Abstract
OBJECTIVE—To test the hypothesis that patients with unstable coronary syndromes show accentuated compensatory vessel enlargement compared with patients with stable angina, and that this may in part be related to increased coronary artery distensibility. DESIGN AND PATIENTS—In 23 patients with unstable coronary syndromes (10 with non-Q wave myocardial infarction and 13 with unstable angina), the culprit lesion was investigated by intravascular ultrasound before intervention. The vessel cross sectional area (VA), lumen area (LA), and plaque area (VA minus LA) were measured at end diastole and end systole at the lesion site and at the proximal and distal reference segments. Similar measurements were made in 23 patients with stable angina admitted during the same period and matched for age, sex, and target vessel. Calculations were made of remodelling index (VA at lesion site ÷ VA at reference site), distensibility index ([(ΔA/A)/ΔP] × 103, where ΔA is the luminal area change in systole and diastole and ΔP the difference in systolic and diastolic blood pressure measured at the tip of the guiding catheter during a cardiac cycle), and stiffness index β ([ln(Psys/Pdias)]/(ΔD/D), where Psys is systolic pressure, Pdias is diastolic pressure, and ΔD is the difference between systolic and diastolic lumen diameters). Positive remodelling was defined as when the VA at the lesion was > 1.05 times larger than at the proximal reference site, and negative remodelling when the VA at the lesion was < 0.95 of the reference site. RESULTS—Mean (SD) LA at the lesion site was similar in both groups (4.03 (1.8) v 4.01 (1.93) mm2), while plaque area was larger in the unstable group (13.29 (4.04) v 8.34 (3.6) mm2, p < 0.001). Remodelling index was greater in the unstable group (1.14 (0.18) v 0.83 (0.15), p < 0.001). Positive remodelling was observed in 15 patients in the unstable group (65%) but in only two (9%) in the stable group (p < 0.001). Negative remodelling occurred only in two patients with unstable symptoms (9%) but in 17 (74%) with stable symptoms. At the proximal reference segment, the difference in LA between systole and diastole was 0.99 (0.66) mm2 in the unstable group and 0.39 (0.3) mm2 in the stable group (p < 0.001), and the calculated coronary artery distensibility was 3.09 (2.69) and 0.94 (0.83) per mm Hg in unstable and stable patients, respectively (p < 0.001). The stiffness index β was lower in patients with unstable angina (1.95 (0.94) v 3.1 (0.96), p < 0.001). CONCLUSIONS—Compensatory vessel enlargement occurs to a greater degree in patients with unstable than with stable coronary syndromes, and is associated with increased coronary artery distensibility. Keywords: coronary artery disease; remodelling; compliance; angina pectoris
Full Text
The Full Text of this article is available as a PDF (160.5 KB).
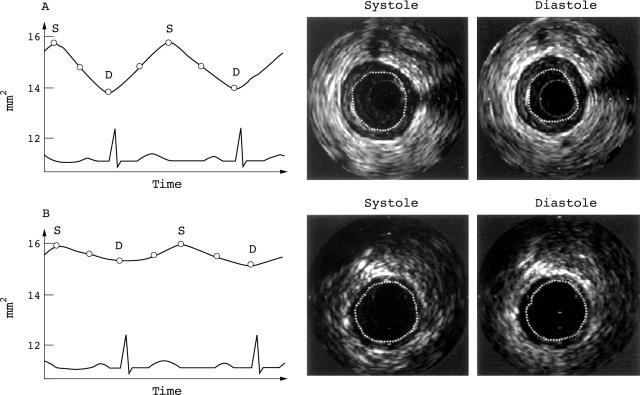
Figure 1 .
Comparison of the pulsatile variation of the cross sectional lumen area of a 77 year old male patient with an unstable lesion in his proximal left anterior descending artery (A) and of an age and target vessel matched patient with stable angina (B). The cyclic variation is graphically illustrated in the left panels, with the corresponding intravascular ultrasound images on the right. The change in luminal area over the cardiac cycle is more pronounced in the unstable patient. Empty circles indicate points of measurements. D, diastole; S, systole.
