Abstract
OBJECTIVE—To determine whether there is evidence of platelet activation following in vivo cocaine administration in humans, as cocaine abuse is associated with myocardial infarction and stroke, and platelet activation leading to thrombosis is a possible mechanism. SETTING—University hospital. DESIGN AND SUBJECTS—Following a randomised, double blind crossover design, 14 healthy volunteers were studied twice, receiving cocaine (2 mg/kg intranasally) once and placebo once. Flow cytometric analysis of P-selectin expression (an α granule membrane protein found on the surface of activated platelets), quantification of the platelet specific proteins platelet factor 4 and β thromboglobulin, and measurement of platelet containing microaggregate and platelet microparticle (fragment) formation were used to assess platelet activation. Circulating von Willebrand factor antigen (vWF) was measured to evaluate a possible role of endothelial stimulation concurrent with platelet activation. RESULTS—There was an increase in both platelet factor 4 (mean (SD), 16 (7) to 39 (22) IU/ml, p = 0.04) and β thromboglobulin (70 (20) to 98 (26) IU/ml, p < 0.01) at 120 minutes following cocaine administration. Platelet containing microaggregate formation was increased at 40 minutes (from 47 (3.2)% to 54 (2.0)%, p < 0.001) and 80 minutes (55 (2.5)%, p = 0.04). Bleeding time decreased following cocaine from 10 (1) to 9 (1) minutes (p = 0.07). No changes in any of the measured variables were noted following placebo administration. CONCLUSIONS—Cocaine exposure causes platelet activation, α granule release, and platelet containing microaggregate formation. These data support the view that cocaine, even at the relatively low doses commonly self administered by occasional abusers, may promote thrombosis and predispose healthy individuals to ischaemic events. Platelet inhibitors should be considered early in any patient with suspected cocaine related ischaemia. Keywords: platelets; cocaine; flow cytometry; myocardial infarction
Full Text
The Full Text of this article is available as a PDF (176.3 KB).
Figure 1 .
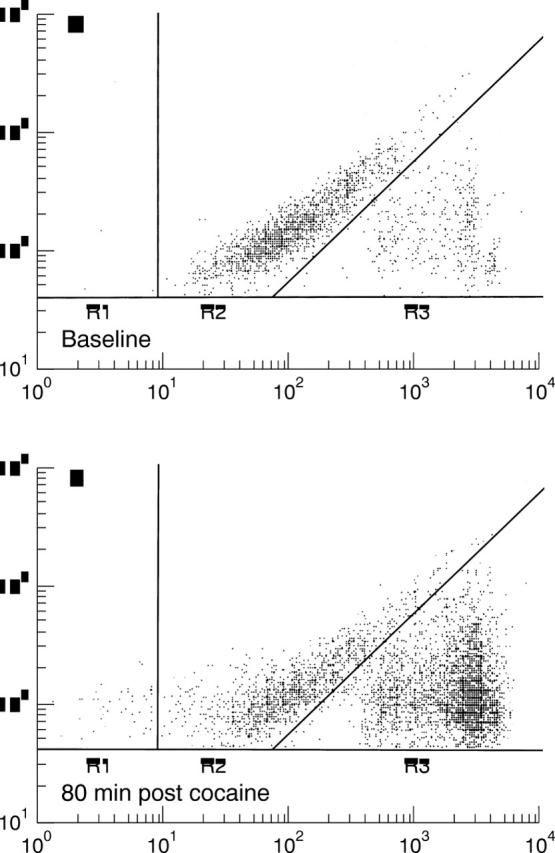
Representative flow cytometric tracing at (A) baseline and (B) 80 minutes after cocaine administration (bottom panel). The y axis measures fluorescence for the platelet marker CD42b, and the x axis measures forward light scatter, both in relative units. The field to the left (R1) represents the platelet fragment population. The middle field (R2) represents the population of single platelets, and platelet containing microaggregates are contained in field R3. There was a pronounced increase in microaggregates following cocaine administration.
