Abstract
We have identified 44 serine protease (SP) and 13 serine protease homolog (SPH) genes in the genome of Apis mellifera. Most of these genes encode putative secreted proteins, but four SPs and three SPHs may associate with the plasma membrane via a transmembrane region. Clip domains represent the most abundant non-catalytic structural units in these SP-like proteins −12 SPs and six SPHs contain at least one clip domain. Some of the family members contain other modules for protein–protein interactions, including disulphide-stabilized structures (LDLrA, SRCR, frizzled, kringle, Sushi, Wonton and Pan/apple), carbohydrate-recognition domains (C-type lectin and chitin-binding), and other modules (such as zinc finger, CUB, coiled coil and Sina). Comparison of the sequences with those from Drosophila led to a proposed SP pathway for establishing the dorsoventral axis of honey bee embryos. Multiple sequence alignments revealed evolutionary relationships of honey bee SPs and SPHs with those in Drosophila melanogaster, Anopheles gambiae, and Manduca sexta. We identified homologs of D. melanogaster persephone, M. sexta HP14, PAP-1 and SPH-1. A. mellifera genome includes at least five genes for potential SP inhibitors (serpin-1 through -5) and three genes of SP putative substrates (prophenoloxidase, spätzle-1 and spätzle-2). Quantitative RT-PCR analyses showed an elevation in the mRNA levels of SP2, SP3, SP9, SP10, SPH41, SPH42, SP49, serpin-2, serpin-4, serpin-5, and spätzle-2 in adults after a microbial challenge. The SP41 and SP6 transcripts significantly increased after an injection of Paenibacillus larva, but there was no such increase after injection of saline or Escherichia coli. mRNA levels of most SPs and serpins significantly increased by 48 h after the pathogen infection in 1st instar larvae. On the contrary, SP1, SP3, SP19 and serpin-5 transcript levels reduced. These results, taken together, provide a framework for designing experimental studies of the roles of SPs and related proteins in embryonic development and immune responses of A. mellifera.
Keywords: Apis mellifera, insect immunity, serine protease homolog, serpin, clip domain, phylogenetic analysis, protease cascade
Introduction
Serine proteases in the S1 family (e.g. chymotrypsin) are involved in various physiological processes, such as digestion, development, and defense responses (Rawlings & Barrett, 1993; Krem & Di Cera, 2002). They are typically synthesized as zymogens, which require proteolysis at a specific site for activation. In some cases, after an initiation protease becomes active upon stimulation, other downstream SP zymogens are sequentially activated in a cascade pathway, which eventually generates effector molecules by limited proteolysis. High specificity of their catalytic domains, interactions among the regulatory regions, and efficient removal of active SPs by irreversible protease inhibitors ensure local, transient reactions to physiological or pathological cues. Human blood coagulation and complement activation are the best known examples of such protease systems (O’Brien & McVey, 1993; Whaley & Lemercier, 1993). The evolutionary history of serine protease pathways can be traced back to the divergence of deuterostomes and arthropods (Iwanaga et al., 1998; Jiang & Kanost, 2000; Krem & Di Cera, 2002; Kanost et al., 2004). Recently, biochemical and genomic analyses revealed that catalytically inactive serine protease homologs are also constituents of these systems (Kwon et al., 2000; Yu et al., 2003). SPHs are similar in sequence to S1 proteases but lack one or more of the catalytic residues in SPs. A human SPH named azurocidin mediates inflammation and has an antimicrobial activity (Watorek, 2003). Invertebrate SPHs participate in acute-phase responses (Kawabata et al., 1996; Huang et al., 2000; Yu et al., 2003).
The horseshoe crab haemolymph clotting system represents the best characterized SP system in invertebrates (Iwanaga et al., 1998). It is composed of four proteases (Factors C, G, B, and clotting enzyme) and one clottable protein (coagulogen). In Drosophila, genetic studies revealed a SP pathway that establishes the dorsoventral axis of embryos (Belvin & Anderson, 1996). This pathway also comprises four proteases, namely nudel, gastrulation defective, Snake, and easter. Easter cleaves spätzle to form an active ligand that binds to the Toll receptor and triggers the intracellular signalling pathway for ventralization. In Drosophila adults, another set of SPs leads to spätzle activation and drosomycin production (Lemaitre et al., 1996). Another insect defense mechanism involving a SP cascade is the proteolytic activation of prophenoloxidase (proPO) (Ashida & Brey, 1998; Ligoxygakis et al., 2002b; Kanost et al., 2004). In Manduca sexta, HP14 and proPO-activating proteases (PAPs) are the first and last components of the proPO activation cascade (Ji et al., 2004; Jiang et al., 1998; Jiang et al., 2003a and 2003b; Lee et al., 1998; Satoh et al., 1999). Our knowledge on composition, order, and regulation of these insect SP cascades has greatly expanded (Levashina et al., 1999; Ligoxygakis et al., 2002a; Kim et al., 2002; Gupta et al., 2004; Tong et al., 2005; Zou & Jiang 2005; Jiang et al., 2005; Wang et al., 2006; Wang & Jiang, 2004 and 2006; Jang et al., 2006).