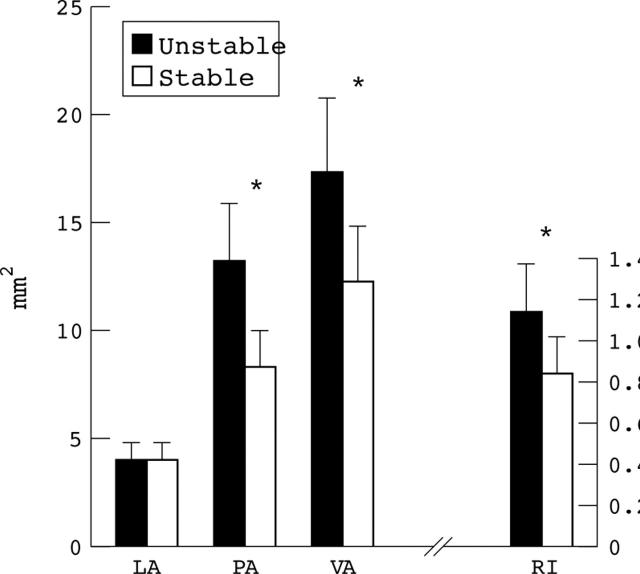
Figure 2 .
Cross sectional lumen area (LA), plaque area (PA), and vessel area (VA) are shown for each patient group at the lesion site. Remodelling index (RI) is calculated as VA at the lesion site divided by VA at the proximal reference segment. *p < 0.001.
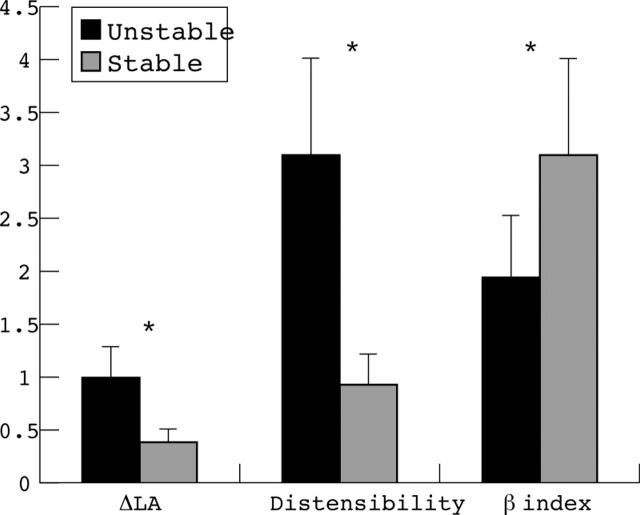
Figure 3 .
Difference in lumen area (ΔLA, mm2), vessel distensibility (per mm Hg), and β stiffness index (B) at the proximal reference site for each patient group. *p < 0.001.
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrose J. A., Winters S. L., Arora R. R., Eng A., Riccio A., Gorlin R., Fuster V. Angiographic evolution of coronary artery morphology in unstable angina. J Am Coll Cardiol. 1986 Mar;7(3):472–478. doi: 10.1016/s0735-1097(86)80455-7. [DOI] [PubMed] [Google Scholar]
- Armstrong M. L., Heistad D. D., Marcus M. L., Megan M. B., Piegors D. J. Structural and hemodynamic response of peripheral arteries of macaque monkeys to atherogenic diet. Arteriosclerosis. 1985 Jul-Aug;5(4):336–346. doi: 10.1161/01.atv.5.4.336. [DOI] [PubMed] [Google Scholar]
- Braunwald E. Unstable angina. A classification. Circulation. 1989 Aug;80(2):410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- Campeau L. Letter: Grading of angina pectoris. Circulation. 1976 Sep;54(3):522–523. [PubMed] [Google Scholar]
- Cox D. A., Vita J. A., Treasure C. B., Fish R. D., Alexander R. W., Ganz P., Selwyn A. P. Atherosclerosis impairs flow-mediated dilation of coronary arteries in humans. Circulation. 1989 Sep;80(3):458–465. doi: 10.1161/01.cir.80.3.458. [DOI] [PubMed] [Google Scholar]
- Davies M. J., Richardson P. D., Woolf N., Katz D. R., Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993 May;69(5):377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galis Z. S., Sukhova G. K., Lark M. W., Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994 Dec;94(6):2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroud D., Li J. M., Urban P., Meier B., Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992 Mar 15;69(8):729–732. doi: 10.1016/0002-9149(92)90495-k. [DOI] [PubMed] [Google Scholar]
- Glagov S., Weisenberg E., Zarins C. K., Stankunavicius R., Kolettis G. J. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987 May 28;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- Hackett D., Davies G., Maseri A. Pre-existing coronary stenoses in patients with first myocardial infarction are not necessarily severe. Eur Heart J. 1988 Dec;9(12):1317–1323. doi: 10.1093/oxfordjournals.eurheartj.a062449. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Handa H., Nagasawa S., Okumura A., Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech. 1980;13(2):175–184. doi: 10.1016/0021-9290(80)90191-8. [DOI] [PubMed] [Google Scholar]
- Hermiller J. B., Tenaglia A. N., Kisslo K. B., Phillips H. R., Bashore T. M., Stack R. S., Davidson C. J. In vivo validation of compensatory enlargement of atherosclerotic coronary arteries. Am J Cardiol. 1993 Mar 15;71(8):665–668. doi: 10.1016/0002-9149(93)91007-5. [DOI] [PubMed] [Google Scholar]
- Hirai T., Sasayama S., Kawasaki T., Yagi S. Stiffness of systemic arteries in patients with myocardial infarction. A noninvasive method to predict severity of coronary atherosclerosis. Circulation. 1989 Jul;80(1):78–86. doi: 10.1161/01.cir.80.1.78. [DOI] [PubMed] [Google Scholar]
