Abstract
OBJECTIVE—To verify the aetiological involvement of enterovirus and identify the viral genomic sequences in Keshan disease. DESIGN—Formalin fixed, paraffin embedded myocardial necropsy tissue samples were collected in Keshan disease endemic regions. Fourteen cases with a histologically confirmed diagnosis of subacute or chronic Keshan disease were studied. Control tissue included 10 samples of myocardium from cases of cerebral trauma and one from accidental acid intoxication. One sample from a case of enteroviral myocarditis was used as a positive control. The presence of viral genomic RNA was investigated using an established reverse transcription nested polymerase chain reaction (PCR) coupled with direct nucleotide sequencing. Further investigations of PCR positive samples included in situ antigen detection or hybridisation to confirm positive results. RESULTS—Nine of 14 myocardial samples from Keshan disease cases and the positive control were positive for the enteroviral RNA. All the controls were negative. Six of the PCR positive samples were investigated further by in situ enteroviral antigen or RNA detection and all were positive. DNA sequencing of six representative PCR products confirmed that they were homologous to the 5' non-translated region of enteroviral genomic RNA. Five had highest homology to coxsackievirus B genotypes and one was identical to poliovirus type 3. CONCLUSIONS—These results support an aetiological role for enteroviral infection in Keshan disease. Nucleotide sequence data suggest that coxsackievirus B or coxsackie B like viruses are often involved in Keshan disease. Keywords: enterovirus; coxsackievirus; cardiomyopathy; Keshan disease
Full Text
The Full Text of this article is available as a PDF (189.9 KB).
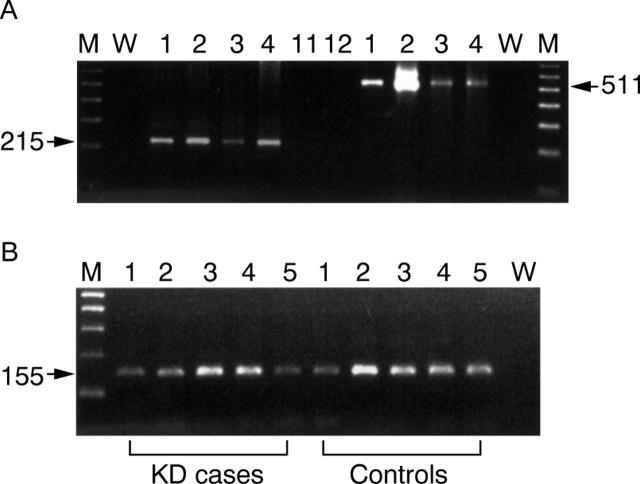
Figure 1 .
Agarose gel electrophoresis of representative reverse transcription nested polymerase chain reaction (RT-NPCR) products. (A) Enteroviral RT-NPCR products amplified with primers F24/R253 (215 bp) or F3/R8 (511 bp). (B) Representative RT-NPCR products of house keeping gene, β actin, from Keshan disease or control heart tissue samples. The first round PCR was with the primer pair HBAF1/R1 and the nested PCR was with the primer pair HBAF2/R2. DNA bands in the 1.5% agarose gel were stained with ethidium bromide. M, 100 bp markers; W, water and reagent control.
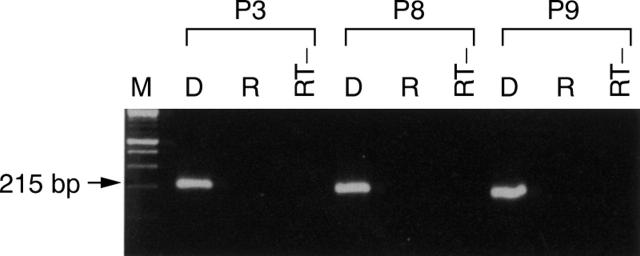
Figure 2 .
Agarose gel electrophoresis of enterovirus reverse transcription nested polymerase chain reaction (RT-NPCR) products from patients 3, 8, and 9. The RNA extracts from these three patients were subjected to DNase (D) or RNase (R) treatment before RT-NPCR. PCR was also performed on these samples directly without reverse transcription (RT−).
Figure 3 .
