Abstract
Recent studies suggest that HIV-1 budding occurs selectively from detergent-insoluble membrane domains, referred to as lipid rafts. Palmitoylation is thought to be one of the factors responsible for targeting membrane proteins to lipid rafts. The cytoplasmic domain of the HIV-1 envelope glycoprotein (gp160) contains two palmitoylated cysteine residues. In this work, we studied the solubility of gp160 after detergent extraction. We show that wild-type gp160 is mostly insoluble after ice-cold Triton X-100 extraction, but that it becomes almost completely soluble at 37°C. In contrast, we find that a mutant gp160, in which the two palmitoylated cysteine residues are replaced by serine, is Triton X-100 soluble even under ice-cold extraction. These findings are consistent with the properties of proteins that localize to lipid rafts and strongly suggest that gp160 is associated with lipid rafts. Further, removal of both palmitoylation sites results in the formation of virus with low levels of gp160 incorporation as well as a decrease in viral infectivity by 60-fold. Our results strongly support the suggestion that HIV-1 buds from lipid rafts and point to a role for rafts as a viral assembly hub.
Detergent-insoluble membrane domains, known as lipid rafts, are thought to exist as microdomains organized in the lateral dimension of the plasma membrane (1, 2). Lipid rafts are thought to play a role in various biological processes such as signal transduction, T cell activation, and virus budding (3). The preferential packing of sphingolipids and cholesterol into these laterally organized domains (2) provides the membrane with a highly ordered state able to resist nonionic detergent extraction at 4°C, allowing the membrane domains to be isolated biochemically (4). The composition of lipid rafts has been shown to be enriched with palmitoylated and glycophosphatidylinositol (GPI)-linked proteins (5, 6).
Like the type C retroviruses and other lentiviruses, HIV-1 budding occurs at the surface of the host cell membrane. A recent study proposed that HIV-1 budding occurs selectively from lipid rafts and showed that whereas HIV-1 particles are highly enriched with proteins known to partition preferentially into lipid rafts, they are deficient in CD45, a highly expressed protein excluded from lipid rafts (7). In addition, other studies show that the lipid composition of HIV-1 is significantly different from its host cell plasma membrane, containing a high percentage of cholesterol and sphingomyelin (8, 9), similar to the composition of lipid rafts (2).
The HIV-1 envelope glycoprotein (Env, gp160) is synthesized as a precursor that is proteolytically cleaved into two subunits: a receptor binding subunit (gp120) and a transmembrane subunit (gp41) (Fig. 1). The gp120/gp41 complex is required for receptor binding and viral entry (10–13). Several lines of evidence suggest that the cytoplasmic domain of gp160 is important for the incorporation of Env (i.e., gp160) into the viral membrane (14–19). A popular model suggests that a specific interaction between the gp160 cytoplasmic domain and the Gag protein matrix domain facilitates the incorporation of Env into virions. Mutations within the matrix domain (20, 21) and deletions in the gp160 cytoplasmic domain (14, 17, 21) block Env incorporation into virions.
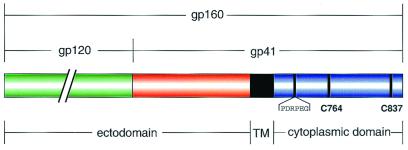
Figure 1.
A schematic representation of HIV-1 gp160. gp160 is proteolytically processed into the receptor-binding domain, gp120 (green), and the transmembrane domain gp41—composed of an ectodomain (red), transmembrane helix (TM, black), and cytoplasmic domain (blue). The cytoplasmic domain contains two palmitoylated cysteine residues at positions 764 and 837 (22). The location of the gp160 mAb (Chessie-8) recognition sequence (PDRPEG) is indicated (27).
In HIV-1, the cytoplasmic domain of Env contains two palmitoylated cysteine residues (22) (Fig. 1). In the influenza virus, palmitoylation of the cytoplasmic domain of hemagglutinin (HA) enhances its association with lipid rafts (23). Removal of all three palmitoylation sites on HA recently was shown to dramatically decrease HA incorporation into virions (24). For HIV-1 Env, removal of the palmitoylation sites was shown to have no effect on envelope expression or cellular trafficking (22). It is unknown, however, if palmitoylation of gp160 plays a role in the virus life cycle. In this study, we generated gp160 mutants with the palmitoylation sites blocked, both singly and in tandem, and examined the correlation between gp160 palmitoylation, detergent solubility, Env incorporation, and viral infectivity. Our results indicate that the state of gp160 palmitoylation plays an important role in the formation of infectious virus.
