or not one considers α subunit glycine receptor homomers, or αβ subunit heteromers. Generally, the pharmacology of glycine receptors can be subdivided into essentially a series of agonists, a few antagonists and modulators. In stark contrast to the GABAA receptor, there are no currently available therapeutically useful ligands that act at the glycine receptor despite its pivotal role in providing inhibition in the spinal cord and brainstem. Glycine receptors can be activated by glycine>β-alanine>taurine, in this approximate potency order, with the latter two agonists often being regarded as partial agonists, although this conclusion can depend on the cell type (Lynch, 2004). In terms of inhibition, strychnine is the most potent and selective competitive glycine receptor antagonist (Curtis et al., 1968; Young & Snyder, 1973), and is used as a diagnostic indicator of the involvement of glycine receptors in physiological processes. Picrotoxin, which is also used as a GABAA receptor antagonist, will also inhibit glycine receptor activation, most likely by interfering in an allosteric manner with operation of the glycine receptor ion channel. Interestingly, picrotoxin appears to be able to distinguish between homomeric and heteromeric glycine receptors, being far more potent on the homomeric receptors (Pribilla et al., 1992). It is therefore a very useful detector of the presence of heteromeric glycine receptors. Three other inhibitors of note are pregnenolone sulphate, tropisetron and colchicines (Lynch, 2004). These antagonists are not selective for the glycine receptor, but can be used as indicators to distinguish between some subtypes of the glycine receptor. For example, the neurosteroid pregnenolone sulphate is marginally more potent on α1 than α2 receptors, but if the β subunit is included the potency is reduced at α2β but hardly affected at α1β receptors. For tropisetron, the potency is reduced at α1 compared to α2, but is increased slightly at both receptors on coexpression of the β subunit. Finally, colchicine is approximately five-fold more potent at α2 receptors compared to α1. These small differences can be experimentally important for a class of receptor that is not noted for its subtype-selective probes.
One of the most interesting allosteric modulators is the divalent cation Zn2+. At low nanomolar concentrations Zn2+ potentiates the action of glycine, while at higher micromolar concentrations it acts as an inhibitor (Bloomenthal et al., 1994; Laube et al., 1995). This dual role is potentially important given that Zn2+ is a naturally occurring cation in the brain and spinal cord and can be released during physiological stimulation (Smart et al., 2004). Moreover, the low concentrations of Zn2+ that are required to cause potentiation are predicted to be easily achieved by basal release of Zn2+, such that some receptors might be tonically modulated by this cation. Structure–function studies have subsequently revealed that Zn2+ is probably binding to discrete sites on the receptor to exert these two roles (Smart et al., 1994). The inhibitory site has now been located to two histidine residues that form a bridge between two α subunits (Harvey et al., 1999; Nevin et al., 2003; Miller et al., 2005). A dual regulation of glycine receptor function is also evident with some other ligands which have been discovered to allosterically inhibit responses activated by low glycine concentrations (<15 μM) and potentiate glycine receptor function at higher glycine concentrations (100 μM). These include dideoxyforskolin and tamoxifen. Furthermore, a selection of modulators is also known to potentiate receptor function, including some general anaesthetic agents (inhalational and intravenous) and ethanol and selected alcohols (Lynch, 2004; see also Franks, this issue). One final example of the pharmacology of glycine receptors which is quite helpful experimentally concerns the interaction of the 5-HT3 antagonist tropisetron. It has variable effects on receptor function, which appear dependent upon the receptor subunit composition and the concentrations of the tropisetron and glycine that are used (Supplisson & Chesnoy-Marchais, 2000). These results suggest that it should be quite possible to design subtype-selective probes for the glycine receptor, which may prove to be of therapeutic significance.
Glycine receptors and disease
Despite the lack of any useful therapeutic ligands for the glycine receptor, there is a considerable amount of evidence suggesting that mutations in the receptor play pivotal roles in a number of diseases concerning motor control. These disorders are often characterized by intense muscle contraction/tone (hypertonia) and normally associated with an exaggerated startle reflex, which leads to the hypertonia. Three naturally occurring mutations in the glycine receptors give rise to the phenotypes known as spastic, spasmodic and oscillator (Harvey & Betz, 2000; Lynch, 2004). The spastic mice seemingly lack a sufficient number of cell surface functional glycine receptors due to disruption in the production of β subunit mRNA. By contrast, the spasmodic mice demonstrate a phenotype that is characterized by a startle reflex to sudden acoustic stimuli. A single mutation (A52S) is sufficient to cause this effect and effectively lowers the potency of glycine at these receptors, making them less efficient as inhibitory receptors. The third mutation, known as oscillator, exhibits rapid, repetitive shaking, eventually leading to a form of rigor. This phenotype has been associated with the drastic loss of adult glycine receptors following the deletion of the large intracellular domain in α1 subunits between TM3 and TM4 and also the loss of TM4 itself.