Genome-wide analyses of SPs and SPHs are available for Drosophila melanogaster and Anopheles gambiae (Christophides et al. 2002; Ross et al., 2003). However, little is known about these proteins in the honey bee. Among ∼1.0 × 104 predicted genes in the genome of A. mellifera, SP and SPH genes form a large family (Honey Bee Genome Sequencing Consortium, 2006; Evans et al., 2006). To begin to understand the potential functions of SPs in immune responses in this beneficial insect, it is necessary to annotate these genes, compare their protein products with homologous molecules from other insects, and predict their functions. In this paper, we report a genome-wide analysis of the structures, evolutionary relationships, and possible physiological functions of A. mellifera SPs and SPHs. Some putative substrates and inhibitors of SPs are also discussed. We hope that these results could provide evolutionary perspectives of the S1 family of protease genes in insects and stimulate interest for in-depth analyses of SP-related proteins (i.e. SPs, SPHs, serpins and SP substrates) in the honey bee.
Results and discussion
Overview of the SP-SPH gene family
Blast searches of the A. mellifera genome yielded 57 sequences with significant similarity to the S1 protease family. Compared with 204 in D. melanogaster (Ross et al., 2003) and 305 in An. gambiae (Christophides et al. 2002), the number of SP-like genes in the honey bee is much smaller. We retrieved and annotated the sequences from Official Gene Set-1 (Honey Bee Genome Sequencing Consortium, 2006). Based on the presence or absence of residues essential for the catalytic activity of SPs, we classify them as SPs or SPHs. We identified 44 SP and 13 SPH genes in the bee genome (Table 1). The ratio of SPs to SPHs is close to that in D. melanogaster, which has 147 SPs and 57 SPHs. A. mellifera SP11, SP29, SPH50 and SPH51 are clustered in Group 9.19–20; SP4, SP5, SP8, SP13 and SP27 in Group 15.3–8; SP25, SP33 and SPH56 in Group 13.1–3. The other genes are widely spread over the genome. In contrast, large clusters of SP/SPH genes are common in the genomes of D. melanogaster and An. gambiae. It appears that this gene family may have undergone a major expansion in the Diptera that did not occur in Hymenoptera after divergence of these orders more than 240 million years ago.
Table 1.
Serine proteases (SPs) and serine proteinase homologs (SPHs) in Apis mellifera
| Homologous proteins | Conserved regionsb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | ID | Drosophila | other arthropodsa | TAAHC | DIAL | GDSGGP | Lengthc (aa) | Activation sited | Enzyme specificitye | Domain structuref |
| cSP1 | 16147 | ea CG1102 | MsHP5 | 376 | TEQK∧IFGG | T(DGA) | C-SP | |||
| cSP2 | 14247 | ea CG1102 | MsHP8 | ∼391 | LSQR∧IIGG | ?(D??) | C-SP | |||
| cSP3 | 11698 | MsHP17 | 353 | SHTR∧VVGG | T(DGG) | C-SP | ||||
| SP4 | 10646 | CG4914 | > 304 | EESR∧IVGG | T(DGG) | SP | ||||
| SP5 | 12300 | CG4386 CG18735 | 329 | VQRR∧IVGG | T(DGG) | SP | ||||
