Abstract
Since the identification of the first chemotactic cytokines 20 years ago, the field has mushroomed, with the discovery of approximately 40 ligands, which interact with 20 different cell surface receptors. At the time of writing this review, a PubMed trawl using the word ‘chemokine' will recover over 28,000 manuscripts. In this article, we will give a short history of the discovery of chemokines and provide examples of the potential for therapeutic targeting of the chemokine network in inflammatory disease.
Keywords: Chemokines, chemotaxis, inflammation, leukocyte
Introduction
Unicellular organisms have the ability to sense a chemical gradient in their environment. This is no mean achievement as it involves detecting a minute concentration difference in the small distance between the margins of the cell and polarizing the machinery involved in locomotion along the direction of the gradient. The detailed study of this process in the single-celled organism, Dictyostelium discoideum, has provided knowledge on the basic mechanisms involved (van Haastert & Devreotes, 2004). Dictyostelium detects cAMP in its environment using a 7-transmembrane region (7TM) G-protein-coupled receptor. The cAMP is released by the organisms themselves during times of nutrient deprivation; this results in the directed migration of cells towards one another, where they aggregate to form a fruiting body with the aim of maintaining spores until conditions are ripe for germination. The degree of G-protein activation on the inside of the cell membrane reflects the cAMP concentration detected on the outside of the cell. Phosphoinositide 3-kinase (PI3K) accumulates with its highest concentration at the region of the cell membrane associated with the highest amount of G-protein activation. The protein PTEN (phosphatase and tensin homolog deleted on chromosome 10) is displaced from this region to the rest of the cell membrane, where it inhibits PI3K. This provides amplification of the polarizing signal and this is followed by cell locomotion up the concentration gradient, a process requiring activation of adhesion molecules at the front of the cell and inactivation at the rear.
This fundamental process of chemotaxis seen in primitive organisms is essential for the organization and physiological functioning of multicellular organisms from conception, through development to adult life. One family of small proteins is recognized as being particularly important in this respect. The discovery of this family came from investigations of inflammatory mechanisms. These proteins, the chemokines, have an essential role in host defence in regulating the production of immune cells, organizing their localization in specialized tissues under basal conditions and controlling their recruitment and activation in response to inflammatory stimuli.
It was an early observation that different types of leukocytes accumulate in different types of inflammatory reaction. Until comparatively recently, it was not known how this was achieved. Chemoattractants had been identified, such as C5a, f-Met-Leu-Phe and leukotriene B4, but these lacked specificity and were mainly recognized for inducing the recruitment of neutrophils, the most numerous leukocyte in the circulation. In the mid to late 1980s the first ‘intercrines' began to emerge under a variety of names (reviewed by Oppenheim et al., 1991). These small proteins had sequence similarities to the earlier discovered platelet factor 4 (platelet anti-heparin factor) and β-thromboglobulin, most notably four well-conserved cysteine residues. These two proteins, released from the α-granules of platelets by treatment with stimuli such as thrombin and ADP, had been characterized (identified, purified and sequenced) by hematologists. The first intercrines to be discovered by immunologists were IP-10, IL-8, macrophage inflammatory protein (MIP)-1, MIP-2, RANTES and MCP-1. Some of these were initially discovered as chemotactic factors by classical techniques, following purification from a cell supernatant and protein sequencing. Others were discovered at the cDNA level by techniques such as the subtractive hybridization of cDNA libraries prepared from stimulated versus unstimulated leukocytes. Subsequent expression of the cDNA then produced a recombinant protein found to be chemotactic for a particular cell type.
In the early days, collaborations between those working in the emerging field were stimulated by international meetings held in the United Kingdom, the first two organized by John Westwick, Ivan Lindley and Steven Kunkel, held at the Royal College of Surgeons in London in 1988 and 1990 (Westwick et al., 1989; 1990).
IL-8 was purified from biological extracts independently by several groups as a neutrophil chemotactic agent and termed monocyte-derived neutrophil chemotactic factor (MDNCF), anionic neutrophil activating factor (ANAP), NAF (neutrophil activating factor) or NAP-1 (neutrophil activating peptide). This human protein was found to be similar to a mouse protein, MIP-2. The gene encoding IP-10 was identified following stimulation of U937 cells with interferon-γ (IFN-γ) in an effort to identify molecules involved in the antiviral response. Its orthologue was subsequently shown to be induced in murine peritoneal macrophages by treatment with lipopolysaccharide. Similarly, the gene encoding RANTES was identified in a cDNA library enriched for T-cell-specific sequences and the resultant protein of approximately 8 kDa found to be expressed in peripheral blood leukocytes stimulated by antigen or mitogen.
Human and mouse orthologues of the MIP-1α (LD78) gene were identified and the expressed protein found to have inhibitory activity on the proliferation of haematopoeitic stem cells and chemotactic activity on neutrophils and monocytes. Specific monocyte chemoattractants were sequenced in supernatants from cytokine- or virus-stimulated cells and their MCAF/MCP-1 genes identified in human and murine cells.
These early studies laid the groundwork for the current knowledge of the chemokine family: there are now known to be approximately 40 chemokines (Table 1) that interact with 18 signalling receptors and two nonsignalling receptors (Table 2). The term chemokine (chemotactic cytokines) was subsequently coined to describe this family of small, typically basic proteins with four conserved cysteine residues. Retrospective analysis of the literature now reveals that platelet factor 4 was actually the first chemokine to be identified although this chemokine is atypical, being only active at micromolar concentrations and, relatively poor at recruiting leukocytes.
Table 1. Human chemokines identified to date.
| Systematic name | Colloquial name | Receptor usage | Systematic name | Colloquial name | Receptor usage |
|---|---|---|---|---|---|
| CCL1 | I-309 | CCR8 | CXCL1 | GROα/MGSA-α | CXCR2 |
| CCL2 | MCP-1/MCAF | CCR2 | CXCL2 | GROβ/MGSA-β | CXCR2 |
| CCL3 | MIP-1α/LD78α | CCR1, 5 | CXCL3 | GROγ/MGSA-γ | CXCR2 |
| CCL3L1 | LD78β | CCR1, 5 | CXCL4 | PF-4 | CXCR3B |
| CCL4 | MIP-1β | CCR5 | CXCL5 | ENA-78 | CXCR2 |
| CCL4L1 | LAG-1 | CCR5 | CXCL6 | GCP-2 | CXCR1, 2 |
| CCL5 | RANTES | CCR1, 3, 5 | CXCL7 | NAP-2 | CXCR2 |
| CCL7 | MCP-3 | CCR1, 2, 3 (CCR5) | CXCL8 | IL-8 | CXCR1, 2 |
| CCL8 | MCP-2 | CCR3 | CXCL9 | Mig | CXCR3 (CCR3) |
| CCL11 | Eotaxin | CCR3 (CCR2) | CXCL10 | IP-10 | CXCR3 (CCR3) |
| CCL13 | MCP-4 | CCR2, 3 | CXCL11 | I-TAC | CXCR3 (CCR3) |
| CCL14 | HCC-1 | CCR1 | CXCL12 | SDF-1α/β | CXCR4 |
| CCL15 | HCC-2/Lkn-1/MIP-1δ/MIP5 | CCR1, 3 | CXCL13 | BLC/BCA-1 | CXCR5 |
| CCL16 | HCC-4/LEC | CCR1 | CXCL14 | BRAK/Bolekine | |
| CCL17 | TARC | CCR4 | CXCL16 | SR-PSOX | CXCR6 |
| CCL18 | DC-CK-1/AMAC-1/MIP5/ PARC/MIP-4 | CCR3 | |||
| CCL19 | MIP-3β/ELC/Exodus-3 | CCR7 | XCL1 | Lymphotactin/ SCM-1α/ATAC | XCR1 |
| CCL20 | MIP3α/LARC/Exodus-1 | CCR6 | XCL2 | SCM-1β | XCR1 |
| CCL21 | SLC/6Ckine/Exodus-2 | CCR7 | |||
| CCL22 | MDC/STCP-1 | CCR4 | CX3CL1 | Fractalkine, neurotactin | CX3CR1 |
| CCL23 | MPIF-1 | CCR1 | |||
| CCL24 | MPIF-2/Eotaxin-2 | CCR3 | |||
| CCL25 | TECK | CCR9 | |||
| CCL26 | Eotaxin-3 | CCR3, (CCR1, 2, 5) | |||
| CCL27 | CTACK/ALP/ILC/ESkine | CCR10 | |||
| CCL28 | MEC | CCR10, 3 |
The systematic names together with the colloquial names and receptor agonist activity are shown. Antagonist activity at other chemokine receptors is shown within parenthesis. Some human chemokines appear to be missing from the list, for example, CCL6. In such instances, while a chemokine of that name has been identified in the mouse, no human orthologue has been documented.
