Abstract
The bond strengths of resins to caries-affected dentin are low. This could be due to weakened organic matrix. The purpose of this work was to determine if the ultimate tensile strength (UTS) of excavated carious dentin is weaker than that of normal dentin. Soft caries was excavated from extracted human molars, and the tooth was vertically sectioned into slabs. Each slab was trimmed to an hourglass shape, parallel or perpendicular to the tubule direction. Half of the specimens were mineralized, while the other half were completely demineralized in EDTA. ANOVA on ranks showed that the three-factor interactions (mineralization, caries, tubule direction) were all significant (p < 0.0001), indicating that mineralization and tubule direction gave different UTS results in normal and caries-affected dentin. No significant differences were seen between the UTS of normal and and that of caries-affected demineralized dentin in the parallel or perpendicular group. The matrix of demineralized caries-affected dentin was as strong as that of normal demineralized dentin when tested in the same direction.
Keywords: dentin, caries, tensile strength, dentinal tubules
INTRODUCTION
Although several reports are available on the ultimate tensile strength (UTS) of human dentin (Craig and Peyton, 1958; Bowen and Rodriguez, 1962; Lehman, 1967; Sano et al., 1994, 1995), several recent studies have emphasized the anisotropy of UTS. The UTS of the mineralized matrix depends on tubule orientation, with the highest strength seen when the load is applied perpendicular to the direction of the tubules (Carvalho et al., 2001; Lertchirakarn et al., 2001; Inoue et al., 2003; Bedran-de-Castro et al., 2004). In dentin, collagen fibrils are largely arranged perpendicular to the long axis of dentinal tubules (Sögaard-Pederson et al., 1990), and apatite crystals tend to be oriented parallel to the long axis of the collagen fibrils. Thus, fractures perpendicular to the tubules occur predominantly with the plane of the collagen network.
The anisotropy of the matrix UTS was recently shown to extend to completely demineralized dentin matrix (Miguez et al., 2004), indicating that differences in the orientation of collagen fibrils and dentinal tubules modify the tensile strength of the matrix. When demineralized dentin is stressed perpendicular to the long axis of the tubules, the collagen fibrils are largely loaded in tension parallel to their long axis, in their strongest direction. If they are stressed parallel to the long axis of the tubules, they are more likely to be loaded perpendicular to their long axis, which would tend to tear the collagen fibril meshwork apart.
A recent report indicated that the matrix of caries-affected dentin has a UTS lower than that of normal dentin (Yoshiyama et al., 2002). The authors found a positive correlation between the UTS of caries-affected dentin and the Knoop hardness of those same specimens. That is, the more demineralized the caries-affected dentin was, the lower was its UTS. Resin-dentin bonds made to caries-affected dentin are lower than bonds made to normal dentin (Nakajima et al., 1995, 1999, 2000; Yoshiyama et al., 2000). The lower bond strengths to caries-affected dentin were attributed to the paucity of resin tags in mineral-filled tubules, and to the weakness of the demineralized matrix (Yoshiyama et al., 2002).
It is possible that the collagen matrix in caries-affected dentin has been weakened by bacterial or host (Pashley et al., 2004) hydrolases. Even though caries-affected dentin contains few bacteria, it may be exposed to soluble bacterial enzymes that could attack the collagen matrix. The purpose of this study was to compare the UTS of mineralized normal and caries-affected dentin matrix, and the UTS of the same tissues after complete demineralization, as a function of tubule orientation, a variable known to affect UTS.
The null hypothesis that was tested was that there is no difference in the UTS of mineralized vs. demineralized normal vs. caries-affected dentin, when the same tubule direction is tested.
