Abstract
Selenomethionine (SeMet) is the chemical form or major component of selenium used for cancer chemoprevention in several clinical trials. However, evidence from experimental studies indicates that SeMet has weaker anticancer effects than most other forms of selenium. Recent studies showed that the anticancer activity of SeMet can be enhanced by methioninase (METase), indicating that SeMet metabolites are responsible for its anticancer activity. In the present study, we demonstrated that wild-type p53-expressing LNCaP human prostate cancer cells were more sensitive to co-treatment with SeMet and METase than p53-null PC3 human prostate cancer cells. SeMet and METase co-treatment significantly increased levels of superoxide and apoptosis in LNCaP cells. Co-treatment with SeMet and METase resulted in increased levels of phosphorylated p53 (serine15), total p53, Bax, and p21Waf1 proteins. LNCaP cells treated with SeMet and METase also showed p53 translocation to mitochondria, decreased mitochondrial membrane potential, cytochrome c release into the cytosol, and activation of caspase 9. The effects of SeMet and METase were suppressed by pre-treatment with a synthetic superoxide dismutase mimic or by knockdown of p53 via RNA interference. Reexpression of wild-type p53 in PC3 cells resulted in increases in superoxide production, apoptosis, and caspase 9 activity, and a decrease in mitochondrial membrane potential following co-treatment with SeMet and METase. Our study demonstrates that apoptosis induced by SeMet plus METase is superoxide-mediated and p53-dependent via mitochondrial pathway(s). These results suggest that superoxide and p53 may play a role in cancer chemoprevention by selenium.
Keywords: apoptosis, superoxide, p53 tumor suppressor, selenomethionine, methioninase, prostate cancer
INTRODUCTION
Experimental and clinical studies have demonstrated that selenium supplementation reduces cancer incidence, particularly prostate cancer (1-3). However, the underlying anticancer mechanism(s) of selenium are still not fully understood. Recent data suggest that selenium may prevent carcinogenesis by inhibiting cancer cell proliferation, promoting apoptosis, and modulating p53 functions (4-12). Induction of apoptosis is postulated to be a key event of cancer chemoprevention by selenium (5). Studies have shown that the effects of selenium on cancer cell growth inhibition and apoptosis in cultures and carcinogenesis in animals depend on the form and dose of selenium (13-16). Evidence from experimental studies suggests that selenium metabolites are responsible for the anticancer action (14,15). In addition, studies have shown that reactive oxygen species (ROS) are produced by several selenium compounds through redox catalysis (7,8,14,17-19). Thus, ROS, particularly superoxide, have been postulated to be key metabolites for induction of cancer cell apoptosis by some selenium compounds (14).
Animal studies have demonstrated that most inorganic and organic forms of selenium compounds have anti-cancer activity (1,2). Selenite and selenomethionine (SeMet) have been used in most experimental and clinical studies. SeMet is the major component in selenized yeast supplements and is the form of selenium used in clinical trials (1,2,6,14). Both selenite and SeMet have anti-cancer activity, but SeMet is less efective than selenite, particularly in vitro (2,14). Our previous studies demonstrated that selenite-induced apoptosis of human prostate cancer cells was superoxide-mediated and p53-dependent via mitochondrial pathways (7,8). A number of studies have demonstrated that selenite-induced apoptosis was mediated by ROS production (14,17-19). In contrast, SeMet has weaker anticancer activity than most other selenium compounds (14). The low anticancer activity of SeMet is most likely associated with its metabolism within cells. Recent studies showed that noneffective concentrations of SeMet in the presence of methioninase (METase) or methinone β-lyase induced apoptosis in human cancer cells (20-24), suggesting that active metabolites are generated from the catalysis of SeMet by these enzymes. Studies showed that overexpression of METase increased apoptosis and superoxide production by SeMet in cancer cells, and co-treatment with METase adenoviral constructs and SeMet inhibited tumor growth in nude mice (20,21). A recent study demonstrated that a mixture of SeMet and METase generated superoxide in an in vitro system (25). These combined data suggest that superoxide may be the one of active metabolites of SeMet responsible for growth inhibition and apoptosis of cancer cells.
The aim of the present study was to investigate the role of superoxide and p53 in SeMet and METase-induced apoptosis in human prostate cancer cells. We compared cellular effects and superoxide production in the wild-type p53-containing LNCaP and p53-null PC3 human prostate cancer cell lines following co-treatment with SeMet and METase. We also analyzed effects of down-regulation or re-expression of p53 on cellular response to SeMet plus METase treatment and the interaction between superoxide and p53 in promoting apoptosis by SeMet plus METase in these two human prostate cancer cell lines. Our study not only confirms the observation of production of superoxide and induction of apoptosis by SeMet plus METase in previous studies, but also demonstrates that induction of apoptosis by SeMet plus METase is p53-dependent via mitochondrial pathway(s) and that superoxide production by SeMet plus METase is p53-dependent. Our results suggest that superoxide acts as both an activator and a down-stream effecter of p53 to promote apoptosis by SeMet plus METase treatment.
MATERIAL AND METHODS
Chemicals and Antibodies
L-selenomethionine (SeMet) was purchased from Sigma Chemical Co. (St. Louis, MO). Methioninase (METase), a recombinant enzyme from the Trichomonas vaginalis gene produced from Escherichia coli, was purchased from Wako Chemicals USA, Inc. (Richmond, VA). Manganese (III) tetrakis (N-methyl-2-pyridyl) porphyrin (MnTMPyP) was purchased from Alexis Biochemicals (San Diego, CA). p53 small interfering RNAs (siRNAs) were purchased from Cell Signaling Technology (Beverly, MA). siRNA Duplex control (non-silencing) and RNAiFect™ Transfection Reagent were purchased from QIAGEN (Valencia, CA). Apoptotic DNA-Ladder kit was purchased from Roche Diagnostic Co. (Indianapolis, IN). Caspase-Glo 9 Assay kit was purchased from Promega Co. (Madison, WI). Lucigenin (bis-N-methylacridinium nitrate) and 5, 5′, 6, 6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) were purchased from Molecular Probes, Inc (Eugene, OR). SuperSignal West Pico Stable Peroxide Solution, SuperSignal West Pico Luminol/Enhancer Solution, M-Per Mammalian Protein Extraction Reagent, and Mitochondria Isolation kit were purchased from Pierce Biotechnology Inc. (Rockford, IL). The other chemicals were all from Fisher Scientific (Fair Lawn, NJ).
Anti-β-actin monoclonal antibody was purchased from Sigma Chemical Co. (St. Louis, MO). Anti-phosphorylated p53 (ser15) antibody was purchased from Cell Signaling Technology (Beverly, MA). Anti-p21Waf1 (C-19) antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-Bax and anti-phosphorylated histone H2AX antibodies were purchased from Upstate USA Inc. (Upstate, Charlottesville, VA).
Cell Culture
LNCaP and PC3 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and routinely maintained in 100-mm tissue culture dishes (Corning, Acton, MA) in RPMI 1640, supplemented with 5% heat-inactivated fetal bovine serum and 1% antibiotic-antimycotic (Life Technologies, Inc., Rockville, MD) at 37°C in a humidified atmosphere of 95% air and 5% CO2.
Superoxide Measurement
Lucigenin-dependent chemiluminescence (CL) in cells was measured by a modified method as described previously (26). The stock solution of lucigenin (10 mM) was prepared in PBS and stored at −20°C in the dark. 100 μM lucigenin was added to 1 × 105 cells in 100 μL PBS, and pre-incubated with or without 5 μM MnTMPyP for 30 min. The reaction was initiated by the addition of lucigenin, SeMet, and METase to the cell suspension and the CL level was measured and recorded as relative light units (RLU) by a luminometer (Lumat, Berthold, LB9501, Oak Ridge, TN) for a total period of 8 min at 30 sec intervals.
