Abstract
Viral protein R (Vpr), one of the human immunodeficiency virus type 1 (HIV-1) accessory proteins, contributes to multiple cytopathic effects, G2 cell cycle arrest and apoptosis. The mechanisms of Vpr have been intensely studied because it is believed that they underlie HIV-1 pathogenesis. We here report a cell-based small molecule screen on Vpr induced cell death in the context of HIV-1 infection. From the screen of 504 bioactive compounds, we identified Damnacanthal (Dam), a component of noni fruit, as an inhibitor of Vpr induced cell death. Our studies illustrate a novel efficient platform for drug discovery and development in anti-HIV therapy which should also be applicable to other viruses.
Keywords: HIV-1 Vpr, screening, Damnacanthal, apoptosis, G2 arrest
Introduction
Therapeutic agents currently available against human immunodeficiency virus type 1 (HIV-1) target viral proteins directly [1]. Although these agents have dramatically reduced the rate of disease progression and improved the lifestyles in HIV infected patients, a fast mutating nature of HIV-1 and persistent production of low level viruses from latently infected T cells gives rise to drug resistant strains and thus urgently necessitates the discovery of more novel agents [2, 3]. An attractive alternative to develop such agents is through modulation of the immune system against HIV. It is clear that immune dysfunction is critical for the development of Acquired Immune Deficiency Syndrome (AIDS). The disease characterized by high viral burden and ongoing and extensive lymphocyte cell death [4-6]. However, the mechanism of cell death remains unclear.
The 14 kDa HIV-1 viral protein R (Vpr) is one of the viral factors that regulate HIV-1 pathogenesis and HIV-1 dependent lymphocyte apoptosis [7]. Vpr is encoded in the HIV genome and packaged in the virion via its association with the structural protein Gag p6. In the past, Vpr was not considered to be biologically important as it was not required for viral replication in established cell lines or in activated or resting T cells [8]. Recent studies clearly showed that Vpr is indispensable for disease progression in rhesus monkeys [9] and in chimpanzees [10]. Non-functional Vpr mutations are also observed in patients with slow progression to AIDS [11-13], suggesting that targeting Vpr inhibition may be a beneficial strategy in AIDS therapy.
Vpr is highly conserved among the primate lentviruses HIV-1, HIV-2, and SIV, suggesting its important role in the viral life cycle. Vpr is a pleiotropic protein with multiple pathobiological mechanisms. These mechanisms include nuclear import of preintegration complex [14], transactivation of several viral promoters including long terminal repeat (LTR) [15, 16], and induction of cell cycle arrest at G2/M phase (G2 arrest) [17] and apoptosis [18, 19]. Apoptosis induced by Vpr is believed to contribute to CD4+ T cell depletion [17, 20]. Virion associated Vpr is sufficient to induce G2 arrest and apoptosis selectively in several proliferating cell lines [21]. Many studies have shown that Vpr induced apoptosis is similar to gamma irradiation induced apoptosis [22], dependent on caspase activation [19] and independent of the p53 tumor suppressor gene [21].
We here show that a small molecule screen can provide signaling probes to dissect Vpr mechanisms and elucidate novel therapeutic agents for anti-HIV therapy. We screened 504 bioactive small molecules and identified Damnacanthal (Dam), a component of noni fruit (Morinda citrifolia), as an inhibitor of Vpr dependent cell death.
Materials and Methods
Cell, virus like particles (VLP) and lentiviral vectors
HeLa cells were cultured and maintained in DMEM containing 10% FBS supplemented with penicillin/streptomycin/glutamine mixture.
