Abstract
This review examines our current understanding of the roles of some of the best known neuropeptides that have played major roles in our combined research programmes. Evidence obtained from over 75 years of research shows involvement of these transmitters in a wide range of organs relevant to cardiovascular, respiratory, cutaneous, neuronal and intestinal systems. There is an increasing understanding of the mechanisms involved in the release of the peptides (substance P and calcitonin gene-related peptide (CGRP)) from sensory nerves or, neuropeptide Y (NPY) from sympathetic, parasympathetic and nonadrenergic, noncholinergic (NANC) neurons. Responses in target tissues result from interactions of the neuropeptides, or related forms, with specific G-protein coupled receptors (GPCRs or 7 transmembrane-spanning, 7TM proteins) that belong to either rhodopsin-like, class 1 (neurokinin (NK) and NPY Y receptors) or secretin-like, class 2 GPCRs (CGRP receptors). The majority of receptors activated by our chosen neuropeptides are now cloned, with knowledge of preferred agonists and selective antagonists for many receptor subtypes within these families. The study of neuropeptides in animal models has additionally revealed physiological and pathophysiological roles that in turn have led to the ongoing development of new drugs, through utilization predominantly of antagonist activities.
Keywords: Neuropeptides, neurokinins, calcitonin gene-related peptide, neuropeptide Y, receptor mechanisms
Introduction
Neuropeptides are contained in and released from a wide range of nerves. Chemically distinct, they exhibit characteristic patterns of localization within the peripheral and central nervous system and possess the ability to stimulate a range of diverse biological activities. The concept of cotransmission within the autonomic nervous system was first suggested by Burnstock (1976), through his study of ATP in sympathetic nerves (see also Burnstock, this issue) followed by the realization that neuropeptides were also contained in both sympathetic and cholinergic nerves. However, while noradrenaline is generally considered to be the classical transmitter in sympathetic nerves and acetylcholine in parasympathetic nerves, the predominant neurotransmitters released from the sensory component of nonadrenergic, noncholinergic (NANC) nerves have been found to be one or more neuropeptides.
Neuropeptide research remains a highly active area despite the fact that evidence for pivotal roles for these peptides is often lacking, due largely to difficulties associated with the development of agents that act in a selective manner to either enhance or block receptor activity, or peptide synthesis or degradation. Furthermore, the field has become increasingly complex, with neuronal plasticity exhibited during physiological events such as development and ageing, and especially in pathophysiological situations where changes in neuropeptide expression patterns and receptor density are regularly observed. It is now realized that structurally identical (such as calcitonin gene-related peptide (CGRP)) or similar peptides (such as peptide YY (PYY) as opposed to neuropeptide Y (NPY)) can also be expressed and released from non-neuronal cells, which can act via the same (or more distant) receptors as their neuronal counterparts. This adds another level of complexity to the understanding of their combined functional significance. Despite this, the knowledge gained in recent years, which has depended on fundamental research, has also resulted in a range of clinical trials of nonpeptide antagonists.
Substance P (SP) was the first neuropeptide to be discovered and was recognized as a sensory neurotransmitter by Lembeck (1953). Other neuropeptides have more recently secured their places in the archives through their potent and varied biological activities, distinct G-protein coupled receptor (GPCR) activation and potential therapeutic targets. In this review, we have utilized our combined experience in neuropeptide research to outline the key advances in our knowledge of the sensory nerve-derived neuropeptides, SP and CGRP, and the nonsensory neuropeptide, NPY (and its hormonal analogue, PYY), that have led to our current understanding of their roles in biology and disease. The structures of these peptides and a brief summary of their pharmacology are shown in Table 1.
Table 1. A summary of the pharmacology and structures of the human neuropeptides discussed in this review.
| Peptide | Pharmacology | Structure |
|---|---|---|
| Substance P (SP) | ||
| Amino acids: 11 Discovered: 1931 Localization: Sensory nerves Related mammalian neuropeptides: Neurokinin A, neurokinin B Related non-neural peptides: Hemokinins, endokinins | Receptors: NK1, NK2, NK3 Antagonists: range of nonpeptide antagonists available for each receptor Therapeutic use: NK1 antagonist in emesis Positive results in clinical trials: NK1 antagonist in depression | Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met-NH2 |
| α-Calcitonin gene related peptide (CGRP) | ||
| Amino acids: 37 Discovered: 1983 Related mammalian neuropeptides: βCGRP, intermedin Related non-neural peptides: Adrenomedullin, amylin | Receptors: CGRP1, CGRP2(?), AM2 (?) Antagonists: CGRP8–37 (peptide) BIBN4096BS (nonpeptide) Positive results in clinical trials: BIBN4096BS in migraine | Ala-Cys-Asp-Thr-Ala-Thr-Cys-Val-Thr-His-Arg-Leu-Ala-Gly-Leu-Leu-Ser-Arg-Ser-Gly-Gly-Val-Val-Lys-Asn-Asn-Phe-Val-Pro-Thr-Asn-Val-Gly-Ser-Lys-Ala-Phe-NH2 |
| Neuropeptide Y (NPY) | ||
| Amino acids: 36 Discovered: 1982 Localization: Sympathetic, cholinergic, sensory, enteric and central neurons Related nonmammalian neuropeptides: PY in bony fish; NPF in D. melanogaster Related non-neural peptides: Peptide YY (PYY), PYY(3–36) and pancreatic polypeptide (PP) | Receptors: Y1, Y2, Y4, Y5 Antagonists: Y1 BIBO3304; Y2 BIIE0246; Y5 L-152,804 Positive results in clinical trials: none to date; Y1 and Y5 antagonists were ineffective against obesity; PYY(3–36) is in development as an anti-obesity agent | Tyr-Pro-Ser-Lys-Pro-Asp-Asn-Pro-Gly-Glu-Asp-Ala-Pro-Ala-Glu-Asp-Met-Ala-Arg-Tyr-Tyr-Ser-Ala-Leu-Arg-His-Tyr-Ile-Asn-Leu-Ile-Thr-Arg-Gln-Arg-Tyr-NH2 |
Sensory nerve-derived neuropeptides
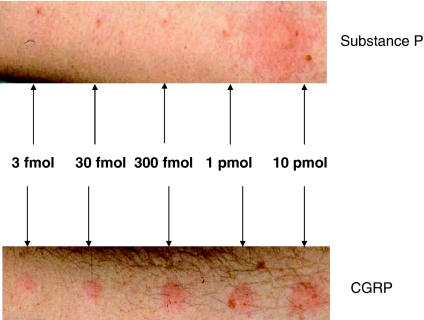
The sensory nerves that contain and release neuropeptides are primarily unmyelinated sensory C-fibres and myelinated Aδ-fibres. They provide a dense innervation to most organs and tissues and, in particular, to blood vessels, where perivascular nerves often terminate in close association with endothelial cells. The classical demonstration of their activity is shown by the flare component of the ‘triple response' in human skin (Lewis, 1927), that is best observed visually in response to insect bites and other punctate injuries. The response comprises a wheal (oedema formation), local reddening (increased blood flow) and a flare (visible in skin of Caucasians for up to several centimetres around the point of injury, due to a spreading increased blood flow). It can also be seen in response to intradermal injection of many mediators including SP and histamine (Figure 1). In skin, histamine released from mast cells acts as a major sensory nerve activating mechanism, due to the presence of H1 receptors on skin mast cells (see Figures 1 and 2). The flare is a consequence of a sensory nerve-mediated axon reflex and involves antidromic stimulation of sensory nerves to release neuropeptides that include SP and CGRP. Histamine appears to play a much smaller role in activating sensory nerves in tissues other than skin and there is now tremendous interest in determining how sensory nerves are activated in vivo. It is now understood that axon reflexes, involving sensory nerves occur in most tissues of the body and, in certain tissues, are central to the proposed proinflammatory role of neuropeptides. Furthermore, there is recognition of a ‘sensory-motor' activity in these nerves (Maggi, 1991).
