Abstract
OBJECTIVE—To identify intracardiac conduction abnormalities in patients with myotonic dystrophy from their clinical, ECG, and genetic features. METHODS—39 consecutive patients (mean (SD) age 42.9 (12.1) years; 16 female, 23 male) underwent clinical examination, genetic studies, resting and 24 hour ambulatory ECG, signal averaged ECG, and electrophysiological studies. RESULTS—23 patients suffered from cardiac symptoms, 23 had one or more cardiac conduction abnormality on resting ECG, one had sinus deficiency, and 21 (53.8%) had prolonged HV intervals. No correlation was found between the severity of the neurological symptoms, onset of disease, cardiac conduction abnormalities on ECG, and the intracardiac conduction abnormalities on electrophysiological study. The size of the DNA mutation was longer in the abnormal HV interval group than in the normal HV interval group (3.5 (1.8) v 2.2 (1.0) kb, p < 0.02). Signal averaged ECG parameters (total QRS duration (QRSD) and duration of low amplitude signals ⩽ 40 µV (LAS 40)) were greater in patients with an abnormal HV interval than in those with a normal HV interval (123.4 (24.6) v 102.8 (12.3) ms and 47.5 (12.8) v 35.3 (8.8) ms, respectively; p < 0.005). Only the association of QRSD ⩾ 100 ms with LAS 40 ⩾ 36 ms identified patients with an abnormal HV interval with good sensitivity (80%) and specificity (83.3%). CONCLUSIONS—Infrahissian conduction abnormalities are common in myotonic dystrophy and can be identified using signal averaged electrocardiography. Keywords: myotonic dystrophy; atrioventricular block; genetic factors; signal averaged ECG
Full Text
The Full Text of this article is available as a PDF (90.5 KB).
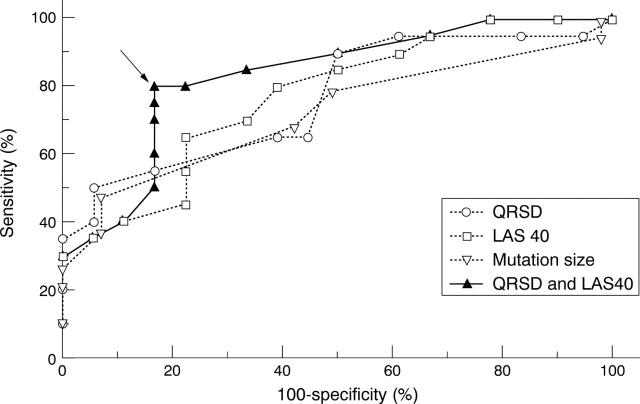
Figure 1 .
Evaluation of the diagnostic value for infrahissian block of size of the DNA mutation, total QRS duration (QRSD), and duration of low amplitude signals of ⩽ 40 µV (LAS 40) on signal averaged ECG in myotonic dystrophy using receiver operating curves. The combination of QRSD ⩾ 100 ms and LAS 40 ⩾ 36 ms identified patients with a prolonged HV interval with a sensitivity of 80% and a specificity of 83.3% (arrow).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annane D., Duboc D., Mazoyer B., Merlet P., Fiorelli M., Eymard B., Radvanyi H., Junien C., Fardeau M., Gajdos P. Correlation between decreased myocardial glucose phosphorylation and the DNA mutation size in myotonic dystrophy. Circulation. 1994 Dec;90(6):2629–2634. doi: 10.1161/01.cir.90.6.2629. [DOI] [PubMed] [Google Scholar]
- Annane D., Merlet P., Radvanyi H., Mazoyer B., Eymard B., Fiorelli M., Junien C., Fardeau M., Ounnoughene Z., Gajdos P. Blunted coronary reserve in myotonic dystrophy. An early and gene-related phenomenon. Circulation. 1996 Sep 1;94(5):973–977. doi: 10.1161/01.cir.94.5.973. [DOI] [PubMed] [Google Scholar]
- Colleran J. A., Hawley R. J., Pinnow E. E., Kokkinos P. F., Fletcher R. D. Value of the electrocardiogram in determining cardiac events and mortality in myotonic dystrophy. Am J Cardiol. 1997 Dec 1;80(11):1494–1497. doi: 10.1016/s0002-9149(97)00742-x. [DOI] [PubMed] [Google Scholar]
- Fragola P. V., Luzi M., Calò L., Antonini G., Borzi M., Frongillo D., Cannata D. Cardiac involvement in myotonic dystrophy. Am J Cardiol. 1994 Nov 15;74(10):1070–1072. doi: 10.1016/0002-9149(94)90864-8. [DOI] [PubMed] [Google Scholar]
- Fragola P. V., Ruscitti G. C., Autore C., Antonini G., Capria A., Fiorito S., Vichi R., Pennisi E., Cannata D. Ambulatory electrocardiographic monitoring in myotonic dystrophy (Steinert's Disease). A study of 22 patients. Cardiology. 1987;74(5):362–368. doi: 10.1159/000174223. [DOI] [PubMed] [Google Scholar]