| cSP6 | 14077 | CG8172 | DVAL | 622 | RSNR∧IVGG | T(DGG) | 2LC-C-SP | |||
| cSP7 | 17145 | CG31728 | 512 | DQER∧IVGG | T(DGG) | C-SP | ||||
| SP8 | 18767 | CG9372 | MsHP21 | DIAV | > 292 | SRSR∧LTGG | T(DGG) | SP | ||
| cSP9 | 18732 | CG11843 | MsHP13 | 423 | DRKL∧IVGG | T(DGG) | pSP-SP | |||
| cSP10 | 17927 | Psh | MsHP21 CfSP | ?751 | PNKF∧IVGG | T(DGG) | 2(C-LC-SP) | |||
| Psh | MsHP21 CfSP | PNKF∧IVGG | T(DGG) | |||||||
| SP11 | 14654 | CG11836 | MsHP1 | DVAL | > 255 | QEDR∧IVGG | T(DGG) | SP | ||
| SP12 | 19856 | CG5255 CG31265 | DIGL | > 237 | EIPK∧IVGG | ?(GGD) | SP | |||
| SP13 | 15640 | CG7996 | MsHP21 Ag18D | > 448 | PNHL∧VIGG | T(DGG) | 3LC(Nr)-SP | |||
| cSP14 | 14044 | CG2056-PB,snake | MsHP6 | DVAI | > 385 | LSFH∧IFNG | T(DGG) | C-SP | ||
| SP15 | 18178 | > 294 | TTGR∧IFNG | ?(GGD) | SP | |||||
| SP16 | 12253 | CG16996 | TAGHC | DLAL | ?1149 | PETR∧IVGG | T(DGG) | 2LC(HTr)-SP | ||
| SP17 | 14603 | CG4316 | TAGHC | ?498 | LEPR∧ITGD | C(SAG) | SP-SP | |||
| CG4316 | TAGHC | FDTR∧IVGG | C(SGS) | |||||||
| SP18 | 10222 | CG31954 | CsSP | DVAL | > 247 | LQPR∧IIGG | T(DGG) | SP | ||
| cSPH19 | 17345 | CG4998 | TtFD | DIAI | GDGGGP | 741 | 4LC-LC(Yr)-C?-SPH | |||
| SP20 | 19590 | nudel corin | BmOvarianSP | DIGM | ?1645 | SQLR∧VVGG | T(DGG) | 3LDLA-SP-3LDLA-pSP-LDLA(RGD)-2LDLA(pSP) | ||
| cSP21 | 16220 | CG7432 | TtPCE | DIAV | > 408 | GKYR∧VVGG | T(DGG) | C-LC-SP | ||
| SP22 | 13791 | CsSP | > 259 | PDTQ∧IVGG | T(DGG) | SP | ||||
| SP23 | 12538 | Tequila CG4821 | Ag22D | ?2323 | IFQK∧VVRG | T(DGG) | 4LC-4CBD-SR-Clect-KR-LDLA-PA-2LDLA-SP | |||
| SP24 | 14233 | CG6865 | DIAI | > 236 | ? | T(DGG) | SP | |||
| cSP25 | 19719 | CG11824 | DLAL | ?942 | PESR∧IVGG | T(DGG) | C-10LC(STr)-SP | |||
| cSP26 | 18450 | CG8170 | CfSP | TAGHC | DVAV | ?667 | AQRR∧IVGG | T(DGG) | TM-2LC-C-SP | |
| SP27 | 11588 | CG31954 | AgTry | ?537 | MDGR∧IVGG | T(DGG) | 2(LC-SP) | |||
| DVAV | PTGQ∧IIGG | T(DGG) | ||||||||
| SP28 | 13489 | CG30375 | DVAL | MDSGGP | > 405 | NPSR∧IVGG | T(DGG) | TM-CUB-SP | ||
| SP29 | 14644 | CG18375 | DIAI | > 224 | ? | T(DGG) | SP | |||
| SP30 | 19649 | corin | TASHC | DVAL | ∼944 | AKTR∧IVGG | T(DGG) | cc-LC-TM-Fri(ZnF)-LDLA-LC-LDLA-SR-SP | ||
| SP31 | 11297 | AaTry | TAGHC | > 291 | EEDR∧IFGG | T(DGG) | SP | |||
| SP32 | 11511 | AaChy | TAGHC | > 260 | RPTR∧IVGG | C(AGS) | SP | |||
| cSP33 | 14309 | CG8213 | OnT2b | DLAL | > 1269 | KSGR∧IVGG | T(DGG) | C-5LC(Tr)-SP | ||
| SP34 | 11552 | CG30371 | CsChy | TAGHC | MGSGGP | ∼405 | NPSR∧IVGG | ?(DGN) | TM-CUB-SP | |
| SP35 | 16021 | CG5255 | DIAI | > 255 | NLEK∧IVGG | ?(GVD) | LC-SP | |||
| SP36 | 19846 | CG5255 | > 263 | PESK∧IVGG | ?(GGD) | SP | ||||
| SPH37 | 18944 | CG13318 | PlMasq | TVAHC | DVAV | GDGGSP | > 307 | pC?-SPH | ||
| SP38 | 16214 | CG10663 | DVAM | ∼481 | YFTR∧IIGG | T(DGG) | 2TSP1-SP | |||
| cSPH39 | 14366 | LD13269p | CrVn50 TmPPAF | DIAV | GDGGGP | ?783 | ZnF-LC-C-LC(Tr)-C-C(LC)-C-SPH | |||
| SP40 | 13263 | CG32808 | PlTry | ?725 | YNPK∧IING | C(GAT) | ZnF-LC-Sina-LC-SP | |||