Figure 1.
Effect of SP and CGRP on erythema in Caucasian human skin. The pictures were taken 10 min after injection of either increasing doses of SP (upper trace) or increasing doses of CGRP (lower trace) in the volar aspect of the same human forearm. The highest dose of SP (10 pmol) induced a typical triple response consisting of oedema formation (wheal) at the injection site, local reddening and a flare, due to a sensory nerve-mediated axon reflex that spread around the flare. Mast cell-derived histamine plays a major role in this response as it is blocked by histamine H1 antagonists. Lower doses of SP induced a minimal response. By comparison, all doses of CGRP induced erythema. While the response to SP was transient, that to CGRP was long lasting (not shown).
Figure 2.
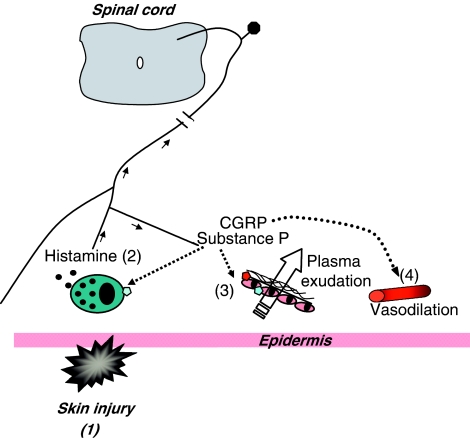
Schematic diagram depicting events following injury to skin. Injury (1) is often followed by activation of mast cells and release of histamine. Histamine then activates sensory neurones via H1 receptors (2) to stimulate orthodromic stimulation to spinal cord and antidromic stimulation to surrounding skin that leads to neuropeptide release. Substance P can act via NK1 receptors on endothelial cells of postcapillary venules or possibly by stimulating further histamine release from mast cells, to mediate plasma extravasation at the site of trauma (3). Substance P can also increase blood flow; however, it is CGRP, acting via CGRP receptors that is best known as a potent and long lasting microvascular dilator (4) and probably the mediator of the flare that surrounds the site of injury.
Capsaicin, the pungent agent from chilli peppers, has played a seminal role in sensory neuropeptide research. For almost 40 years it has been known to activate sensory nerves and the original finding was followed 10 years later by a more detailed study, showing that repeated application of capsaicin leads to desensitization and under some circumstances sensory nerve degeneration (Jancsó et al., 1977). Evidence from capsaicin depletion studies in rodents especially has done much to further the concept that sensory nerves play a role in inflammatory disease with respect to both pain and inflammatory mechanisms in animal models and humans. It is now known that the transient receptor potential vanilloid 1 (TRPV1) is activated by vanilloids such as capsaicin (Caterina et al., 1997). However, the first ‘capsaicin antagonist', capsazepine, was described 5 years before the receptor was formally identified (Bevan et al., 1992). Ligands for TRPV1, especially antagonists, are now under intense study as novel analgesic/anti-inflammatory therapies. The endogenous agonists for this receptor include protons, noxious heat and a range of mediators, including molecules as diverse as cannabinoids and hydrogen sulphide, although their relative importance in pathology is unclear. Furthermore, presynaptic/prejunctional receptors on sensory nerves can modulate neuropeptide release. These receptors include those for 5-hydroxytryptamine (5-HT1 receptor), γ-aminobutyric acid (GABAB receptor), histamine (H3 receptor), and also to neuropeptides such as NPY, endogenous opioid peptides, somatostatin, vasoactive intestinal peptide and galanin (Maggi, 1991). Again, their pathological importance is presently not clear, but is under active investigation.
SP and related peptides
In the year the BPS was founded, SP was characterized by von Euler and Gaddum, through use of bioassays to detect biological activity in extracts of equine intestine and brain. It is a member of the tachykinin family (referring to a rapid contractile effect upon smooth muscle) and later, these peptides were recognized by their common carboxy-terminal amino-acid sequence (see Table 1; Maggi, 1995). Other mammalian tachykinins include neurokinin A (NKA), neuropeptide K (NPK), neuropeptide-γ (NPγ) and neurokinin B (NKB; Table 1). Two separate genes encode for this family, the preprotachykinin I (PPT-I) and preprotachykinin II (PPTII).
Biological activities of neurokinins are mediated via NK receptors, which are divided into at least three types, NK1, NK2 and NK3, all of which are G-protein linked, with species homologues for each receptor type (Maggi, 1995). SP is the preferred binding ligand for NK1 receptors, NKA for NK2 receptors and NKB for NK3 receptors, although they do not act as exclusive agonists for the respective receptors. It is now realized that there are other members of the tachykinin family, namely hemokinins and endokinins (Page, 2004). These are found in non-neuronal tissues and may have been previously identified as non-neuronal sources of SP, due to the crossreactivity of antibodies used for immuno-histochemical studies. They act upon the canonical NK receptors, especially the NK1 receptor, to mediate their activities. The full relevance of these non-neuronal agonists is not yet known but will undoubtedly be a focus of future investigations. Interestingly, NK1 knockout mice have now been used in a range of studies relating to inflammation and pain and give similar responses to those observed in mice lacking the PPT-I gene that encodes SP and NKA. These mice breed and reproduce normally while predictably showing reduced responses to certain inflammatory stimuli and to stimuli inducing moderate pain.
SP is commonly found in C-fibre sensory nerves and is well known for a wide range of peripheral and central activities that include inflammation and pain, emesis and mood. It is a vasodilator but is perhaps better known for its ability to increase microvascular permeability leading to inflammatory oedema formation (Holzer, 1998). SP affects microvascular permeability via two main mechanisms: via endothelial NK1 receptors to increase plasma leakage and via mast cells, which SP activates to release oedema-inducing mast cell amines (Foreman et al., 1983). Surprisingly, it is now questioned whether, in human conditions, endogenous levels of SP are sufficient to increase microvascular permeability. NKA, which lacks a basic amino acid as its N terminus, only acts via NK1 receptors to mediate increased microvascular permeability.
The tachykinins, and the NK1 receptor, especially, were proposed, from studies in animal models, to be involved in a range of inflammatory and pain conditions. Thus, tachykinins and their receptors are involved in arthritis (Keeble & Brain, 2004) and the NK1 receptor is upregulated at inflamed sites in a range of tissues that include joints (arthritis) and intestine (inflammatory bowel disease). The first nonpeptide NK1 antagonist, CP96,345, was discovered by Pfizer and this compound was soon superseded by other NK1 antagonists with improved selectivity and oral bioavailability. However, published results to date are disappointing and it is now generally accepted that NK1 antagonists do not have a beneficial effect in the treatment of human inflammatory pain conditions such as osteoarthritis. One possible reason for this failure is that, as these agents penetrate the CNS relatively poorly, they were not able to establish an adequate blockade of NK1 receptors at the doses used (Urban & Fox, 2000).
During the 1980s and 1990s, the neurogenic theory of migraine postulated that neurogenic oedema formation was an essential pathological feature in migraine. A number of nonpeptide receptor antagonists for the major vasoactive receptor for SP (the NK1 receptor) were shown to inhibit neurogenic oedema formation in the dura following trigeminal ganglion stimulation in the rat. Surprisingly, results from clinical studies for migraine have shown little beneficial effect of these agents. This may be because plasma protein extravasation may not occur in humans during migraine attacks. It is now suggested that the functional sensory nerves in the trigeminal system in humans include A-fibres that contain and release CGRP rather than SP and that this component of the trigemino-vascular system may be the more important factor in migraine (see below).