Flow Cytometric Analysis
Cell samples were prepared and analyzed as described previously (8). After trypsinization, 1 × 106 cells were washed with PBS/EDTA/BSA buffer (PBS, 1 mM EDTA, and 0.1% BSA) and fixed in 100 μl of PBS/EDTA/BSA buffer plus 900 μl of 70% ethanol for 30 min at −20°C. After washing with phosphate-citric acid buffer (0.192 M Na2HPO4 and 4 mM citric acid, pH 7.8), the cells were stained in 500 μl of propidium iodide staining solution (33 μg/ml propidium iodide, 200 μg/ml DNase-free RNase A, and 0.2% Triton X-100) overnight at 4°C. Both cell cycle distribution and sub-G1 cells were simultaneously measured in a FACScan flow cytometer (Becton Dickinson, Franklin Lakes, NJ) using 488-nm laser excitation.
To measure mitochondrial membrane potential (MMP), cells were resuspended in 1 mL of serum-free medium containing 2.5 mM JC-1 dye and incubated at 37 °C for 20 min. After washing twice with PBS, fluorescence in cells was immediately measured in a flow cytometer. Mitochondrial depolarization is indicated by the decrease in the ratio of the red signal at emission 590 nm to the green signal at emission 530 nm.
Apoptotic DNA Ladder Analysis
DNA isolation and gel electrophoresis were performed according to manufacturer's instructions. Briefly, cells were scraped in PBS buffer and harvested by centrifugation at 500g for 5 min at room temperature and lysed in 400 μL lysis buffer for 10 min at room temperature. Following the addition of 100 μL isopropanol, the lysate was centrifuged through a filter and washed with the washing buffer. Genomic DNA was eluted with 100 μL elution buffer. Equal amounts of DNA were loaded onto a 1.5% agarose gel containing 0.1 mg/ml ethidium bromide and electrophoresed. The gel were photographed with Kodak Image Station 2000R (Eastman Kodak Company, Rochester, NY) using UV illumination and digitized with Kodak 1D 3.6 software.
MTT assay
Cells were seeded at 1 × 105 cells/well in 24-well plates overnight and then treated with different agents for an additional 5 days. MTT (3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2 tetrazolium bromide) solution (10 μl, 5 mg/ml in PBS) was added to each well of the plate and incubated for 3 hr at 37°C. MTT lysis buffer (100 μl of 10% SDS, 45% dimethyl formamide, adjusted to pH 4.5 by glacial acid) was then added to dissolve the formazan. The optical density was measured at 570 nm using a Beckman Coulter DU-640 Spectrophotometer (Beckman Coulter, Inc. Fullerton, CA). The percentage of viable cells was calculated as the relative ratio of optical density to the control.
Western blot analysis
Cell pellets were lysed with M-PER mammalian protein extraction reagent (Pierce, Rockford, IL) and protein concentrations were determined by the Bradford assay (Bio-Rad, Hercules, CA). Cell lysates (20-50 μg) were electrophoresed in 12.5 % SDS polyacrylamide gels and then transferred onto nitrocellulose membranes. After blotting in 5% nonfat dry milk in Tween-20 Tris-buffered saline (TTBS), the membranes were incubated first with primary antibodies at 1:1,000–2,000 dilutions in TTBS overnight at 4°C and then with secondary antibodies conjugated with horseradish peroxidase at 1:10,000 dilution in TTBS for 1 hr at room temperature. Protein bands were visualized on X-ray film using an enhanced chemiluminescence system (Pierce Biotechnology, Rockford, IL).
Small interfering RNA (siRNA) transfection
Cells were seeded at 1 × 105 cells/well in 6-well plates, and allowed to grow to 60% confluence. Cells were transfected with 50 nM of p53 siRNA and 2 μL RNAiFect transfection reagent in 1 ml serum-free medium for 12 hr, and then 1 ml fresh medium with 10% FBS was added to each well for 24 hr before SeMet and MET treatment. Cells were also transfected with the non-silencing, negative control siRNA which has no known homology to mammalian genes and allows assessment of non-specific gene silencing effects.
Adenoviral transduction
PC3 cells were seeded at 4 × 105 in 60 mm tissue culture dishes for western blot analysis and at 1 × 105/well in 24-well plates for viability assay. Approximately 20 hr later, cells were infected with the indicated multiplicity of infection (MOI) of recombinant Ad5 CMV wt p53-GFP adenoviral constructs (p53-Ad) or empty control adenoviral constructs (control-Ad) in serum-free medium. After 12 hr, an equal volume of fresh medium with 10% FBS was added to each dish or well for 24 hr before SeMet and METase treatment.
Caspase 9 activity assay
Cells were seeded at 3 × 104 cells/well in a 96-well plate with 100 μL medium. Approximately 16 hr later, cells were treated with SeMet and METase for 18 hr to induce apoptosis. Caspase-Glo™ 9 Reagent (100 μl) was directly added into each well to a final volume of 200 μl/well. Chemiluminescence was measured using a Tropix TR717™ Microplate Luminometer (Applied Biosystems, Bedford, MA).
Mitochondria fractionation
Cells were seeded at 6 × 105 cells in 100 mm tissue culture dishes and allowed to grow to 60% confluence. Cells were treated with 3 μM SeMet and METase for 18 hr to induce apoptosis, and then mitochondria and cytosol fractions were separated from cells according to the manufacturer's instructions (Pierce Biotechnology Inc., Rockford, IL).
Statistical analysis
All data were presented as means ± standard deviation (SD) from at least three sets of independent experiments. ANOVA analysis with Tukey's multiple comparisons was used to determine the significance of statistical differences between data at the level of p <0.05 using SPSS computer statistics software (SPSS, Inc., Chicago, IL)
RESULTS
Induction of superoxide production and apoptosis by SeMet and METase in LNCaP cells
LNCaP cells were treated with different doses of SeMet plus 0.1U/mL METase for different time periods and cell viability was assessed by the MTT assay. As shown in Fig. 1A and B, SeMet and METase co-treatment decreased cell viability in a dose- and time-dependent manner. Significant cell viability decreases occurred in cells treated with 1.5 μM and higher doses of SeMet with 0.1U/mL METase (Fig. 1A) or in cells treated with 3.0 μM SeMet with 0.1U/mL METase for 36 hr and longer times (Fig. 1B) with an IC50 of 2.5 μM after 72 hr of treatment. METase alone did not cause significant cell death (Fig. 1B). Analyses of apoptosis by flow cytometry and gel electrophoresis showed that cells treated with 3.0 μM SeMet plus 0.1U/mL METase showed a 45-fold increase in the sub-G1 cell population compared to the control, and DNA laddering (fragmentation) was observed in cells treated with 3.0 μM SeMet plus 0.1U/mL METase (Fig. 1C and D). These data demonstrated that cells underwent apoptosis following treatment with SeMet plus METase. To assess the involvement of superoxide in apoptosis, cells were pre-treated with a chemical superoxide dismutase (SOD) mimic, MnTMPyP. Pre-treatment with 3 μM MnTMPyP significantly reduced SeMet plus METase-induced DNA fragmentation (Fig. 1D) and cell death (Fig. 1E). Treatment with SeMet, METase, or MnTMPyP alone did not cause significant cell death (Fig. 1E). Lucigenin-dependent chemiluminescence assay showed that treatment with 3.0 μM SeMet and 0.1U/mL METase resulted in an increase in intracellular chemiluminescence in 4 min with a peak value at 6 min (Fig. 1F). SeMet or METase alone did not cause significant increases in chemiluminescence. Chemiluminescence produced by SeMet and METase treatment was suppressed by MnTMPyP pre-treatment. There was only minimal chemiluminescence detected in the mixture of the culture medium and lucigenin in the absence of cells (data not shown). These combined results indicate that SeMet and METase treatment triggers cell apoptosis by producing superoxide.
Figure 1.