With regard to humans, the rare but potentially fatal disorder of hyperekplexia or human startle disease also involves the mutation of glycine receptors (Rajendra & Schofield, 1995). Essentially, the disorder presents as a severe muscle rigidity which is initiated by abrupt stimuli that can take various forms, including light, sound or physical contact. The muscle contraction that results impedes ambulation and can also lead to postural instability. Molecular genetics studies have enabled many mutations in the α1 subunit TM1–TM2 linker, TM2 itself and the TM2–TM3 linker to be implicated as the underlying causes of hyperekplexia (Rajendra & Schofield, 1995). Curiously, no hyperekplexia mutations have been detected in human α2 or α3 subunits to date. The mutations have various effects that range from reducing the sensitivity of the receptor to glycine, to reducing the single-channel current or the number of functional receptors in the cell membrane. Sometimes single mutations may cause no phenotype, but when they are combined in one individual they reduce receptor expression (Lynch, 2004). A combined mutation in the β subunit has also been reported that reduces the α1β glycine receptor sensitivity to glycine, giving rise to a hyperekplexia phenotype (Rees et al., 2002). Generally, taking all these observations into consideration, the reduced sensitivity to glycine and the reduced numbers of glycine receptors all contribute to the main effect of decreasing the amplitude of glycine-activated currents. Therefore, the dysfunction of other proteins associated with the glycinergic synapse, which impact on the concentration of glycine in the synapse, could have consequences for the expression of hyperekplexia. It is therefore not surprising that mutations in gephyrin can cause hyperekplexia (Rees et al., 2003) and the ablation of the glycine transporter (GlyT2) also produces a hyperekplexia phenotype (Gomeza et al., 2003) because this transporter is required to sequester glycine for future release at inhibitory synapses.
Conclusions
History indicates that there are many similarities between GABA and glycine as neurotransmitters. Their ubiquitous distribution and involvement in disease bears testimony to their importance for moderating the excitability of neurones. One of the next major steps in this field will be to target particular receptor isoforms to achieve, until recently considered an almost unfeasible objective, ‘sculptured' therapeutic effects on particular neural networks avoiding many inconveniencing side effects.
Acknowledgments
T.G.S. acknowledges the support of the MRC and Wellcome Trust.
References
- APRISON M.H., WERMAN R. The distribution of glycine in cat spinal cord and roots. Life Sci. 1965;4:2075–2083. doi: 10.1016/0024-3205(65)90325-5. [DOI] [PubMed] [Google Scholar]
- BARBER R.P., VAUGHN J.E., SAITO K., MCLAUGHLIN B.J., ROBERTS E. GABAergic terminals are presynaptic to primary afferent terminals; in the substantia gelatinosa of the rat spinal cord. Brain Res. 1978;141:35–55. doi: 10.1016/0006-8993(78)90615-7. [DOI] [PubMed] [Google Scholar]
- BECKER C.M., HOCH W., BETZ H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BETZ H., HARVEY R.J., SCHLOSS P.2000Structures, diversity and pharmacology of glycine receptors and transporters Pharmacology of GABA and Glycine Neurotransmissioned. Mohler, H. pp 375–401.Berlin, Heidelberg: Springer-Verlag [Google Scholar]
- BLOOMENTHAL A.B., GOLDWATER E., PRITCHETT D.B., HARRISON N.L. Biphasic modulation of the strychnine-sensitive glycine receptor by Zn2+ Mol. Pharmacol. 1994;46:1156–1159. [PubMed] [Google Scholar]