MATERIALS & METHODS
We collected normal and carious teeth after obtaining the patients’ informed consent under a protocol reviewed and approved by the Human Assurance Committee of the Medical College of Georgia. Teeth with caries lesions extending one-third the distance through the dentin were stained with Caries Detector Solution (Kuraray Medical, Tokyo, Japan), and rinsed. Caries was then excavated with a round bur at slow speed until there was no more bright-red-staining dentin. Caries-affected dentin was defined as dentin that was colorless to light pink, firm, and opaque. Multiple mesiodistal vertical serial sections 0.5 mm thick were prepared parallel to the long axis of the tooth. The middle 2 or 3 slabs were through the sclerotic, opaque, caries-affected dentin. Sclerotic dentin was ranked according to a scale provided by the Clinical Research Unit in the University of North Carolina’s Department of Operative Dentistry (see Nakajima et al., 1999). This is a 1–4 scale: 1, no sclerosis, no transparency or translucency, no discoloration; 2, more opaque than #1, but less than 50% of the area is affected; 3, less than #4, but more than 50% is affected; and 4, dentin is glassy, dark yellow or brownish, and significant translucency and transparency are evident. The caries-affected dentin used in this study was classified as #2 and #3. Previous work has shown that such caries-affected dentin is largely free of bacteria (Yoshiyama et al., 2003; Doi et al., 2004). Caries-free teeth were prepared similarly (Fig.). Using an ultrafine diamond bur in a highspeed handpiece with copious air-water spray, we created hourglass-shaped specimens (Fig.) where the test site was 0.54 ± 0.06 mm wide and 0.51 ± 0.04 mm thick, giving a cross-sectional area of 0.28 mm2. Those specimens that were demineralized were covered with nail varnish except for the 0.5 mm × 1.5 mm test sites. The test sites were loaded parallel or perpendicular to the direction of the tubules (Fig.). Demineralized specimens were immersed in 1 L of 0.5 M ethylenediamine-tetraacetic acid, pH 7.4, containing 4 protease inhibitors (mmol/L): benzamidine HCl (2.5), ɛ-amino-n-caproic acid (50), N-ethylmaleimide (0.5), and phenylmethylsulfonyl fluoride (0.3). After demineralization of the center section for 6 days at 25°C with constant stirring, the varnish on the mineralized ends was scraped off prior to tensile testing. The trimmed specimens were glued to a Bisco microtensile test jig by means of cyanoacrylate cement (Zapit, Dental Ventures of America, Corona, CA, USA). The jig was pulled in tension at 0.6 mm/min in a Vitrodyne tester (John Chatillon & Sons, Greensboro, NC, USA).
Figure.
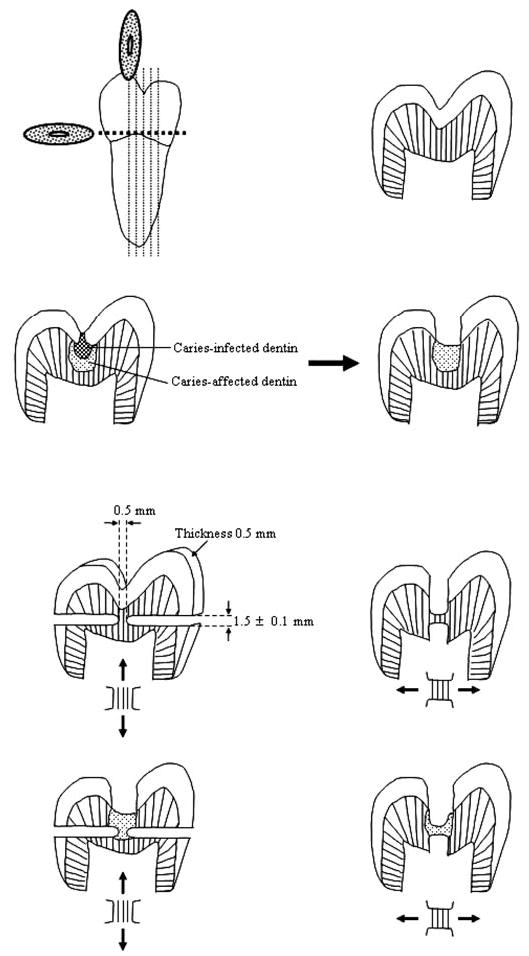
Schematic illustrating how caries lesions were excavated to the level of caries-affected dentin, how vertical serial sections were made, how all but the test dentin was ground away in two different directions on adjacent slabs to permit tensile testing parallel (↕) or (↔) perpendicular to tubule long axis.
Statistical Analyses
A full-factorial three-factor analysis of variance was performed on the ranks of the ultimate tensile strength (UTS) data, due to non-normality of the UTS and a violation of equal variance in the groups. We ranked the data by giving the lowest value of the dependent variable a value of 1 and so on, with tied values receiving the average of the ranks that would have corresponded to the tied values. We ranked data without taking their subgroup into account, using PROC Rank in the SAS software. The 3 factors examined were dentin type (normal, caries-affected), mineralization (mineralized, demineralized), and direction to the dentinal tubule axis (parallel, perpendicular). All main effects, two-factor interactions, and the three-factor interaction were included in the model. We used a Bonferroni adjustment to the overall alpha level of 0.05 to examine post hoc differences. All statistical analyses were performed with SAS 8.2.