- Kok W. E., Peters R. J., Prins M. H., Piek J. J., Koch K. T., David G. K., Visser C. A. Contribution of age and intimal lesion morphology to coronary artery wall mechanics in coronary artery disease. Clin Sci (Lond) 1995 Sep;89(3):239–246. doi: 10.1042/cs0890239. [DOI] [PubMed] [Google Scholar]
- Lee R. T., Schoen F. J., Loree H. M., Lark M. W., Libby P. Circumferential stress and matrix metalloproteinase 1 in human coronary atherosclerosis. Implications for plaque rupture. Arterioscler Thromb Vasc Biol. 1996 Aug;16(8):1070–1073. doi: 10.1161/01.atv.16.8.1070. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Kent K. M., Pichard A. D., Satler L. F., Popma J. J., Leon M. B. Contribution of inadequate arterial remodeling to the development of focal coronary artery stenoses. An intravascular ultrasound study. Circulation. 1997 Apr 1;95(7):1791–1798. doi: 10.1161/01.cir.95.7.1791. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Painter J. A., Pichard A. D., Kent K. M., Satler L. F., Popma J. J., Chuang Y. C., Bucher T. A., Sokolowicz L. E., Leon M. B. Atherosclerosis in angiographically "normal" coronary artery reference segments: an intravascular ultrasound study with clinical correlations. J Am Coll Cardiol. 1995 Jun;25(7):1479–1485. doi: 10.1016/0735-1097(95)00088-l. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Popma J. J., Pichard A. D., Kent K. M., Satler L. F., Chuang Y. C., DeFalco R. A., Leon M. B. Limitations of angiography in the assessment of plaque distribution in coronary artery disease: a systematic study of target lesion eccentricity in 1446 lesions. Circulation. 1996 Mar 1;93(5):924–931. doi: 10.1161/01.cir.93.5.924. [DOI] [PubMed] [Google Scholar]
- Mintz G. S., Potkin B. N., Keren G., Satler L. F., Pichard A. D., Kent K. M., Popma J. J., Leon M. B. Intravascular ultrasound evaluation of the effect of rotational atherectomy in obstructive atherosclerotic coronary artery disease. Circulation. 1992 Nov;86(5):1383–1393. doi: 10.1161/01.cir.86.5.1383. [DOI] [PubMed] [Google Scholar]
- Nakatani S., Yamagishi M., Tamai J., Goto Y., Umeno T., Kawaguchi A., Yutani C., Miyatake K. Assessment of coronary artery distensibility by intravascular ultrasound. Application of simultaneous measurements of luminal area and pressure. Circulation. 1995 Jun 15;91(12):2904–2910. doi: 10.1161/01.cir.91.12.2904. [DOI] [PubMed] [Google Scholar]
- Nishioka T., Luo H., Eigler N. L., Berglund H., Kim C. J., Siegel R. J. Contribution of inadequate compensatory enlargement to development of human coronary artery stenosis: an in vivo intravascular ultrasound study. J Am Coll Cardiol. 1996 Jun;27(7):1571–1576. doi: 10.1016/0735-1097(96)00071-x. [DOI] [PubMed] [Google Scholar]
- Nissen S. E., Gurley J. C., Grines C. L., Booth D. C., McClure R., Berk M., Fischer C., DeMaria A. N. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. 1991 Sep;84(3):1087–1099. doi: 10.1161/01.cir.84.3.1087. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G., Schoneveld A. H., van der Wal A. C., Haudenschild C. C., Clarijs R. J., Becker A. E., Hillen B., Borst C. Relation of arterial geometry to luminal narrowing and histologic markers for plaque vulnerability: the remodeling paradox. J Am Coll Cardiol. 1998 Sep;32(3):655–662. doi: 10.1016/s0735-1097(98)00304-0. [DOI] [PubMed] [Google Scholar]
- Pasterkamp G., Wensing P. J., Post M. J., Hillen B., Mali W. P., Borst C. Paradoxical arterial wall shrinkage may contribute to luminal narrowing of human atherosclerotic femoral arteries. Circulation. 1995 Mar 1;91(5):1444–1449. doi: 10.1161/01.cir.91.5.1444. [DOI] [PubMed] [Google Scholar]