VSV-G envelope pseudotyped RNA(-) VLP with or without Vpr (Vpr+/VLP or Vpr-/VLP, respectively) were generated by cotransfection with either pCMVΔR8.2 or pCMVΔR8.2ΔVpr, and pCMV VSV-G, respectively, as described previously [21]. Flagtagged Vpr encoding lentiviral vector (Vpr+/CCR-X) or control vector (Vpr-/CCR-X) were produced by cotransfection of pRRL-cPPT-CMV-PRE-SIN [23], which carries Flag-tagged Vpr sequence [24] with or without stop codon right after Flag tag sequence, pCMVΔR8.2ΔVpr, and pCMV VSV-G, respectively. VLPs and the lentiviral vectors were harvested, concentrated as described previously [25], and titratedto ensure that approximately 50 % (for Vpr+/VLP) and 90% (for Vpr+/CCR-X) of HeLa cells were arrested inG2 phase at 24 h postinfection.
Screening protocol
1 × 106 HeLa cells were infected with 10 μg (p24 equivalent) of Vpr+/VLP or Vpr-/VLP in the presence of 8 μg/ml polybrene for 2 hrs. Cells were rinsed with fresh medium for six times, trypsinized, and 500 cells in 25 μl of medium were replated in each well of a 384-well (white) microtiter plate (Corning). Small molecule libraries were obtained from Biomol International LP. The molecules include 72 ion channel inhibitors (Cat No. 2805), 84 kinase/phosphatase inhibitors (Cat No. 2831), 84 orphan ligands (Cat No. 2825), 60 endocannabinoids (Cat No. 2801) and 204 bioactive lipids (Cat No. 2800). The compounds were dissolved in DMSO or H2O and stored at -80° C in 384 wells. They were transferred to the plates using 384 pin replicators (Genetix). The cells were incubated at 37°C under 5% CO2 for 72 hrs before cell viability was accessed.
Cell viability assay
Cell viability was accessed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega) following the manufacturer’s instructions. Briefly, 25 μl of reagent was added to each well with Multidrop 384 (Thermo Electron). The cells were lysed on a rotating shaker for 2 minutes and incubated at RT on a table top for 10 minutes. Luminescence levels were measured using Analyst HT (Molecular Devices).
Apoptosis assay
Briefly, 2 × 104 HeLa cells were infected with 200 ng (p24 equivalent) of Vpr+/VLP or Vpr-/VLP in the presence of 8 μg/ml polybrene for 2 hrs in a 12-well plate. The cells were treated with 5 μM of Dam (EMD Biosciences) or DMSO (Sigma-Aldrich) as a control. At 60 hrs postinfection, cells were collected so that none of the suspended cells were lost during the collection process. 5 × 104 cells were stained with 2.5 μl of Alexa Fluor® 488 annexin V solution, acquired on a FACScan (BD Biosciences) and analyzed with the Cellquest software (BD Biosciences).
Cell cycle profiles and sub-G1 peak analyses
HeLa cells were infected with VLPs (1μg of p24 per 1 × 105 cells) or with lentiviral vectors (200 ng of p24 per 1 x 105 cells) and collected using the protocol similar to that used for annexin V staining. The cell pellets were then fixed with ice cold 70% ethanol and stained with propidium iodide (Sigma-Aldrich). A total of 10,000 events were collected by FACScan for cell cycle status and sub-G1 peak as previously described [26] and analyzed with ModFit LT™ software (Verity Software House).
Statistical Analysis
The raw data of untreated samples were converted to the frequency of luminescent units using the histogram function in Microsoft Excel Data Analysis ToolPak (Microsoft). Normal distribution function was used to fit the frequency values to Gaussian distribution and to test significance of treated data from untreated samples.
Results and discussion
A phenotype-based small molecule screen for inhibitors of Vpr cytotoxicity
To identify novel small molecule probes that inhibit Vpr induced cell growth cessation which is mainly caused by apoptosis and G2 arrest, we screened small molecule libraries against VSV-G pseudotyped VLP containing Vpr in the virion. As previously described, VLP lacks the RNA genome necessary for de novo synthesis of viral proteins, but contains Vpr in association with virion Gag p6. Virion associated Vpr is sufficient to induce G2 arrest as well as apoptosis [21]. We used HeLa cells for the screen because HeLa cells are most sensitive to Vpr-induced apoptosis [21].