Materials and Methods
Cell Culture and Plasmid Constructs.
The wild-type (WT) gp160 gene, derived from the HXB-2D sequence (25), was cloned into a pcDNA expression vector (Invitrogen). Point mutants—in which cysteine 764, 837, or both were replaced with serine (denoted as C764S, C837S, and C764/837S, respectively)—were constructed by using PCR-mediated, site-directed mutagenesis (QuikChange, Stratagene). The entire coding region of each construct was sequenced and confirmed.
Expression of WT and mutant HIV-1 Env genes were analyzed in transiently transfected human embryonic kidney cells (293T). Cells were transfected by using a standard calcium phosphate method (Promega). 293T and CD4-expressing human osteosarcoma (HOS T4) cell lines were cultured at 37°C, 5% CO2 in DMEM (GIBCO/BRL) supplemented with 10% FCS.
Viral Infectivity and Envelope Protein Incorporation Assay.
Virus was produced by cotransfection of 293T cells with the Env-deficient, luciferase-expressing HIV-1 genome (NL43LucR−E−) (26) and an Env expression vector (WT, C764S, C837S, or C764/837S) using standard calcium phosphate methods. The medium was replaced 18–24 h posttransfection and supernatants were harvested after 2 days. Virus-containing supernatants were cleared of cellular debris by centrifugation at 500 g for 5 min. Target cells (HOS T4) were infected with virus supernatants for 2 days at 37°C. The cells then were washed with PBS and lysed with 100 μl of luciferase cell culture lysis buffer (Promega). A 20-μl sample of each lysate was assayed for photon emission with a luminometer (AutoLumat, EG&G, Wellesley, MA). The concentration of viral core antigen, p24, was determined by ELISA (NEN Life Sciences), and results were normalized to p24 levels. The normalization factors for all samples were between 1.0 and 1.3.
To measure the amount of gp160 found in the virion particles, supernatants containing equal amounts of p24 antigen were centrifuged through a sucrose cushion (20% sucrose/100 mM NaCl/10 mM Tris buffer, pH 7.5) at 14,000 g for 2 h. Virus pellets were resuspended in 4% SDS sample buffer, heated to 95°C, and separated by SDS/PAGE. Virus lysates then were transferred to poly(vinylidene difluoride) membranes and Western-blotted with a mAb against gp160 (Chessie-8; ref. 27).
Cell Extraction with Triton X-100.
293T cells expressing gp160 were grown in 35-mm plates. After three washes with PBS, cells were incubated in 1 ml of ice-cold or 37°C extraction buffer (0.5% Triton X-100/10 mM Pipes/0.1 M KCl/3 mM MgCl2/10 mM EGTA/0.3 M sucrose, pH 7.5) for 3 min. The soluble and the insoluble fractions were collected in 1 ml of extraction buffer. The insoluble fraction then was sonicated and cleared of cellular debris by centrifugation at 400 g for 2 min. The soluble and insoluble fractions were each incubated with 5 μg of Chessie-8 at 4°C for 1 h. Forty microliters of protein G-conjugated Sepharose beads (Amersham Pharmacia) was added to the suspension and incubated for an additional 30 min at 4°C. The beads were pelleted in a microfuge and washed three times with extraction buffer. Immunoprecipitated material was eluted by boiling with 1% SDS and visualized by using Chessie-8 as the primary antibody in Western blots.
Results
To test whether gp160 is associated with lipid rafts we studied its solubility in 0.5% Triton X-100. When cells expressing WT gp160 were extracted by using ice-cold Triton X-100, we found the majority of gp160 in the insoluble pellet (Fig. 2). However, when the extraction temperature was elevated to 37°C, a significant amount of gp160 shifted from the insoluble to the soluble fraction. The temperature dependence of gp160 solubility in Triton X-100 is similar to that reported for proteins that localize to lipid rafts (4, 23, 28).
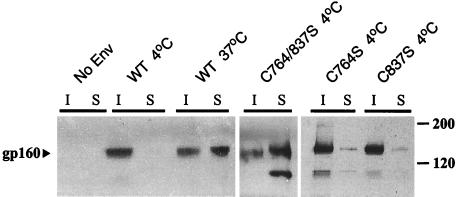
Figure 2.