Nevertheless, two NK1 antagonists (aprepitant and L-759274) have been shown to have significant beneficial effects in placebo-controlled clinical trials of patients with moderate and severe depression (Kramer et al., 2004). The incidence of side effects is low, indicating an important therapeutic potential of these compounds. However, the first registered clinical use for NK1 antagonists has been found in the treatment of emesis, associated with cancer therapies, following results in animal models, involving NK1 receptors in emesis. The addition of an NK1 antagonist to standard antiemetic therapy improved the incidence of emesis side effects in the acute, and especially in the delayed phase, by approximately 20% (Chawla et al., 2003). The possibility that NK2 and NK3 receptor antagonists may also have therapeutic roles, especially in intestinal inflammation, has also been debated. Furthermore, dual NK1/NK2 antagonists have been proposed as treatments for inflammatory bowel disease and for asthma. Thus, 75 years after their discovery, the tachykinins have been shown to possess biological activity in a wide variety of physiological and pathological systems, but the therapeutic potential of the tachykinin antagonists has, to date, not been realized when compared with expectations.
CGRP
The 37 amino-acid peptide, CGRP (Table 1) is produced following the expression of CGRP mRNA from the calcitonin gene in a tissue specific manner in nerves (Brain & Grant, 2004). This widespread form of CGRP is known as αCGRP (or CGRPI). βCGRP (or CGRPII) is also produced, for example, in the enteric nerves of the intestine, and has >90% structural similarity to αCGRP, although it is formed from a distinct gene. CGRP belongs to a family that include adrenomedullin, predominantly found in non-neuronal tissues (such as vascular tissues) and amylin, produced in the β cells of the pancreas. They share 25–40% structural homology and some overlapping biological activities. CGRP has a wide distribution throughout the central and peripheral nervous systems and is usually colocalized in C-fibres with a range of other peptides that include SP. A new member of the family has been more recently described, intermedin, which exists as a 40- and as a 47 amino-acid peptide and also has a wide distribution, with interestingly, a presence in the anterior pituitary. The structure of intermedin is highly conserved across species, but has only about 15% sequence similarity to the other CGRP peptides (Roh et al., 2004).
CGRP has been suggested to be involved in an increasing number of biological activities. It was first described as a vasodilator, and is active throughout the cardiovascular system but with the most pronounced effects in the microcirculation. This was initially observed in skin where the intradermal injection of low femtomolar concentrations led to an increased blood flow, (see Figure 1) while larger amounts caused increases in skin blood flow that in Caucasian skin could be observed for several hours (Brain et al., 1985). CGRP causes facial flushing when given intravenously at low doses and only reduces blood pressure when given at higher doses (Brain & Grant, 2004). These findings have led to the assumption that endogenous CGRP will mainly act close to its site of release in vivo. Evidence suggests that CGRP does not play a pivotal role in the physiological regulation of blood pressure, although it is suggested that changes in CGRP levels are involved in the pathophysiology of a number of vascular disorders including Raynaud's disease and migraine (Brain & Grant, 2004). Plasma levels of CGRP are low in normal subjects, but increased in a number of diseases that include migraine. In addition, they are also increased in pregnancy and it is suggested that CGRP receptor components are lacking in pre-eclamptic pregnancies, usually associated with high blood pressure (Dong et al., 2005).
The study of CGRP receptors resulted in the discovery of a unique family of receptors. Pharmacological analysis led to the initial classification of two receptors, CGRP1 and CGRP2. The CGRP1 receptor is now considered to be the primary and predominant cardiovascular receptor. A fragment of CGRP, CGRP(8−37), was found to be a selective antagonist for this receptor and is still widely used (Brain & Grant, 2004). The ligands used to classify the CGRP2 receptor are now considered to lack selectivity and the knowledge of the relevance of this receptor, especially at the molecular level, remains poor. The CGRP family of receptors are each composed of the same GPCR, calcitonin receptor-like receptor CL, which is involved in ligand binding, and which interacts in an essential manner with a single membrane spanning receptor activity modifying protein (RAMP; McLatchie et al., 1998). This interaction with a RAMP is critical for the phenotype of the receptor and its presence at the cell surface in an active form. This heterodimer complex links to a further CGRP-receptor component (RCP) that is considered to promote receptor coupling and signal transduction. Three RAMPs (RAMP1, RAMP2 and RAMP3) are known. Thus, when CL interacts with RAMP1, the heterodimer functions as a CGRP receptor. Interaction of CL with RAMP2 produces an adrenomedullin (AM) receptor. CL with RAMP3 leads to a CGRP/AM receptor, of which least is known functionally. Vascular relaxation is mediated via activation of the CGRP receptor (CL/RAMP1). This is effected via a range of signalling mechanisms that principally include either nitric oxide (NO)-dependent endothelium-dependent mechanisms or cAMP-mediated endothelium-independent pathways. David Poyner and his co-workers (Hay et al., 2004) have systematically analysed the pharmacology of these receptors in cell systems.
One particular problem in the analysis of CGRP receptors has been their marked species differences. Thus, the low molecular weight, nonpeptide CGRP antagonist, BIBN4096BS, is a potent competitive antagonist of the human and marmoset CGRP1 receptor, but has approximately 200-fold less affinity in rodent tissues (Doods et al., 2000). A structurally similar compound, Compound 1, has also been utilized in bioassay studies, but is a weak antagonist of vascular responses mediated via human and guinea-pig CGRP receptors. A further nonpeptide CGRP1 receptor antagonist, SB-273779, is much less potent than BIBN4096BS in human tissues, but it has the advantage of being the first CGRP antagonist that is not species dependent (Aiyar et al., 2001).
The most exciting data that relates to studies of CGRP in disease obtained so far relates to migraine. Migraine is a common condition, experienced by up to 15% of the population (predominantly females) at some point, and is composed of a debilitating primary headache with a unilateral pulsating pain and a range of associated symptoms. CGRP is localized to the trigeminovascular system, where it has potent vasoactive and nociceptive effects. BIBN4096BS was shown to inhibit trigeminal nerve-mediated neurogenic vasodilatation in rodents and primates (Doods et al., 2000). It is proposed that intracranial, extracerebral blood vessels (such as the middle meningeal artery and related arterioles) that supply the dura mater layer, exhibit vasodilatation that is causally related to the pain. However, it is now becoming increasingly likely that CGRP is not acting purely via its vasodilator activity. There is evidence for a sensory neuronal site of action for CGRP in migraine (Storer et al., 2004). Results showing that CGRP may affect neural activity directly are increasing, compatible with earlier work in other systems, and supported by the recent demonstration of the abundant presence of CGRP receptors on sensory nerves (Cottrell et al., 2005).
The CGRP antagonist, BIBN4096BS, given by the intravenous route, has been shown to be successful in phase II clinical trials for migraine (Olesen et al., 2004) and a potent, orally available, CGRP antagonist is now awaited. It is perhaps surprising, considering the large number of nonpeptide, orally active, NK1 antagonists, that more non-peptide CGRP antagonists are not available. However, it would appear, following research programmes in several pharmaceutical companies, that it is difficult to develop a suitable orally active CGRP antagonist with high affinity, for use in humans.