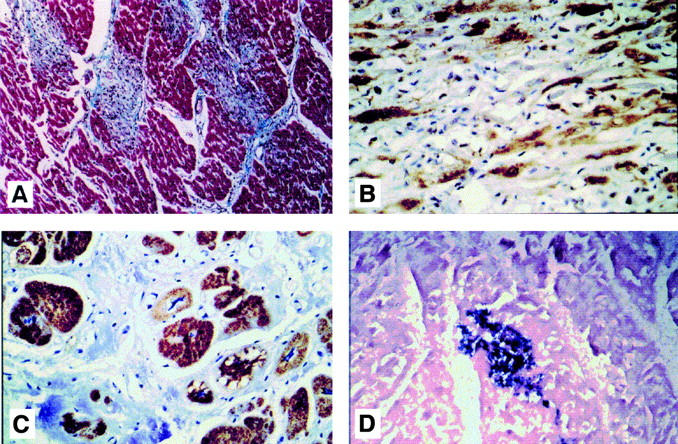
Photomicrographs of heart sections of Keshan disease. (A) Trichrome staining of a section from patient 3 with subacute Keshan disease (× 100, cytoplasm and muscle fibres stain red, nuclei stain black, and collagen stains blue). (B) Immunohistochemistry staining of a section from the same patient. The brown staining indicates the presence of enteroviral capsid protein VP1 reactive with Mab 5-D8/1 (haematoxylin counterstaining × 200). (C) Immunohistochemistry staining of a section from patient 8 with chronic Keshan disease. The brown staining indicates the presence of enteroviral capsid protein VP1 reactive with Mab 5-D8/1 (haematoxylin counterstaining × 200). (D) In situ hybridisation on a section from patient 10 with chronic Keshan disease. The dark purple signals indicate the presence of enteroviral genomic RNA probed by the digoxigenin labelled primer mixture (no counterstaining × 100).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Bowles N. E., Cunningham L., Freeke C. A., Olsen E. G., Rose M. L., Meany B., Why H. J., Richardson P. J. Molecular probes for detection of persisting enterovirus infection of human heart and their prognostic value. Eur Heart J. 1991 Aug;12 (Suppl 500):56–59. doi: 10.1093/eurheartj/12.suppl_d.56. [DOI] [PubMed] [Google Scholar]
- Archard L. C., Khan M. A., Soteriou B. A., Zhang H., Why H. J., Robinson N. M., Richardson P. J. Characterization of Coxsackie B virus RNA in myocardium from patients with dilated cardiomyopathy by nucleotide sequencing of reverse transcription-nested polymerase chain reaction products. Hum Pathol. 1998 Jun;29(6):578–584. doi: 10.1016/s0046-8177(98)80006-3. [DOI] [PubMed] [Google Scholar]
- Badorff C., Lee G. H., Lamphear B. J., Martone M. E., Campbell K. P., Rhoads R. E., Knowlton K. U. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat Med. 1999 Mar;5(3):320–326. doi: 10.1038/6543. [DOI] [PubMed] [Google Scholar]
- Beck M. A., Esworthy R. S., Ho Y. S., Chu F. F. Glutathione peroxidase protects mice from viral-induced myocarditis. FASEB J. 1998 Sep;12(12):1143–1149. doi: 10.1096/fasebj.12.12.1143. [DOI] [PubMed] [Google Scholar]
- Beck M. A., Shi Q., Morris V. C., Levander O. A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in selenium-deficient mice results in selection of identical virulent isolates. Nat Med. 1995 May;1(5):433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Richardson P. J., Olsen E. G., Archard L. C. Detection of Coxsackie-B-virus-specific RNA sequences in myocardial biopsy samples from patients with myocarditis and dilated cardiomyopathy. Lancet. 1986 May 17;1(8490):1120–1123. doi: 10.1016/s0140-6736(86)91837-4. [DOI] [PubMed] [Google Scholar]
- Cann A. J., Stanway G., Hughes P. J., Minor P. D., Evans D. M., Schild G. C., Almond J. W. Reversion to neurovirulence of the live-attenuated Sabin type 3 oral poliovirus vaccine. Nucleic Acids Res. 1984 Oct 25;12(20):7787–7792. doi: 10.1093/nar/12.20.7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham L., Bowles N. E., Lane R. J., Dubowitz V., Archard L. C. Persistence of enteroviral RNA in chronic fatigue syndrome is associated with the abnormal production of equal amounts of positive and negative strands of enteroviral RNA. J Gen Virol. 1990 Jun;71(Pt 6):1399–1402. doi: 10.1099/0022-1317-71-6-1399. [DOI] [PubMed] [Google Scholar]
- Huttunen J. K. Selenium and cardiovascular diseases--an update. Biomed Environ Sci. 1997 Sep;10(2-3):220–226. [PubMed] [Google Scholar]
- JUNGEBLUT C. W., EDWARDS J. E. Isolation of poliomyelitis virus from the heart in fatal cases. Am J Clin Pathol. 1951 Jul;21(7):601–623. doi: 10.1093/ajcp/21.7.601. [DOI] [PubMed] [Google Scholar]
- Klump W. M., Bergmann I., Müller B. C., Ameis D., Kandolf R. Complete nucleotide sequence of infectious Coxsackievirus B3 cDNA: two initial 5' uridine residues are regained during plus-strand RNA synthesis. J Virol. 1990 Apr;64(4):1573–1583. doi: 10.1128/jvi.64.4.1573-1583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton K. U., Jeon E. S., Berkley N., Wessely R., Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996 Nov;70(11):7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander O. A., Beck M. A. Interacting nutritional and infectious etiologies of Keshan disease. Insights from coxsackie virus B-induced myocarditis in mice deficient in selenium or vitamin E. Biol Trace Elem Res. 1997 Jan;56(1):5–21. doi: 10.1007/BF02778980. [DOI] [PubMed] [Google Scholar]