- BOWERY N.G., HILL D.R., HUDSON A.L., DOBLE A., MIDDLEMISS D.N., SHAW J., TURNBULL M.J. (−)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980;283:92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- BOWERY N.G., HILL D.R., HUDSON A.L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br. J. Pharmacol. 1983;78:191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COUSINS M.S., ROBERTS D.C.S., DE WIT H. GABAB receptor agonists for the treatment of drug addiction: a review of recent findings. Drug Alcohol Depend. 2002;65:209–220. doi: 10.1016/s0376-8716(01)00163-6. [DOI] [PubMed] [Google Scholar]
- CURTIS D.R. Pharmacological investigations upon inhibition of spinal motoneurones. J. Physiol. 1959;145:175–192. doi: 10.1113/jphysiol.1959.sp006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D.R. Direct extracellular application of drugs. Biochem. Pharmacol. 1962;9:205–212. doi: 10.1016/0006-2952(62)90029-1. [DOI] [PubMed] [Google Scholar]
- CURTIS D.R., HOSLI L., JOHNSTON G.A. A pharmacological study of the depression of spinal neurones by glycine and related amino acids. Exp. Brain Res. 1968;6:1–18. doi: 10.1007/BF00235443. [DOI] [PubMed] [Google Scholar]
- CURTIS D.R., WATKINS J.C. The excitation and depression of spinal neurones by structurally related amino acids. J. Neurochem. 1960;6:117–141. doi: 10.1111/j.1471-4159.1960.tb13458.x. [DOI] [PubMed] [Google Scholar]
- DAVIDOFF R.A., APRISON M.H., WERMAN R. The effects of strychnine on the inhibition of interneurons by glycine and gamma-aminobutyric acid. Int. J. Neuropharmacol. 1969;8:191–194. doi: 10.1016/0028-3908(69)90013-6. [DOI] [PubMed] [Google Scholar]
- DREIFUSS J.J., KELLY J.S., KRNJEVIC K. Cortical inhibition and gamma-aminobutyric acid. Exp. Brain Res. 1969;9:137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- DUTAR P., NICOLL R.A. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- ELLIOTT K.A.C., VAN GELDER N.M. Occlusion and metabolism of γ-aminobutyric acid by brain tissue. J. Neurochem. 1958;3:28–40. doi: 10.1111/j.1471-4159.1958.tb12606.x. [DOI] [PubMed] [Google Scholar]
- FLOREY E. An inhibitory and an excitatory factor of mammalian central nervous system, and their action on a single sensory neuron. Arch. Int. Physiol. 1954;62:33–53. doi: 10.3109/13813455409145367. [DOI] [PubMed] [Google Scholar]
- FLOREY E., MCLENNAN H. The effects of factor I and of gamma-aminobutyric acid on smooth muscle preparations. J. Physiol. 1959;145:66–76. doi: 10.1113/jphysiol.1959.sp006127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FROESTL W., GALLAGHER M., JENKINS H., MADRID A., MELCHER T., TEICHMAN S., MONDADORI C., PEARLMAN R. SGS742: the first GABAB antagonist in clinical trials. Biochem. Pharmacol. 2004;68:1479–1487. doi: 10.1016/j.bcp.2004.07.030. [DOI] [PubMed] [Google Scholar]
- GOMEZA J., OHNO K., HULSMANN S., ARMSEN W., EULENBURG V., RICHTER D.W., LAUBE B., BETZ H. Deletion of the mouse glycine transporter 2 results in a hyperekplexia phenotype and postnatal lethality. Neuron. 2003;40:797–806. doi: 10.1016/s0896-6273(03)00673-1. [DOI] [PubMed] [Google Scholar]
- GRAHAM D., PFEIFFER F., BETZ H. Photoaffinity-labelling of the glycine receptor of rat spinal cord. Eur. J. Biochem. 1983;131:519–525. doi: 10.1111/j.1432-1033.1983.tb07292.x. [DOI] [PubMed] [Google Scholar]
- GRAHAM D., PFEIFFER F., SIMLER R., BETZ H. Purification and characterization of the glycine receptor of pig spinal cord. Biochemistry. 1985;24:990–994. doi: 10.1021/bi00325a027. [DOI] [PubMed] [Google Scholar]