| cSPH41 | 10943 | masquerade CG15002 | GDGGGP | 735 | 5[C-LC(STr)]-SPH | |||||
| cSPH42 | 11298 | CG5390 | BmMasq CrVn50 | DFAI | GDGGSP | 417 | LC-C-SPH | |||
| SP43 | 18530 | CG9564 | DVAV | 268 | PTGQ∧IIGG | T(DGG) | SP | |||
| SP44 | 15453 | DITI | GDSGGG | > 340 | LIGR∧IVNG | T(DGG) | SP | |||
| SP45 | 17654 | SAAHC | DIAM | 1748 | RRSR∧IVGG | ?(D??) | TM?-6LC-3LDLA-SP | |||
| SP46 | 16367 | CG13461 stubble gd | MsHP19 | DLAV | GDSGSG | 439 | FNLL∧VAGG | E(GSI) | SP | |
| SP47 | 14774 | ----- | > 157 | ? | ?(DI?) | pSP | ||||
| SP48 | 12379 | CG32376 | MsHP3 | TALHC | > 257 | ATIK∧IIGG | T(DGG) | SP | ||
| SP49 | 15317 | CG31217 | MsHP14 | DIAI | GDSGGG | ∼628 | SKTL∧IVNG | E(SSS) | LC-4LDLA-Sushi-Wonton-SP | |
| cSPH50 | 14001 | CG14945 | MsPAP1 | TTANC | NIAM | GYNGSP | 707 | TM-LC-PLCXc-C-SPH | ||
| SPH51 | 13397 | CG18735 CG4386 | TCGNC | ---- | LDVSSS | > 296 | SPH | |||
| SPH52 | 19292 | ----- | ---- | > 136 | pSP | |||||
| SPH53 | 15702 | TSAQC | NIAL | GNPGSP | > 294 | LC-SPH | ||||
| SPH54 | 15980 | ASYSC | NDEGAP | ?2733 | TM-LC-EGF-13LC(HEPSr)-5LDLA-SR-SPH | |||||
| cSPH55 | 15254 | CG11066 | TAANC | DLAT | TDIGSP | > 539 | C-SPH | |||
| SPH56 | 13019 | CG1632 | TTASC | TTVL | EFAGSP | ∼777 | LC-TM-SEA-LC-FRI-2LDLA-SPH | |||
| SPH57 | 16038 | CG31954 | ----- | ------ | > 159 | pSPH | ||||
Aa, Aedes aegypti; Ag, Anopheles gambiae; Bm, Bombyx mori; Cf, Ctenocephalides felis; Cr, Cotesia rubecula; Cs, Culicoides sonorensis; Ms, Manduca sexta; On, Ostrinia nubilalis; Pl, Pacifastacus leniusculus; Tm, Tenebrio molitor; Tt, Tachypleus tridentatus.
If not listed, sequences are identical to the conserved TAAHC, DIAL, or GDSGGP. -----: conserved region not identified.
>, incomplete sequence due to prediction errors; ∼, nearly complete (e.g. partial signal peptide); ?, prediction error?
∧, putative activation cleavage site; ?, not predicted; blank, not applicable (SPH).
Enzyme specificity predicted based on Perona and Craik (1995). T, trypsin; C, chymotrypsin; E, elastase; ?: not predictable; blank: not applicable (SPH). Letters in parentheses: amino acid residues determining the primary specificity of a serine proteinase.
C, clip domain; CBD: chitin-binding domain; cc, coiled coil region; Clect, C-type lectin domain; CUB, a domain identified in Complement 1r/s, Uegf, and Bmp1; EGF, Ca2+-binding EGF domain; FRI, frizzle domain; KR: kringle domain; LC, low complexity region; LDLA: low-density lipoprotein receptor class A domain; p, partial; PA, pan-apple domain; PLCXc, phospho-lipase C catalytic domain; SEA, a ∼120-residue domain in Sperm protein, Enterokinase and Agrin; Sina, a domain identified in Drosophilasevenin absentia; SP, serine proteinase catalytic domain; SPH, serine proteinase-like domain; SR: scavenger receptor cysteine-rich domain; Sushi, Sushi domain, also known as CCP or SCR. Wonton: a disulfide knotted domain found in M. sexta HP14; TSP1, thrombospondin type I repeat; TM, transmembrane region; XYr, regions rich in amino acid residues X and Y; ZnF, Zinc finger domain.