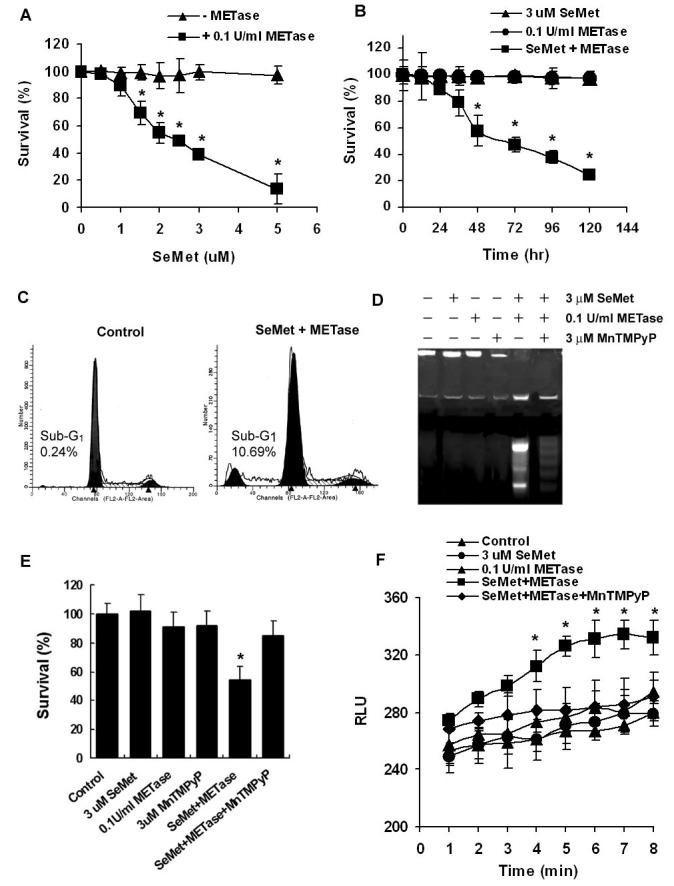
Effects of SeMet and METase on apoptosis and superoxide production in LNCaP cells. A, MTT assay demonstrating a dose-dependent effect of SeMet and METase on cell viability. Cells were treated with SeMet and METase for 5 days. B, MTT assay showing a time-dependent effect of SeMet, METase, or combination on cell viability. C, Flow cytometric analysis demonstrating apoptosis (sub-G1 cell population) induced by SeMet and METase. Cells were treated with 3 μM SeMet and 0.1 U/ml METase for 24 hr. D, Agarose gel electrophoretic detection of DNA fragmentation as a marker of cell apoptosis induced by SeMet and METase. Cells were treated with 3 μM SeMet, 0.1 U/ml METase, and 3 μM MnTMPyP alone or in combinations for 24 hr. E, Protection by MnTMPyP against cytotoxicity of SeMet and METase. Cells were treated with 3.0 μM SeMet, 0.1U/ml METase, 3.0 μM MnTMPyP alone or in combinations for 5 days and cell survival was measured by the MTT assay. F, Superoxide production in cells co-treated with SeMet and METase. Cells were treated with 3.0 μM SeMet and 0.1U/ml METase with/without 3.0 μM MnTMPyP and superoxide was measured using a chemiluminescence assay. RLU, relative light units. Data are presented as means ± SD of three independent experiments. * p<0.05 compared to no METase (A), SeMet or METase alone (B), and control or SeMet or METase (E and F).
p53 regulation and p53-dependent cell death by SeMet plus METase in LNCaP cells
To determine whether p53 is activated by SeMet and METase treatment, western blot analysis was used to detect immunoreactive levels of total p53 and phosphorylated p53 at serine 15 (P-p53 Ser15) and p53 target genes p21Waf1 and Bax. As shown in Fig. 2A and B, co-treatment with SeMet and METase resulted in elevations of total p53 and P-p53 Ser15 in LNCaP cells in a dose- and time-dependent pattern. Protein levels of both p21Waf1 and Bax were also elevated corresponding to the elevation of p53 observed following SeMet plus METase treatment. Detectable elevations of total and phosphorylated p53 occurred at 1.5 μM and higher concentrations of SeMet, while elevations of p21Waf1 and Bax were detected at the 0.5 μM concentration. Elevations of total p53, P-p53 Ser15, and Bax were observed at 1 hr and thereafter following treatment with SeMet and METase, while an elevation of p21Waf1 was first observed at 3 hr. To exclude an involvement of DNA damage by SeMet and METase treatment, a DNA damage marker, phosphorylated histone H2AX at serine 139 (H2AX), was analyzed by western blot analysis (27). As shown in Fig. 2A and B, there were no significant changes in this phosphorylated protein in cells treated with SeMet plus METase, indicating that DNA damage is not the major factor causing p53 activation in this study. Fig. 2C shows that only co-treatment with SeMet and METase resulted in significant elevations of total p53, P-p53 Ser15, p21Waf1, and Bax while pre-treatment with MnTMPyP inhibited the effect of SeMet and METase on these proteins. These results suggest that treatment with SeMet and METase produces superoxide which subsequently activates p53 via a nonDNA damage pathway.
Figure 2.
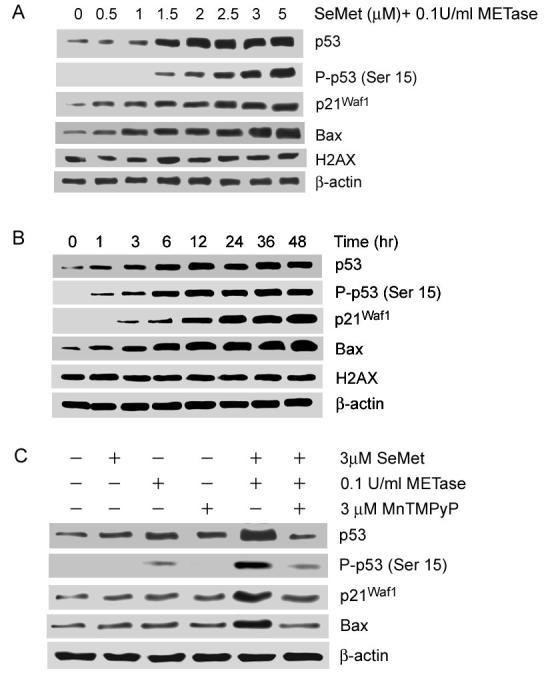
Western blot analysis of effects of SeMet and METase on the expression of p53, p21Waf1, and Bax and phosphorylation of p53 (Ser15) and histone (Ser 139) (H2AX) in LNCaP cells. A, Dose-dependent effect of SeMet and METase. Cells were treated with SeMet + 0.1U/ml METase for 18 hr. B, Time-dependent effect of SeMet and METase. Cells were treated with 3.0 μM SeMet + 0.1U/ml METase. C, Suppression of effects of SeMet and METase on p53, p21Waf1, and Bax by SOD mimic MnTMPyP. Cells were treated for 18 hr. Protein loading: 40 μg for p53, P-p53 Ser15, p21Waf1, Bax, and H2AX and 20 μg for β-actin.
We next determined the role of p53 in SeMet and METase-induced cell death using RNA interference to reduce levels of p53 protein. As shown in Fig. 3A, transfection with p53 siRNA inhibited up-regulation of total p53, P-p53 Ser15, and p21Waf1 proteins by co-treament with SeMet and METase in LNCaP cells, while transfection with the negative control siRNA did not affect the results of SeMet plus METase treatment. However, up-regulation of Bax was not affected by p53 siRNA transfection, suggesting that Bax regulation by SeMet and METase is not completely p53-dependent. Cells transfected with p53 siRNA showed decreased sensitivity to SeMet plus METase compared to SeMet or METase, while the sensitivity did not change in cells transfected with the control siRNA (Fig. 3B). These results demonstrate that cell death induced by SeMet plus METase is p53-dependent.
Figure 3.