NPY and related peptides
NPY was purified a decade after the sequencing of SP, in 1982 by Tatemoto et al. (1982) using a novel identification process that selectively detected carboxy-terminal amidated peptides. Investigations over the ensuing 20 years have shown that, in mammals, the 36 amino-acid neuropeptide NPY is distributed widely. It is located in, and released from specific central neurons and peripheral neurons that include NANC (particularly enteric nerves), sympathetic and sensory nerves. Its structural analogues, pancreatic polypeptide (PP, the first of this family to be discovered, a by-product of insulin purification) and PYY are located in neuroendocrine cells particularly in the pancreas and terminal intestine, respectively. However, PYY is also expressed in a minor population of gastric neurons, distinct from those expressing NPY and only in certain species (not human or mouse). It is important to note at this juncture that many ‘neuro'-peptides are also expressed in glia, including NPY and SP, although the functional significance of this expression is currently unknown. In certain species (such as rats), extra-neuronal NPY is found in high concentrations in platelets and this source contributes significantly to plasma NPY levels, which otherwise are derived predominantly from sympathetic neuron activity.
Once NPY is released from dense-core vesicles (Lundberg, 1996) and enters the blood, it becomes susceptible to dipeptidyl peptidase IV activity (Mentlein et al., 1993). The resultant product, NPY(3–36), has significant activity at Y2 and Y5 receptors, typically activated by peptide fragments of NPY. The ‘inactivation' of circulating PYY follows a similar path and the circulating product, PYY(3–36), is acknowledged as a major, physiologically and clinically significant factor mediating the feeling of satiety (McGowan & Bloom, 2004). Shorter NPY and PYY fragments lose their biological activities with decreasing length, and loss of the amide group at the C-terminus of all full-length NPY-like peptides abolishes their activities.
A fourth family member, PY exists in fish, which lack PP. Found in the pancreas of several bony fishes, PY is also found in zebrafish, but it is unlikely that a mammalian orthologue exists (Larhammar et al., 2004). Drosophila melanogaster has an NPY-like peptide called neuropeptide F (NPF), which is present in neurons and intestinal endocrine cells, in contrast with the exclusive neuronal localization of NPY in all vertebrates studied to date, all of which express PYY in their endocrine cells.
NPY was initially shown to exhibit a surprisingly weak direct vasoconstrictor response but a powerful potentiation of noradrenaline-induced vasoconstriction in a variety of rabbit isolated blood vessels. This developed swiftly into the ‘two receptor model' proposed by Rolf Håkanson's group in Lund (Wahlestedt et al., 1987) and concurrent in vivo studies identified prolonged (PYY-preferred rather than NPY) vasoconstriction of canine hepatic vascular beds. A unique feature of these 36 amino-acid long peptides is their stable antiparallel ‘hair-pin' structure despite the lack of Cys residues. This is known as the PP-fold (Schwartz et al., 1990) and it is crucial for the activation of some Y receptors (in particular, Y1) but not others (Y2 and Y5). Substitution of Glu34 in NPY (see Table 1) or in PYY, by a proline residue (which is the 34th amino acid of PP) resulted in analogues that were Y1-, rather than Y2-preferring (Fuhlendorff et al., 1990). At that time, the 1980s and 1990s, there were no useful Y receptor antagonists and this meant that structure–activity studies were somewhat frustratingly slow processes, using a selection of neuropeptide fragments and substituted analogues of NPY or PYY.
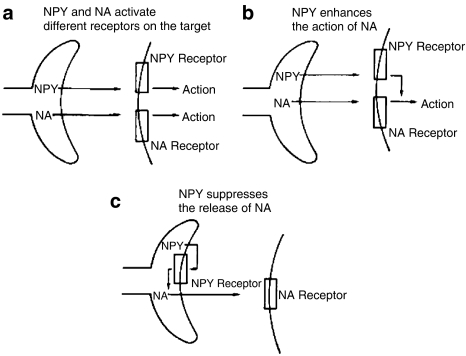
Nevertheless, these strategies allowed the discrimination and accurate characterization of Y1- from Y2-receptor mediated mechanisms underpinning NPY-mediated vasoconstriction (Figure 3). In fact, NPY has been shown to exert a range of neuromodulatory effects including the suppression of cholinergic and sensory neurotransmission as well as the paradigmatic effects at sympathetic neuro-effector junctions (Edvinsson et al., 1987; Wahlestedt et al., 1987). In most sympathetic neurons, NPY coexists with noradrenaline and ATP (but not in the same vesicles) and the three main mechanisms of NPY action are: (a) direct Y1-mediated vascular contractile effects; (b) amplification of adrenoceptor (α1)-mediated vasoconstriction, and (c) prejunctional Y2-mediated suppression of noradrenaline, ATP and NPY release (Figure 3, taken from Edvinsson et al., 1987).
Figure 3.
The multiple actions of NPY, together with circulating PYY, PYY(3–36) upon perivascular sympathetic nerve terminals. There are three mechanisms of interaction between NPY and noradrenaline (NA) at this neuroeffector junction: (a) NPY and NA act independently and postjunctionally at different receptors to initiate vasoconstriction; (b) NPY enhances the post-junctional effect of NA and in (c) NPY suppresses the release of NA (and NPY also, not shown). Taken from Edvinsson et al. (1987).
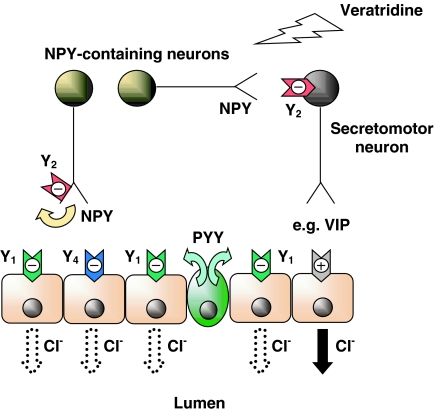
Together, NPY and the other three major mammalian peptides in this group, PYY, PYY(3–36) and PP, exert wide-ranging effects in central and peripheral target tissues. In the CNS, NPY is an inhibitory neurotransmitter and exhibits anxiolytic, anti-stress, anti-depressant, anti-convulsant and anti-nociceptive actions, in addition to its hypertensive, and potent appetite-stimulating effects and its capacity to shift circadian rhythms. Pharmacological investigations have also revealed certain similarities between the sympathetic neuro-effector junction and the NANC enteric neuro-effector junction in the colon (Figure 4). In the intestine, NPY (and PYY) are potent anti-secretory agents in rodent and human tissues (Hyland & Cox, 2004) and in patients infused with PYY (Playford et al., 1990). The receptors that mediate the mucosal antisecretory response in isolated tissues from human and mouse colon are a clear combination of (a) post-junctional Y1 receptors activated by enteric NPY (or neuroendocrine PYY) together with (b) a pre-junctional Y2-neuromodulatory mechanism that ultimately results in attenuated ion and electrolyte secretion (Figure 4). Additionally, in the intestine of mouse and human colon, Y4 receptors are located on the basolateral surface of epithelial cells and these are stimulated by PP (and possibly also by high levels of PYY) that reaches the lamina propria layer via the circulation to elicit antisecretory (pro-absorptive) responses.
Figure 4.
Schematic diagram depicting the sites of action of NPY from enteric neurons and endocrine PYY upon different targets in normal mouse colon mucosa. Direct activation of epithelial Y1 receptors by NPY and PYY will inhibit epithelial anion (Cl−) secretion. Veratridine nonselectively stimulates all intrinsic submucosal neurons. NPY released from submucosal secretomotor neurons can autoinhibit NPY release (a Y2 receptor-mediated effect) and also, when released from interneurons can inhibit (again via Y2 receptors) other NANC secretomotor (e.g. VIP-ergic) neurons. Endocrine PYY may coactivate neuronal Y2 receptors as well as the epithelial Y1 receptors (predominant in mouse and human colon). Both mechanisms result in a sustained inhibition of epithelial Cl− secretion. The NANC neurotransmitter in the final secretomotor neuron has not yet been positively identified, but it is most likely to be VIP that causes the cAMP-dependent epithelial Cl− secretion.