- Li G. S., Wang F., Kang D., Li C. Keshan disease: an endemic cardiomyopathy in China. Hum Pathol. 1985 Jun;16(6):602–609. doi: 10.1016/s0046-8177(85)80110-6. [DOI] [PubMed] [Google Scholar]
- Lindberg A. M., Stålhandske P. O., Pettersson U. Genome of coxsackievirus B3. Virology. 1987 Jan;156(1):50–63. doi: 10.1016/0042-6822(87)90435-1. [DOI] [PubMed] [Google Scholar]
- Minor P. D., John A., Ferguson M., Icenogle J. P. Antigenic and molecular evolution of the vaccine strain of type 3 poliovirus during the period of excretion by a primary vaccinee. J Gen Virol. 1986 Apr;67(Pt 4):693–706. doi: 10.1099/0022-1317-67-4-693. [DOI] [PubMed] [Google Scholar]
- Nicholson F., Ajetunmobi J. F., Li M., Shackleton E. A., Starkey W. G., Illavia S. J., Muir P., Banatvala J. E. Molecular detection and serotypic analysis of enterovirus RNA in archival specimens from patients with acute myocarditis. Br Heart J. 1995 Nov;74(5):522–527. doi: 10.1136/hrt.74.5.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberste M. S., Maher K., Kilpatrick D. R., Pallansch M. A. Molecular evolution of the human enteroviruses: correlation of serotype with VP1 sequence and application to picornavirus classification. J Virol. 1999 Mar;73(3):1941–1948. doi: 10.1128/jvi.73.3.1941-1948.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang X., Zhang H., Bayston T. A., Archard L. C. Detection of Coxsackievirus B3 RNA in mouse myocarditis by nested polymerase chain reaction. Clin Diagn Virol. 1995 Mar;3(3):233–245. doi: 10.1016/s0928-0197(94)00040-9. [DOI] [PubMed] [Google Scholar]
- Pöyry T., Kinnunen L., Hyypiä T., Brown B., Horsnell C., Hovi T., Stanway G. Genetic and phylogenetic clustering of enteroviruses. J Gen Virol. 1996 Aug;77(Pt 8):1699–1717. doi: 10.1099/0022-1317-77-8-1699. [DOI] [PubMed] [Google Scholar]
- Stanway G., Cann A. J., Hauptmann R., Hughes P., Clarke L. D., Mountford R. C., Minor P. D., Schild G. C., Almond J. W. The nucleotide sequence of poliovirus type 3 leon 12 a1b: comparison with poliovirus type 1. Nucleic Acids Res. 1983 Aug 25;11(16):5629–5643. doi: 10.1093/nar/11.16.5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trembleau A., Bloom F. E. Enhanced sensitivity for light and electron microscopic in situ hybridization with multiple simultaneous non-radioactive oligodeoxynucleotide probes. J Histochem Cytochem. 1995 Aug;43(8):829–841. doi: 10.1177/43.8.7622844. [DOI] [PubMed] [Google Scholar]
- Wessely R., Klingel K., Santana L. F., Dalton N., Hongo M., Jonathan Lederer W., Kandolf R., Knowlton K. U. Transgenic expression of replication-restricted enteroviral genomes in heart muscle induces defective excitation-contraction coupling and dilated cardiomyopathy. J Clin Invest. 1998 Oct 1;102(7):1444–1453. doi: 10.1172/JCI1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Why H. J., Meany B. T., Richardson P. J., Olsen E. G., Bowles N. E., Cunningham L., Freeke C. A., Archard L. C. Clinical and prognostic significance of detection of enteroviral RNA in the myocardium of patients with myocarditis or dilated cardiomyopathy. Circulation. 1994 Jun;89(6):2582–2589. doi: 10.1161/01.cir.89.6.2582. [DOI] [PubMed] [Google Scholar]
- Xu G. L., Wang S. C., Gu B. Q., Yang Y. X., Song H. B., Xue W. L., Liang W. S., Zhang P. Y. Further investigation on the role of selenium deficiency in the aetiology and pathogenesis of Keshan disease. Biomed Environ Sci. 1997 Sep;10(2-3):316–326. [PubMed] [Google Scholar]
- Yousef G. E., Brown I. N., Mowbray J. F. Derivation and biochemical characterization of an enterovirus group-specific monoclonal antibody. Intervirology. 1987;28(3):163–170. doi: 10.1159/000150012. [DOI] [PubMed] [Google Scholar]
- Zhang H. Y., Yousef G. E., Cunningham L., Blake N. W., OuYang X., Bayston T. A., Kandolf R., Archard L. C. Attenuation of a reactivated cardiovirulent coxsackievirus B3: The 5'-nontranslated region does not contain major attenuation determinants. J Med Virol. 1993 Oct;41(2):129–137. doi: 10.1002/jmv.1890410208. [DOI] [PubMed] [Google Scholar]
- Zhang H., Soteriou B., Knowlson S., Theodoridou A., Archard L. C. Characterisation of genomic RNA of Coxsackievirus B3 in murine myocarditis: reliability of direct sequencing of reverse transcription-nested polymerase chain reaction products. J Virol Methods. 1997 Dec;69(1-2):7–17. doi: 10.1016/s0166-0934(97)00122-5. [DOI] [PubMed] [Google Scholar]