6ckine=chemokine with 6 cysteines; ALP=amino-terminal alanine-leucine-proline chemokine; AMAC=alternative macrophage activation-associated CC chemokine; ATAC=activation-induced, chemokine-related molecule; BCA=B-cell-attracting chemokine; BRAK=breast- and kidney-expressed chemokine; CTACK; cutaneous T-cell-activating chemokine; DARC=Duffy antigen receptor for chemokines; DC-CK1=dendritic cell-derived CC chemokine; ELC=EBL-1 ligand chemokine; ENA-78=epithelial neutrophil activating peptide 78; ESkine=embryonal stem cell chemokine; GCP=granulocyte chemotactic protein; GRO=growth-regulated oncogene; HCC=human CC chemokine; ILC=IL-11 receptor α-locus chemokine; IP, IFNγ-inducible protein; I-TAC=IFNγ-inducible T-cell chemoattractant; LAG=lymphocyte activation gene-1; LARC=liver- and activation-regulated chemokine; LEC=liver-expressed chemokine; Lkn=leukotactin; MCP=monocyte chemoattractant protein; MCAF=monocyte chemotactic and activating factor; MDC=monocyte-derived chemokine; MEC=mucosae-associated epithelial chemokine; MGSA=melanoma growth stimulatory activity; Mig=monokine-induced by IFNγ; MIP=macrophage inflammatory protein; MPIF=myeloid progenitor inhibitory factor; NAP=neutrophil-activating peptide; PARC=pulmonary- and activation-regulated chemokine; PF=platelet factor; RANTES=regulated on activation, normal T-cells expressed and secreted; SCM=single C motif; SDF-1=stromal cell-derived factor 1; SLC=secondary lymphoid tissue chemokine; SR-PSOX=scavenger receptor for phosphatidylserine and oxidized lipoprotein; STCP=stimulated T-cell chemoattractant protein; TARC=thymus- and activation-regulated chemokine; TECK=thymus-expressed chemokine.
Table 2. Human chemokine receptors and their cellular expression.
| Chemokine receptor | Cellular expression | Chemokine receptor | Cellular expression |
|---|---|---|---|
| CCR1 | Mo, DC, Eo, Bs, T, PMN, NK | CXCR1 | No, Mo |
| CCR2 | Mo, DC, T, Bs | CXCR2 | No, Mo |
| CCR3 | Eo, T, Bs, Mc | CXCR3 | T, B |
| CCR4 | DC, T, Bs, NK | CXCR4 | T, B, DC, Mo |
| CCR5 | Mo, DC, T | CXCR5 | T, B |
| CCR6 | DC, T | CXCR6 | T |
| CCR7 | DC, T, B, NK | ||
| CCR8 | Mo, T, NK | XCR1 | T, NK |
| CCR9 | T | ||
| CCR10 | T | CX3CR1 | T, NK, DC, Mo |
| D6 | LEC | ||
| DARC | RBC, VEC |
This table shows the distribution of chemokine receptors over a range of cell types. Although there are reports of chemokine receptors on nearly every type of cell, those shown above represent the commonly agreed attributions.
B=B-lymphocyte; Bs=basophil; DC=dendritic cell; Eo=eosinophil; Mc=mast cell; Mo=monocyte; NK=natural killer cell; No=neutrophil; LEC=lymphatic endothelial cell; RBC=red blood cell; T=T-lymphocyte; VEC=vascular endothelial cell.
The majority of chemokines reside within two major classes, namely the CXC or α chemokines (in which the two amino-terminal cysteine residues are separated by a single amino acid residue) or the CC or β chemokines (in which the two amino terminal cysteine residues are adjacent). There are also two other minor classes, the CX3C and C chemokine families, which have only three members between them. One consequence of the intensive research which identified many of the chemokines was the fact that they were often simultaneously identified by several different groups, each laboratory giving the same protein a different name. An extreme example of this is the chemokine pulmonary and activation-regulated chemokine (PARC), which has also been named MIP-4, alternative macrophage activation-associated CC chemokine-1 (AMAC-1), and dendritic cell chemokine-1 (DC-CK-1). Following healthy discussion at the Keystone Chemotactic Cytokine Conference in 1999, chemokines were designated a systematic number to distinguish between group members, updating the anecdotal method of defining a chemokine upon its function (Zlotnik & Yoshie, 2000). Chemokines are given the names CCL (representing CC ligand), CXCL (CXC ligand), CX3CL (CX3C ligand) and XCL (C ligand), together with an identifying number. Although some of the romance of the old nomenclature may have been lost, in many cases, the new system is an improvement on what went before and has been rapidly taken up by the research community.
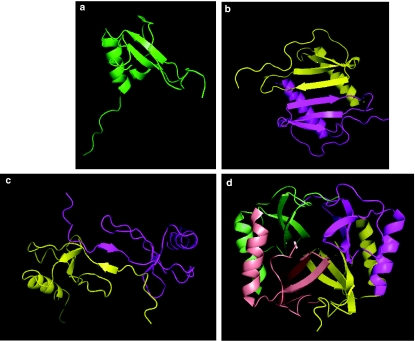
All chemokines analysed at the structural level to date have been shown to share the same protein fold, known as a ‘Greek key' motif, in which three antiparallel β-pleated sheets are overlaid by a c-terminal α-helix (Figure 1). With a few exceptions, four conserved cysteine residues within the primary sequence serve to hold the protein together via two disulphide bonds giving stability to the tertiary structure. The majority of chemokines are also able to form dimers and higher-order oligomers. In the case of CC chemokines, this is achieved by the juxtaposition of residues within the amino terminus of each monomer, while in contrast, the dimer interface of CXC chemokines such as CXCL8 is between the first β strands (Figure 1). The functional relevance of chemokine dimerization is poorly understood, as the majority of chemokines have maximal activity in vivo at nanomolar concentrations, in stark contrast to their association constants for dimerization, which are usually in the micromolar range. Moreover, the biochemical generation of obligate monomers has been shown to have little effect upon biological activity (Paavola et al., 1998). The chemokines CX3CL1 and CXCL16 are unusual in that they are expressed in a membrane-bound form on the end of a cleavable mucin-like stalk which allows them to function as adhesion molecules when intact, or as soluble chemoattractants when cleaved from the stalk by the action of metalloproteinases.