Four different human Y receptor types exist currently; the Y1, Y2, Y4 and Y5 and the International Union of Pharmacology recommended that the overtly NPY-preferring (not cloned) Y3 receptor should remain undesignated, while the y6 receptor is a pseudogene in mammals (Michel et al., 1998). An unusual feature of the Y receptor family is their lack of sequence identity, particularly between Y1, Y2 and Y5 receptors, that are only 30% identical. Subtypes of the Y1 receptor are known to exist in fish but not in mammals, while a further Y2-like receptor, named Y7, exists in amphibia, sharks and bony fish, but has apparently been lost from the mammalian lineage (Larhammar et al., 2004). From evolutionary considerations and from some recently published unusual pharmacological profiles (Dumont et al., 2005), additional members of this divergent GPCR family might still be found.
This research field does now benefit from some selective tools that have recently been used to discriminate between different Y receptor-mediated activities. Proof of Y1-mediated vasoconstrictor effects, as distinct from fragment-activated Y2-mediated responses, was achieved using low molecular weight, nonpeptide, Y receptor antagonists. In 1994, the first selective Y1 antagonist became available, BIBP3226; whose structure is based on the final two C-terminal amino acids of NPY (Michel et al., 1998). This compound blocked, competitively, postjunctional vasoconstriction and exogenous and endogenous NPY-induced vasoconstriction in vivo. A few years later the one, and so far only, selective high affinity Y2 antagonist, BIIE0246, was described (Doods et al., 1999), as antagonizing PYY(3–36)-mediated contraction in prototypical Y2 receptor bioassays, but not Y1 receptor-mediated contractile effects. BIIE0246 has since been used widely and successfully to distinguish actions mediated by Y2 receptors in a mixed population of Y receptor types (El Bahh et al., 2005), that could (and have!) erroneously been interpreted as being nonselective effects and thus either Y5 (or y6) receptor-mediated.
Selectivity studies for the Y1 receptor (blocked by BIBP3226 or BIBO3304; Michel et al., 1998; Wieland et al., 1998) and Y2 receptor (utilizing BIIE0246) have allowed clear differentiation of these activities from Y5 receptor-mediated mechanisms (blocked by CGP71683A, and more selectively by L-152,804). To date, there are no Y4 receptor antagonists. A homo-dimeric peptide GR231118 (also known as 1229U91; Michel et al., 1998) exhibits a combination of potent Y1 receptor antagonism, paired with agonist activity at the Y4 receptor (Schober et al., 1998). The signalling pathway activated by all Y receptor types is most commonly Gi/o protein-coupled, but elevations in Ca2+ are known to be responsible for the Y1-mediated vasopressor effects of NPY. The multiplicity of signalling pathways utilized by Y receptors has been recently reviewed (Holliday et al., 2004). The presynaptic inhibitory effects of NPY upon central and peripheral neurons occur via the activation of G-protein activated inwardly rectifying K+ channels or inhibition of N- and P/Q-type Ca2+ channels, thus inhibiting neurotransmitter release. In fact, NPYs effects upon neurotransmission closely resemble those of activating μ-opioid receptors (see also Corbett et al., this issue). In the spinal cord, NPY acts presynaptically via Y1 receptors to attenuate inhibitory currents induced by GABA or glycine, in combination with presynaptic Y2-mediated inhibition of excitatory (glutamatergic) neurotransmission (Moran et al., 2004). These mechanisms occur at the same synapses as those at which μ-opioid agonists such as DAMGO are active and underpin the previously documented analgesic effects of NPY, indicating that NPY(3–36) or better still, a synthetic Y2-selective agonist has promise as an analgesic treatment for neuropathic pain (Moran et al., 2004). In the hippocampus, the anticonvulsant actions of NPY are mediated via presynaptic Y2 receptors attenuating glutamate release from CA1 and CA3 regions and thus Y2 agonists could be novel anticonvulsants (El Bahh et al., 2005).
Homozygous mutant mice lacking NPY from central, peripheral (and enteric) neurones fed and grew normally, exhibited a normal response to starvation (Erickson et al., 1996) although they were hypersensitive to the satiety factor, leptin (which normally inhibits NPY release). NPY null mice were also more susceptible to status epilepticus and death than their wild-type littermates (Baraban et al., 1997), implying that loss of this major inhibitory neuropeptide resulted in overexcitation of specific central pathways. NPY−/− mice also drank more ethanol and were more resistant to its sedative effects. Recent elegant studies from Freidman's group (Roseberry et al., 2004) have utilized NPY tagged with the green fluorescent protein (NPY-GFP) and pro-opiomelanocortin (POMC)-GFP transgenic mice to show how selectively NPY hyperpolarizes POMC neurons in the arcuate nucleus of the hypothalamus via activation of Y1 receptors and GIRK K+ channels. The ‘soft-wiring' (Horvath, 2005) of neural circuits that regulate feeding behaviour and energy expenditure continue to challenge those working in this area. The dissection of these complex peptidergic pathways may yet provide novel targets in the treatment of obesity, despite earlier set-backs with Y1 and Y5 receptor antagonists and the phenotypes exhibited by germline knockout mice.
Mutant mice that lack NPY in specific tissues, as exemplified in recent studies from Steve Bloom's group (Bewick et al., 2005), offer a new approach to determining the neuropeptide's full biological activities. In the last few months, a mutant mouse lacking PYY (together with its product, PYY(3–36), but also inadvertently PP) has been shown to exhibit normal growth, food intake, energy expenditure, sensitivity to infused PYY(3–36) and terminal differentiation of enteroendocrine cells (Schonhoff et al., 2005), further emphasising the multifactorial and plastic nature of intrinsic mechanisms controlling these end points. Future postembryonic ablation models (Bewick et al., 2005) that are single (or double) peptide null mutants will be invaluable for testing with validated methodologies, to clarify and establish the full range of physiological activities.
Germline, single Y receptor knockout mice have however been revealing. For example, Y1−/− mice exhibit reduced daily food intake, higher leptin levels and a marked reduction in fast-induced refeeding, implying a role for the Y1 receptors in the control of food intake (Pedrazzini et al., 1998). New Y1 antagonists were developed as potential novel antiobesity drugs but they did not live up to expectations. Basal cardiovascular parameters (and circulating NPY levels) were unaltered in Y1−/− mice, indicating that Y1 receptors are not critical for maintaining basal heart rate and blood pressure. Y1−/− mice did however lose all sensitivity to infused NPY, thus reinforcing the involvement of Y1 receptors in cardiovascular responses to NPY (Pedrazzini et al., 1998). In the Y1−/− mouse colon, NPY (and PYY) antisecretory responses were severely blunted and any sign of Y1-absorptive tone was lost. Studies focused on the CNS have shown that the Y1 receptor is the main mediator of NPY's anxiolytic actions and that lack of signalling through this receptor leads to an increase in anxiety and aggressive behaviours, the latter by modulation of central 5-HT pathways (Thiele & Heilig, 2004). However, the Y2 receptor, when activated by NPY, also will mediate tonic autoinhibition and thus a competitive Y2 antagonist (such as BIIE0246) enhances NPY release in central and peripheral tissues. Selective agonists at the Y2 receptor are anxiogenic, whereas NPY is anxiolytic, having effects not only on Y2 but also upon Y1 receptors. Behaviourally, Y2−/− mice are more adventurous and better able to deal with stressful situations than their wild-type littermates (Tschenett et al., 2003). Thus, it is possible that a centrally acting Y2 antagonist may yet be a valuable anxiolytic, but one potential peripheral side effect, diarrhoea (see below), may limit its possible benefit.