The screen identified Damnacanthal (Dam), an anthraquinone derivative, as an inhibitor of Vpr induced cell growth cessation (Fig.1). Dam was first isolated from noni fruit, a traditional Tahaitian fruit commonly used as a folk medicine by Polynesians for over 2000 years [27]. It has been reported that Dam reverts the morphology of K-ras transformed cells to normal one [28] and that Dam inhibits p56lck tyrosine kinase in in vitro kinase assay [29]. Dam inhibited Vpr induced cell growth cessation by twofold with more than 7 standard deviations from untreated Vpr infected cells (Fig.1 B & C). This effect of Dam was confirmed both in a replicate secondary screen and in assays using lentiviral vector encoded Vpr (Vpr+/CCR-X) (data not shown). The effect on cell viability was also measured in a different assay using traditional Trypan blue staining, which indicated that Dam increased total viable cell number 1.5 fold compared to control Vpr+/VLP or Vpr+/CCR-X infected cells.
Figure 1.
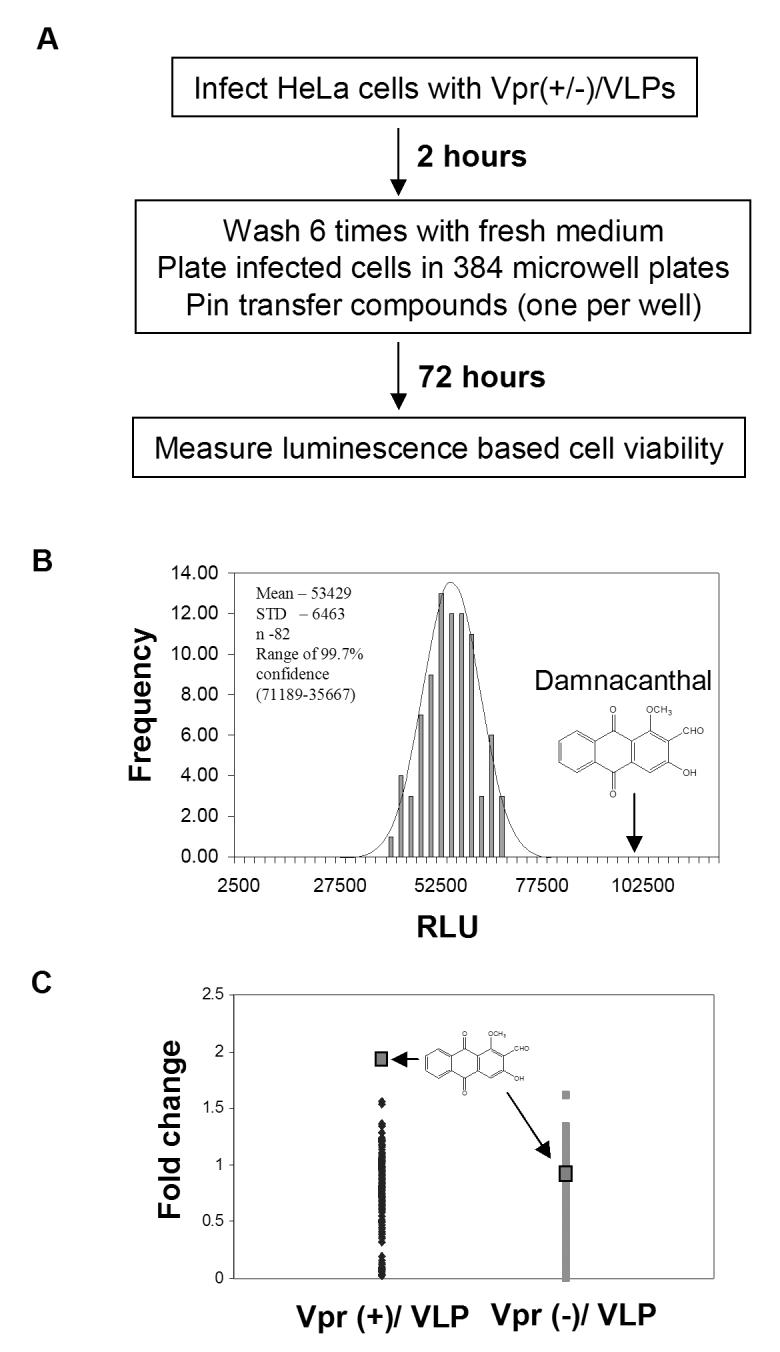
A small molecule screen for inhibitors of Vpr dependent cytotoxicity.A) A schematic diagram of a screen for small molecule modulators of Vpr induced cell growth cessation. B) The frequency distribution of luminescent activities of untreated Vpr+/VLP infected HeLa cells. The arrow indicates the luminescent value of Dam treated cells. Mean, standard deviations (STD) and range of 3 STDs of untreated samples are shown. C) Fold change in cell viability via Dam in Vpr+/VLP or Vpr-/VLP infected cells compared to other compounds. Fold change was calculated as compound treatment value/mean untreated value.