RESULTS
The three-factor interaction was statistically significant, indicating that the effects of mineralization and tubule direction were different in the normal and caries-affected dentin types (Table). The post hoc tests revealed several significant pairwise differences. For normal mineralized dentin (p < 0.0001), normal demineralized dentin (p < 0.0001), and caries-affected demineralized dentin (p < 0.0001), the ranked UTS was significantly higher for the perpendicular direction than the parallel direction. No statistically significant difference in UTS was seen in the carious mineralized dentin. All dentin and direction combinations (normal parallel p < 0.0001, normal perpendicular p < 0.0001, caries-affected parallel p < 0.0001, and caries-affected perpendicular p < 0.0001) in mineralized dentin had significantly higher ranked UTS than in completely demineralized dentin. Significant differences in UTS were seen between caries-affected and normal dentin in the mineralized parallel (p < 0.0001) and mineralized perpendicular (p < 0.0001) groups, with the normal dentin having significantly higher ranked UTS than caries-affected dentin. No statistically significant differences between normal and caries-affected dentin were seen in the demineralized parallel or demineralized perpendicular groups (p = 0.252).
Table.
Three-factor ANOVA on Ultimate Tensile Strength (UTS) of Mineralized and Demineralized Normal and Caries-affected Dentin vs. Tubule Orientation
| Raw UTS (MPa) | Ranked UTS (MPa) | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Median | F-value | p-value |
| Dentin | 46.92 | < 0.0001 | |||||
| Carious | 33.69 | (22.93) | 36.05 | (18.36) | 26.79 | ||
| Normal | 53.37 | (44.13) | 44.95 | (26.76) | 45.42 | ||
| Mineralization | 947.68 | < 0.0001 | |||||
| Demineralized | 12.53 | (5.98) | 20.50 | (11.69) | 11.41 | ||
| Mineralized | 74.53 | (25.79) | 60.50 | (11.69) | 70.03 | ||
| Direction | 95.53 | < 0.0001 | |||||
| Parallel | 36.93 | (31.56) | 34.15 | (25.03) | 25.45 | ||
| Perpendicular | 50.14 | (39.83) | 46.85 | (19.62) | 29.24 | ||
| Dentin × Mineralization | 56.88 | < 0.0001 | |||||
| Carious | |||||||
| Demineralized | 12.90 | (6.26) | 20.95 | (12.80) | 12.64 | ||
| Mineralized | 54.49 | (11.42) | 51.15 | (6.95) | 52.92 | ||
| Normal | |||||||
| Demineralized | 12.17 | (5.82) | 20.05 | (10.78) | 11.41 | ||
| Mineralized | 94.58 | (19.72) | 69.85 | (6.95) | 98.87 | ||
| Dentin × Direction | 1.33 | 0.2521 | |||||
| Carious | |||||||
| Parallel | 31.12 | (25.43) | 30.45 | (22.34) | 25.07 | ||
| Perpendicular | 36.27 | (20.46) | 41.65 | (11.28) | 26.79 | ||
| Normal | |||||||
| Parallel | 42.74 | (36.43) | 37.85 | (27.53) | 37.20 | ||
| Perpendicular | 64.00 | (49.32) | 52.05 | (24.61) | 63.60 | ||
| Mineralization × Direction | 31.56 | < 0.001 | |||||
| Demineralized | |||||||
| Parallel | 7.61 | (1.94) | 10.50 | (5.92) | 7.44 | ||
| Perpendicular | 17.46 | (4.30) | 30.50 | (5.92) | 16.99 | ||
| Mineralized | |||||||
| Parallel | 66.25 | (15.21) | 57.80 | (8.55) | 69.60 | ||
| Perpendicular | 82.81 | (31.46) | 63.20 | (13.86) | 88.85 | ||
| Dentin × Mineralization × Direction | 11.47 | < 0.0012 | |||||
| Carious | |||||||
| Demineralized | |||||||
| Parallel | 7.27 | (1.86) | 9.50 | (6.02) | 7.28 | 1.33 | 0.252 |
| Perpendicular | 18.53 | (2.97) | 32.40 | (4.27) | 17.17 | ||
| Mineralized | |||||||
| Parallel | 54.97 | (9.93) | 51.40 | (6.45) | 52.49 | 1.33 | 0.252 |
| Perpendicular | 54.01 | (13.36) | 60.90 | (7.75) | 53.46 | ||
| Normal | |||||||
| Demineralized | |||||||
| Parallel | 7.95 | (2.06) | 11.50 | (5.95) | 7.46 | 1.33 | 0.252 |
| Perpendicular | 16.39 | (5.26) | 28.60 | (6.90) | 15.21 | ||
| Mineralized | |||||||
| Parallel | 77.54 | (10.32) | 64.20 | (4.66) | 76.90 | 9.78 | < 0.001 |
| Perpendicular | 111.62 | (8.32) | 75.50 | (3.03) | 113.62 | ||
Values are mean ± 1 (SD) in Mpa, n = 10. There were 5 teeth per subgroup and 2 slabs taken from each tooth.