Figure 1.
Tertiary and quaternary structures of chemokines. Panel a shows the secondary structural ‘Greek Key' motif of chemokines, as typified by the obligate monomer CCL7/MCP-3. Three antiparallel β-pleated sheets overlay a C-terminal, α-helical domain. In panels b and c, dimers of the CXC chemokine CXCL8/IL-8 and CCL2/MCP-1 are shown. Note the elongation of the CCL2 dimer in comparison with the CXCL8 dimer. Panel d shows the CXCl4/PF-4 tetramer. The images were constructed using the program Pymol (http://www.pymol.org) using the respective pdb files (1BO0 1IL8, 1DOM and 1RHP) retrieved from the protein data Bank (http://www.rcsb.org/pdb).
Chemokine receptors
Chemokines exert their effects by binding to chemokine receptors on the surface of cells, predominantly leukocytes. Back-to-back Science papers in 1991 described the first chemokine receptors (CXCR1 and CXCR2), which, like all signalling chemokine receptors identified since, belong to the family of G protein-coupled receptors (Holmes et al., 1991; Murphy & Tiffany, 1991). To date, 18 signalling chemokine receptors have been identified in humans (Table 2), with CCR and CXCR being used to define receptors for CC and CXC chemokines respectively. Promiscuity is common, with chemokine receptors typically interacting with several different ligands, although their ligand repertoire is typically class restricted. This means that CC chemokine receptors will interact with only CC chemokines and, likewise, CXC chemokine receptors will bind only CXC chemokines. Two exceptions to this rule of thumb are the chemokines CXCL9, 10 and 11, which have agonist activity at CXCR3 and also antagonist activity at CCR3 (Loetscher et al., 2001). Another is the Duffy antigen receptor complex (DARC), which binds both CC and CXC chemokines (Lu et al., 1995) and which will be discussed later. As more and more chemokines were identified, intensive efforts were undertaken to match these with the many ‘orphan' receptors previously identified as being expressed by leukocytes; for example, interrogation of transfectants expressing a variety of orphan chemokines lead to the pairing of CXCL12 with its receptor CXCR4 (Bleul et al., 1996). CXCR4 acts as a coreceptor for HIV-1 (discussed later) and is also important in homeostasis; for example, the homing of senescent neutrophils to the bone marrow (Martin et al., 2003).
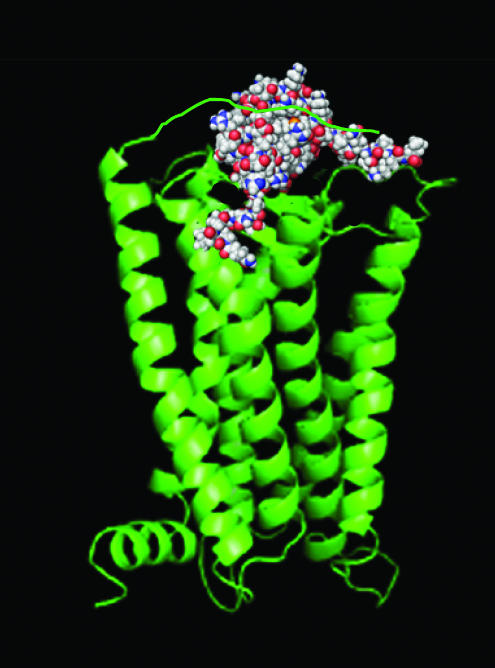
Chemokine receptors are typically 340–380 amino acids in length with an extracellular acidic N-terminus important in tethering the basic chemokine ligand to the receptor. This facilitates a second interaction of lower affinity in which the chemokine is delivered to the remainder of the receptor, leading to the activation of heterotrimeric G proteins and intracellular signaling. Recent studies of CCR5 have refined the model further (Blanpain et al., 2003), with the notion that the disruption of hydrophobic interactions between the side chains of helix II and helix III initiates the conformational changes needed for receptor activation and G-protein signaling (Figure 2).
Figure 2.
The two-step model of receptor activation of chemokine receptor activation. The amino-terminus of the receptor (green ribbon diagram) is thought to tether the chemokine (space filled model) with high affinity, following which the chemokine N-terminus activates a ligand-binding pocket with the TM helices. The resulting conformational changes result in the recruitment of heterotrimeric G proteins and subsequent downstream signalling.
Chemokine receptors can be broadly divided into those that are expressed exclusively by a particular subset of leukocytes or those which are more widely expressed (see Table 2). For example, CCR6 expression is largely restricted to immature dendritic cells and memory T-lymphocytes, facilitating their migration to lymphoid organs expressing the ligand CCL20. In contrast, the receptor for CCL11/eotaxin, CCR3, is expressed on a variety of cells involved in allergic responses, such as eosinophils, basophils and mast cells (see below). T-helper lymphocytes of either the Th1 or Th2 subset also exhibit differential chemokine receptor expression profiles. Th1 lymphocytes selectively express CCR1, CCR5 and CXCR3, whereas CCR3, CCR4 and CCR8 are found on Th2 lymphocytes (Bonecchi et al., 1998). Such dynamic expression is thought to enable the T cells to respond to a variety of chemokines, undoubtedly providing flexibility in the adaptive immune response, and explains many of the earlier observations, such as CCL3 and CCL4 attracting distinct populations of lymphocytes (Schall et al., 1993).
Chemokine receptors, like other members of the GPCR family, transduce signals via heterotrimeric G-proteins (see also Milligan & Kostenis, this issue). Initial experiments employing Pertussis toxin blockade suggested that Gαi proteins were primarily responsible for downstream signalling as physiological responses such as chemotaxis were readily inhibited by preincubation of cells with the toxin (Thelen et al., 1988). The use of PI3K inhibitors in vitro has demonstrated a significant role for PI3K in leukocyte chemotaxis, which has been supported by studies using mice deficient in PI3K γ (Li et al., 2000), although more recent data obtained using T-lymphocytes suggest that PI3K is not an absolute requirement for all chemokine-mediated chemotaxis (Cronshaw et al., 2004).
Another early observation was that CXCL8 downregulated over 90% of its cell surface receptor on neutrophils within 10 min at 37°C (Samanta et al., 1990). We now know this process to be mediated by phosphorylation of the receptor C-terminus and subsequent recruitment of the arrestins, which impede G-protein signalling and facilitate endocytosis via clathrin-coated pits (reviewed by Neel et al., 2005). This was thought to be solely a means of desensitizing chemokine receptors until the unexpected finding that T and B cells from arrestin-2-deficient mice exhibited impaired chemotactic responses to CXCL12, suggestive of a role for arrestins in signalling. Arrestins are now thought to additionally function as adaptors, allowing the docking of molecules such as c-Jun amino-terminal kinase 3 (JNK3) and the subsequent activation of additional signalling pathways (reviewed by Lefkowitz & Shenoy, 2005).