The effect of palmitoylation on gp160 resistance to Triton X-100 extraction. Cells expressing gp160 (WT, C764S, C837S, or C764/837S) were lysed in 0.5% Triton X-100. The soluble (S) and insoluble (I) fractions were immunoprecipitated (after solubilization of the insoluble fraction by sonication) with a gp160 antibody (Chessie-8), and subjected to Western blot analysis (see text).
Palmitoylation has been shown to be essential for the association of some proteins with lipid rafts (5, 6). To investigate the role of the palmitoylated cysteine residues in gp160, we replaced the cysteines with serines. Both single mutants (C764S and C837S) remain insoluble to detergent extraction. The palmitoylation-deficient double mutant (C764/837S), however, is almost completely soluble after ice-cold membrane extraction (Fig. 2). These results, together with the temperature dependence of gp160 solubility, suggest that gp160 associates with lipid rafts.
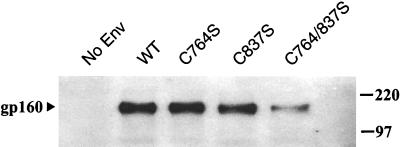
We then tested whether gp160 palmitoylation is important for proper incorporation of Env into virus particles. 293T cells were cotransfected with an Env-deficient, luciferase-expressing HIV-1 genome (NL43LucR−E−) (26) and an envelope protein expression vector (WT, C764S, C837S, or C764/837S). Viral supernatants were normalized by using p24 ELISA and subjected to Western blot analysis with Chessie-8, an anti-gp160 mAb [the Chessie-8 epitope (27) does not include either of the palmitoylated cysteine residues (Fig. 1)]. The results show that mutation of either one of the palmitoylation sites has little, if any, effect on the amount of Env detected on virions (Fig. 3). Removal of both sites, however, results in a significant decrease in the quantity of envelope protein located on the virus membrane.
Figure 3.
The state of gp160 palmitoylation affects the amount of Env in virus. Virus-containing supernatants were produced by cotransfection of 293T cells with an Env-deficient, HIV-1 genome (NL43LucR−E−) and an Env expression vector, as indicated. Supernatants containing equal amounts of virus (as determined by p24 ELISA) then were centrifuged through a 20% sucrose cushion. Virus pellets were resuspended in SDS and subjected to Western blot analysis using a gp160 mAb (Chessie-8).
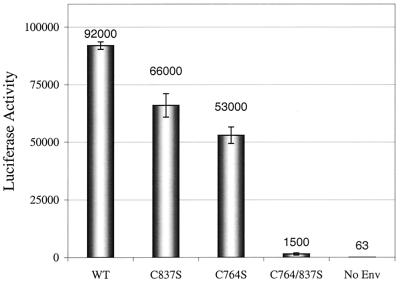
We further studied whether the decrease in virus envelope protein levels influences viral infectivity. Removal of one palmitoylation site (C764S or C837S) decreases viral infectivity by approximately 2-fold, relative to WT gp160. However, elimination of both palmitoylation sites (C764/837S) leads to a 60-fold decrease in viral infectivity (Fig. 4).
Figure 4.
gp160 palmitoylation is critical for viral infectivity. Luciferase-expressing viruses carrying Env mutants were used to infect HOS T4 (CD4+) cells. The infectivity of the virions was measured with a luciferase assay of the infected cell lysates at 48 h postinfection. Raw luciferase data then were normalized to the concentration of the input virus as measured by p24 ELISA (see text). Error bars represent the standard deviation of triplicate experiments.
Discussion
Our detergent-extraction results suggest that palmitoylation targets gp160 into detergent-insoluble membrane domains. We find that in contrast to WT gp160, the double-palmitoylation-deficient mutant of Env is not resistant to detergent extraction. This association of Env with lipid rafts correlates with the incorporation of gp160 into virus particles. Although gp160 palmitoylation has essentially no effect on cell–cell fusion, intracellular transport, or surface expression of gp160 (22), our results demonstrate that Env palmitoylation is critical for the formation of infectious virus.