- GRENNINGLOH G., PRIBILLA I., PRIOR P., MULTHAUP G., BEYREUTHER K., TALEB O., BETZ H. Cloning and expression of the 58 kD beta subunit of the inhibitory glycine receptor. Neuron. 1990a;4:963–970. doi: 10.1016/0896-6273(90)90149-a. [DOI] [PubMed] [Google Scholar]
- GRENNINGLOH G., RIENITZ A., SCHMITT B., METHFESSEL C., ZENSEN M., BEYREUTHER K., GUNDELFINGER E.D., BETZ H. The strychnine-binding subunit of the glycine receptor shows homology with nicotinic acetylcholine receptors. Nature. 1987;328:215–220. doi: 10.1038/328215a0. [DOI] [PubMed] [Google Scholar]
- GRENNINGLOH G., SCHMIEDEN V., SCHOFIELD P.R., SEEBURG P.H., SIDDIQUE T., MOHANDAS T.K., BECKER C.M., BETZ H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990b;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUDZINSKA J., SCHEMM R., HAEGER S., NICKE A., SCHMALZING G., BETZ H., LAUBE B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- HAEFELY W., KULCSAR A., MOHLER H., PIERI L., POLC P., SCHAFFNER R. Possible involvement of GABA in the central actions of benzodiazepines. Adv. Biochem. Psychopharmacol. 1975;11:131–151. [PubMed] [Google Scholar]
- HARVEY R.J., BETZ H.2000Structure, diversity, pharmacology and pathology of glycine receptor chloride channels Pharmacology of Ionic Channel Function: Activators and Inhibitorseds. Endo, M., Kurachi, Y. & Mishina, M. pp 479–499.Berlin: Springer-Verlag [Google Scholar]
- HARVEY R.J., DEPNER U.B., WASSLE H., AHMADI S., HEINDL C., REINOLD H., SMART T.G., HARVEY K., SCHUTZ B., ABO-SALEM O.M., ZIMMER A., POISBEAU P., WELZL H., WOLFER D.P., BETZ H., ZEILHOFER H.U., MULLER U. GlyR alpha3: an essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- HARVEY R.J., THOMAS P., JAMES C.H., WILDERSPIN A., SMART T.G. Identification of an inhibitory Zn2+ binding site on the human glycine receptor a1 subunit. J. Physiol. 1999;520:53–64. doi: 10.1111/j.1469-7793.1999.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYASHI T. Inhibition and excitation due to gamma-aminobutyric acid in the central nervous system. Nature. 1958;182:1076–1077. doi: 10.1038/1821076a0. [DOI] [PubMed] [Google Scholar]
- HILL D.R., BOWERY N.G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABAB sites in rat brain. Nature. 1981;290:149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- HOPKIN J.M., NEAL M.J. Thr release of 14C-glycine from electrically stimulated rat spinal cord slices. Br. J. Pharmacol. 1970;40:136P. [PMC free article] [PubMed] [Google Scholar]
- HOSIE A.M., DUNNE E.L., HARVEY R.J., SMART T.G. Zinc-mediated inhibition of GABAA receptors: discrete binding sites underlie subtype specificity. Nat. Neurosci. 2003;6:362–369. doi: 10.1038/nn1030. [DOI] [PubMed] [Google Scholar]
- HOUAMED K.M., BILBE G., SMART T.G., CONSTANTI A., BROWN D.A., BARNARD E.A., RICHARDS B.M. Expression of functional GABA, glycine and glutamate receptors in Xenopus oocytes injected with rat brain mRNA. Nature. 1984;310:318–321. doi: 10.1038/310318a0. [DOI] [PubMed] [Google Scholar]
- IVERSEN L.L. The Uptake and Storage of Noradrenaline in Sympathetic Nerves. Cambridge: Cambridge University Press; 1967. [Google Scholar]
- IVERSEN L.L., NEAL M.J. The uptake of [3H]GABA by slices of rat cerebral cortex. J. Neurochem. 1968;15:1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- KAUPMANN K., HUGGEL K., HEID J., FLOR P.J., BISCHOFF S., MICKEL S.J., MCMASTER G., ANGST C., BITTIGER H., FROESTL W., BETTLER B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- KRAVITZ E.A., KUFFLER S.W., POTTER D.D. Gamma-aminobutyric acid and other blocking compounds in crostacea. iii. Their relative concentrations in separated motor and inhibitory axons. J. Neurophysiol. 1963;26:739–751. doi: 10.1152/jn.1963.26.5.739. [DOI] [PubMed] [Google Scholar]