One area of chemokine research still courting controversy is that of receptor dimerization. While the oligomerization of many GPCRs is a well-established phenomenon (reviewed in Milligan et al., 2003; see also Hill, this issue), the ability of chemokine receptors to dimerize has only recently undergone close scrutiny. Homodimerization following ligand activation has been shown by the same laboratory to occur for CCR2 (Rodriguez-Frade et al., 1999a), CCR5 (Rodriguez-Frade et al., 1999b) and CXCR4. While many have considered the evidence for receptor dimerization to be unconvincing without detection of receptor re-distribution and oligomerization in real time (Thelen & Baggiolini, 2001), more recent studies using fluorescence resonance energy transfer (FRET) techniques have provided more evidence for chemokine receptor dimerization. One report has suggested that ligand-induced dimerization of CCR5 is via an interface between transmembrane helices 1 and 4 (Hernanz-Falcon et al., 2004), although the exact nature of this interaction is still a topic for debate (Lemay et al., 2005).
Chemokine binding to glycosaminoglycans (GAGs)
In addition to binding to their receptors, chemokines are also capable of binding to proteoglycans, in an electrostatic interaction facilitated by the highly acidic GAG side chains (reviewed by Handel et al., 2005). The affinity of chemokines for GAG chains is typically in the micromolar range, although CXCL4/platelet factor 4 can interact with nanomolar affinity with GAGs and was originally purified on the basis of its high affinity for heparin sepharose. The interaction of chemokines with endothelial cell-expressed proteoglycans has been proposed to immobilize a high concentration of locally generated chemokine on the luminal surface of the microvascular endothelium. It is proposed that leukocytes roll along the endothelium by virtue of the tethering effect of selectin molecules. Chemokines are presented on the endothelial surface GAGs and, on encountering an appropriate receptor, trigger signalling in the leukocyte, resulting in activated integrin adhesion molecules. The integrins lock onto complementary molecules on the endothelium, resulting in firm adhesion of the leukocytes, followed by migration through the barrier and into the tissue. Thus, chemokines are able to recruit specific leukocyte types by virtue of the differential expression of chemokine receptors.
The GAG-binding domains of some CC chemokines have been extensively characterized by mutagenesis and a variant of CCL5, lacking its GAG-binding motif, retains its in vitro chemotactic activity. However, the same mutant is unable to recruit cells when administered to mice in vivo, suggesting that GAG binding is essential for the in vivo activity of some chemokines. This suggests the possibility that antagonism of the chemokine GAG interaction may be a useful therapeutic target (Lever & Page, 2002).
Microbial subversion of the chemokine system
While the specific task of each chemokine and its receptor in vivo is being gradually teased apart by the generation of mice deficient in either a specific chemokine or receptor, it is interesting that this has been pre-empted by microbes in their ability to manipulate the immune system to achieve both evasion of the host defence systems and increase their own propagation. This ongoing process has been put forward as the driving force behind the generation of host defence protein diversity (Murphy, 1993). Perhaps the most infamous example of exploitation of the chemokine network is the use of chemokine receptors by HIV-1 to gain cellular entry into leukocytes (reviewed by Berger et al., 1999). For many years it was known that HIV-1 utilized the cell surface protein CD4 to enter and infect cells, but cell transfectants engineered to express CD4 did not allow viral entry, suggesting the required presence of an unknown cofactor. By means of an elegant molecular cloning approach, Feng and colleagues identified an orphan 7TM receptor, which they named ‘fusin' by reference to the fact that, in conjunction with CD4, the receptor facilitated fusion of host cells with those expressing envelope proteins prepared from T-lymphocyte (T)-tropic l HIV-1 strains. Subsequent work identified the ligand for fusin as CXCL12/SDF1-α, immediately cementing the link between the chemokine field with that of HIV-1 research and providing a further impetus to chemokine research. In keeping with the nomenclature, fusin was renamed CXCR4. Further work showed that, the related receptor CCR5 facilitates the entry of macrophage (M)-tropic strains. Blockade of HIV-1 entry via either coreceptor can be blocked by their specific ligands, a point which explained the earlier identification of CCR5 ligands as HIV-suppressive factors produced by CD8+ T cells. The importance of CCR5 in HIV-1 infection is highlighted by homozygous inheritance of the naturally occurring CCR5Δ32 mutation, which results in truncation of the CCR5 protein and, as a consequence, an absence of CCR5 upon the cell surface. The mutation renders individuals highly resistant to infection by M-tropic HIV-1 viruses. Individuals lacking CCR5 on their cell surface show no overt physiological abnormalities, except, interestingly, a significant delay in their ability to reject renal transplants (Fischereder et al., 2001). The origins of such a mutation are still unaccounted for and a popular theory that it originally provided protection against bubonic plague in people of northern European ancestry has been questioned by recent experimentation (Mecsas et al., 2004). Likewise, the importance of CXCR4 in HIV-1 infection is supported by the finding that infection can be facilitated and maintained by viral strains exclusively utilizing CXCR4. In addition to these receptors, other coreceptors, such as CXCR6, CCR3, CCR8 and CX3CR1, have been shown to facilitate viral entry in vitro. A role for these receptors in HIV-1 pathogenesis has not been clearly demonstrated, although CCR3 is implicated in viral entry into the CNS.
The non-signalling chemokine receptors, DARC and D6
The two receptors Duffy antigen receptor for chemokines (DARC) and D6 have been termed ‘interceptors' as they constitutively facilitate the uptake of chemokines into cells without an ensuing signal (reviewed by Mantovani et al., 2003; Nibbs et al., 2003). The biology of DARC closely mirrors that of CXCR4 and CCR5 in that the protozoan Plasmodium vivax, the causative agent of human malaria, uses the receptor to enter erythrocytes. Duffy was originally defined in the 1950s as a minor red blood cell antigen and over 25 years elapsed before it was identified as a host factor required for vivax malaria by correlation of the absence of the Duffy antigen in most black Africans with natural resistance. Molecular characterization of DARC was accelerated by parallel studies from two different groups which identified an erythrocyte receptor for the chemokine CXCL8. They showed that the receptor was absent from Duffy negative individuals and unable to bind chemokine in the presence of anti-Duffy monoclonal antibodies, suggesting that the Duffy antigen and the erythrocyte chemokine receptor were one and the same. Subsequent cloning of the cDNA unveiled a putative 7TM protein with homology to chemokine receptors, which is additionally found on the venular endothelial cells of several organs. In keeping with the parallel with HIV-1 and chemokines, a mutation in the DARC promoter has been identified, which confers high-level resistance to infection by P. vivax, leading to a lack of expression in the erythroid lineage. This mutation is thought to be fixed at high levels in Africans, presumably due to positive selective pressure exerted by P. vivax malaria; indeed, more than 95% of Africans in regions where malaria is endemic and ∼70% of African Americans lack erythroid expression of DARC. The physiological role of DARC has been more difficult to unravel, as no signal appears to be transduced upon chemokine binding. Studies of mice in which the DARC gene has been deleted suggest that the receptor functions as a biological ‘sink' for chemokines, with both an anti-inflammatory role and an anti-angiogenic role. To date, there has been no confirmed association of DARC loss with susceptibility to disease, although some have postulated that it may predispose African American males to a greater incidence of prostate cancer, perhaps by reducing their ability to clear angiogenic chemokines (Lentsch, 2002).