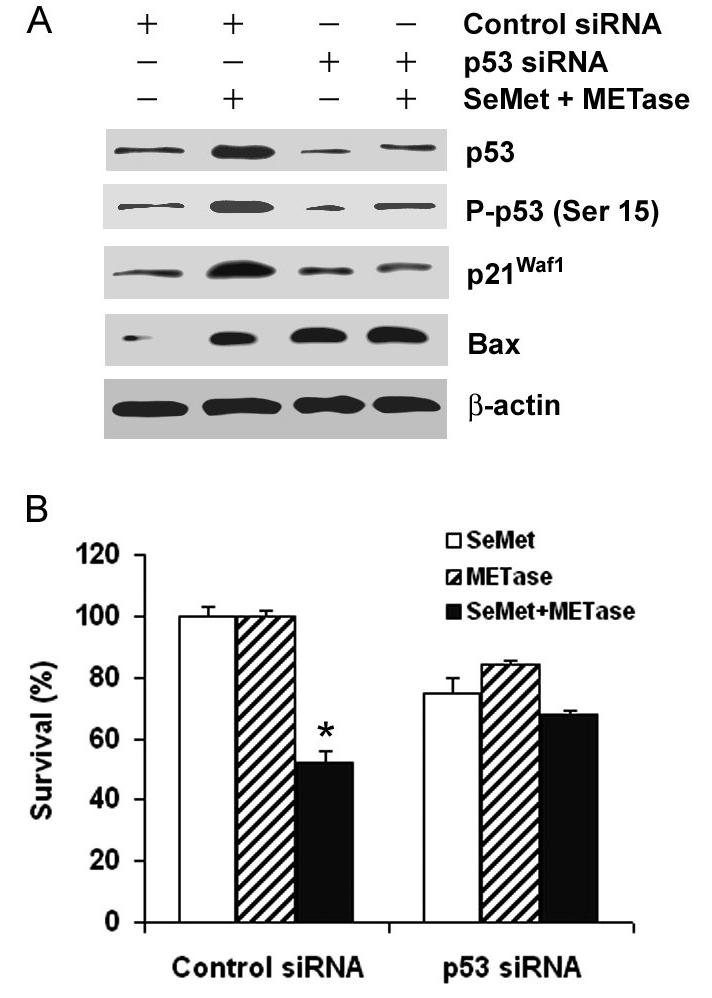
Suppressive effects of p53 siRNA transfection on cellular response to SeMet and METase in LNCaP cells. A, Western blot analysis of suppression of SeMet and METase-induced up-regulation of p53, p21Waf1, and Bax by p53 siRNA transfection. Cells were transfected with 50 nM p53 siRNA or control siRNA for 36 hr and then treated with 3 μM SeMet plus 0.1 U/ml METase for 18 hr. Protein loading: 40 μg for p53, P-p53 Ser15, p21Waf1, and Bax, and 20 μg for β-actin. B, MTT assay of viability of LNCaP cells with p53 siRNA transfection and treatment with 3.0 μM SeMet and/or 0.1U/ml METase. Cells were transfected with 50 nM p53 siRNA or control siRNA for 36 hr and then treated with SeMet and/or METase for 5 days. The data were obtained from three independent experiments and the results shown are the mean ± SD. * p<0.05 in comparison of control siRNA with SeMet or METase only or p53 siRNA with SeMet, METase or SeMet + METase.
Effect of p53 on cellular response to SeMet and METase in p53-null PC3 cells
To further verify that cellular sensitivity to SeMet and METase is dependent on p53, we next tested the sensitivity of p53-null PC3 cells to SeMet and METase before and after re-expression of wild type (wt)-p53. Dose-dependent and time-course studies showed that PC3 cells were much less sensitive to SeMet plus METase than LNCaP cells (Fig. 4A and B). The IC50 was 2.5 μM SeMet for LNCaP cells (Fig. 1A), while treatment with 5 μM SeMet induced only about 20% cell death in PC3 cells (Fig. 4A). The time course study showed that IC50 was achieved at 78 hr in LNCaP cells (Fig. 1B), while only 10% of PC3 cells died at 72 hr and less than 20% cell died at 120 hr at the same dose of SeMet (Fig. 4B). Western blot analysis showed that PC3 cells had no detectable p53 and very low levels of p21 and Bax at the protein loading levels (40 μg) analyzed (Fig. 4C). Following transduction of p53 adenoviral constructs (p53-Ad), p53 was re-expressed and p21Waf1 and Bax were elevated in PC3 cells. SeMet plus METase treatment further increased levels of total p53 and P-p53 Ser15, p21Waf1, and Bax in PC3 cells transduced with p53-Ad. SeMet and METase treatment also increased levels of p21Waf1 and Bax proteins in PC3 cells without p53-Ad transduction, indicating that p21Waf1 and Bax regulation can be both p53-dependent and p53-independent. Fig. 4D shows that re-expression of p53 enhanced the sensitivity of PC3 cells to SeMet plus METase only, while transduction of control adenoviral constructs (Control-Ad) did not alter the cellular sensitivity to SeMet plus METase. These results clearly demonstrate that cell death induced by SeMet plus METase is p53-dependent.
Figure 4.
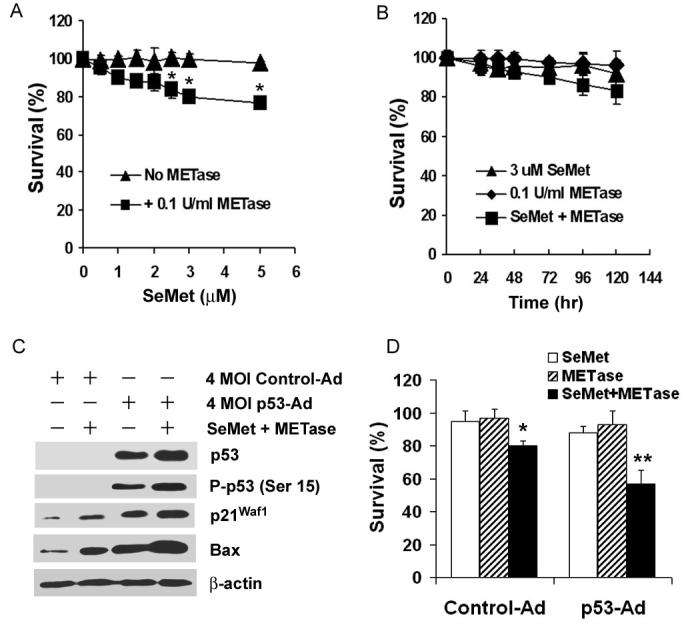
Effects of p53 on cellular response to SeMet and METase in PC3 cells. A, MTT assay of dose-dependent effect of SeMet plus METase on cell viability. Cells were treated with SeMet alone or with 0.1 U/ml METase for 5 days. B, MTT assay of time-dependent effect of SeMet and METase on cell viability. Cells were treated with SeMet or METase alone or in combination. C, Western blot analysis of levels of p53, P-p53 Ser 15, p21Waf1, and Bax in cells following transduction of 4 multiplicity of infection (MOI) units of empty control adenovirus (control-Ad) or p53 cDNA adenovirus (p53-Ad) constructs for 36 hr and subsequent treatment with 3 μM SeMet and 0.1 U/ml METase for 18 hr. Protein loading: 40 μg for p53, P-p53 Ser15, p21Waf1, and Bax, and 20 μg for β-actin. D, MTT assay of effect of p53 on viability of cells with or without SeMet and METase treatment. Cells were transduced with 4 MOI control-Ad or p53-Ad constructs for 36 hr and then treated with 3.0 μM SeMet, 0.1U/ml METase, or SeMet plus METase for 5 days. The data were obtained from three independent experiments and the results shown are the mean± SD. *p<0.05 compared with control-Ad with SeMet or METase only. ** p<0.05 compared with control-Ad with SeMet + METase and p53-Ad with SeMet or METase only.