- KRNJEVIC K., PHILLIS J.W. Iontophoretic studies of neurones in the mammalian cerebral cortex. J. Physiol. 1963;165:274–304. doi: 10.1113/jphysiol.1963.sp007057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRNJEVIC K., SCHWARTZ S. The action of γ-aminobutyric acid on cortical neurones. Exp. Brain Res. 1967;3:320–326. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- KUFFLER S.W. Mechanisms of activation and motor control of stretch receptors in lobster and crayfish. J. Neurophysiol. 1954;17:558–574. doi: 10.1152/jn.1954.17.6.558. [DOI] [PubMed] [Google Scholar]
- KUHSE J., LAUBE B., MAGALEI D., BETZ H. Assembly of the inhibitory glycine receptor: identification of amino acid sequence motifs governing subunit stoichiometry. Neuron. 1993;11:1049–1056. doi: 10.1016/0896-6273(93)90218-g. [DOI] [PubMed] [Google Scholar]
- KUHSE J., SCHMIEDEN V., BETZ H. Identification and functional expression of a novel ligand binding subunit of the inhibitory glycine receptor. J. Biol. Chem. 1990;265:22317–22320. [PubMed] [Google Scholar]
- LANGOSCH D., THOMAS L., BETZ H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl. Acad. Sci. U.S.A. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAUBE B., KUHSE J., RUNDSTROM N., KIRSCH J., SCHMIEDEN V., BETZ H. Modulation by zinc ions of native rat and recombinant human inhibitory glycine receptors. J. Physiol. 1995;483:613–619. doi: 10.1113/jphysiol.1995.sp020610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEGENDRE P. The glycinergic inhibitory synapse. Cell. Mol. Life Sci. 2001;58:760–793. doi: 10.1007/PL00000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LYNCH J.W. Molecular structure and function of the glycine receptor chloride channel. Physiol. Rev. 2004;84:1051–1095. doi: 10.1152/physrev.00042.2003. [DOI] [PubMed] [Google Scholar]
- MANGIN J.M., BALOUL M., PRADO D.C., ROGISTER B., RIGO J.M., LEGENDRE P. Kinetic properties of the α2 homo-oligomeric glycine receptor impairs a proper synaptic functioning. J. Physiol. 2003;553:369–386. doi: 10.1113/jphysiol.2003.052142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATZENBACH B., MAULET Y., SEFTON L., COURTIER B., AVNER P., GUENET J.L., BETZ H. Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J. Biol. Chem. 1994;269:2607–2612. [PubMed] [Google Scholar]
- MILLER P.S., BEATO M., HARVEY R.J., SMART T.G. Molecular determinants of glycine receptor αβ subunit sensitivities to Zn2+ mediated inhibition. J. Physiol. 2005;566:657–670. doi: 10.1113/jphysiol.2005.088575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEVIN S.T., CROMER B.A., HADDRILL J.L., MORTON C.J., PARKER M.W., LYNCH J.W. Insights into the structural basis for zinc inhibition of the glycine receptor. J. Biol. Chem. 2003;278:28985–28992. doi: 10.1074/jbc.M300097200. [DOI] [PubMed] [Google Scholar]
- OLSEN R.W., TOBIN A.J. Molecular biology of GABAA receptors. FASEB J. 1990;4:1469–1480. doi: 10.1096/fasebj.4.5.2155149. [DOI] [PubMed] [Google Scholar]
- OTSUKA M., IVERSEN L.L., HALL Z.W., KRAVITZ E.A. Release of gamma-aminobutyric acid from inhibitory nerves of lobster. Proc. Natl. Acad. Sci. U.S.A. 1966;56:1110–1115. doi: 10.1073/pnas.56.4.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEIFFER F., GRAHAM D., BETZ H. Purification by affinity chromatography of the glycine receptor of rat spinal cord. J. Biol. Chem. 1982;257:9389–9393. [PubMed] [Google Scholar]