- Richardson P. D., Davies M. J., Born G. V. Influence of plaque configuration and stress distribution on fissuring of coronary atherosclerotic plaques. Lancet. 1989 Oct 21;2(8669):941–944. doi: 10.1016/s0140-6736(89)90953-7. [DOI] [PubMed] [Google Scholar]
- Shimazu T., Hori M., Mishima M., Kitabatake A., Kodama K., Nanto S., Inoue M. Clinical assessment of elastic properties of large coronary arteries: pressure-diameter relationship and dynamic incremental elastic modulus. Int J Cardiol. 1986 Oct;13(1):27–45. doi: 10.1016/0167-5273(86)90077-x. [DOI] [PubMed] [Google Scholar]
- Sudhir K., Mullen W. L., Hausmann D., Fitzgerald P. J., Chou T. M., Yock P. G., Chatterjee K. Contribution of endothelium-derived nitric oxide to coronary arterial distensibility: an in vivo two-dimensional intravascular ultrasound study. Am Heart J. 1995 Apr;129(4):726–732. doi: 10.1016/0002-8703(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Sumner D. S., Hokanson D. E., Strandness D. E., Jr Arterial walls before and after endarterectomy. Stress-strain characteristics and collagen-elastin content. Arch Surg. 1969 Nov;99(5):606–611. doi: 10.1001/archsurg.1969.01340170058013. [DOI] [PubMed] [Google Scholar]
- Tagawa H., Tomoike H., Nakamura M. Putative mechanisms of the impairment of endothelium-dependent relaxation of the aorta with atheromatous plaque in heritable hyperlipidemic rabbits. Circ Res. 1991 Feb;68(2):330–337. doi: 10.1161/01.res.68.2.330. [DOI] [PubMed] [Google Scholar]
- Tauth J., Pinnow E., Sullebarger J. T., Basta L., Gursoy S., Lindsay J., Jr, Matar F. Predictors of coronary arterial remodeling patterns in patients with myocardial ischemia. Am J Cardiol. 1997 Nov 15;80(10):1352–1355. doi: 10.1016/s0002-9149(97)00682-6. [DOI] [PubMed] [Google Scholar]
- Tobis J. M., Mallery J., Mahon D., Lehmann K., Zalesky P., Griffith J., Gessert J., Moriuchi M., McRae M., Dwyer M. L. Intravascular ultrasound imaging of human coronary arteries in vivo. Analysis of tissue characterizations with comparison to in vitro histological specimens. Circulation. 1991 Mar;83(3):913–926. doi: 10.1161/01.cir.83.3.913. [DOI] [PubMed] [Google Scholar]
- Villanueva F. S., Jankowski R. J., Klibanov S., Pina M. L., Alber S. M., Watkins S. C., Brandenburger G. H., Wagner W. R. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998 Jul 7;98(1):1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- Villanueva F. S., Jankowski R. J., Klibanov S., Pina M. L., Alber S. M., Watkins S. C., Brandenburger G. H., Wagner W. R. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998 Jul 7;98(1):1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- Yamagishi M., Umeno T., Hongo Y., Tsutsui H., Goto Y., Nakatani S., Miyatake K. Intravascular ultrasonic evidence for importance of plaque distribution (eccentric vs circumferential) in determining distensibility of the left anterior descending artery. Am J Cardiol. 1997 Jun 15;79(12):1596–1600. doi: 10.1016/s0002-9149(97)00205-1. [DOI] [PubMed] [Google Scholar]
- Zeiher A. M., Drexler H., Wollschläger H., Just H. Modulation of coronary vasomotor tone in humans. Progressive endothelial dysfunction with different early stages of coronary atherosclerosis. Circulation. 1991 Feb;83(2):391–401. doi: 10.1161/01.cir.83.2.391. [DOI] [PubMed] [Google Scholar]
- de Korte C. L., van der Steen A. F., Céspedes E. I., Pasterkamp G. Intravascular ultrasound elastography in human arteries: initial experience in vitro. Ultrasound Med Biol. 1998 Mar;24(3):401–408. doi: 10.1016/s0301-5629(97)00280-9. [DOI] [PubMed] [Google Scholar]