Circulating PYY levels are reduced in obesity (le Roux et al., 2005) and this peptide's conversion to the Y2-preferred satiety signal, PYY(3–36) could offer an effective antiobesity therapy, despite the complexity of the hypothalamic circuitry mentioned above (McGowan & Bloom, 2004; Horvath, 2005). Y2 receptor ablation has, in two different mutants, resulted in different effects upon mouse body weight. The lean phenotype demonstrated by Sainsbury et al. (2002a) (a complete Y2 receptor knock-out) highlighted the requirement for tissue-specific knockouts in the future. Hypothalamic Y2 receptor knockout not only resulted in a relatively transient reduction in body weight linked to increased expression of NPY in these animals (Sainsbury et al., 2002a), but also to stimulated bone formation through an autonomic mechanism relayed via the hypothalamus. In the intestine, loss of actions mediated by Y2 receptors, whether through genetic ablation of the receptor or treatment with the Y2 antagonist, BIIE0246, results in a neuronal dis-inhibition and consequently increased mucosal ion secretion (Figure 4). Notably, the competitive Y2 antagonist also revealed significant Y2 receptor-mediated absorptive tone in human and mouse colon (Hyland et al., 2003). Thus, a Y2 agonist may not only be a novel antiobesity drug and antinociceptive (Moran et al., 2004, see above) but it could also be antidiarrhoeal. In the latter instance, a Y2 agonist lacking central activity could be a useful adjunct to oral rehydration therapy (ORT), since ORT alone does not treat intestinal hypersecretion resulting from viral (rotavirus) or specific bacterial (cholera) infections. In these pathologies, there is therefore a clinical requirement for a metabolically stable, peripherally effective, Y2 receptor agonist.
The third Y receptor stimulated by endogenous NPY is the brain-specific Y5 receptor, known for a while as the ‘feeding' receptor. However, the absence of the predicted hypophagia, coupled with unpredicted mild late-onset obesity exhibited by Y5 knockout mice dampened early excitement in the field and Y5 antagonists developed as antiobesity drugs (Block et al., 2002) failed in clinical trials. Interest in the Y4 receptor as a therapeutic target currently lags behind that of the other members of the Y receptor family. The gut-specific expression of the Y4 receptor population in normal animals could be practically advantageous as Y4 agonists should be effective as antidiarrhoeal agents with little or no additional effects via the other Y receptors in the body (see mechanism presented in Figure 4). Germline ablation of Y4 receptors resulted in increased plasma levels of PP and a lean phenotype. Despite unchanged food intake, these animals gained weight more slowly than wild-type mice (Sainsbury et al., 2002b). In addition, male Y4−/− mice displayed overtly aggressive behaviour. Interestingly, when these mice were cross bred with the leptin deficient ob/ob mice that are infertile, the resulting double null mutants were fertile (Sainsbury et al., 2002b), suggesting an inhibitory role for central Y4 receptors in reproductive function. Recent observations also show that Y4−/− mouse intestine is more prone to hypersecretion initiated by cholera toxin (Cox et al., unpublished), further advancing the possibility that a Y4 selective agonist that does not penetrate the CNS, may be a useful antidiarrhoeal. In the presence of Y1 and Y2 antagonists, isolated colons from Y4−/− mice lost all sensitivity to NPY, PYY and other Y agonists, thus proving that the trio of Y1, Y2 and Y4 receptors account fully for the mucosal responses mediated by Y receptors in mouse colon (Figure 4).
In stark contrast to clinical progress made in the NK and CGRP receptor fields, to date, no clinical trials with Y antagonists have been successful. A significant challenge in the NPY-Y receptor area is the requirement for, and synthesis of metabolically stable, low molecular weight, analogues that are selective agonists. Research programmes in several pharmaceutical companies are ongoing, such as assessing PYY(3–36) as an antiobesity agent, but the experimental evidence suggests that selective and stable Y agonists should be more efficacious as therapeutic agents.
Conclusion
The study of these neuropeptides and their receptors, through innovative research, has increased considerably our understanding of their mechanisms of action in a wide range of physiological and pathophysiological situations. Intriguingly, the field has yet to provide a major therapeutic agent. This has provoked much discussion and some suggestions that the neuropeptides may only act as ‘modulators'. In our view, this dearth of clinically useful compounds is more likely to be a reflection of their complex pharmacologies, significant species differences and plasticity of neuro-effector junctions and circuitry, both in terms of peptide and receptor expression levels. Nevertheless, we are at very interesting, though different, stages in the clinical development of pharmaceuticals, based on these neuropeptides. Research with tachykinin antagonists is continuing, while recent findings with the TRPV1 and CGRP antagonists are potentially promising. However, clinically useful compounds based on NPY- have yet to be developed, despite recent feverish activity in the antiobesity area. Here the challenge is for high affinity, selective peptide or nonpeptide agonists that are orally bioavailable or at least relatively stable and in specific cases are also able to penetrate the CNS. Overall, it would appear that despite the high level of research activity in both the academic and industrial research environment over the previous century, we find that we still are in the early stages of learning about the importance of neuropeptides in biology.
Glossary
- 5-HT
5-hydroxytryptamine
- 7TM
7 transmembrane spanning
- AM
adrenomedullin
- CGRP
calcitonin gene-related peptide
- CL
calcitonin receptor-like receptor
- CNS
central nervous system
- DAMGO
[D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin
- GABA
γ-aminobutyric acid
- GFP
green fluorescent protein
- GPCRs
G-protein coupled receptors
- NK
neurokinin
- NKA
neurokinin A
- NKB
neurokinin B
- NPγ
neuropeptide-γ
- NA
noradrenaline
- NANC
nonadrenergic, noncholinergic
- NO
nitric oxide
- NPF
neuropeptide F
- NPY
neuropeptide Y
- ORT
oral rehydration therapy
- POMC
pro-opiomelanocortin
- PNS
peripheral nervous system
- PP
pancreatic polypeptide
- PPT-I
preprotachykinin I
- PPT-II
preprotachykinin II
- PYY
peptide YY
- RAMP
receptor activity modifying protein
- RCP
receptor component
- SP
substance P
- TRPV1
transient receptor potential vanilloid 1
- VIP
vasoactive intestinal polypeptide
References
- AIYAR N., DAINES R.A., DISA J., CHAMBERS P.A., SAUERMELCH C.F., QUINIOU M., KHANDOUDI N., GOUT B., DOUGLAS S.A., WILLETTE R.N. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J. Pharmacol. Exp. Ther. 2001;296:768–775. [PubMed] [Google Scholar]