p53-dependent superoxide production by SeMet plus METase treatment in LNCaP and PC3 cells
Since SeMet and METase-induced cell apoptosis is dependent on p53, we further analyzed the role of superoxide in SeMet plus METase-induced, p53-dependent apoptosis. We first reduced levels of p53 in LNCaP cells by siRNA transfection. As shown in Fig. 5A, transfection of p53 siRNA suppressed SeMet plus METase-induced elevation of superoxide, though p53 siRNA transfection alone also increased levels of superoxide compared to the control. The latter was mostly likely due to superoxide production from RNAiFect and siRNA transfection which was observed in our previous study (8). Conversely, re-expression of wt-p53 in PC3 cells significantly increased superoxide production following SeMet and METase treatment (Fig. 5B). Over-expression of p53 or treatment with SeMet or METase only also increased superoxide levels in PC3 cells, but the magnitudes were much lower than their combination. These results demonstrate that superoxide production by SeMet and METase treatment can be enhanced by p53, suggesting that superoxide may act as a p53 activator and downstream mediator of p53-dependent apoptosis. It is well known that ROS can cause oxidative stress and cell apoptosis and that p53 can be activated by oxidative tress to regulate cell cycle arrest and apoptosis (28,29). It has been reported that ROS were downstream effecters of p53 (30), which is consistent with our observation in this study.
Figure 5.
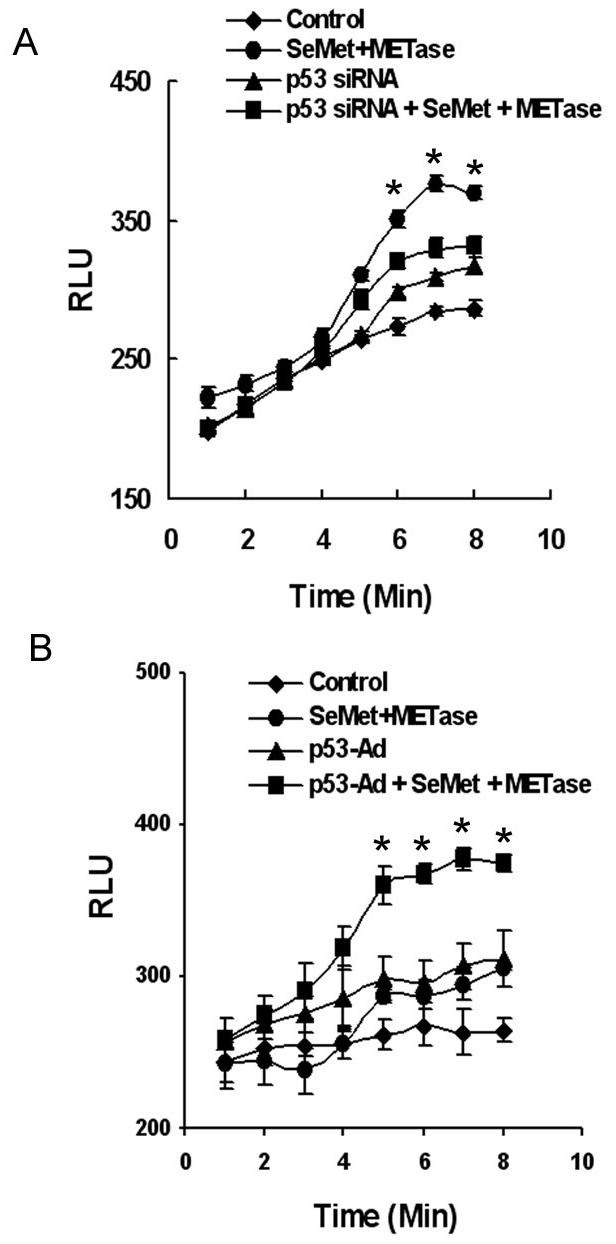
Effects of p53 on SeMet and MET-induced production of superoxide in LNCaP and PC3 cells. A, Chemiluminescence assay showing suppression of superoxide production by p53 siRNA transfection in LNCaP cells treated with SeMet and METase. B, Elevation of superoxide production by co-treatment with SeMet and METase in PC3 cells transduced with Ad-p53. Cells were transfected with 50 nM p53 siRNA or transduced with 4 MOI Ad-p53 for 36 hr and then treated with 3.0 μM SeMet and 0.1U/ml METase in suspension. Superoxide was immediately measured using a luminometer. The data were obtained from three independent experiments and the results shown are the mean ± SD. *p<0.05 compared with control.
p53-dependent, superoxide-mediated mitochondrial pathways of apoptosis induced by SeMet plus METase treatment
It has been known that p53 can execute apoptosis through mitochondria via transcription-dependent and –independent pathways (31,32). To explore mitochondrial-dependent apoptosis, mitochondria were isolated from the cytosol of LNCaP cells. Mitochondrial translocation of p53 and cytochrome c release from mitochondria were assessed by western blot analysis. As shown in Fig. 6A, protein levels of p53, P-p53 Ser15, p21Waf1, and Bax increased in LNCaP cells following treatment with 3.0 μM SeMet plus 0.1U/mL METase for 18 hr. In addition, a substantial amount of p53 translocated to mitochondria. The levels of cytochrome c in mitochondria also dramatically increased with a substantial amount released into the cytosol. Levels of Bax were elevated in the cytosol, but no significant changes were observed in mitochondria. To further assess the mitochondrial pathway of apoptosis, mitochondrial membrane potential (MMP) was studies by JC-1 fluorescent dye staining, a procedure that analyzes depolarization of mitochondrial membranes. As shown in Fig. 6B, a significant decrease in MMP occurred only in LNCaP cells treated with 3.0 μM SeMet plus 0.1U/mL METase for 18 hr. The depolarization of MMP by SeMet plus METase was suppressed by transfection with p53 siRNA. In contrast, PC3 cells showed no significant change in MMP following SeMet, METase, or combine treatment (Fig. 6C). After re-expression of wt-p53 by andenoviral transduction, only SeMet plus METase treatment significantly decreased MMP in PC3 cells.
Figure 6.