The catalytic triad of S1 proteases is composed of His57, Asp102 and Ser195 (chymotrypsin numbering). In most cases, these residues are present in highly conserved sequence motifs of TAAHC, DIAL and GDSGGP (Table 1). One or more of the catalytic residues are replaced in SPHs. GDSGGP is present in 32 of the honey bee SPs. In the 13 SPHs, 5 contain GDGG in the context of GDGGGP or GDGGSP. His57, also critical for protease activity, is located in TAAHC or its analogs: TAAHC and TAGHC are present in 67% and 12% of the SP/SPH family members, respectively. Asp102, the 3rd member of the catalytic site, is located in DIAL (28), DVAL (5), DVAV (4), DIAI (4), DLAL (3) or DIAV (3), where the number in parentheses indicates its occurrence in the SP-like sequences. While most SPs or SPHs are expected to be extracellular proteins, we only found 13 with a complete signal peptide for secretion. The gene prediction programs apparently failed to locate exons encoding such short sequences, which lack particular structural features other than having a stretch of hydrophobic residues.
Single domain SPs
Digestive SPs (e.g. trypsin) have a relatively simple structure, containing ∼240 residues. Fourteen A. mellifera SPs, shorter than 300 residues, may function in digestion, a process that does not require sophisticated protein–protein interactions. The bee has far fewer single domain SPs compared with ∼80 in D. melanogaster and ∼140 in An. gambiae. This could be related to its relatively simple food source, nectar and pollen. Nearly all of these putative digestive proteases reside in one branch of the honey bee SP-SPH phylogenetic tree, representing descendents of a simple ancestral SP gene (data not shown). On the other hand, 39 (or 69%) of the A. mellifera SPs and SPHs are longer than 300 residues. Only 1/2 and 1/3 of the family members in D. melanogaster and An. gambiae may contain additional regulatory domains. These proteins are probably involved in more complex physiological processes in which other structural units are needed for molecular recognition.
Clip-domain SPs and SPHs
In arthropods, clip-domain SPs mediate innate immunity and embryonic development (Jiang & Kanost, 2000; Kanost & Clarke, 2005). Each clip domain contains three disulphide bonds, and many SPs and SPHs between 300 and 400 residues contain one such domain. Although clip domain sequences are hypervariable, we have identified 12 cSPs and six cSPHs in the honey bee by locating the conserved pattern of Cys residues. Consistent with the small overall family size, the total number of A. mellifera cSPs and cSPHs is ∼1/3 of that in the Drosophila or Anopheles. In the bee, we did not find any dual clip-domain SPs, which serve as PAPs in M. sexta and Bombyx mori (Satoh et al., 1999; Jiang et al., 2003a and 2003b).
The clip domains in A. mellifera SPs/SPHs range from 30 to 70 residues between Cys1 and Cys6, with an average size of 45 residues (Fig. 1A). The regions between Cys2 and Cys3 are exactly five residues, except for cSPH41. The lengths between Cys3 and Cys4 of cSPs are similar to those in cSPHs. According to our previous analyses (Jiang & Kanost, 2000; Ross et al., 2003), clip domains can be divided into two groups based on the number of residues between Cys3 and Cys4. Group 1 contains less than 16 residues whereas Group 2 is longer (average size: ∼23 residues). All Group 1a cSPs in the honey bee are predicted to be activated by proteolytic cleavage between Arg and Ile (Table 1). They form one clade in the phylogenetic tree (Fig. 1B), except for SP7. In Group 1b, the Arg residue before the scissile bond is replaced by Phe or Leu. The corresponding position is occupied by Arg in Group 2 SPs, except for cSP14 – cSP14 is probably cut after a His residue, and it lacks the signature Cys pair present in most Group 2 cSPs (Ross et al., 2003).
Figure 1.

Sequence comparison and phylogenetic relationships among the Apis mellifera clip-domain SPs and SPHs. A. alignment of the clip domain sequences. Six conserved Cys residues form 3 disulphide bonds. B. phylogenetic tree based on an alignment of the catalytic and protease-like domains. Vertical bars and numbers indicate the clip domain groups.
A multiple sequence alignment of their catalytic domains suggests that all of the cSPs have a trypsin-like specificity, based on residues predicted to form the primary substrate-binding site (Table 1). A highly conserved Cys after the active site Asp in the context of PICLP is predicted to form a disulphide bond with a Cys in the linker between the clip and catalytic domains (based on horseshoe crab clotting enzyme). The phylogenetic analysis also indicates that clip-domain SPs and SPHs are more closely related to each other than to other members of the family. The divergence of A. mellifera clip-domain proteins was apparently an early evolutionary event with no shuffling of clip and protease domains thereafter. Moreover, since members of the each subgroup (group-1a, −1b or −2) are clustered with each other, they may represent the three lineages emerged from ancient splits of the gene family.