- BARABAN S.C., HOLLOPETER G., SCHWARTZKROIN P.A., PALMITER R.D. Knock-out mice reveal a critical antiepileptic role for neuropeptide Y. J. Neurosci. 1997;17:8927–8936. doi: 10.1523/JNEUROSCI.17-23-08927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEWICK G.A., GARDINER J.V., DHILLO W.S., WHITE N.E., WEBSTER Z., GHATEI M.A., BLOOM S.R. Postembryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J. 2005;19:1680–1682. doi: 10.1096/fj.04-3434fje. [DOI] [PubMed] [Google Scholar]
- BLOCK M.H., BOYER S., BRAILSFORD W., BRITTAIN D.R., CARROLL D., CHAPMAN S., CLARKE D.S., DONALD C.S., FOOTE K.M., GODFREY L., LADNER A., MARSHAM P.R., MASTERS D.J., MEE C.D., O'DONOVAN M.R., PEASE J.E., PICKUP A.G., RAYNER J.W., ROBERTS A., SCHOFIELD P., SULEMAN A., TURNBULL A.V. Discovery and optimization of a series of carbazole ureas as NPY5 antagonists for the treatment of obesity. J. Med. Chem. 2002;45:3509–3523. doi: 10.1021/jm011125x. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., GRANT A.D. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol. Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- BRAIN S.D., WILLIAMS T.J., TIPPINS J.R., MORRIS H.R., MACINTYRE I. Calcitonin gene-related peptide is a potent vasodilator. Nature. 1985;313:54–56. doi: 10.1038/313054a0. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Do some nerve cells release more than one transmitter. J. Neurosci. 1976;1:239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHAWLA S.P., GRUNBERG S.M., GRALLA R.J., HESKETH P.J., RITTENBERG C., ELMER M.E., SCHMIDT C., TAYLOR A., CARIDES A.D., EVANS J.K., HORGAN K.J. Establishing the dose of the oral NK1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting. Cancer. 2003;97:2290–2300. doi: 10.1002/cncr.11320. [DOI] [PubMed] [Google Scholar]
- COTTRELL G.S., ROOSTERMAN D., MARVIZON J.C., SONG B., WICK E., PIKIOS S., WONG H., BERTHELIER C., TANG Y., STERNINI C., BUNNETT N.W., GRADY E.F. Localization of calcitonin receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J. Comp. Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- DONG Y.L., GREEN K.E., VEGIRAGU S., HANKINS G.D., MARTIN E., CHAUHAN M., THOTA C., YALLAMPALLI C. Evidence for decreased calcitonin gene-related peptide (CGRP) receptors and compromised responsiveness to CGRP of fetoplacental vessels in preeclamptic pregnancies. J. Clin. Endocrinol. Metab. 2005;90:2336–2343. doi: 10.1210/jc.2004-1481. [DOI] [PubMed] [Google Scholar]
- DOODS H., GAIDA W., WIELAND H.A., DOLLINGER H., SCHNORRENBERG G., ESSER F., ENGEL W., EBERLEIN W., RUDOLF K. BIIE0246: a selective and high affinity neuropeptide Y Y(2) receptor antagonist. Eur. J. Pharmacol. 1999;384:R3–R5. doi: 10.1016/s0014-2999(99)00650-0. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., WU D., ENTZEROTH M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUMONT Y., MOYSE E., FOURNIER A., QUIRION R. Evidence for the existence of an additional class of neuropeptide Y receptors in the rat brain. J. Pharmacol. Exp. Therap. 2005;315:99–108. doi: 10.1124/jpet.105.089300. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., HÅKANSON R., WAHLESTEDT C., UDDMAN R. Effects of neuropeptide Y on the cardiovascular system. Trends Pharmacol. Sci. 1987;8:231–235. [Google Scholar]
- EL BAHH B., BALOSSO S., HAMILTON T., HERZOG H., BECK-SICKINGER A., SPERK G., GEHLERT D.R., VEZZANI A., COLMERS W. The anti-epileptic actions of neuropeptide Y in the hippocampus are mediated by Y2 and not Y5 receptors. Eur. J. Neurosci. 2005;22:1417–1430. doi: 10.1111/j.1460-9568.2005.04338.x. [DOI] [PubMed] [Google Scholar]
- ERICKSON J.C., CLEGG K.E., PALMITER R.D. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–418. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- FOREMAN J.C., JORDAN C.C., OEHME P., RENNER H. Structure–activity relationships for some substance P-related peptides that cause wheal and flare reactions in human skin. J. Physiol. 1983;335:449–465. doi: 10.1113/jphysiol.1983.sp014543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUHLENDORFF J., GETHER U., AAKERLUND L., LANGELAND-JOHANSEN N., THORGERSEN H., MELBERG S.G., OLSEN U.B., THASTRUP O., SCHWARTZ T.W. [Leu31,Pro34]neuropeptide Y: a specific Y1 receptor agonist, Proc. Natl. Acad. Sci. U.S.A. 1990;87:182–186. doi: 10.1073/pnas.87.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAY D.L., CONNER A.C., HOWITT S.G., SMITH D.M., POYNER D.R. The pharmacology of adrenomedullin receptors and their relationship to CGRP receptors. J. Mol. Neurosci. 2004;22:105–113. doi: 10.1385/JMN:22:1-2:105. [DOI] [PubMed] [Google Scholar]
- HOLZER P. Neurogenic vasodilatation and plasma leakage in the skin. Gen. Pharmacol. 1998;30:5–11. doi: 10.1016/s0306-3623(97)00078-5. [DOI] [PubMed] [Google Scholar]
- HOLLIDAY N.D., MICHEL M.C., COX H.M.2004NPY receptor subtypes and their signal transduction Neuropeptide Y and Related Peptides, Handbook of Experimental Pharmacologyed. Michel M.C. Vol. 162, pp45–74.Berlin: Springer-Verlag [Google Scholar]
- HORVATH T.L. The hardship of obesity: a soft-wired hypothalamus. Nature Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- HYLAND N.P., COX H.M.2004NPY-like peptides, Y receptors and gastrointestinal function Neuropeptide Y and Related Peptides, Handbook of Experimental Pharmacologyed. Michel M.C. Vol. 162, pp. 389–408.Berlin: Springer-Verlag [Google Scholar]
- HYLAND N.P., SJÖBERG F., TOUGH I.R., HERZOG H., COX H.M. Functional consequences of neuropeptide Y Y2 receptor knockout and Y2 antagonism in mouse and human colonic tissues. Br. J. Pharmacol. 2003;139:863–871. doi: 10.1038/sj.bjp.0705298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCSó G., KIRALY E., JANSCó-GABOR A. Pharmacologically induced selective degeneration of chemosensitive primary sensory neurones. Nature. 1977;270:741–743. doi: 10.1038/270741a0. [DOI] [PubMed] [Google Scholar]
- KEEBLE J.E., BRAIN S.D. A role for substance P in arthritis. Neurosci. Lett. 2004;361:176–179. doi: 10.1016/j.neulet.2003.12.020. [DOI] [PubMed] [Google Scholar]
- KRAMER M.S., WINOKUR A., KELSEY J., PRESKORN S.H., ROTHSCHILD A.J., SNAVELY D., GHOSH K., BALL W.A., REINES S.A., MUNJACK D., APTER J.T., CUNNINGHAM L., KLING M., BARI M., GETSON A., LEE Y. Demonstration of the efficacy and safety of a novel SP (NK1) receptor antagonist in major depression. Neuropsychopharmacology. 2004;29:385–392. doi: 10.1038/sj.npp.1300260. [DOI] [PubMed] [Google Scholar]
- LARHAMMAR D., FREDRIKSSON R., LARSON E.T., SALANECK E.2004Phylogeny of NPY-family peptides and their receptors Neuropeptide Y and Related Peptides, Handbook of Experimental Pharmacologyed. Michel M.C. Vol. 162, pp76–100.Berlin: Springer-Verlag [Google Scholar]
- LEMBECK F. Central transmission of afferent impulses. III. Incidence and significance of the substance P in the dorsal roots of the spinal cord. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 1953;219:197–213. [PubMed] [Google Scholar]