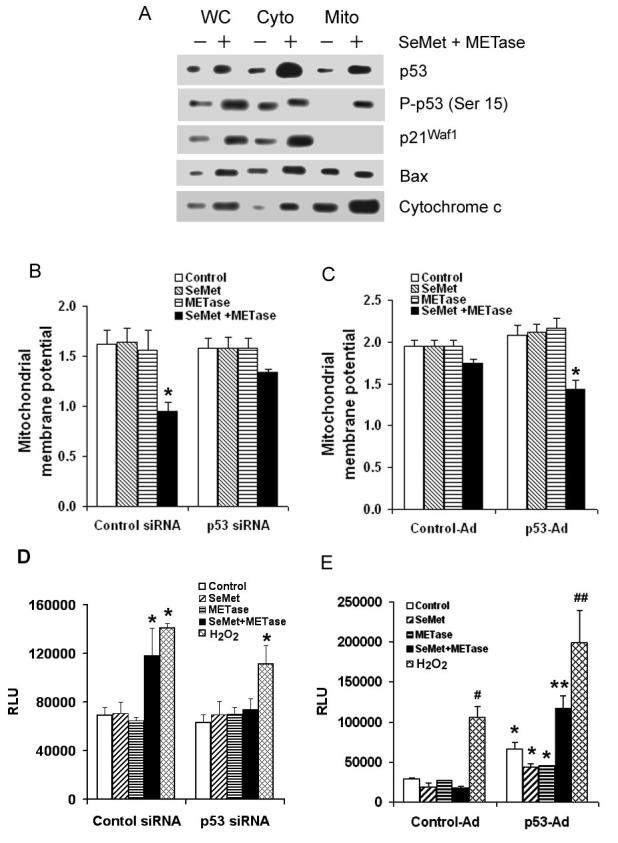
Effects of SeMet and METase on p53 mitochondrial translocation, cytochrome c release from mitochondria, mitochondrial membrane potential, and caspase 9 activation in LNCaP and PC3 cells. A, Western blot analysis of SeMet and METase-induced p53 accumulation in mitochondria and release of cytochrome c into the cytosol in LNCaP cells. WC, whole cell lysate; Cyto, cytosol fraction; Mito, mitochondrial fraction. Cells were treated with 3.0 μM SeMet and 0.1U/ml METas for 18 hr. p21Waf1 was used as a control for the purity of mitochondrial extracts and also as a possible marker for p53 transcriptional activity. Protein loading: 40 μg/lane. B. JC-1 fluorescence analysis of effect of SeMet and METase on mitochondrial membrane potential in LNCaP cells with or without p53 siRNA transfection. *p<0.05 compared to control siRNA with SeMet or METase alone and p53 siRNA with SeMet, METase, or SeMet + METase. C, JC-1 fluorescence analysis of alteration of mitochondrial membrane potential by treatment with SeMet and METase in PC3 cells. *p<0.05 compared to control-Ad and p53-Ad + SeMet or METase alone. D, Chemiluminescence assay of activation of caspase 9 in LNCaP cells treated with 3 μM SeMet, 0.1U/ml METase, SeMet + METase, or 0.1 mM H2O2 and the suppressive effect of p53 siRNA transfection. *p<0.05 compared to control siRNA only or p53 siRNA only. E, Chemiluminescence assay of activation of caspase 9 in PC3 cells treated with 3 μM SeMet, 0.1U/ml METase, SeMet + METase, or 0.1 mM H2O2 and the enhancing effect of p53-Ad transduction. *p<0.05 compared with corresponding control-Ad only and treatments with SeMet and/or METase. **p<0.05 compared with p53-Ad with SeMet or METase alone. #p<0.05 compared to control-Ad only. ##p< 0.05 compared with control-Ad treated with H2O2. LNCaP cells were transfected with 50 nM control or p53 siRNAs and PC3 cells were transduced with 4 MOI control-Ad or p53-Ad constructs for 36 hr and both LNCaP and PC3 cells were then treated with SeMet, METase, SeMet plus METase, or H2O2 for 18 hr. Chemiluminescence was measured using a luminometer, while JC-1 fluorescence was measured using a flow cytometer. All the data were obtained from three independent experiments and the results shown are the mean ± SD.
We next analyzed caspase 9 activity with a chemiluminescence assay for mitochondrial-dependent apoptosis. As shown in Fig. 6D, only treatment with 3.0 μM SeMet plus 0.1U/mL METase increased the caspase 9 activity in LNCaP cells. Activation of caspase 9 by SeMet plus METase treatment was suppressed by p53 siRNA transfection. In contrast, PC3 cells showed no significant change in caspase 9 activity following SeMet plus METase treatment (Fig. 6E). After re-expression of wt-p53 by andenoviral transduction, SeMet plus METase treatment significantly increased caspase 9 activity in PC3 cells. Fig. 6D and E also show that caspase 9 activation by H2O2 was only partially dependent on p53. These data demonstrate that apoptosis induced by SeMet plus METase is p53-dependent via mitochondrial pathway(s).
DISCUSSION
Selenium is an essential trace element for human health and has been shown to be an anticancer agent in animal and clinical studies (1-3). Maintenance of maximal levels of selenium-containing antioxidant enzymes requires only nutritional levels of selenium supplementation, while cancer chemoprevention requires supranutritional levels of selenium supplementation, indicating that other mechanism(s) may be involved in cancer chemoprevention by selenium in addition to its antioxidant effects. Combs suggested that nutritional levels of selenium supplementation provide antioxidant protection against oxidative stress, while supranutritional levels may cause subtoxic effects to induce cell growth inhibition and/or apoptosis for cancer prevention (1).
Accumulating evidence from experimental studies indicates that active metabolites, particularly redox cycling ones, play an important role in inhibition of proliferation and induction of apoptosis by some selenium compounds (2,14). ROS, particularly superoxide, are produced by several selenium compounds when they interact with glutathione (GSH), and induction of apoptosis was associated with ROS production. Spallholz suggested that the anticarcinogenic property of these selenium compounds is likely due to the toxicity of superoxide generated from redox cycling of certain metabolites (14). It has been well documented that metabolism of selenite is involved in oxidation of GSH and superoxide production in biologic systems. Selenite reacts with GSH to form selenodiglutathione (GSSeSG) and glutathione disulfide (GSSG). GSSeSG reacts with NADPH or GSH to produce hydrogen selenide (H2Se). Hydrogen selenide is oxidized by O2 to produce elemental selenium and superoxide. The intermediate metabolite selenotrisulfide generated from interaction of selenite with GSH may also produce superoxide and other ROS (14). One study reported that selenocystamine (RSeSeR) can interact with GSH to form reduced diselenide (RSe−) that interacts with O2 to produce superoxide (33), suggesting that the selenopersulfide anion (GSSe−) formed from selenite may react with O2 to produce superoxide in a similar pathway. Therefore, superoxide is most likely to play a major role in the pro-oxidant effects by some selenium compounds. Studies also found that different chemical forms of selenium compounds have different efficacy in cancer prevention and selenium compounds with superoxide production generally have better anticancer activity (14,25), suggesting that the subtoxic yet pro-oxidative effect of these selenium compounds may be the mechanism by which selenium induces cell growth inhibition and apoptosis. Selenite-induced cell death can be inhibited by treatment with a SOD mimic or by overexpression of MnSOD (8,34). Recent studies demonstrated that normal prostate epithelial cells had high levels of MnSOD and low sensitivity to selenite compared to prostate cancer cells (35,36), suggesting that high levels of MnSOD protect normal epithelial cells against superoxide toxicity from selenium compounds. These data support the concept that superoxide is responsible for apoptosis induced by certain selenium compounds.
Unlike selenite, SeMet is very ineffective in vitro though it can reduce cancer incidence in vivo, but has lower in vivo anticarcinogenic effect than selenite (3,14). Recent studies showed that in vitro anticancer activity of SeMet was significantly enhanced in cancer cells with over-expression of METase or with METase treatment (20,21). This enhanced activity of SeMet by METase was suppressed by SOD treatment. Spallholz et al. showed that SeMet plus METase generated superoxide in an in vitro chemiluminescence assay (25). METase is an enzyme that can convert SeMet to methyselenol and has been found in bacteria and the protozoan Trichomonas vaginalis (37). Other studies have demonstrated analogous enzymes to METase in tissues of humans and mice (37-41). These data suggest that superoxide production by SeMet may contribute to its anticancer action in vivo because METase is present in tissues. Experimental evidence indicates that methyselenol is the selenium metabolite responsible for cancer chemoprevention (42). A recent study showed that methylselenol generated superoxide from the direct reduction of both dimethyldiselenide and methylseleninic acid in the presence of GSH (43). One study showed that the toxic pro-oxidant methylselenol was released from SeMet by cancer cells transformed with the adenoviral METase gene (20). Methylselenol damaged the mitochondria via oxidative stress, and caused cytochrome c release into the cytosol, thereby activating caspases and promoting apoptosis. Accordingly, superoxide production from the catalysis of SeMet by METase is postulated to be associated with the reaction of methylselenol or other selenium radicals with oxygen (25). Our study clearly showed that superoxide was produced only in the presence of both SeMet and METase. Low activity of SeMet in vitro is most likely due to lack or low activity of METase in cancer cells. Low anticancer activity of SeMet may also be due to its direct incorporation into proteins in place of methionine and therefore is unable to undergo redox cycling to produce active metabolites, including superoxide, in cancer cells.