Several inflammatory CC chemokines, including CCL2, CCL4, CCL5 and CCL11, bind with high affinity to the receptor D6, which is expressed at high levels on the surface of lymphatic endothelial cells and also by synctitial trophoblasts of the placenta. Despite much investigation, no intracellular signals have been reported to be transduced following chemokine binding, leading to the suggestion that D6 acts as a scavenger of inflammatory chemokines. D6 undergoes rapid constitutive internalization, enabling it to rapidly remove chemokines from the endothelial cell surface. The fate of the internalized chemokine appears to be proteolytic degradation, while the receptor is recycled back to the cell surface. Interestingly, D6 does not bind homeostatic chemokines such as CCL19 and CCL20, which are involved in cell trafficking to lymphoid organs. In this way, D6 is seen as a ‘gatekeeper', preserving the integrity of lymphoid tissue (Bonecchi et al., 2004).
Chemokines in disease
Extensive knowledge of chemokines and chemokine receptors has had an impact on the many diseases where inflammation is a component. The differential distribution of chemokine receptors on different leukocyte types provides a template for determining the role of individual chemokines in particular diseases. In patients and in disease models in animals, the levels of particular chemokines have been measured using immunoassays. Gene deletion and neutralizing antibodies have been employed to determine the importance of particular chemokines or receptors. Potential small-molecule chemokine receptor antagonists have been tested on human chemokine receptors in high-throughput screens of chemical libraries and promising candidate compounds have been discovered. Unfortunately, these are often of low affinity on the equivalent animal receptor owing to sequence differences in ligands and receptors. This has caused problems using traditional animal testing in the chemokine field.
To illustrate the approaches utilized, two diseases will be considered in this article, firstly, rheumatoid arthritis (RA) and secondly allergic asthma.
Rheumatoid arthritis
A marked infiltration of T- and B-lymphocytes, macrophages and neutrophils within the synovial lining is a hallmark of the inflammation clinically observed in RA, with Th1-associated chemokines thought to play a pivotal role (reviewed by Koch, 2005). In rheumatoid patients, CCL3/MIP-1α is readily detectable in synovial fluid and increased levels have been reported to correlate with the severity of RA. In rodent models of RA, the murine orthologue of CCL3 has been found within arthritic joints during the evolution of both collagen-induced arthritis and adjuvant-induced arthritis and neutralizing antibodies observed to provide a significant degree of protection, supportive of a role for the CCR1 axis in RA pathogenesis.
Likewise, CCL2/MCP-1, a potent chemoattractant for monocytes, has been shown to be produced by both synovial cells and infiltrated cells in RA with its specific receptor, CCR2, also attracting interest as a target for anti-inflammatory therapeutics. The murine orthologue of CCR2 is also upregulated in the joints from arthritic mice and the use of a mutated form of the CCL2 molecule with antagonist properties led to a marked reduction in both the symptoms and histopathological damage observed.
In contrast to peripheral blood T-cells, of which 40% are CXCR3 positive, almost all T-cells located within the inflammatory synovium of RA patients express CXCR3, which can be dissected further into increased numbers of memory CD3+/CD4+ and CD3+/CD8+ lymphocytes. This has led to the hypothesis that CXCR3 is important for T-lymphocyte homing to the RA joint, where the cells may orchestrate subsequent inflammatory events. Supportive of this, increased levels of CXCR3 agonists in the synovial tissue of RA patients have been reported in clinical studies. Thus, with respect to future RA treatments, at least three chemokine receptors present themselves as possible targets for the development of specific antagonists.
Allergic asthma
Allergic diseases have an underlying immune mechanism driven by Th2 lymphocytes in common with responses to helminth parasite infection. Allergic asthma has an early phase of bronchoconstriction resulting from activation of tissue mast cells and a delayed response associated with the activation of Th2 lymphocytes and a marked infiltration of granulocytes, characteristically eosinophils as the predominant cells. Eosinophils are able to release cationic proteins from granules and reactive oxygen species that are involved in host defence against helminth parasites. It is thought that allergy is an aberration of this host defence system and that these eosinophil-derived products are also important in the lung tissue damage and airway hyper-responsiveness, typical features of asthma. Several animal models of allergic airways disease, involving IL-5 gene deletion or neutralizing antibodies to IL-5, have supported the view that the eosinophil is important (IL-5 being necessary for eosinophil differentiation and proliferation in the bone marrow). There has been considerable interest in the endogenous chemoattractants responsible for recruiting eosinophils into the asthmatic lung and into other tissues during allergic reactions. In the early 1990s, when we became interested in this area, there were no known selective eosinophil chemoattractant agents. This led to our experiments on eotaxin, the last chemokine to be found by traditional biochemical techniques and the only chemokine to be discovered using in vivo bioassay (Jose et al., 1994). The generating system was also unique in being an in vivo system. This work was given as an oral communication at the 1993 Winter Meeting of the BPS held at Charing Cross Hospital. Sensitized guinea pigs were challenged with an aerosol of ovalbumin and bronchoalveolar lavage (BAL) fluid collected at intervals. The fluid was then injected intradermally into bioassay guinea-pigs previously injected intravenously with 111In-labelled eosinophils. BAL fluid samples taken 1–3 h after challenge were found to induce the accumulation of radiolabelled eosinophils in the skin. The activity was purified by HPLC using the skin assay throughout to locate the fractions containing chemoattractant. Subsequent peptide sequencing revealed a novel CC chemokine that we named eotaxin. Research in other laboratories revealed that eotaxin signalled through a unique receptor, CCR3. Further work revealed eotaxin orthologues in several species, including man, and two other human eotaxins were discovered with low sequence similarity, but the same selective agonist activity on CCR3.
The eotaxins are now known as eotaxin-1/CCL11, eotaxin-2/CCL24 and eotaxin-3/CCL26. Eotaxins and CCR3 have been the subject of intensive investigation, because knowledge of their biology provides the opportunity to block eosinophil trafficking and its sequelae selectively (reviewed in Pease & Williams, 2001). Eotaxin-1 was found to be expressed in the lungs in allergen-challenged animals in early studies. Eotaxin-1 was also found in the lungs of asthmatic patients and its expression correlated with lung dysfunction. Several companies who successfully developed small-molecule antagonists suspended work in this area because of the lack of efficacy of IL-5 antibodies on lung function in asthmatic patients, implying that the eosinophil is not important in this respect. However, follow-up work in these trials showed that the antibody depleted eosinophils from the blood, but not effectively from the lungs. Interestingly, in support of work in mouse models, the antibodies were found to suppress indices of airway remodelling, suggesting that eosinophils are important in this respect. CCR3 is highly expressed on eosinophils, but also on other cells involved in allergic reactions, such as basophils, mast cells and a subpopulation of Th2 lymphocytes. This may have a bearing on the potential clinical activity of CCR3 antagonists (see later section).