- PRIBILLA I., TAKAGI T., LANGOSCH D., BORMANN J., BETZ H. The atypical M2 segment of the b subunit confers picrotoxinin resistance to inhibitory glycine receptor channels. EMBO J. 1992;11:4305–4311. doi: 10.1002/j.1460-2075.1992.tb05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIOR P., SCHMITT B., GRENNINGLOH G., PRIBILLA I., MULTHAUP G., BEYREUTHER K., MAULET Y., WERNER P., LANGOSCH D., KIRSCH J. Primary structure and alternative splice variants of gephyrin, a putative glycine receptor–tubulin linker protein. Neuron. 1992;8:1161–1170. doi: 10.1016/0896-6273(92)90136-2. [DOI] [PubMed] [Google Scholar]
- RAJENDRA S., SCHOFIELD P.R. Molecular mechanisms of inherited startle syndromes. Trends Neurosci. 1995;18:80–82. [PubMed] [Google Scholar]
- REES M.I., HARVEY K., WARD H., WHITE J.H., EVANS L., DUGUID I.C., HSU C.C., COLEMAN S.L., MILLER J., BAER K., WALDVOGEL H.J., GIBBON F., SMART T.G., OWEN M.J., HARVEY R.J., SNELL R.G. Isoform heterogeneity of the human gephyrin gene (GPHN), binding domains to the glycine receptor, and mutation analysis in hyperekplexia. J. Biol. Chem. 2003;278:24688–24696. doi: 10.1074/jbc.M301070200. [DOI] [PubMed] [Google Scholar]
- REES M.I., LEWIS T.M., KWOK J.B., MORTIER G.R., GOVAERT P., SNELL R.G., SCHOFIELD P.R., OWEN M.J. Hyperekplexia associated with compound heterozygote mutations in the beta-subunit of the human inhibitory glycine receptor (GLRB) Hum. Mol. Genet. 2002;11:853–860. doi: 10.1093/hmg/11.7.853. [DOI] [PubMed] [Google Scholar]
- SCHMITT B., KNAUS P., BECKER C.M., BETZ H. The Mr 93,000 polypeptide of the postsynaptic glycine receptor complex is a peripheral membrane protein. Biochemistry. 1987;26:805–811. doi: 10.1021/bi00377a022. [DOI] [PubMed] [Google Scholar]
- SHANK R.P., APRISON M.H. The metabolism in vivo of glycine and serine in eight areas of the rat central nervous system. J. Neurochem. 1970;17:1461–1475. doi: 10.1111/j.1471-4159.1970.tb00513.x. [DOI] [PubMed] [Google Scholar]
- SMART T.G., HOSIE A.M., MILLER P.S. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- SMART T.G., HOUAMED K.M., VAN RENTERGHEM C., CONSTANTI C. mRNA-directed synthesis and insertion of functional amino acid receptors in Xenopus laevis oocytes. Biochem. Soc. Trans. 1987;15:117–122. doi: 10.1042/bst0150117. [DOI] [PubMed] [Google Scholar]
- SMART T.G., XIE X., KRISHEK B.J. Modulation of inhibitory and excitatory amino acid receptor ion channels by zinc. Prog. Neurobiol. 1994;42:393–441. doi: 10.1016/0301-0082(94)90082-5. [DOI] [PubMed] [Google Scholar]
- SUPPLISSON S., CHESNOY-MARCHAIS D. Glycine receptor β subunits play a critical role in potentiation of glycine responses by ICS-205,930. Mol. Pharmacol. 2000;58:763–770. doi: 10.1124/mol.58.4.763. [DOI] [PubMed] [Google Scholar]
- TRILLER A., CLUZEAUD F., PFEIFFER F., BETZ H., KORN H. Distribution of glycine receptors at central synapses: an immunoelectron microscopy study. J. Cell Biol. 1985;101:683–688. doi: 10.1083/jcb.101.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R. Criteria for identification of a central nervous system transmitter. Comp. Biochem. Physiol. 1966;18:745–766. doi: 10.1016/0010-406x(66)90209-x. [DOI] [PubMed] [Google Scholar]
- WERMAN R., DAVIDOFF R.A., APRISON M.H. Inhibition of motoneurones by iontophoresis of glycine. Nature. 1967;214:681–683. doi: 10.1038/214681a0. [DOI] [PubMed] [Google Scholar]
- YEVENES G.E., PEOPLES R.W., TAPIA J.C., PARODI J., SOTO X., OLATE J., AGUAYO L.G. Modulation of glycine-activated ion channel function by G-protein betagamma subunits. Nat. Neurosci. 2003;6:819–824. doi: 10.1038/nn1095. [DOI] [PubMed] [Google Scholar]
- YOUNG A.B., SNYDER S.H. Strychnine binding associated with glycine receptors of the central nervous system. Proc. Natl. Acad. Sci. U.S.A. 1973;70:2832–2836. doi: 10.1073/pnas.70.10.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]