DISCUSSION
Our UTS results were very similar to previously published work. Sano et al. 1994 compared the UTS of mineralized coronal dentin (0.5 × 0.5 mm) pulled perpendicular to the long axis of the tubules. They obtained values of 104 ± 27.6 MPa. Bedran-de-Castro et al. 2004 reported a UTS for bovine coronal dentin of 114.9 ± 26.5 MPa perpendicular to the tubules (compared with our current unranked values of 111.6 ± 8.3 MPa, Table) and 68.6 ± 29.7 MPa when loaded parallel to the tubule axis (compared with our unranked values of 77.5 ± 10.3 MPa, Table). In human coronal dentin, Inoue et al. 2003 reported UTS for mineralized dentin of 99.8 ± 27.9 MPa when loaded perpendicular to the tubule axis and 77.6 ± 24.7 MPa when loaded parallel to the tubules. There are no statistically significant differences between our values, at any given tubule direction, and those reported in previously published work. Our contribution is that we measured the UTS of caries-affected dentin, at various mineralized states and fully demineralized, and compared it with that of normal dentin as a function of tubule orientation.
Because caries-affected dentin had a UTS lower than that of normal dentin (Yoshiyama et al., 2003), many had assumed that the collagen matrix might have been weakened. However, others contend that, since caries-affected dentin matrix is capable of remineralizing, the collagen must be normal and would be expected to have the same UTS as normal demineralized dentin. Whether the data were evaluated in the raw or ranked UTS, there was no significant difference between the UTS for normal demineralized dentin vs. caries-affected demineralized dentin when tested parallel to the tubule axis (7.95 ± 2.06 vs. 7.27 ± 1.86 MPa raw, or 11.50 ± 5.95 vs. 9.50 ± 6.02 MPa on ranked data, respectively). When tested perpendicular to the tubule axis, the UTS for normal demineralized dentin vs. caries-affected demineralized dentin matrix was 16.39 ± 5.26 vs. 18.53 ± 2.97 MPa in raw values and 28.60 ± 6.90 vs. 32.40 ± 4.27 MPa in ranked data (Table). Our raw UTS data are very similar to those obtained by Miguez et al. 2004.
Thus, it is clear that while the UTS for “mineralized” caries-affected dentin is less than half that for normal dentin, this difference is lost after the matrices are completely demineralized. The lower bond strengths of adhesive resins to caries-affected dentin (Nakajima et al., 1995, 1999; Yoshiyama et al., 2000, 2002) cannot be due to weakened matrices, but must be associated with a lack of mineral around and within the collagen fibrils (Kinney et al., 2003).
The relative contributions of the mineral and collagen phases to the overall UTS of dentin can be determined by examination of the interaction of dentin and mineralization, independent of tubule orientation (Table). In normal dentin, the ranked demineralized dentin UTS divided by the ranked mineralized UTS equals 0.287. This means that 28.7% of the total strength of normal dentin was due to the contribution of the collagen matrix, while the remaining (100–28.7) 71.3% was due to the mineral phase of dentin. However, in carious dentin, the ranked UTS of partially demineralized dentin (as evidenced by the ranked UTS for mineralized carious dentin being 51.2 MPa instead of 69.9 MPa in normal dentin) was already lower than normal. Since the demineralized carious matrix had a normal ranked UTS (i.e., 20.9 MPa), the ratio of the demineralized to “mineralized” ranked UTS was 0.410 (Table). That is, in caries-affected dentin, 41% of the UTS was due to the collagen matrix, and 59% was due to the mineral phase.
If resin does not infiltrate demineralized matrices as far as did the acid etchant, then the retention of bonded resin composites would be limited to the weakest link in the substrate, namely, the residual naked matrix at about 20–21 MPa, depending on whether the substrate was normal or carious.
Acknowledgments
This study was supported by grants R01 DE 014911 and R01 DE 015306 from the National Institute of Dental and Craniofacial Research (David Pashley, PI). The authors are grateful to Mrs. Michelle Barnes for secretarial support.