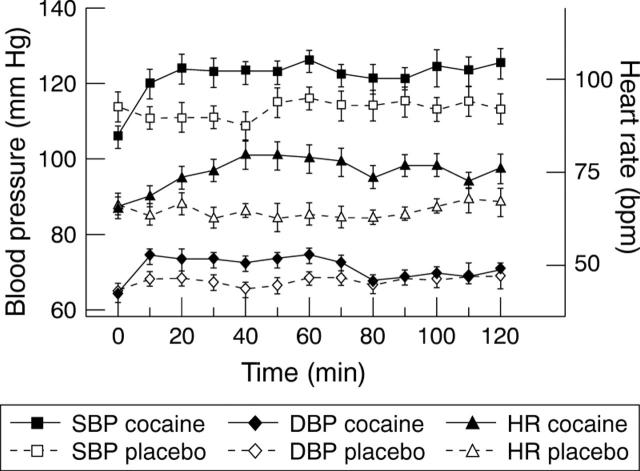
Figure 2 .
Changes in heart rate (HR), systolic blood pressure (SPB), and diastolic blood pressure (DBP) preceding and following the administration of cocaine (black symbols) or placebo (white symbols). Values are means, error bars = SEM.
Figure 3 .
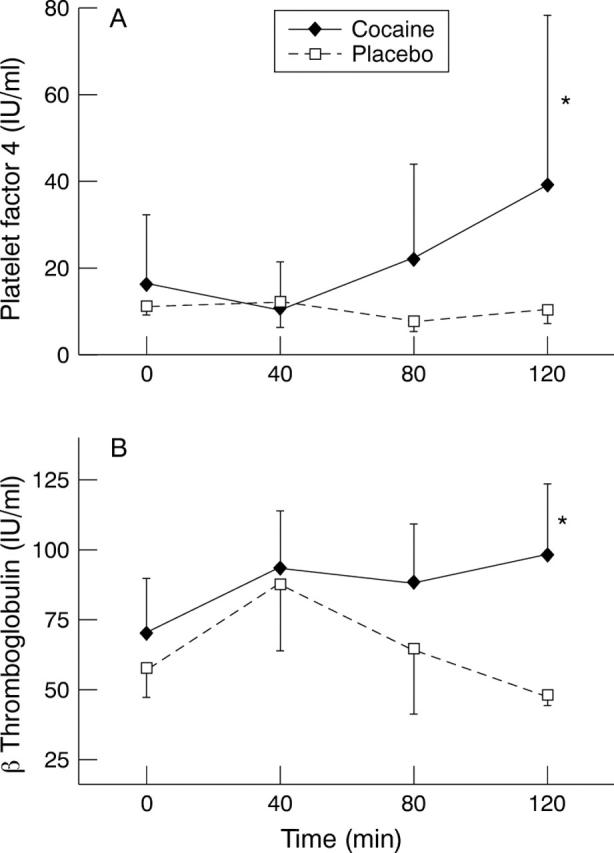
Changes in (A) platelet factor 4 and (B) β thromboglobulin at 120 minutes; both variables were significantly increased from baseline. Values are means, error bars = SEM.
Figure 4 .