Incorporation of Env during viral assembly involves an interaction between the gp160 cytoplasmic domain and Gag (14–16, 18, 19, 29, 30) that consequently leads to the formation of infectious virus (14, 19, 29, 31, 32). The region of Env thought to be responsible for interaction with Gag contains one of the two palmitoylation sites (C837) of Env (14, 19). Nonetheless, gp160 incorporation and viral infectivity are only slightly affected by a single mutation that blocks palmitoylation at this site (C837S) or at the second site (C764S), suggesting that neither palmitoyl group is a key determinant of the Env-Gag interaction. We cannot rule out the formal possibility that removal of both palmitoylation sites somehow disrupts the interaction between the Env cytoplasmic domain and Gag. Importantly, however, our results illustrate that even in the absence of Gag, the gp160 cytoplasmic domain targets the envelope protein to specialized lipid domains. This targeting function requires palmitolyation of Env and appears to be essential for efficient Env incorporation during viral assembly and viral infectivity. Thus, in the absence of palmitoylation, the Env-Gag interaction is inadequate to recruit gp160 to the virus particles.
Several recent studies suggest that lipid rafts act as platforms for virus assembly and budding, particularly for HIV-1 and influenza (7–9, 24). For the influenza membrane-fusion protein, HA, palmitoylation targets the protein to lipid rafts (23, 24). Moreover, removal of all three palmitoylation sites in HA dramatically decreases HA incorporation into virions (24). For HIV-1, chains of virions have been observed extending from the plasma membrane (33), suggesting that virus budding occurs from distinct sites on cell surfaces. Analysis of the viral membrane lipid composition (8, 9) suggests that these budding sites are lipid rafts.
Our results indicate that gp160, in the absence of other viral proteins, is localized into lipid rafts. Several studies have shown that Gag is insoluble after Triton X-100 extraction (34, 35) and therefore is likely to also be associated with lipid rafts. Env and Gag recently have been shown to colocalize when coexpressed on the cell membrane (36). Nef, another viral protein that is packaged into virus particles, also appears to be associated with lipid rafts by itself, as judged by detergent-extraction tests (37). Thus, our results, together with earlier findings, lead to a model for the role of lipid rafts as a viral assembly hub, in which the targeting of viral proteins to lipid rafts serves to concentrate them in a local manner on the cell surface. Recently, Lamb and coworkers (24) proposed an analogous role for lipid rafts in the influenza virus. Indeed, it seems likely that targeting of viral proteins to lipid rafts is an important step in the assembly process of many enveloped viruses. We note that the cytoplasmic domain of the envelope protein from the Moloney murine leukemia retrovirus is palmitoylated (38), and the viral-membrane fusion proteins from many diverse viruses (including retroviruses, filoviruses, and paramyxoviruses) contain at least one cysteine residue in their cytoplasmic domain. Finally, our results raise the possibility that a new class of antiviral agents might be developed based on the inhibition of palmitoylation.
Acknowledgments
We thank Heng Chhay for assistance with the viral-infectivity assays, Dr. Michael S. Kay and Dr. Michael J. Root for helpful discussions, Leslie Gaffney for assistance in preparation of the manuscript, and the Kim lab for comments on the manuscript. I.R. was supported by a Merck/Massachusetts Institute of Technology collaboration program postdoctoral fellowship. M.B.M. was supported by an American Cancer Society National postdoctoral fellowship. This research was supported by the National Institutes of Health (PO1 GM56552).
Abbreviations
- Env
HIV-1 envelope glycoprotein
- HA
hemagglutinin
- WT
wild type
Note Added in Proof.