Damnacanthal inhibits Vpr dependent apoptosis without affecting the induction of G2 arrest
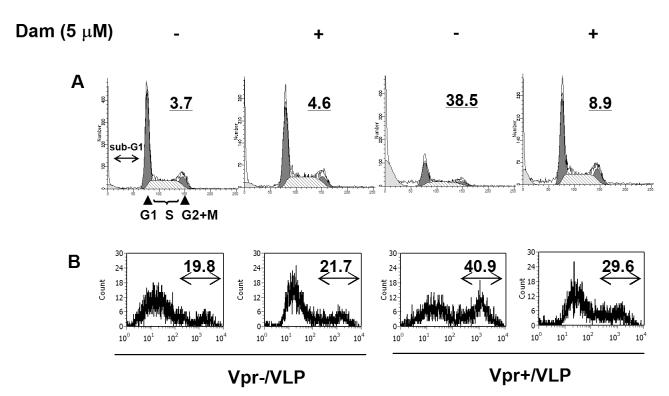
To determine inhibitory mechanisms of Dam on Vpr induced cell growth cessation, we first examined cell cycle profiles of VLP-infected cells. 44.3% of Vpr+/VLP infected cells arrest at G2 phase at 24 hrs postinfection and 44.7 % of Vpr+/VLP infected cells remained arrested at G2 in the presence of Dam. Beyond 24 hrs, the VLP system did not allow us to determine whether Dam affects Vpr induced G2 arrest because Vpr effect is relieved over time in this system (Fig.2 A). However, Dam significantly inhibited Vpr induced accumulation of sub-G1 cells at 60 hrs postinfection by approximately 30%. Annexin-V staining indicated that these sub-G1 cells were partly derived from dead cells by apoptosis and that Dam suppressed approximately 11% of Vpr induced apoptosis (Fig.2 B). The discrepancy between sub-G1 measurement and annexin V staining can be explained by Dam’s inhibition of multiple cell death pathways in addition to apoptosis.
Figure 2.
Damnacanthal inhibits HIV-1 Vpr dependent cell death. HeLa cells were infected with Vpr+/VLP or Vpr-/VLP in the presence of Dam (5 μM) or same volume of DMSO control (final DMSO concentration = 0.1%). After 60 hrs postinfection, cells were stained with propidium iodide (A) or with Alexa488 conjugated Annexin-V (B) and analyzed with a FACScan cell sorter. A total of 10,000 events were collected and analyzed with FACScan. The % of sub-G1 (A) or Annexin-V positive cells (B) is indicated at the upper right corner of each diagram.