We identified putative Drosophila orthologs for many A. mellifera clip-domain proteins (Table 1). cSP10 has a four-domain structure of clip-catalytic-clip-catalytic, and both halves of the molecule are highly similar to Drosophila persephone. Persephone is a component of the fungal-responsive branch of the SP system that triggers the Toll pathway for induced synthesis of drosomycin (Ligoxygakis et al., 2002a). A. mellifera SP17 and SP20 also contain more than one catalytic domain. Further analyses are needed to verify whether these three genes indeed encode proteins with such unusual domain structures. A. mellifera cSP14 and cSP2, most similar to Drosophila Snake and easter, may participate in the early development of honey bee embryos. All of the cSPHs are located in one clade of the phylogenetic tree (Fig. 1B). cSPH39 contains 4 clip domains, and cSPH41, a homolog of Drosophila masquerade, has 5 clip domains.
SPs and SPHs with complex domain structures
Many of the SP/SPH family members contain other structural modules predicted to function in protein–protein interactions. These include several types of disulphide-stabilized domains (e.g. LDLrA, SRCR, frizzled, kringle, Sushi, Wonton and Pan/apple), carbohydrate-recognition domains (C-type lectin, chitin-binding), and other domains (e.g. zinc finger, CUB, coiled coil, and Sina) (Table 1 and Fig. 2A). SP20, SP23, SP30, SP45, SP49 and SP54 contain LDLrA repeats, which are ∼40-residue-long Cys-rich sequences first identified in the ligand-binding domain of low-density lipoprotein receptor (LDLr). SP23 is most similar to An. gambiae SP22D (Danielli et al., 2000; Gorman et al., 2000), but also resemble D. melanogaster Tequila in domain architecture (Fig. 2). Tequila has 15 chitin-binding domains, two scavenger receptor Cys-rich (SRCR) domains, 2 LDLr Cys-rich domains and one SP domain (Munier et al., 2004). It also contains His- and Pro-rich regions and NGGYQPP repeats. At least three spliced forms of Tequila are detected throughout Drosophila development. Although there was no phenotype in the null mutant, its up-regulation in the wild-type fly upon fungal or bacterial infection suggests a role in innate immunity. In the mosquito, SP22D binds to chitin but not bacteria. The functions of A. mellifera SP23 and its orthologs in the fly and mosquito are unclear. A. mellifera SP49 is orthologous to M. sexta HP14, An. gambiae CP12488 and D. melanogaster AY118964 (Ji et al., 2004). These mosaic proteases have an identical domain structure: 4–5 LDLrA repeats, a Sushi domain, a Wonton domain and a SP catalytic domain (Wang & Jiang, 2006). M. sexta HP14 is an initiation enzyme activated upon pathogen recognition, and it triggers the SP pathway for proPO activation. A. mellifera SP49 may have the same function.
Figure 2.
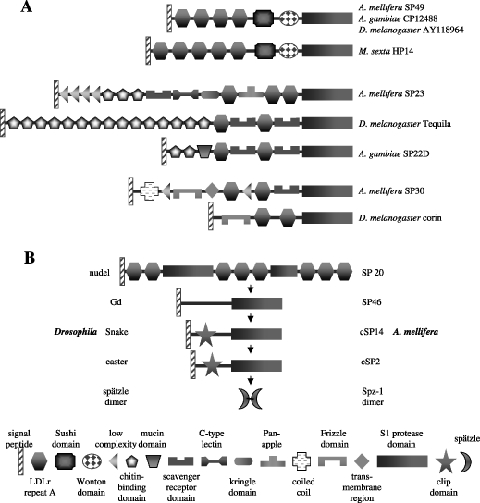
Domain organization of some SPs in Apis mellifera and other insects. A. Apis mellifera SP49 is orthologous to Manducta sexta HP14, An. gambiae AgCP12488 and Drosophila melanogaster AY118964. Apis mellifera SP23 is similar to Anopheles gambiae SP22D and D. melanogaster Tequila, whereas honey bee SP30 is homologous to D. melanogaster corin. B. A proposed SP cascade (left) for establishing the dorsal-ventral axis of A. mellifera embryo, in comparison to a similar system discovered in D. melanogaster.
A. mellifera cSPH41 is orthologous to Drosophila masquerade, which is essential in the development of embryonic nerve tissues (Murugasu-Oei et al., 1995). SPH39 is identical in domain structure to Drosophila LD13269p (Table 1). SP30 and Drosophila corin are apparent orthologs, both containing a frizzle domain, LDLrA repeats and a type II transmembrane region (Fig. 2A). A. mellifera SP46 is an ortholog of Drosophila Stubble, a transmembrane SP required for leg and wing morphogenesis, which functions through a RhoA intracellular signalling pathway (Bayer et al., 2003).