Our detergent-extraction experiments do not distinguish between cell-surface gp160 and intracellular gp160. However, immunofluorescence microscopy experiments show that gp160 is detected on cell surfaces after TX-100 extraction at 4°C but not at 37°C (I.R. and P.S.K., unpublished results). We thank Dr. E. Hunter for discussion of this issue.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240459697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240459697
References
- 1.Kusumi A, Sako Y. Curr Opin Cell Biol. 1996;8:566–574. doi: 10.1016/s0955-0674(96)80036-6. [DOI] [PubMed] [Google Scholar]
- 2.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 3.Brown D A, London E. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder R J, Ahmed S N, Zhu Y, London E, Brown D A. J Biol Chem. 1998;273:1150–1157. doi: 10.1074/jbc.273.2.1150. [DOI] [PubMed] [Google Scholar]
- 5.Resh M D. Biochim Biophys Acta. 1999;1451:1–16. doi: 10.1016/s0167-4889(99)00075-0. [DOI] [PubMed] [Google Scholar]
- 6.Moffett S, Brown D A, Linder M E. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen D H, Hildreth J E. J Virol. 2000;74:3264–3272. doi: 10.1128/jvi.74.7.3264-3272.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aloia R C, Jensen F C, Curtain C C, Mobley P W, Gordon L M. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aloia R C, Tian H, Jensen F C. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freed E O, Martin M A. J Biol Chem. 1995;270:23883–23886. doi: 10.1074/jbc.270.41.23883. [DOI] [PubMed] [Google Scholar]
- 11.Moore J P, Binley J. Nature (London) 1998;393:630–631. doi: 10.1038/31359. [DOI] [PubMed] [Google Scholar]
- 12.Chan D C, Kim P S. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 13.Berger E A, Murphy P M, Farber J M. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 14.Dubay J W, Roberts S J, Hahn B H, Hunter E. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brody B A, Rhee S S, Hunter E. J Virol. 1994;68:4620–4627. doi: 10.1128/jvi.68.7.4620-4627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosson P. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 17.Spies C P, Ritter G D, Jr, Mulligan M J, Compans R W. J Virol. 1994;68:585–591. doi: 10.1128/jvi.68.2.585-591.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitt M A, Chong L, Rose J K. J Virol. 1989;63:3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu X, Yuan X, McLane M F, Lee T H, Essex M. J Virol. 1993;67:213–221. doi: 10.1128/jvi.67.1.213-221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Freed E O, Martin M A. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freed E O, Martin M A. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang C, Spies C P, Compans R W. Proc Natl Acad Sci USA. 1995;92:9871–9875. doi: 10.1073/pnas.92.21.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melkonian K A, Ostermeyer A G, Chen J Z, Roth M G, Brown D A. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J, Pekosz A, Lamb R A. J Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw G M, Hahn B H, Arya S K, Groopman J E, Gallo R C, Wong-Staal F. Science. 1984;226:1165–1171. doi: 10.1126/science.6095449. [DOI] [PubMed] [Google Scholar]
- 26.Chen B K, Saksela K, Andino R, Baltimore D. J Virol. 1994;68:654–660. doi: 10.1128/jvi.68.2.654-660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abacioglu Y H, Fouts T R, Laman J D, Claassen E, Pincus S H, Moore J P, Roby C A, Kamin-Lewis R, Lewis G K. AIDS Res Hum Retroviruses. 1994;10:371–381. doi: 10.1089/aid.1994.10.371. [DOI] [PubMed] [Google Scholar]
- 28.Brown D A, Rose J K. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 29.Yu X, Yuan X, Matsuda Z, Lee T H, Essex M. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaedigk-Nitschko K, Schlesinger M J. Virology. 1991;183:206–214. doi: 10.1016/0042-6822(91)90133-v. [DOI] [PubMed] [Google Scholar]
- 31.Gabuzda D H, Lever A, Terwilliger E, Sodroski J. J Virol. 1992;66:3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston P B, Dubay J W, Hunter E. J Virol. 1993;67:3077–3086. doi: 10.1128/jvi.67.6.3077-3086.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klimkait T, Strebel K, Hoggan M D, Martin M A, Orenstein J M. J Virol. 1990;64:621–629. doi: 10.1128/jvi.64.2.621-629.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perrin-Tricaud C, Davoust J, Jones I M. Virology. 1999;255:20–25. doi: 10.1006/viro.1998.9573. [DOI] [PubMed] [Google Scholar]
- 35.Simon J H, Carpenter E A, Fouchier R A, Malim M H. J Virol. 1999;73:2667–2674. doi: 10.1128/jvi.73.4.2667-2674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermida-Matsumoto L, Resh M D. J Virol. 2000;74:8670–8679. doi: 10.1128/jvi.74.18.8670-8679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J K, Kiyokawa E, Verdin E, Trono D. Proc Natl Acad Sci USA. 2000;97:394–399. doi: 10.1073/pnas.97.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang C, Compans R W. Virology. 1996;221:87–97. doi: 10.1006/viro.1996.0355. [DOI] [PubMed] [Google Scholar]