As mentioned above, allergic reactions have common features with host defence reactions to worm parasites and the eosinophil has probably evolved as an effector cell specialized for this role. Accordingly, local eotaxin-1 generation has been observed in several parasite infection models. Interestingly, the hookworm Necator americanus produces a protease that selectively cleaves and inactivates eotaxin-1, another example of subversion of host-defence reaction by pathogenic organisms (Culley et al., 2000).
Antagonism of chemokine receptors
The fact that chemokines and their receptors are implicated in the recruitment of leukocytes during the inflammatory phase of several clinically important diseases has led to intensive efforts directed at antagonizing the receptors. The serendipitous discovery that the recombinant production of CCL5/RANTES in Escherichia coli produced a potent antagonist of CCR1 paved the way for initial studies directed at antagonizing chemokine receptors, both in vitro and in vivo (reviewed in Proudfoot, 2002). The antagonistic properties of the protein result from retention of the initiating methionione residue at the amino-terminus. Met-RANTES, as it is commonly known, is also able to bind to CCR3 and CCR5 and functions as a competitive antagonist at all three receptors. Chemical modification of the amino-terminus by the addition of an aminoxypentane group leads to the second-generation molecule AOP-RANTES, with increased affinity for CCR1. Amino-terminally modified RANTES analogues have been shown to have beneficial effects in a variety of in vivo disease models, including mouse models of collagen induced arthritis (CIA) and allergic airway inflammation.
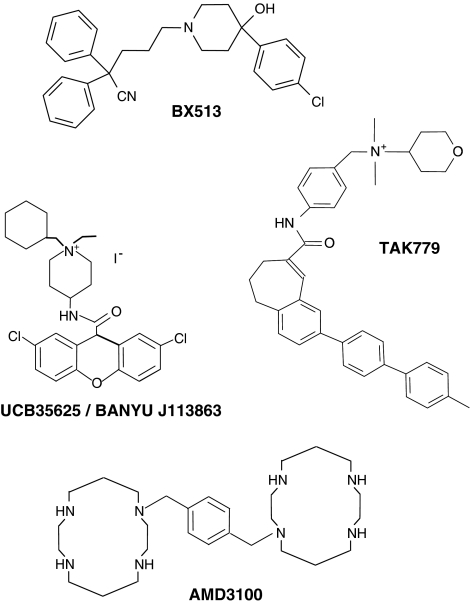
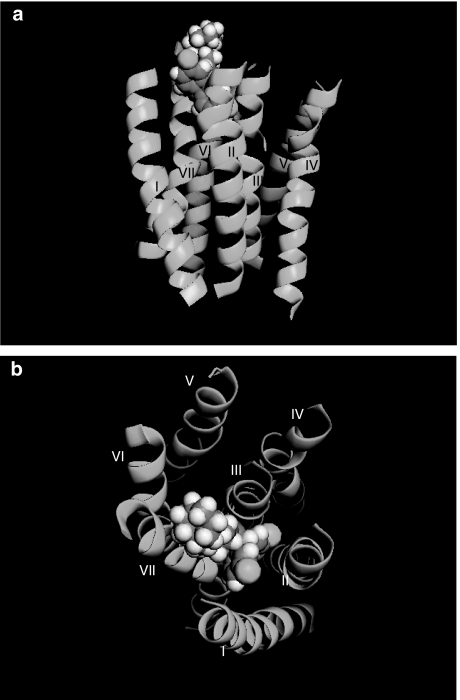
The first small-molecule chemokine receptor antagonists to be described in the literature were also antagonists of CCR1 and were identified by scientists at Berlex Biosciences. These molecules belonged to a family of 4-hydroxypiperidines and included the compound 2-2-diphenyl-5-(4-chlorophenyl)piperidinyl)valeronitrite (BX 513) (Figure 3), which exhibited low nanomolar potency in its ability to inhibit CCL3-induced responses from human CCR1 transfectants (Hesselgesser et al., 1998). Antagonists of several other chemokine receptors quickly followed. Fuelled by the discovery that CXCR4 and CCR5 served as co-receptors for HIV-1 entry, the respective antagonists phenylbis(methylene)-bis-(1,4,8,11-tetraazacyclotetradecane) (AMD3100) and N,N-dimethyl-N-(4-[[[2-(4-methylphenyl)-6,7-dihydro-5H-benzocyclohepten-8-yl]carbon-yl]amino]benzyl)-tetrahydro-2H-pyran-4-aminium chloride (TAK-779) were also described (Figure 3), the binding pocket for the latter compound being determined by an extensive mutagenesis programme (Dragic et al., 2000). Our own efforts focused upon a bi-specific small-molecule antagonist UCB 35625 (1-cycloheptenylmethyl-1-ethyl-4(2,7-dichloroxanthene-9-carboxamido)-piperidinium iodine) of CCR1 and CCR3 (originally discovered by scientists at Banyu Pharmaceutical Company) (Sabroe et al., 2000). Unlike other chemokine receptor antagonists identified at the time, the compound was a potent inhibitor of biological function at low nanomolar concentrations, despite an apparent inability to displace ligands from either receptor. This led us to hypothesize that the molecule exerts its antagonistic effects by interacting with amino acids within an intrahelical binding pocket of the receptor and stabilizes the receptor in an inactive conformation that allows ligand binding but not signal transduction. A recent mutagenesis study directly examined this hypothesis and found three intrahelical residues that, when mutated, conferred resistance to the compound in assays of chemotaxis (de Mendonca et al., 2005). Docking of the compound to this binding site suggests that access to the second and third transmembrane helices is likely to be severely restricted (Figure 4), a region postulated to interact with the chemokine receptor amino-terminus during the process of chemokine receptor activation.
Figure 3.
Chemical structures of some known chemokine receptor antagonists. The chemical structures of the CCR1 antagonist BX-471, the CCR1/3 dual antagonist UCB 35625, the CCR5//2 specific antagonist TAK779 and the CXCR4 antagonist AMD3100 are shown.
Figure 4.
Modeling of the UCB 35625–CCR1 interaction. The transmembrane helices of CCR1 (numbered) are shown as green ribbons with the specific antagonist UCB 35625 as a space-filled representation, docked into its intrahelical binding site. The helices are viewed from the side (a) and the extracellular face (b). Images were constructed using the program Pymol with the pdb file 1Y5D.
Despite several successes in the laboratory, relatively few small-molecule chemokine receptor antagonists have subsequently exhibited corresponding in vivo activity, let alone efficacy in a clinical setting. As mentioned earlier, a major drawback has been a lack of activity of antagonists developed against human receptors at blocking the corresponding rodent receptor, resulting in a bottleneck in the drug discovery pipeline. Without data from surrogate animal efficacy models, it is often difficult to justify further development of the drug, given the considerable risks and costs involved. Deficiencies in other areas, such as unwanted activity at the hERG channel with resulting cardiac complications, have also sounded the death knell for some programmes (Hodgson et al., 2004). Although there is, at present, a lack of understanding regarding the molecular basis of antagonist selectivity, it is hoped that recent efforts at molecular modelling coupled with receptor mutagenesis may shed some light upon this and aid the rational design of antagonists in the future.