References
- Bedran-de-Castro AK, Pereira PN, Thompson JY. Influence of load cycling and tubule orientation on ultimate tensile strength of dentin. J Adhes Dent. 2004;6:191–194. [PubMed] [Google Scholar]
- Bowen RE, Rodriguez MS. Tensile strength and modulus of elasticity of tooth structure and several restorative materials. J Am Dent Assoc. 1962;64:378–387. doi: 10.14219/jada.archive.1962.0090. [DOI] [PubMed] [Google Scholar]
- Carvalho RM, Fernandes CA, Villanueva R, Wang L, Pashley DH. Tensile strength of human dentin as a function of tubule orientation and density. J Adhes Dent. 2001;3:309–314. [PubMed] [Google Scholar]
- Craig RG, Peyton FA. Elastic and mechanical properties of human dentin. J Dent Res. 1958;37:710–718. doi: 10.1177/00220345580370041801. [DOI] [PubMed] [Google Scholar]
- Doi J, Itota T, Yoshiyama M, Tay FR, Pashley DH. Bonding to root caries by a self-etching adhesive system containing MDPB. Am J Dent. 2004;17:89–93. [PubMed] [Google Scholar]
- Inoue S, Pereira PN, Kawamoto C, Nakajima M, Koshiro K, Tagami J, et al. Effect of depth and tubule direction on ultimate tensile strength of human coronal dentin. Dent Mater J. 2003;22:39–47. doi: 10.4012/dmj.22.39. [DOI] [PubMed] [Google Scholar]
- Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82:957–961. doi: 10.1177/154405910308201204. [DOI] [PubMed] [Google Scholar]
- Lehman ML. Tensile strength of human dentin. J Dent Res. 1967;46:197–201. doi: 10.1177/00220345670460011001. [DOI] [PubMed] [Google Scholar]
- Lertchirakarn V, Palamara JEA, Messer HH. Anisotropy of tensile strength of root dentin. J Dent Res. 2001;80:453–456. doi: 10.1177/00220345010800021001. [DOI] [PubMed] [Google Scholar]
- Miguez PA, Pereira PNR, Atsawasuwan P, Yamauchi M. Collagen cross-linking and ultimate tensile strength of dentin. J Dent Res. 2004;83:807–810. doi: 10.1177/154405910408301014. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Sano H, Burrow MF, Tagami J, Yoshiyama M, Ebisu S, et al. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesive. J Dent Res. 1995;74:1679–1688. doi: 10.1177/00220345950740100901. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Sano H, Zheng L, Tagami J, Pashley DH. Effect of moist vs. dry bonding to normal vs. caries-affected dentin with Scotchbond Multi-Purpose Plus. J Dent Res. 1999;78:1298–1303. doi: 10.1177/00220345990780070301. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Sano H, Urabe I, Tagami J, Pashley DH. Bond strengths of single-bottle dentin adhesives to caries-affected dentin. Oper Dent. 2000;25:2–10. [PubMed] [Google Scholar]
- Pashley DH, Tay FR, Hashimoto M, Breschi L, Carvalho RM, Ito S. Degradation of dentin collagen by host-derived enzymes during aging. J Dent Res. 2004;83:216–221. doi: 10.1177/154405910408300306. [DOI] [PubMed] [Google Scholar]
- Sano H, Ciucchi B, Matthews WG, Pashley DH. Tensile properties of mineralized and demineralized human and bovine dentin. J Dent Res. 1994;73:1205–1211. doi: 10.1177/00220345940730061201. [DOI] [PubMed] [Google Scholar]
- Sano H, Takatsu T, Ciucchi B, Russell CM, Pashley DH. Tensile properties of resin-infiltrated demineralized human dentin. J Dent Res. 1995;74:1093–1102. doi: 10.1177/00220345950740041001. [DOI] [PubMed] [Google Scholar]
- Sögaard-Pedersen B, Boye H, Matthiessen ME. Scanning electron microscope observations on collagen fibers in human dentin and pulp. Scand J Dent Res. 1990;98:89–95. doi: 10.1111/j.1600-0722.1990.tb00945.x. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Urayama A, Kimochi T, Matsuo T, Pashley DH. Comparison of conventional vs self-etching adhesive bonds to caries-affected dentin. Oper Dent. 2000;25:163–169. [PubMed] [Google Scholar]
- Yoshiyama M, Tay FR, Doi J, Nishitani Y, Yamada T, Itou K, et al. Bonding of self-etch and total-etch adhesives to carious dentin. J Dent Res. 2002;81:556–560. doi: 10.1177/154405910208100811. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Tay FR, Torii Y, Nishitani Y, Doi J, Itou K, et al. Resin adhesion to carious dentin. Am J Dent. 2003;16:47–52. [PubMed] [Google Scholar]