SP-mediated extracellular signal transduction
Formation of SP pathways is a common strategy employed by animals to respond to physiological or pathological stimuli. Genetic and biochemical analyses of protease cascades in model insects (e.g. D. melanogaster), when combined with genome sequences, may provide useful insights on similar processes in other arthropod species. Therefore, we compared the SP genes in the honey bee genome with Nudel, gastrulation defective (Gd), Snake, and easter, which establish the dorsoventral axis of Drosophila embryo (Belvin & Anderson, 1996). A. mellifera SP20 and SP46 are orthologous to Nudel and Gd, respectively (Fig. 2B). While high sequence similarity (identity: 26% and 39%) and identical domain structure suggest cSP14 and cSP2 may be honey bee Snake and easter, respectively, we are unable to assign unambiguous orthologous relationships due to the existence of other Apis clip-domain SPs with the same domain structure. Future experiments are needed to test whether A. mellifera SP20, SP46, cSP14 and cSP2 are involved in the early embryonic development. We have identified possible substrates for this proposed SP pathway, namely spätzle-1 (GB15688) and spätzle-2 (GB13503). A. mellifera spätzle-1 and −2 are 47% and 40% similar in sequence (identities: 28% and 22%) to Drosophila spätzle (Fig. 3). The numbers and positions of their Cys residues are conserved in most cases.
Figure 3.

Alignment of Drosophila spätzle and Apis spätzle-1 and −2. The first 127 residues at the amino terminus of the fly protein were not shown. *, identical:, similar.
Proteolytic activation of proPO is a common defense mechanism in insects and crustaceans (Ashida & Brey, 1998). Active PO is involved in melanotic encapsulation and wound healing. In the last decade, this SP pathway has been extensively studied in B. mori, M. sexta and Holotrichia diomphalia. As described above, A. mellifera SP23, the ortholog of M. sexta HP14, may be an initiation protease of the pathway. While intermediate steps of the cascade are still unknown, we found A. mellifera cSP1 and cSPH42 are similar in sequence and domain structure to M. sexta PAP-1 and SPH-1, respectively. M. sexta PAP-1, SPH-1 and other clip-domain proteins participate in the proPO cleavage and activation (Tong et al., 2005; Zou & Jiang 2005). A. mellifera GB18313, 56% identical in amino acid sequence to M. sexta proPO-1, is the only proPO gene identified in the genome (Lourenco et al., 2005). Like most proPOs known so far, the honey bee proPO lacks a signal peptide and has the consensus sequence of NR51*F52G around the proteolytic activation site (*). These data suggest there is a conserved SP pathway to activate proPO in the bee.
Serpins
SP inhibitors of the serpin superfamily are present in insect haemolymph to remove excess proteases and maintain homeostasis (Kanost, 1999). They are 45–55 kDa proteins with a conserved tertiary structure. Serpins regulate haemolymph coagulation, melanization and antimicrobial protein synthesis in arthropods. The reactive site loop near the carboxyl terminus is critical for inhibitory selectivity. Seven annotated genes in the honey bee genome encode five serpins and two serpin-like proteins with unusual insertions or extensions that may represent errors in gene prediction (Table 2). The ratio of SPs to serpins is 6.3 in A. mellifera, similar to that in D. melanogaster (5.3).
Table 2.
Serine protease inhibitors (serpins) in Apis mellifera
| Homologous proteins | |||||||
|---|---|---|---|---|---|---|---|
| Accession number | GenBank ID | Drosophila | Other arthropodsa | Length (aa) | Signal peptide | Predicted reactive site | Target enzyme specificitya |
| serpin-1 | GB17012 | serpin-4 | MsSerpin-1,2/AgSRPN-10 | 334 | No | LR*RC | T |
| serpin-2 | GB16472 | serpin-4 | MsSerpin-1,2 | 342 | QG-ET | PL*SS | C |
| serpin-3 | GB12279 | spn-27 A | MsSerpin-3/AgSRPN-2 | 466 | DG-KE | NK*NQ | T |
| serpin-4 | GB13578 | CG7219 | AgSRPN-6 | 469 | FG-QL | ER*DG | T |
| serpin-5 | GB19582 | serpin-5 | MsSerpin-6 | 451 | SA-QC | FR*SG | T |
| GB10078b | CG14470 | 1543 | VG-SP | ER*AE | T | ||
| GB15070b | CG12807 | 612 | YC-VD | ER*AG | T | ||
Ag, Anopheles gambiae; Ms, Manduca sexta; C, chymotrypsin; T, trypsin.