- Griggs R. C., Davis R. J., Anderson D. C., Dove J. T. Cardiac conduction in myotonic dystrophy. Am J Med. 1975 Jul;59(1):37–42. doi: 10.1016/0002-9343(75)90319-8. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Ikeda U., Kojo T., Nishinaga M., Miyashita H., Kuroda T., Inoue K., Nishizawa M., Shimada K. Cardiac abnormalities and cytosine-thymine-guanine trinucleotide repeats in myotonic dystrophy. Am Heart J. 1997 Aug;134(2 Pt 1):292–297. doi: 10.1016/s0002-8703(97)70137-6. [DOI] [PubMed] [Google Scholar]
- Hiromasa S., Ikeda T., Kubota K., Hattori N., Nishimura M., Watanabe Y., Maldonado C., Palakurthy P. R., Kupersmith J. Myotonic dystrophy: ambulatory electrocardiogram, electrophysiologic study, and echocardiographic evaluation. Am Heart J. 1987 Jun;113(6):1482–1488. doi: 10.1016/0002-8703(87)90665-x. [DOI] [PubMed] [Google Scholar]
- Komajda M., Frank R., Vedel J., Fontaine G., Petitot J. C., Grosgogeat Y. Intracardiac conduction defects in dystrophia myotonica. Electrophysiological study of 12 cases. Br Heart J. 1980 Mar;43(3):315–320. doi: 10.1136/hrt.43.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melacini P., Villanova C., Menegazzo E., Novelli G., Danieli G., Rizzoli G., Fasoli G., Angelini C., Buja G., Miorelli M. Correlation between cardiac involvement and CTG trinucleotide repeat length in myotonic dystrophy. J Am Coll Cardiol. 1995 Jan;25(1):239–245. doi: 10.1016/0735-1097(94)00351-p. [DOI] [PubMed] [Google Scholar]
- Merino J. L., Carmona J. R., Fernández-Lozano I., Peinado R., Basterra N., Sobrino J. A. Mechanisms of sustained ventricular tachycardia in myotonic dystrophy: implications for catheter ablation. Circulation. 1998 Aug 11;98(6):541–546. doi: 10.1161/01.cir.98.6.541. [DOI] [PubMed] [Google Scholar]
- Milner M. R., Hawley R. J., Jachim M., Lindsay J., Jr, Fletcher R. D. Ventricular late potentials in myotonic dystrophy. Ann Intern Med. 1991 Oct 15;115(8):607–613. doi: 10.7326/0003-4819-115-8-607. [DOI] [PubMed] [Google Scholar]
- Moorman J. R., Coleman R. E., Packer D. L., Kisslo J. A., Bell J., Hettleman B. D., Stajich J., Roses A. D. Cardiac involvement in myotonic muscular dystrophy. Medicine (Baltimore) 1985 Nov;64(6):371–387. doi: 10.1097/00005792-198511000-00002. [DOI] [PubMed] [Google Scholar]
- Olofsson B. O., Forsberg H., Andersson S., Bjerle P., Henriksson A., Wedin I. Electrocardiographic findings in myotonic dystrophy. Br Heart J. 1988 Jan;59(1):47–52. doi: 10.1136/hrt.59.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perloff J. K., Stevenson W. G., Roberts N. K., Cabeen W., Weiss J. Cardiac involvement in myotonic muscular dystrophy (Steinert's disease): a prospective study of 25 patients. Am J Cardiol. 1984 Nov 1;54(8):1074–1081. doi: 10.1016/s0002-9149(84)80147-2. [DOI] [PubMed] [Google Scholar]
- Prystowsky E. N., Pritchett E. L., Roses A. D., Gallagher J. The natural history of conduction system disease in myotonic muscular dystrophy as determined by serial electrophysiologic studies. Circulation. 1979 Dec;60(6):1360–1364. doi: 10.1161/01.cir.60.6.1360. [DOI] [PubMed] [Google Scholar]
- Redman J. B., Fenwick R. G., Jr, Fu Y. H., Pizzuti A., Caskey C. T. Relationship between parental trinucleotide GCT repeat length and severity of myotonic dystrophy in offspring. JAMA. 1993 Apr 21;269(15):1960–1965. [PubMed] [Google Scholar]
- Shelbourne P., Winqvist R., Kunert E., Davies J., Leisti J., Thiele H., Bachmann H., Buxton J., Williamson B., Johnson K. Unstable DNA may be responsible for the incomplete penetrance of the myotonic dystrophy phenotype. Hum Mol Genet. 1992 Oct;1(7):467–473. doi: 10.1093/hmg/1.7.467. [DOI] [PubMed] [Google Scholar]
- Simson M. B. Use of signals in the terminal QRS complex to identify patients with ventricular tachycardia after myocardial infarction. Circulation. 1981 Aug;64(2):235–242. doi: 10.1161/01.cir.64.2.235. [DOI] [PubMed] [Google Scholar]