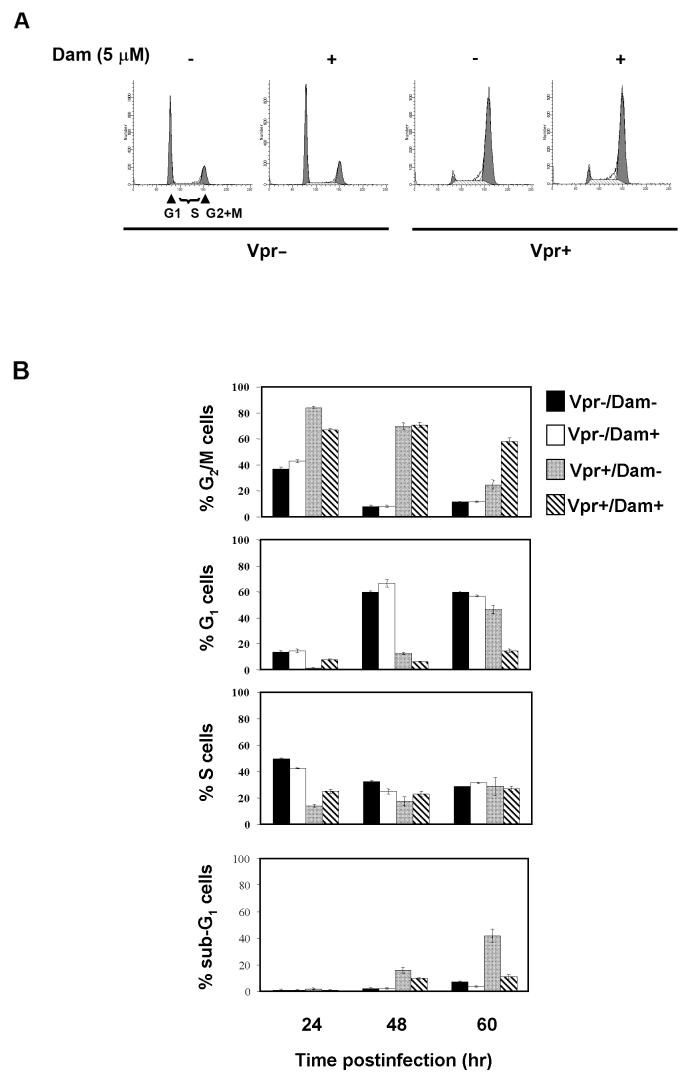
To determine whether Dam affects induction of G2 arrest by Vpr more clearly, we used a recombinant lentiviral vector encoding Vpr (Vpr+/CCR-X). We infected a population of synchronized HeLa cells released from a double thymidine block at the G1/S border as previously described [22]. Infection of Vpr+/CCR-X arrested a majority of infected cells inG2+M phase at 12 hrs postinfection (Fig.3 A). We added Dam to the infected cell culture at the time of infection and analyzed cell cycle profiles at 12 hrs postinfection. Dam had no effect on induction of G2 arrest in Vpr+/CCR-X infected cells since the percentage of G2+M population of cells in these cultures were 77.2% without Dam and 73.9% with Dam.
Figure 3.
Damnacanthal inhibits HIV-1 Vpr induced cell death independent of Vpr’s G2 arrest induction or maintenance. HeLa cells were synchronized at G1/S by a double thymidine block and then infected with equivalent amounts of lentiviral vectors, Vpr+/CCR-X or Vpr-/CCR-X (200 ng of viral p24/1x 105 cells). 5 μM of Dam or DMSO control was added at the time of infection (A) or 12 hrs postinfection (B). At 12 hrs postinfection (A) or at 24, 48 and 60 hrs postinfection (B), a total of 10,000 events were collected and analyzed by a FACScan cell sorter. The percent DNA histograms values of corresponding cell cycle phases calculated with the ModFit™ software were shown.
Damnacanthal inhibits cell death without affecting Vpr’s G2 maintenance
To determine the effect of Dam on Vpr’s G2 maintenance, we infected HeLa cells released from double thymidine block with Vpr+/CCR-X or Vpr-/CCR-X viruses. We added Dam or DMSO control at 12 hrs postinfection, when approximately 70-80% of cells have accumulated in G2+M phase of the cell cycle as shown in Fig 3. A. The profiles at 24, 48 and 60 hrs postinfection indicated that the number of Vpr+/CCR-X infected cells at G2+M phase decreased over time and the numbers of G1 and sub-G1 cells accumulated at the same time (Fig.3 B). The increased number of G1 population is not a consequence of perturbation on G2 arrest because the total cell number decreased and more fragmented DNAs increased over time (data not shown).