Conclusion
Based on clinical observations, cell and molecular biology and elegant animal modelling, we now have a detailed picture of the roles of different leukocytes in inflammatory disease, their interactions with one another and with other cell types. Fundamental to this grand scheme is the process of cell migration. Extensive investigation of the chemokine field has provided the knowledge about how particular cell types move between the compartments of the body, in health and disease. Small molecules that can selectively block this process to provide potential selective anti-inflammatory therapy have been developed. It has to be said that this aspiration is largely unfulfilled, for some of the reasons discussed in this article. The ability to produce selective therapeutic agents has exposed our ignorance of the relative importance of different inflammatory mechanisms in disease processes in man. We feel that these obstacles will be overcome, but will demand more precise knowledge of such mechanisms. An important source of this knowledge will be data obtained from the clinical trials of selective agents. Despite the attraction of selective intervention, it may be that combinations of chemokine receptor antagonists will ultimately provide the effective treatments for some inflammatory diseases in the future.
Acknowledgments
We thank Peter Jose for helpful discussions and Enid Goodman for her help in preparing the manuscript. We are also grateful to Asthma U.K., the Wellcome Trust, the Medical Research Council, the British Heart Foundation and the Arthritis Research Campaign for their funding of our research.
Glossary
- CIA
collagen-induced arthritis
- CNS
central nervous system
- DARC
Duffy antigen receptor for chemokines
- PI3K
phosphoinositide 3 kinase
- PMN
polymorphonuclear leukocyte
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- RA
rheumatoid arthritis
References
- BERGER E.A., MURPHY P.M., FARBER J.M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- BLANPAIN C., DORANZ B.J., BONDUE A., GOVAERTS C., DE LEENER A., VASSART G., DOMS R.W., PROUDFOOT A., PARMENTIER M. The core domain of chemokines binds CCR5 extracellular domains while their amino terminus interacts with the transmembrane helix bundle. J. Biol. Chem. 2003;278:5179–5187. doi: 10.1074/jbc.M205684200. [DOI] [PubMed] [Google Scholar]
- BLEUL C.C., FARZAN M., CHOE H., PAROLIN C., CLARK-LEWIS I., SODROSKI J., SPRINGER T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- BONECCHI R., BIANCHI G., BORDIGNON P.P., D'AMBROSIO D., LANG R., BORSATTI A., SOZZANI S., ALLAVENA P., GRAY P.A., MANTOVANI A., SINIGAGLIA F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1 s) and Th2 s. J. Exp. Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONECCHI R., LOCATI M., GALLIERA E., VULCANO M., SIRONI M., FRA A.M., GOBBI M., VECCHI A., SOZZANI S., HARIBABU B., VAN DAMME J., MANTOVANI A. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J. Immunol. 2004;172:4972–4976. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- CRONSHAW D.G., OWEN C., BROWN Z., WARD S.G. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for T lymphocyte chemotaxis. J. Immunol. 2004;172:7761–7770. doi: 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- CULLEY F.J., BROWN A., CONROY D.M., SABROE I., PRITCHARD D.I., WILLIAMS T.J. Eotaxin is specifically cleaved by hookworm metalloproteases preventing its action in vitro and in vivo. J. Immunol. 2000;165:6447–6453. doi: 10.4049/jimmunol.165.11.6447. [DOI] [PubMed] [Google Scholar]
- DE MENDONCA F.L., DA FONSECA P.C., PHILLIPS R.M., SALDANHA J.W., WILLIAMS T.J., PEASE J.E. Site-directed mutagenesis of CC chemokine receptor 1 reveals the mechanism of action of UCB 35625, a small molecule chemokine receptor antagonist. J. Biol. Chem. 2005;280:4808–4816. doi: 10.1074/jbc.M412267200. [DOI] [PubMed] [Google Scholar]
- DRAGIC T., TRKOLA A., THOMPSON D.A., CORMIER E.G., KAJUMO F.A., MAXWELL E., LIN S.W., YING W., SMITH S.O., SAKMAR T.P., MOORE J.P. A binding pocket for a small molecule inhibitor of HIV-1 entry within the transmembrane helices of CCR5. Proc. Natl. Acad. Sci. U.S.A. 2000;97:5639–5644. doi: 10.1073/pnas.090576697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISCHEREDER M., LUCKOW B., HOCHER B., WUTHRICH R.P., ROTHENPIELER U., SCHNEEBERGER H., PANZER U., STAHL R.A., HAUSER I.A., BUDDE K., NEUMAYER H., KRAMER B.K., LAND W., SCHLONDORFF D. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- HANDEL T.M., JOHNSON Z., CROWN S.E., LAU E.K., PROUDFOOT A.E. Regulation of protein function by glycosaminoglycans–as exemplified by chemokines. Annu. Rev. Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- HERNANZ-FALCON P., RODRIGUEZ-FRADE J.M., SERRANO A., JUAN D., DEL SOL A., SORIANO S.F., RONCAL F., GOMEZ L., VALENCIA A., MARTINEZ A.C., MELLADO M. Identification of amino acid residues crucial for chemokine receptor dimerization. Nat. Immunol. 2004;5:216–223. doi: 10.1038/ni1027. [DOI] [PubMed] [Google Scholar]
- HESSELGESSER J., NG H.P., LIANG M., ZHENG W., MAY K., BAUMAN J.G., MONAHAN S., ISLAM I., WEI G.P., GHANNAM A., TAUB D.D., ROSSER M., SNIDER R.M., MORRISSEY M.M., PEREZ H.D., HORUK R. Identification and characterization of small molecule functional antagonists of the CCR1 chemokine receptor. J. Biol. Chem. 1998;273:15687–15692. doi: 10.1074/jbc.273.25.15687. [DOI] [PubMed] [Google Scholar]
- HODGSON S., CHARLTON S., WARNE P. Chemokines and drug discovery. Drug News Perspect. 2004;17:335–338. [PubMed] [Google Scholar]
- HOLMES W.E., LEE J., KUANG W.-J., RICE G.C., WOOD W.I. Structure and functional expression of a human interleukin-8 receptor. Science. 1991;253:1278–1283. doi: 10.1126/science.1840701. [DOI] [PubMed] [Google Scholar]
- JOSE P.J., GRIFFITHS-JOHNSON D.A., COLLINS P.D., WALSH D.T., MOQBEL R., TOTTY N.F., TRUONG O., HSUAN J.J., WILLIAMS T.J. Eotaxin: a potent eosinophil chemoattractant cytokine detected in a guinea-pig model of allergic airways inflammation. J. Exp. Med. 1994;179:881–887. doi: 10.1084/jem.179.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOCH A.E. Chemokines and their receptors in rheumatoid arthritis: future targets. Arthritis Rheum. 2005;52:710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J., SHENOY S.K. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- LEMAY J., MARULLO S., JOCKERS R., ALIZON M., BRELOT A.2005On the dimerization of CCR5 Nat Immunol 6535author reply 535–536. [DOI] [PubMed] [Google Scholar]