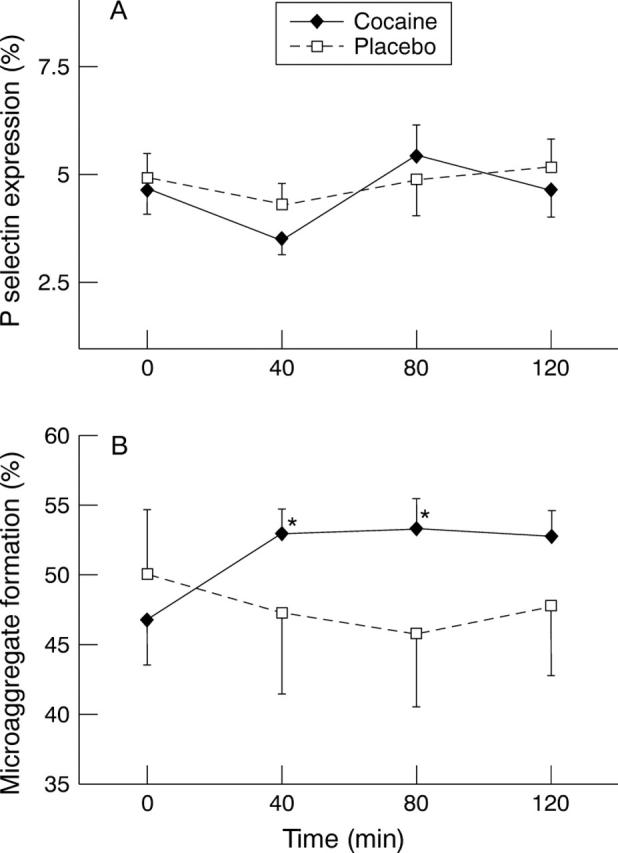
Changes in (A) P-selectin expression (per cent of single platelet population also positive for CD62) and (B) microaggregate formation (expressed as per cent of all CD42b positive event). No changes were seen in P-selectin expression, whereas microaggregates were increased at 40 and 80 minutes. Values are means, error bars = SEM.
Figure 5 .
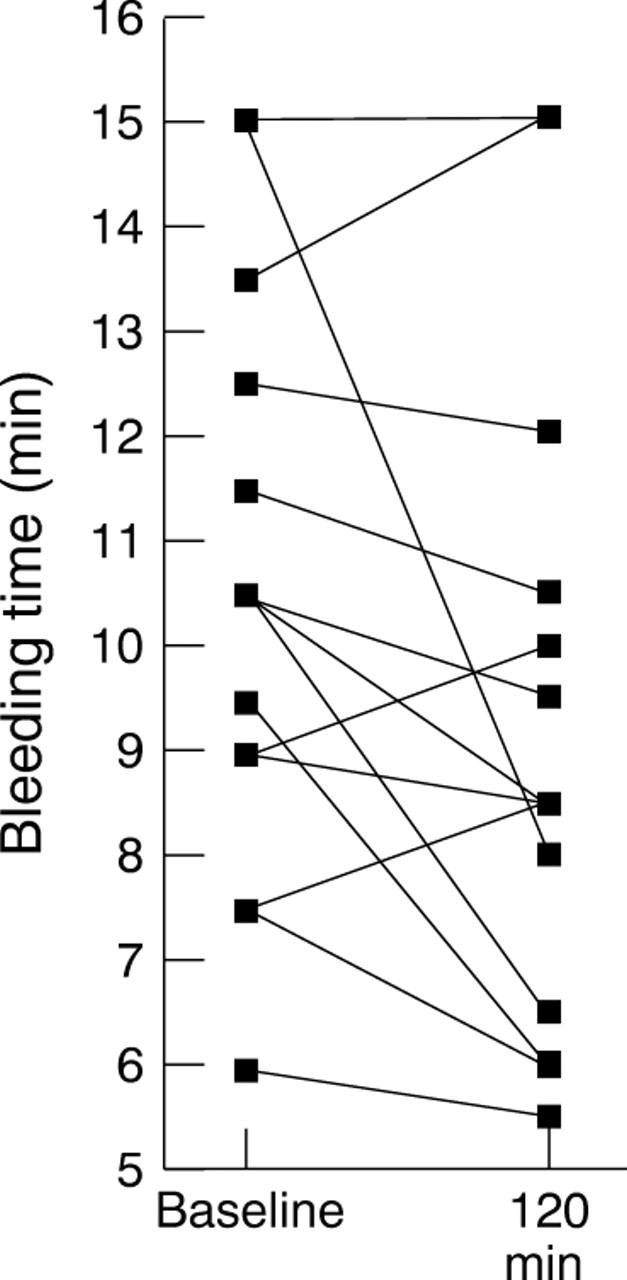
Results of bleeding time determinations for each individual at baseline and 120 minutes after the administration of cocaine.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams C. S., Ellison N., Budzynski A. Z., Shattil S. J. Direct detection of activated platelets and platelet-derived microparticles in humans. Blood. 1990 Jan 1;75(1):128–138. [PubMed] [Google Scholar]
- Aledort L. M., Niemetz J. Dissociation of platelet aggregation from clot retraction, potassium loss, and adenosine triphosphatase activity. Proc Soc Exp Biol Med. 1968 Jul;128(3):658–661. doi: 10.3181/00379727-128-33091. [DOI] [PubMed] [Google Scholar]
- Anderton J. M., Nassar W. Y. Topical cocaine and general anaesthesia: an investigation of the efficacy and side effects of cocaine on the nasal mucosae. Anaesthesia. 1975 Nov;30(6):809–817. doi: 10.1111/j.1365-2044.1975.tb00962.x. [DOI] [PubMed] [Google Scholar]
- Bailey M. E., Fraire A. E., Greenberg S. D., Barnard J., Cagle P. T. Pulmonary histopathology in cocaine abusers. Hum Pathol. 1994 Feb;25(2):203–207. doi: 10.1016/0046-8177(94)90279-8. [DOI] [PubMed] [Google Scholar]
- Blann A. D. Is raised von Willebrand factor a marker of endothelial cell damage? Med Hypotheses. 1993 Nov;41(5):419–424. doi: 10.1016/0306-9877(93)90118-a. [DOI] [PubMed] [Google Scholar]
- Blann A. D., McCollum C. N. von Willebrand factor, endothelial cell damage and atherosclerosis. Eur J Vasc Surg. 1994 Jan;8(1):10–15. doi: 10.1016/s0950-821x(05)80112-4. [DOI] [PubMed] [Google Scholar]
- Blann A. D., Taberner D. A. A reliable marker of endothelial cell dysfunction: does it exist? Br J Haematol. 1995 Jun;90(2):244–248. doi: 10.1111/j.1365-2141.1995.tb05143.x. [DOI] [PubMed] [Google Scholar]
- Boehrer J. D., Moliterno D. J., Willard J. E., Snyder R. W., 2nd, Horton R. P., Glamann D. B., Lange R. A., Hillis L. D. Hemodynamic effects of intranasal cocaine in humans. J Am Coll Cardiol. 1992 Jul;20(1):90–93. doi: 10.1016/0735-1097(92)90142-a. [DOI] [PubMed] [Google Scholar]