In the presence of Dam, the percentage of cells in G2+M phase hardly changed over time postinfection. A slight decrease in G2+M cells at 60 hrs may explain Dam’s inability to completely inhibit Vpr induced cell death. Furthermore, the majority of Vpr-/CCR-X infected cells remained at G1 phase with or without Dam treatment, showing that Dam does not affect the cell cycle of normal cells growing in the absence of Vpr. Dam treatment did not affect the number of S phase cells throughout the course of the experiment both in Vpr+/CCR-X and in Vpr-/CCR-X infected cells.
Interestingly, a previous screen by Ali et al. identified Dam as an agent that protects against cytotoxicity caused by HIV [30]. Their screen was performed on CEM-SS cell infected with HIV and the mechanism of inhibition has remained unknown. Here, we identified Dam directly as an inhibitor of cell death caused by a specific HIV protein, Vpr, and demonstrated that Dam inhibits Vpr induced cell death partly by the anti-apoptotic function. Furthermore, our HIV infection experiment using human peripheral blood lymphocytes showed that Dam inhibited p24 production by 52% and 33 % at day 5 and 7 after the infection, respectively (data not shown), suggesting that Dam might work as an anti-AIDS agent by suppressing HIV production and inhibiting CD4+ cell depletion in patients.
Vpr has been shown to induce apoptosis by inducing prolonged cell cycle arrest [18, 31]. On the other hand, Vpr has also been shown to induce apoptosis independent of G2 arrest [32]. We here provide evidence using an inhibitor of Vpr dependent cell death that small molecules can be used to divide Vpr induced cell death mechanisms. The mechanism(s) on how Dam inhibits Vpr induced apoptosis is currently unknown and will need to be elucidated. Identification of small molecules that inhibit Vpr induced cell death not only provides mechanistic insights into how Vpr regulated cell cycle arrest and apoptosis, but it may also enhance our understanding and treatment for other diseases with deregulated apoptosis and cell cycle.
Acknowledgments
We thank Owen Witte for acquiring the Biomol Library and helping to establish the screening center. Funded in part by NIH grant CA070018-10 (I.S.Y.C.), the Department of Molecular and Medical Pharmacology, Jonsson Comprehensive Cancer Center, and UCLA. R.P.W. is a recipient of an NIH Interdisciplinary Training in Virology and Gene Therapy training grant, J.P.S. was a recipient of an NIH Research Training in Pharmacological Sciences training grant. R.P.W. thanks Winston Wu for advice on statistical analysis.
References
- [1].Turpin JA. The next generation of HIV/AIDS drugs: novel and developmental anti HIV drugs and targets. Expert Rev Anti Infect Ther. 2003;1:97–128. doi: 10.1586/14787210.1.1.97. [DOI] [PubMed] [Google Scholar]
- [2].Richman DD. HIV chemotherapy. Nature. 2001;410:995–1001. doi: 10.1038/35073673. [DOI] [PubMed] [Google Scholar]
- [3].Simon V, Ho DD. HIV-1 dynamics in vivo: implications for therapy. Nat Rev Microbiol. 2003;1:181–190. doi: 10.1038/nrmicro772. [DOI] [PubMed] [Google Scholar]
- [4].Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324:961–964. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- [5].Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331–340. doi: 10.1084/jem.175.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Meyaard L, Otto SA, Jonker RR, Mijnster MJ, Keet RP, Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992;257:217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- [7].Gougeon ML. Apoptosis as an HIV strategy to escape immune attack. Nat Rev Immunol. 2003;3:392–404. doi: 10.1038/nri1087. [DOI] [PubMed] [Google Scholar]