- LENTSCH A.B. The Duffy antigen/receptor for chemokines (DARC) and prostate cancer. A role as clear as black and white. FASEB J. 2002;16:1093–1095. doi: 10.1096/fj.02-0066hyp. [DOI] [PubMed] [Google Scholar]
- LEVER R., PAGE C.P. Novel drug development opportunities for heparin. Nat. Rev. Drug Discov. 2002;1:140–148. doi: 10.1038/nrd724. [DOI] [PubMed] [Google Scholar]
- LI Z., JIANG H., XIE W., ZHANG Z., SMRCKA A.V., WU D. Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- LOETSCHER P., PELLEGRINO A., GONG J.H., MATTIOLI I., LOETSCHER M., BARDI G., BAGGIOLINI M., CLARK-LEWIS I. The ligands of CXC chemokine receptor 3, I-TAC, Mig and IP10, are natural antagonists for CCR3. J. Biol. Chem. 2001;276:2986–2991. doi: 10.1074/jbc.M005652200. [DOI] [PubMed] [Google Scholar]
- LU Z.H., WANG Z.X., HORUK R., HESSELGESSER J., LOU Y.C., HADLEY T.J., PEIPER S.C. The promiscuous chemokine binding profile of the Duffy antigen/receptor for chemokines is primarily localized to sequences in the amino-terminal domain. J. Biol. Chem. 1995;270:26239–26245. doi: 10.1074/jbc.270.44.26239. [DOI] [PubMed] [Google Scholar]
- MANTOVANI A., BONECCHI R., MARTINEZ F.O., GALLIERA E., PERRIER P., ALLAVENA P., LOCATI M. Tuning of innate immunity and polarized responses by decoy receptors. Int. Arch. Allergy Immunol. 2003;132:109–115. doi: 10.1159/000073711. [DOI] [PubMed] [Google Scholar]
- MARTIN C., BURDON P.C.E., BRIDGER G., GUTIERREZ-RAMOS J-C., WILLIAMS T.J., RANKIN S.M. The balance between chemokines acting via CXCR4 and CXCR2 determines the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- MECSAS J., FRANKLIN G., KUZIEL W.A., BRUBAKER R.R., FALKOW S., MOSIER D.E. Evolutionary genetics: CCR5 mutation and plague protection. Nature. 2004;427:606. doi: 10.1038/427606a. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G., RAMSAY D., PASCAL G., CARRILLO J.J. GPCR dimerization. Life Sci. 2003;74:181–188. doi: 10.1016/j.lfs.2003.09.005. [DOI] [PubMed] [Google Scholar]
- MURPHY P.M. Molecular mimicry and the generation of host defense protein diversity. Cell. 1993;72:823–826. doi: 10.1016/0092-8674(93)90571-7. [DOI] [PubMed] [Google Scholar]
- MURPHY P.M., TIFFANY H.L. Cloning of complementary DNA encoding a functional human interleukin-8 receptor. Science. 1991;253:1280–1283. doi: 10.1126/science.1891716. [DOI] [PubMed] [Google Scholar]
- NEEL N.F., SCHUTYSER E., SAI J., FAN G.H., RICHMOND A. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 2005;16:637–658. doi: 10.1016/j.cytogfr.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIBBS R., GRAHAM G., ROT A. Chemokines on the move: control by the chemokine ‘interceptors' Duffy blood group antigen and D6. Semin Immunol. 2003;15:287–294. doi: 10.1016/j.smim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- OPPENHEIM J.J., ZACHARIAE C.O.C., MUKAIDA N., MATSUSHIMA K. Properties of the novel proinflammatory supergene ‘intercrine' cytokine family. Ann. Rev. Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- PAAVOLA C.D., HEMMERICH S., GRUNBERGER D., POLSKY I., BLOOM A., FREEDMAN R., MULKINS M., BHAKTA S., MCCARLEY D., WIESENT L., WONG B., JARNAGIN K., HANDEL T.M. Monomeric monocyte chemoattractant protein-1 (MCP-1) binds and activates the MCP-1 receptor CCR2B. J. Biol. Chem. 1998;273:33157–33165. doi: 10.1074/jbc.273.50.33157. [DOI] [PubMed] [Google Scholar]
- PEASE J.E., WILLIAMS T.J. Eotaxin and asthma. Curr. Opin. Pharmacol. 2001;1:248–253. doi: 10.1016/s1471-4892(01)00044-3. [DOI] [PubMed] [Google Scholar]
- PROUDFOOT A.E. Chemokine receptors: multifaceted therapeutic targets. Nat. Rev. Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-FRADE J.M., VILA-CORO A.J., DE ANA A.M., ALBAR J.P., MARTINEZ A., MELLADO M. The chemokine monocyte chemoattractant protein-1 induces functional responses through dimerization of its receptor CCR2. Proc. Natl. Acad. Sci. U.S.A. 1999a;96:3628–3633. doi: 10.1073/pnas.96.7.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-FRADE J.M., VILA-CORO A.J., MARTIN A., NIETO M., SANCHEZ-MADRID F., PROUDFOOT A.E., WELLS T.N., MARTINEZ A., MELLADO M. Similarities and differences in RANTES- and (AOP)-RANTES-triggered signals: implications for chemotaxis. J. Cell. Biol. 1999b;144:755–765. doi: 10.1083/jcb.144.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SABROE I., PECK M.J., JAN VAN KEULEN B., JORRITSMA A., SIMMONS G., CLAPHAM P.R., WILLIAMS T.J., PEASE J.E. A small molecule antagonist of the chemokine receptors CCR1 and CCR3: potent inhibition of eosinophil function and CCR3-mediated HIV-1 entry. J. Biol. Chem. 2000;275:25985–25992. doi: 10.1074/jbc.M908864199. [DOI] [PubMed] [Google Scholar]
- SAMANTA A.K., OPPENHEIM J.J., MATSUSHIMA K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J.Biol.Chem. 1990;265:183–189. [PubMed] [Google Scholar]
- SCHALL T.J., BACON K., CAMP R.D.R., HERBERT C., GOEDDEL D.V. Human macrophage inflammatory protein α (MIP-1α) and MIP-1β chemokines attract distinct populations of lymphocytes. J.Exp.Med. 1993;177:1821–1825. doi: 10.1084/jem.177.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THELEN M., BAGGIOLINI M. Is dimerization of chemokine receptors functionally relevant. Sci. STKE. 2001;2001:PE34. doi: 10.1126/stke.2001.104.pe34. [DOI] [PubMed] [Google Scholar]
- THELEN M., PEVERI P., KERNEN P., VON TSCHARNER V., WALZ A., BAGGIOLINI M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988;2:2702–2706. [PubMed] [Google Scholar]
- VAN HAASTERT P.J., DEVREOTES P.N. Chemotaxis: signalling the way forward. Nat Rev Mol Cell Biol. 2004;5:626–634. doi: 10.1038/nrm1435. [DOI] [PubMed] [Google Scholar]
- WESTWICK J., LI S.W., CAMP R.D. Novel neutrophil-stimulating peptides. Immunol. Today. 1989;5:146–147. doi: 10.1016/0167-5699(89)90164-3. [DOI] [PubMed] [Google Scholar]
- WESTWICK J., LINDLEY I.J.D., KUNKEL S.L.1990Chemotactic cytokines Biology of the inflammatory peptide supergene family. In: Advances in Experimental Medicine and BiologyVol 305. New York, NY: Plenum Press; [PubMed] [Google Scholar]
- ZLOTNIK A., YOSHIE O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]