- Boelsterli U. A., Göldlin C. Biomechanisms of cocaine-induced hepatocyte injury mediated by the formation of reactive metabolites. Arch Toxicol. 1991;65(5):351–360. doi: 10.1007/BF02284256. [DOI] [PubMed] [Google Scholar]
- Brogan W. C., 3rd, Lange R. A., Glamann D. B., Hillis L. D. Recurrent coronary vasoconstriction caused by intranasal cocaine: possible role for metabolites. Ann Intern Med. 1992 Apr 1;116(7):556–561. doi: 10.7326/0003-4819-116-7-556. [DOI] [PubMed] [Google Scholar]
- Brogan W. C., 3rd, Lange R. A., Kim A. S., Moliterno D. J., Hillis L. D. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991 Aug;18(2):581–586. doi: 10.1016/0735-1097(91)90617-i. [DOI] [PubMed] [Google Scholar]
- Eichhorn E. J., Demian S. E., Alvarez L. G., Willard J. E., Molina S., Bartula L. L., Prince M. D., Inman L. R., Grayburn P. A., Myers S. I. Cocaine-induced alterations in prostaglandin production in rabbit aorta. J Am Coll Cardiol. 1992 Mar 1;19(3):696–703. doi: 10.1016/s0735-1097(10)80295-5. [DOI] [PubMed] [Google Scholar]
- Geerdink P., Levy-Toledano S., Wessels H., Caen J., Haanen C. Influence of lithium on aggregation, release-reaction and function of human platelets. Pathol Biol (Paris) 1972 Dec;(Suppl):15–27. [PubMed] [Google Scholar]
- Heesch C. M., Negus B. H., Steiner M., Snyder RW I. I., McIntire D. D., Grayburn P. A., Ashcraft J., Hernández J. A., Eichhorn E. J. Effects of in vivo cocaine administration on human platelet aggregation. Am J Cardiol. 1996 Jul 15;78(2):237–239. doi: 10.1016/s0002-9149(96)90406-3. [DOI] [PubMed] [Google Scholar]
- Heesch C. M., Steiner M., Hernandez J. A., Ashcraft J., Eichhorn E. J. Effects of cocaine on human platelet aggregation in vitro. J Toxicol Clin Toxicol. 1996;34(6):673–684. doi: 10.3109/15563659609013828. [DOI] [PubMed] [Google Scholar]
- Heng M. C., Haberfeld G. Thrombotic phenomena associated with intravenous cocaine. J Am Acad Dermatol. 1987 Feb;16(2 Pt 2):462–468. doi: 10.1016/s0190-9622(87)70062-0. [DOI] [PubMed] [Google Scholar]
- Hollander J. E. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995 Nov 9;333(19):1267–1272. doi: 10.1056/NEJM199511093331907. [DOI] [PubMed] [Google Scholar]
- Jennings L. K., White M. M., Sauer C. M., Mauer A. M., Robertson J. T. Cocaine-induced platelet defects. Stroke. 1993 Sep;24(9):1352–1359. doi: 10.1161/01.str.24.9.1352. [DOI] [PubMed] [Google Scholar]