- [8].Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206:935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- [9].Gibbs JS, Lackner AA, Lang SM, Simon MA, Sehgal PK, Daniel MD, Desrosiers RC. Progression to AIDS in the absence of a gene for vpr or vpx. J Virol. 1995;69:2378–2383. doi: 10.1128/jvi.69.4.2378-2383.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goh WC, Rogel ME, Kinsey CM, Michael SF, Fultz PN, Nowak MA, Hahn BH, Emerman M. HIV-1 Vpr increases viral expression by manipulation of the cell cycle: a mechanism for selection of Vpr in vivo. Nat Med. 1998;4:65–71. doi: 10.1038/nm0198-065. [DOI] [PubMed] [Google Scholar]
- [11].Lum JJ, Cohen OJ, Nie Z, Weaver JG, Gomez TS, Yao XJ, Lynch D, Pilon AA, Hawley N, Kim JE, Chen Z, Montpetit M, Sanchez-Dardon J, Cohen EA, Badley AD. Vpr R77Q is associated with long-term nonprogressive HIV infection and impaired induction of apoptosis. J Clin Invest. 2003;111:1547–1554. doi: 10.1172/JCI16233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mologni D, Citterio P, Menzaghi B, Poma BZ, Riva C, Broggini V, Sinicco A, Milazzo L, Adorni F, Rusconi S, Galli M, Riva A. Vpr and HIV-1 disease progression: R77Q mutation is associated with long-term control of HIV-1 infection in different groups of patients. AIDS. 2006;20:567–574. doi: 10.1097/01.aids.0000210611.60459.0e. [DOI] [PubMed] [Google Scholar]
- [13].Rajan D, Wildum S, Rucker E, Schindler M, Kirchhoff F. Effect of R77Q, R77A and R80A changes in Vpr on HIV-1 replication and CD4 T cell depletion in human lymphoid tissue ex vivo. AIDS. 2006;20:831–836. doi: 10.1097/01.aids.0000218546.31716.7f. [DOI] [PubMed] [Google Scholar]
- [14].Popov S, Rexach M, Zybarth G, Reiling N, Lee MA, Ratner L, Lane CM, Moore MS, Blobel G, Bukrinsky M. Viral protein R regulates nuclear import of the HIV-1 pre-integration complex. EMBO J. 1998;17:909–917. doi: 10.1093/emboj/17.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Agostini I, Navarro JM, Rey F, Bouhamdan M, Spire B, Vigne R, Sire J. The human immunodeficiency virus type 1 Vpr transactivator: cooperation with promoter-bound activator domains and binding to TFIIB. J Mol Biol. 1996;261:599–606. doi: 10.1006/jmbi.1996.0485. [DOI] [PubMed] [Google Scholar]
- [16].Forget J, Yao XJ, Mercier J, Cohen EA. Human immunodeficiency virus type 1 vpr protein transactivation function: mechanism and identification of domains involved. J Mol Biol. 1998;284:915–923. doi: 10.1006/jmbi.1998.2206. [DOI] [PubMed] [Google Scholar]
- [17].Jowett JB, Planelles V, Poon B, Shah NP, Chen ML, Chen IS. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J Virol. 1995;69:6304–6313. doi: 10.1128/jvi.69.10.6304-6313.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stewart SA, Poon B, Jowett JB, Chen IS. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. J Virol. 1997;71:5579–5592. doi: 10.1128/jvi.71.7.5579-5592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Stewart SA, Poon B, Song JY, Chen IS. Human immunodeficiency virus type 1 vpr induces apoptosis through caspase activation. J Virol. 2000;74:3105–3111. doi: 10.1128/jvi.74.7.3105-3111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bouzar AB, Villet S, Morin T, Rea A, Genestier L, Guiguen F, Garnier C, Mornex JF, Narayan O, Chebloune Y. Simian immunodeficiency virus Vpr/Vpx proteins kill bystander noninfected CD4+ T-lymphocytes by induction of apoptosis. Virology. 2004;326:47–56. doi: 10.1016/j.virol.2004.05.016. [DOI] [PubMed] [Google Scholar]