The tumor suppressor p53 protein plays an important role in apoptosis (44,45). Induction of apoptosis is considered to be central to the tumor suppressive function of p53. p53 can translocate to mitochondria in response to DNA damage or other stressors, resulting in apoptosis via alteration of the mitochondrial membrane potential and cytochrome c release into the cytosol with resultant caspase activation (31,46,47). p53-dependent apoptosis has also been demonstrated to be mediated by ROS (28). Apoptosis triggered by p53 has been reported to be dependent on an increase in ROS and the release of apoptotic factors from mitochondrial damage (47). These studies suggest that ROS are downstream mediators in p53-dependent apoptosis in transcription-dependent or independent pathways. ROS are known to play an important role in apoptosis. When cells are exposed to oxidative stress, p53 is expressed at high levels by post-translational modifications, including phosphorylation, acetylation and glycosylation (48,49). These modifications occur rapidly and lead to the activation of p53, resulting in cell cycle arrest or apoptosis. Therefore, ROS can function as p53 activators or p53 downstream effectors.
Our data demonstrated that wt-p53 expressing LNCaP cells were more sensitive to SeMet plus METase treatment than p53-null PC3 cells. SeMet plus METase treatment resulted in increased intracellular superoxide, p53 activation, and cell apoptosis. SeMet and METase treatment also resulted in translocation of p53 to mitochondria, cytochrome c release into the cytosol, and activation of caspase 9. The effects in LNCaP cells were suppressed by the SOD mimic MnTMPyP or by knockdown of p53 via RNA interference. On the other hand, the effects of SeMet plus METase were enhanced by restoration of wt-p53 expression in p53-null PC3 cells. In addition, our study showed that superoxide production by SeMet and METase treatment was enhanced by restoration of p53 expression in PC3 cells and decreased by knockdown of p53 in LNCaP cells. These results indicate that induction of apoptosis by SeMet plus METase treatment is superoxide-mediated and p53-dependent via mitochondrial pathway(s) in association with translocation of p53 to mitochondria. The results also suggest that superoxide is a p53 activator and a downstream mediator of p53-dependent apoptosis. These effects of SeMet and METase are identical to those of selenite observed in our previous study (8). One should note that selenium may prevent cancer via multiple mechanisms and cancer cell response to selenium may also depend on other factors, such as androgen dependence. In addition to the difference of the p53 status between LNCaP and PC3 cells, LNCaP cells express androgen receptor and respond to androgen treatment. Recent studies have demonstrated that the selenium compounds can suppress the androgen receptor and its signaling in LNCaP and LAPC-4 cells (50,51). Thus, we believe that superoxide-mediated, p53-dependent apoptosis is only one of the mechanisms by which selenium exerts its anticancer activity.
In summary, Results from this study and others indicate that superoxide production from SeMet catalysis by METase plays a role in induction of cancer cell apoptosis, suggesting that production of superoxide from SeMet metabolism may be responsible, at least in part, for anticancer action in vivo. The results from our previous and current studies demonstrate that superoxide and p53 play an important role in selenite- and SeMet-induced apoptosis and apoptosis induced by these two selenium compounds is triggered via mitochondrial pathway(s). Our studies suggest that superoxide generated from redox metabolism of selenite and SeMet may account, at least in part, for the mechanism of anticancer action of selenium. Our results also suggest that anticancer efficacy depends on not only the dose and form of selenium, but also metabolism of selenium, superoxide production from selenium metabolites, and the antioxidant capacity and p53 status of cancer cells.
Abbreviations List
- H2AX
phosphorylated histone H2AX (serine 139)
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
glutathione disulfide
- METase
methioninase
- MMP
mitochondrial membrane potential
- MnSOD
manganese-containing superoxide dismutase
- MnTMPyP
manganese (III) tetrakis (N-methyl-2-pyridyl) porphyrin
- MOI
multiplicity of infectivity
- MTT
3-[4, 5-dimethyl-2-thiazolyl]-2, 5-diphenyl-2 tetrazolium bromide
- RLU
relative light units
- ROS
reactive oxygen species
- SeMet
selenomethionine
- siRNA
small interfering RNA
- SOD
superoxide dismutase
Footnotes
Grant support: NIH grants CA114281 and CA73612, Department of Veterans Administration Merit Review Award, and the American Cancer Society.
REFERENCES
- 1.Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–92. doi: 10.1016/s0163-7258(98)00014-x. [DOI] [PubMed] [Google Scholar]
- 2.Ip C. Lessons from basic research in selenium and cancer prevention. J Nutr. 1998;128:1845–54. doi: 10.1093/jn/128.11.1845. [DOI] [PubMed] [Google Scholar]
- 3.Clark LC, Combs GF, Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. J Am Med Assoc. 1996;276:1957–63. [PubMed] [Google Scholar]
- 4.Clark LC, Dalkin B, Krongrad A, et al. Decreased incidence of prostate cancer with selenium supplementation: results of a double-blind cancer prevention trial. Br J Urol. 1998;81:730–4. doi: 10.1046/j.1464-410x.1998.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.Sinha R, El-Bayoumy K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr Cancer Drug Targets. 2004;4:13–28. doi: 10.2174/1568009043481614. [DOI] [PubMed] [Google Scholar]
- 6.Combs GF. Status of selenium in prostate cancer prevention. Br J Cancer. 2004;91:195–9. doi: 10.1038/sj.bjc.6601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer Res. 2002;61:7071–8. [PubMed] [Google Scholar]
- 8.Zhao R, Xiang N, Domann FE, et al. Expression of p53 Enhances Selenite-Induced Superoxide Production and Apoptosis in Human Prostate Cancer Cells. Cancer Res. 2006;66:2296–304. doi: 10.1158/0008-5472.CAN-05-2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Cao X, Qu Y, et al. SeO(2) induces apoptosis with down-regulation of Bcl-2 and up-regulation of p53 expression in both immortal human hepatic cell line and hepatoma cell line. Mut Res. 2001;490:113–21. doi: 10.1016/s1383-5718(00)00149-2. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C, Hu H, Malewicz B, et al. Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells. Mol Cancer Ther. 2004;3:877–84. [PubMed] [Google Scholar]
- 11.Seo YR, Kelley MR, Smith ML. Selenomethionine regulation of p53 by a ref1-dependent redox mechanism. Proc Natl Acad Sci USA. 2002;99:14548–53. doi: 10.1073/pnas.212319799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanfear J, Fleming J, Wu L, et al. The selenium metabolite selenodiglutathione induces p53 and apoptosis: relevance to the chemopreventive effects of selenium? Carcinogenesis. 1994;15:1387–92. doi: 10.1093/carcin/15.7.1387. [DOI] [PubMed] [Google Scholar]
- 13.Ip C, Ganther HE. Relationship between the chemical forms of selenium and anticarcinogenic activity. In: Wattenberg L, Lipkin M, Boone CW, Kelloff GJ, editors. Cancer Chemoprevention. CRC; Boca Raton, FL: 1992. pp. 479–88. [Google Scholar]
- 14.Spallholz JE. On the nature of selenium toxicity and carcinostatic activity. Free Radic Biol Med. 1994;17:45–64. doi: 10.1016/0891-5849(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 15.Ip C, Hayes C, Budnick RM, Ganther HE. Chemical form of selenium, critical metabolites and cancer prevention. Cancer Res. 1991;51:595–600. [PubMed] [Google Scholar]