- LE ROUX C.W., BATTERHAM R.L., AYLWIN S.J.B., PATTERSON M., BORG C.M., WYNNE K.J., KENT A., VINCENT R.P., GHATEI M.A., BLOOM S.R.2005Attenuated peptide YY release in obese subjects is associated with reduced satiety Endocrinology(in press). [DOI] [PubMed]
- LEWIS T.(ed). (1927Released substances the cause of the triple response The Blood Vessels of the Human Skin and their Responses London: Shaw and Son [Google Scholar]
- LUNDBERG J.M. Pharmacology of cotransmission in the autonomic nervous system: integrative aspects on amines, neuropeptides, ATP, amino acids and nitric oxide. Pharmacol. Rev. 1996;48:113–178. [PubMed] [Google Scholar]
- MAGGI C.A. The pharmacology of the efferent function of sensory nerves. J. Auton. Pharmacol. 1991;11:173–208. doi: 10.1111/j.1474-8673.1991.tb00317.x. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A. The mammalian tachykinin receptors. Gen. Pharmacol. 1995;26:911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- MCGOWAN B.M.C., BLOOM S.R. Peptide YY and appetite control. Curr. Opin. Pharmacol. 2004;4:583–588. doi: 10.1016/j.coph.2004.06.007. [DOI] [PubMed] [Google Scholar]
- MCLATCHIE L.M., FRASER N.J., MAIN M.J., WISE A., BROWN J., THOMPSON N., SOLARI R., LEE M.G., FOORD S.M. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- MENTLEIN R., DAHMS P., GRANDT D., KRUGER R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Reg. Peptides. 1993;49:133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- MICHEL M.C., BECK-SICKINGER A., COX H.M., DOODS H.N., HERZOG H., LARHAMMAR D., QUIRION R., SCHWARTZ T., WESTFALL T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, and pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50:143–150. [PubMed] [Google Scholar]
- MORAN T.D., COLMERS W.F., SMITH P.A. Opioid-like actions of neuropeptide Y in rat substantia gelatinosa: Y1 suppression of inhibition and Y2 suppression of excitation. J. Neurophysiol. 2004;92:3266–3275. doi: 10.1152/jn.00096.2004. [DOI] [PubMed] [Google Scholar]
- OLESEN J., DIENER H.C., HUSSTEDT I.W., GOADSBY P.J., HALL D., MEIER U., POLLENTIER S., LESKO L.M. BIBN 4096 BS Clinical Proof of Concept Study Group. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- PAGE N.M. Hemokinins and endokinins. Cell Mol. Life Sci. 2004;61:1652–1663. doi: 10.1007/s00018-004-4035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEDRAZZINI T., SEYDOUX J., KUNSTNER P., AUBERT J.-F., GROUZMANN E., BEERMANN F., BRUNNER H.-R. Cardiovascular response, feeding behaviour and locomotion in mice lacking the NPY Y1 receptor. Nat. Med. 1998;4:722–726. doi: 10.1038/nm0698-722. [DOI] [PubMed] [Google Scholar]
- PLAYFORD R.J., DOMIN J., BEACHAM J., PARMAR K.B., TATEMOTO K., BLOOM S.R., CALAM J. Preliminary report: role of peptide YY in defence against diarrhoea. Lancet. 1990;335:1555–1557. doi: 10.1016/0140-6736(90)91378-n. [DOI] [PubMed] [Google Scholar]
- ROH J., CHANG C.L., BHALLA A., KLEIN C., HSU S.Y. Intermedin is a calcitonin/calcitonin gene-related peptide family peptide acting through the calcitonin receptor-like receptor/receptor activity-modifying protein receptor complexes. J. Biol. Chem. 2004;279:7264–7274. doi: 10.1074/jbc.M305332200. [DOI] [PubMed] [Google Scholar]
- ROSEBERRY A.G., LUI H., JACKSON A.C., CAI X., FRIEDMAN J.M. Neuropeptide Y-mediated inhibition of proopiomelanocortin neurons in the arcuate nucleus shows enhanced desensitisation in ob/ob mice. Neuron. 2004;41:711–722. doi: 10.1016/s0896-6273(04)00074-1. [DOI] [PubMed] [Google Scholar]
- SAINSBURY A., SCHWARZER C., COUZENS M., FETISSOV S., FURTINGER S., JENKINS A., COX H.M., SPERK G., HÖKFELT T., HERZOG H. Important role of hypothalamic Y2 receptors in body weight regulation revealed in conditional knockout mice. Proc. Natl. Acad. Sci. U.S.A. 2002a;99:8938–8943. doi: 10.1073/pnas.132043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAINSBURY A., SCHWARZER C., COUZENS M., JENKINS A., OAKES S.R., ORMANDY C.J., HERZOG H. Y4 receptor knockout rescues fertility in ob/ob mice. Genes Dev. 2002b;16:1077–1088. doi: 10.1101/gad.979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOBER D.A., VAN ABBEMA A.M., SMILEY D.L., BRUNS R.F., GEHLERT D.R. The neuropeptide Y Y1 antagonist, 1229U91, a potent agonist for the human pancreatic polypeptide-preferring (NPY Y4) receptor. Peptides. 1998;19:537–542. doi: 10.1016/s0196-9781(97)00455-5. [DOI] [PubMed] [Google Scholar]
- SCHONHOFF S., BAGGIO L., RATINEAU C., RAY S.K., LINDNER J., MAGNUSON M.A., DRUCKER D.J., LEITER A.B. Energy homeostasis and gastrointestinal endocrine differentiation do not require the anorectic hormone peptide YY. Mol. Cell. Biol. 2005;25:4189–4199. doi: 10.1128/MCB.25.10.4189-4199.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ T.W., FUHLENDORFF J., KJEMS L.L., KRISTENSEN M.S., VERVELDE M., O'HARE M., KRSTENANSKY J.K., BJORNHOLM B. Signal epitopes in the three-dimensional structure of neuropeptide Y. Ann. NY Acad. Sci. 1990;611:35–47. doi: 10.1111/j.1749-6632.1990.tb48920.x. [DOI] [PubMed] [Google Scholar]
- STORER R.J., AKERMAN S., GOADSBY P.J. Calcitonin gene-related peptide (CGRP) modulates nociceptive trigeminovascular transmission in the cat. Br. J. Pharmacol. 2004;142:1171–1181. doi: 10.1038/sj.bjp.0705807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TATEMOTO K., CARLQUIST M., MUTT V. Neuropeptide Y- a novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature. 1982;296:659–660. doi: 10.1038/296659a0. [DOI] [PubMed] [Google Scholar]
- TSCHENETT A., SINGEWALD N., CARLI M., BALDUCCI C., SALCHNER P., VEZZANI A., HERZOG H., SPERK G. Reduced anxiety and improved stress coping ability in mice lacking NPY-Y2 receptors. Eur J Neurosci. 2003;18:143–148. doi: 10.1046/j.1460-9568.2003.02725.x. [DOI] [PubMed] [Google Scholar]
- THIELE T.E., HEILIG M.2004Behavioural effects of neuropeptide Y Neuropeptide Y and Related Peptides, Handbook of Experimental Pharmacologyed. Michel M.C. Vol. 162, pp. 251–282.Berlin: Springer-Verlag [Google Scholar]
- URBAN L.A., FOX A.J. NK1 receptor antagonists – are they really without effect in the pain clinic. Trends Pharmacol. Sci. 2000;21:462–464. doi: 10.1016/s0165-6147(00)01578-9. [DOI] [PubMed] [Google Scholar]
- VON EULER U.S., GADDUM J.H. An unidentified depressor substance in certain tissue extracts. J. Physiol. 1931;72:74–87. doi: 10.1113/jphysiol.1931.sp002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHLESTEDT C., EDVINSSON L., EKBLAD E., HÅKANSON R.1987Effects of neuropeptide Y at sympathetic neuroeffector junctions: existence of Y1- and Y2-receptors Neuronal Messengers in Vascular Functioned. Nobin, A., Owman, C. & Arneklo-Nobin, B. pp. 231–242.Amsterdam: Elsevier Science Publishers [Google Scholar]
- WIELAND H.A., ENGEL W., EBERLEIN W., RUDOLF K., DOODS H.N. Subtype selectivity of the novel nonpeptide neuropeptide Y Y1 receptor antagonist BIBO3304 and its effect on feeding in rodents. Br. J. Pharmacol. 1998;125:549–555. doi: 10.1038/sj.bjp.0702084. [DOI] [PMC free article] [PubMed] [Google Scholar]