- Kloner R. A., Hale S., Alker K., Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992 Feb;85(2):407–419. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- Kolodgie F. D., Farb A., Virmani R. Pathobiological determinants of cocaine-associated cardiovascular syndromes. Hum Pathol. 1995 Jun;26(6):583–586. doi: 10.1016/0046-8177(95)90160-4. [DOI] [PubMed] [Google Scholar]
- Kolodgie F. D., Virmani R., Cornhill J. F., Herderick E. E., Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J Am Coll Cardiol. 1991 Jun;17(7):1553–1560. doi: 10.1016/0735-1097(91)90646-q. [DOI] [PubMed] [Google Scholar]
- Kugelmass A. D., Oda A., Monahan K., Cabral C., Ware J. A. Activation of human platelets by cocaine. Circulation. 1993 Sep;88(3):876–883. doi: 10.1161/01.cir.88.3.876. [DOI] [PubMed] [Google Scholar]
- Kugelmass A. D., Shannon R. P., Yeo E. L., Ware J. A. Intravenous cocaine induces platelet activation in the conscious dog. Circulation. 1995 Mar 1;91(5):1336–1340. doi: 10.1161/01.cir.91.5.1336. [DOI] [PubMed] [Google Scholar]
- Kugelmass A. D., Ware J. A. Cocaine and coronary artery thrombosis. Ann Intern Med. 1992 May 1;116(9):776–777. doi: 10.7326/0003-4819-116-9-776_2. [DOI] [PubMed] [Google Scholar]
- Lange R. A., Cigarroa R. G., Yancy C. W., Jr, Willard J. E., Popma J. J., Sills M. N., McBride W., Kim A. S., Hillis L. D. Cocaine-induced coronary-artery vasoconstriction. N Engl J Med. 1989 Dec 7;321(23):1557–1562. doi: 10.1056/NEJM198912073212301. [DOI] [PubMed] [Google Scholar]
- Michelson A. D., Barnard M. R., Hechtman H. B., MacGregor H., Connolly R. J., Loscalzo J., Valeri C. R. In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11877–11882. doi: 10.1073/pnas.93.21.11877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moliterno D. J., Lange R. A., Gerard R. D., Willard J. E., Lackner C., Hillis L. D. Influence of intranasal cocaine on plasma constituents associated with endogenous thrombosis and thrombolysis. Am J Med. 1994 Jun;96(6):492–496. doi: 10.1016/0002-9343(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Albin R. J., Mergner W., Criner G. J. Diffuse alveolar hemorrhage temporally related to cocaine smoking. Chest. 1988 Feb;93(2):427–429. doi: 10.1378/chest.93.2.427. [DOI] [PubMed] [Google Scholar]
- O'BRIEN J. R. The adhesiveness of native platelets and its prevention. J Clin Pathol. 1961 Mar;14:140–149. doi: 10.1136/jcp.14.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien J. R., Etherington M. D., Shuttleworth R. D., Calwell W. H. Platelet function in acute myocardial infarction patients compared with controls. Thromb Haemost. 1980 Oct 31;44(2):96–99. [PubMed] [Google Scholar]