- 16.Rizky A, Kaori Mi, Minato N, et al. Chemical forms of selenium for cancer prevention. J Trace Elem Med Biol. 2005;19:141–50. doi: 10.1016/j.jtemb.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Shen H-M, Yang C-F, Ong C-N. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. Int J Cancer. 1999;81:820–8. doi: 10.1002/(sici)1097-0215(19990531)81:5<820::aid-ijc25>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Jung U, Zheng X, Yoon SO, Chung AS. Se-methylselenocysteine induces apoptosis mediated by reactive oxygen species in HL-60 cells. Free Radic Biol Med. 2001;31:479–89. doi: 10.1016/s0891-5849(01)00604-9. [DOI] [PubMed] [Google Scholar]
- 19.Kim TS, Yun BY, Kim IY. Induction of the mitochondrial permeability transition by selenium compounds mediated by oxidation of the protein thiol groups and generation of the superoxide. Biochem Pharmacol. 2003;66:2301–11. doi: 10.1016/j.bcp.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 20.Miki K, Xu M, Gupta A, et al. Methioninase cancer gene therapy with selenomethionine as suicide prodrug substrate. Cancer Res. 2001;61:6805–10. [PubMed] [Google Scholar]
- 21.Miki K, Al-Refaie W, Xu M, et al. Methioninase gene therapy of human cancer cells is synergistic with recombinant methioninase treatment. Cancer Res. 2000;60:2696–702. [PubMed] [Google Scholar]
- 22.Gupta A, Miki K, Xu M, et al. Combination efficacy of doxorubicin and adenoviral methioninase gene therapy with prodrug selenomethionine. Anticancer Res. 2003;23:1181–8. [PubMed] [Google Scholar]
- 23.Yamamoto N, Gupta A, Xu M, et al. Methioninase gene therapy with selenomethionine induces apoptosis in bcl-2-overproducing lung cancer cells. Cancer Gene Ther. 2003;10:445–50. doi: 10.1038/sj.cgt.7700587. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z, Jeang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–20. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- 25.Spallholz JE, Palace VP, Reid TW. Methioninase and selenomethionine but not Semethylselenocysteine generate methylselenol and superoxide in an in vitro chemiluminescent assay: implications for the nutritionalcarcinostatic activity of selenoamino acids. Biochem Pharmacol. 2004;67:547–54. doi: 10.1016/j.bcp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Peters TR, Tosk JM, Goulbourne EA., Jr Lucigenin chemiluminescence as a probe for measuring reactive oxygen species production in Escherichia coli. Anal Biochem. 1990;186:316–9. doi: 10.1016/0003-2697(90)90087-p. [DOI] [PubMed] [Google Scholar]
- 27.Rogakou EP, Pilch DR, Orr AH, et al. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 1998;273:5858–68. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- 28.Macip S, Igarashi M, Berggren P, Yu J, Lee SW. Aaronson SA. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol Cell Biol. 2003;23:8576–85. doi: 10.1128/MCB.23.23.8576-8585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofseth LJ, Hussain SP, Harris CC. p53:25 years after its discovery. Trends Pharmacol Sci. 2004;25:177–81. doi: 10.1016/j.tips.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Johnson TM, Yu ZX, Ferrans VJ, et al. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc Natl Acad Sci USA. 1996;93:11848–52. doi: 10.1073/pnas.93.21.11848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marchenko ND, Zaika A, Moll UM. Death signal-induced localization of p53 protein to mitochondria. A potential role in apoptotic signaling. J Biol Chem. 2000;275:16202–12. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 32.Polyak K, Xia Y, Zweier JL, et al. A model for p53-induced apoptosis. Nature. 1997;389:300–5. doi: 10.1038/38525. [DOI] [PubMed] [Google Scholar]
- 33.Chaudiere J, Courtin O, Leclaire J. Glutathione oxidase activity of selenocystamine: a mechanistic study. Arch Biochem Biophys. 1992;296:328–36. doi: 10.1016/0003-9861(92)90580-p. [DOI] [PubMed] [Google Scholar]
- 34.Zhong W, Yan T, Webber MM, et al. Alteration of cellular phenotype and responses to oxidative stress by manganese superoxide dismutase and a superoxide dismutase mimic in RWPE-2 human prostate adenocarcinoma cells. Antioxid Redox Signal. 2004;6:513–22. doi: 10.1089/152308604773934279. [DOI] [PubMed] [Google Scholar]
- 35.Menter DG, Sabichi AL, Lippman SM. Selenium effects on prostate cell growth. Cancer Epidemiol Biomarkers Prev. 2000;9:1171–82. [PubMed] [Google Scholar]
- 36.Husbeck B, Nonn L, Peehl DM, Knox SJ. Tumor-selective killing by selenite in patient-matched pairs of normal and malignant prostate cells. Prostate. 2005;66:218–25. doi: 10.1002/pros.20337. [DOI] [PubMed] [Google Scholar]
- 37.Amanda EM, Thomas E, John W, et al. The primitive protozoon trichomonas vaginalis contains two methionine γ-lyase genes that encode members of the γ-family of pyridoxal 5′-phosphate-dependent enzymes. J Biol Chem. 1998;273:5549–56. doi: 10.1074/jbc.273.10.5549. [DOI] [PubMed] [Google Scholar]
- 38.Tomofumi O, Tomoyuki K, Tomonori K, et al. Contribution of enzymic α,γ-elimination reaction in detoxification pathway of selenomethionine in mouse liver. Toxicol Appl Pharmacol. 2001;176:18–23. doi: 10.1006/taap.2001.9260. [DOI] [PubMed] [Google Scholar]
- 39.Rooseboom M, Commandeur JN, Vermeulen NP. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 40.Rooseboom M, Vermeulen NP, Groot EJ, Commandeur JN. Tissue distribution of cytosolic beta-elimination reactions of selenocysteine Se-conjugates in rat and human. Chem Biol Interact. 2002;140:243–64. doi: 10.1016/s0009-2797(02)00039-x. [DOI] [PubMed] [Google Scholar]
- 41.Tomofumi O, Shinji M, Hitoshi U, et al. Purification and characterization of mouse hepatic enzyme that converts selenomethionine to methylselenol by its alpha,gamma-elimination. Biol Trace Elem Res. 2005;106:77–94. doi: 10.1385/bter:106:1:077. [DOI] [PubMed] [Google Scholar]
- 42.Ip C, Thompson HJ, Zhu Z, et al. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Res. 2000;60:2882–86. [PubMed] [Google Scholar]
- 43.Spallholz JE, Shriver BJ, Reid TW. Dimethyldiselenide and methylseleninic acid generate superoxide in an in vitro chemiluminescence assay in the presence of glutathione: implications for the anticarcinogenic activity of L-selenomethionine and L-Se-methylselenocysteine. Nutr Cancer. 2001;40:34–41. doi: 10.1207/S15327914NC401_8. [DOI] [PubMed] [Google Scholar]
- 44.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 45.Burns TF, El-Deiry WS. The p53 pathway and apoptosis. J Cell Physiol. 1999;181:231–9. doi: 10.1002/(SICI)1097-4652(199911)181:2<231::AID-JCP5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Kroemer G, Zamzami N, Susin SA. Mitochondrial control of apoptosis. Immunol Today. 1997;18:44–51. doi: 10.1016/s0167-5699(97)80014-x. [DOI] [PubMed] [Google Scholar]
- 47.Li PF, Dietz R, von Harsdorf R. p53 regulates mitochondrial membrane potential through reactive oxygen species and induces cytochrome c-independent apoptosis blocked by Bcl-2. EMBO J. 1999;18:6027–36. doi: 10.1093/emboj/18.21.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chandel NS, Vander Heiden MG, Thompson CB, et al. Redox regulation of p53 during hypoxia. Oncogene. 2000;19:3840–8. doi: 10.1038/sj.onc.1203727. [DOI] [PubMed] [Google Scholar]
- 49.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human Bax gene. Cell. 1995;80:293–9. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 50.Dong Y, Lee SO, Zhang H, et al. Prostate specific antigen expression is down-regulated by selenium through disruption of androgen receptor signaling. Cancer Res. 2004;64:19–22. doi: 10.1158/0008-5472.can-03-2789. [DOI] [PubMed] [Google Scholar]
- 51.Husbeck B, Bhattacharyya RS, Feldman D, et al. Inhibition of androgen receptor signaling by selenite and methylseleninic acid in prostate cancer cells: two distinct mechanisms of action. Mol Cancer Ther. 2006;5:2078–85. doi: 10.1158/1535-7163.MCT-06-0056. [DOI] [PubMed] [Google Scholar]