- [21].Stewart SA, Poon B, Jowett JB, Xie Y, Chen IS. Lentiviral delivery of HIV-1 Vpr protein induces apoptosis in transformed cells. Proc Natl Acad Sci U S A. 1999;96:12039–12043. doi: 10.1073/pnas.96.21.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yuan H, Kamata M, Xie YM, Chen IS. Increased levels of Wee-1 kinase in G2 are necessary for Vpr- and γ-irradiation-induced G2 arrest. J Virol. 2004;78:8183–8190. doi: 10.1128/JVI.78.15.8183-8190.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Barry SC, Harder B, Brzezinski M, Flint LY, Seppen J, Osborne WR. Lentivirus vectors encoding both central polypurine tract and posttranscriptional regulatory element provide enhanced transduction and transgene expression. Hum Gene Ther. 2001;12:1103–1108. doi: 10.1089/104303401750214311. [DOI] [PubMed] [Google Scholar]
- [24].Kamata M, Aida Y. Two putative α-helical domains of human immunodeficiency virus type 1 Vpr mediate nuclear localization by at least two mechanisms. J Virol. 2000;74:7179–7186. doi: 10.1128/jvi.74.15.7179-7186.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Poon B, Jowett JB, Stewart SA, Armstrong RW, Rishton GM, Chen IS. Human immunodeficiency virus type 1 vpr gene induces phenotypic effects similar to those of the DNA alkylating agent, nitrogen mustard. J Virol. 1997;71:3961–3971. doi: 10.1128/jvi.71.5.3961-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Nishizawa M, Kamata M, Katsumata R, Aida Y. A carboxy-terminally truncated form of the human immunodeficiency virus type 1 Vpr protein induces apoptosis via G1 cell cycle arrest. J Virol. 2000;74:6058–6067. doi: 10.1128/jvi.74.13.6058-6067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wang MY, West BJ, Jensen CJ, Nowicki D, Su C, Palu AK, Anderson G. Morinda citrifolia (Noni): a literature review and recent advances in Noni research. Acta Pharmacol Sin. 2002;23:1127–1141. [PubMed] [Google Scholar]
- [28].Hiramatsu T, Imoto M, Koyano T, Umezawa K. Induction of normal phenotypes in ras-transformed cells by damnacanthal from Morinda citrifolia. Cancer Lett. 1993;73:161–166. doi: 10.1016/0304-3835(93)90259-c. [DOI] [PubMed] [Google Scholar]
- [29].Faltynek CR, Schroeder J, Mauvais P, Miller D, Wang S, Murphy D, Lehr R, Kelley M, Maycock A, Michne W, et al. Damnacanthal is a highly potent, selective inhibitor of p56lck tyrosine kinase activity. Biochemistry. 1995;34:12404–12410. doi: 10.1021/bi00038a038. [DOI] [PubMed] [Google Scholar]
- [30].Ali A, Ismail N, Mackeen M, Yazan L, Mohamed S, Ho A, Lajis N. Antiviral, cytotoxic and antimicrobial activities of anthraquinones isolated from the roots of Morinda Elliptica. Pharmaceutical Biology. 2000;38:298–301. doi: 10.1076/1388-0209(200009)3841-AFT298. [DOI] [PubMed] [Google Scholar]
- [31].Zhu Y, Gelbard HA, Roshal M, Pursell S, Jamieson BD, Planelles V. Comparison of cell cycle arrest, transactivation, and apoptosis induced by the simian immunodeficiency virus SIVagm and human immunodeficiency virus type 1 vpr genes. J Virol. 2001;75:3791–3801. doi: 10.1128/JVI.75.8.3791-3801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Nishizawa M, Kamata M, Mojin T, Nakai Y, Aida Y. Induction of apoptosis by the Vpr protein of human immunodeficiency virus type 1 occurs independently of G2 arrest of the cell cycle. Virology. 2000;276:16–26. doi: 10.1006/viro.2000.0534. [DOI] [PubMed] [Google Scholar]