- O'brien J. R. Platelet aggregation: Part I Some effects of the adenosine phosphates, thrombin, and cocaine upon platelet adhesiveness. J Clin Pathol. 1962 Sep;15(5):446–452. doi: 10.1136/jcp.15.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezkalla S. H., Mazza J. J., Kloner R. A., Tillema V., Chang S. H. Effects of cocaine on human platelets in healthy subjects. Am J Cardiol. 1993 Jul 15;72(2):243–246. doi: 10.1016/0002-9149(93)90173-a. [DOI] [PubMed] [Google Scholar]
- Riggs D., Weibley R. E. Acute hemorrhagic diarrhea and cardiovascular collapse in a young child owing to environmentally acquired cocaine. Pediatr Emerg Care. 1991 Jun;7(3):154–155. doi: 10.1097/00006565-199106000-00006. [DOI] [PubMed] [Google Scholar]
- Rinder H. M., Ault K. A., Jatlow P. I., Kosten T. R., Smith B. R. Platelet alpha-granule release in cocaine users. Circulation. 1994 Sep;90(3):1162–1167. doi: 10.1161/01.cir.90.3.1162. [DOI] [PubMed] [Google Scholar]
- Rinder H. M., Bonan J. L., Rinder C. S., Ault K. A., Smith B. R. Activated and unactivated platelet adhesion to monocytes and neutrophils. Blood. 1991 Oct 1;78(7):1760–1769. [PubMed] [Google Scholar]
- Rinder H. M., Bonan J. L., Rinder C. S., Ault K. A., Smith B. R. Dynamics of leukocyte-platelet adhesion in whole blood. Blood. 1991 Oct 1;78(7):1730–1737. [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Slack S. M., Jennings L. K., Turitto V. T. Platelet size distribution measurements as indicators of shear stress-induced platelet aggregation. Ann Biomed Eng. 1994 Nov-Dec;22(6):653–659. doi: 10.1007/BF02368290. [DOI] [PubMed] [Google Scholar]
- Tofler G. H., Brezinski D., Schafer A. I., Czeisler C. A., Rutherford J. D., Willich S. N., Gleason R. E., Williams G. H., Muller J. E. Concurrent morning increase in platelet aggregability and the risk of myocardial infarction and sudden cardiac death. N Engl J Med. 1987 Jun 11;316(24):1514–1518. doi: 10.1056/NEJM198706113162405. [DOI] [PubMed] [Google Scholar]
- Togna G., Tempesta E., Togna A. R., Dolci N., Cebo B., Caprino L. Platelet responsiveness and biosynthesis of thromboxane and prostacyclin in response to in vitro cocaine treatment. Haemostasis. 1985;15(2):100–107. doi: 10.1159/000215129. [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Rolsten C., Ruiz B., Davis C. M. Pharmacokinetics of cocaine: basic studies of route, dosage, pregnancy and lactation. Neurotoxicology. 1989 Fall;10(3):367–381. [PubMed] [Google Scholar]