GB10078 contains a carboxyl-terminal serpin domain; GB15070 contains a split serpin domain (maleszka3).
While there is no experimental report on honey bee serpins, these inhibitors have been extensively investigated in moth, fly, and mosquito (Kanost et al., 2004). Through sequence alignment, we have identified putative orthologs of individual honey bee serpins and suggested their possible functions in the development and immunity (Fig. 4). A. mellifera serpin-1, −4 and −5 have an Arg at the predicted P1 site, the residue N-terminal to the cleavage site (Table 2), and A. mellifera serpin-3 has a Lys at the putative P1 position, suggesting that they may inhibit SPs with trypsin-like specificity. Consistent with the prediction that a few of the honey bee SPs are chymotrypsin-like (Table 1), one serpin (A. mellifera serpin-2) has a Leu at the putative P1 site.
Figure 4.
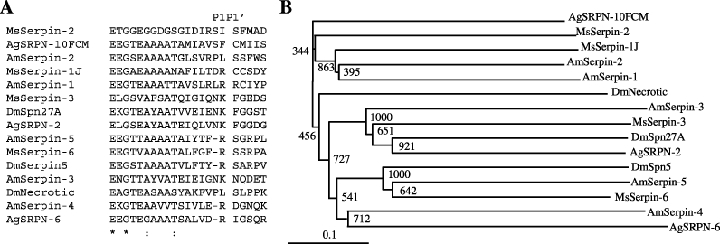
Sequence alignment and phylogenetic relationships of serpins from Apis mellifera and other insects. A. Amino acid sequence alignment of the P17-P4′ region. Identical residues are indicated by ‘*’, and similar residues by ‘:’. B. Phylogenetic tree based on alignment of full-length serpins selected from A. mellifera, Anopheles gambiae, Drosophila melanogaster and Manducta sexta.
We did not identify honey bee ortholog of Necrotic, a Drosophila serpin that controls the Toll pathway activation and spontaneous melanization. A. mellifera serpin-1 and −2 have a relatively high similarity (identity: 39%) to M. sexta serpin-1. M. sexta serpin gene-1 encodes 12 reactive site loop variants through alternative exon 9 usage (Kanost, 1999). Serpin-1 J blocks proPO activation by inhibiting PAP-1, −2 and −3 (Jiang et al., 2003b). At a high concentration, M. sexta serpin-1I partly inhibited haemolymph protease 14, an initiation protease of the proPO activation cascade (Wang & Jiang, 2006). We identified A. mellifera serpin-3 (GB12279) as the ortholog of D. melanogaster Spn27A and M. sexta serpin-3, which inhibit PAPs to regulate melanization (Ligoxygakis et al., 2002b; Zhu et al., 2003). During embryonic development, Spn27A inhibits easter and suppresses activation of the Toll pathway that establishes the dorsoventral axis. The honey bee serpin-5 (GB19582) may also be a negative regulator of melanization, since its ortholog M. sexta serpin-6 formed stable complexes with PAP-3 and HP8 (Zou & Jiang, 2005). Although experimental data are unavailable to support the proposed functions of the bee serpins, the observed sequence similarity provides useful working hypotheses to test.
Gene expression
To investigate transcriptional regulation of the SP-related genes upon microbial infection, we injected adult workers with saline, E. coli or a honey bee pathogen (Paenibacillus larva). Real-time RT-PCR indicated that SP2, SP9, SP10 and SP23 mRNA levels increased after the saline injection (Fig. 5A). SP3, SPH42, SP49, serpin-2, serpin-4, serpin-5 and spätzle-2 transcripts were elevated after the saline or E. coli injection. We detected increases in the SP1, SP2, SP3, SP6, SP41, SPH42, SP49 and serpin-2 transcript levels after the P. larva injection. Compared with the injection of saline or E. coli, the pathogen challenge gave rise to a much stronger induction of SP41 and SP6 gene transcription.
Figure 5.

Quantitative RT-PCR analyses of Apis mellifera SP-related transcripts. A. RNA samples from adult workers at 24 h after injections of Paenibacillus larvae (P.l.), E. coli (E.c.), or saline and uninjected control. B. RNA from the 2nd instar larvae at 24 h or 48 h after feeding on the regular diet (–) or diet with an infective dose of P. larvae. Gene expression is shown in grey scale, with darker squares indicating higher expression levels. SP10N and SP10C: N- and C-terminal halves.
In contrast, mRNA level changes in the honey bee larvae were subtle at 24 h after the larvae fed on a diet containing P. larva spores (Fig. 5B). At 48 h, some SP and serpin transcripts became more abundant. Strong induction was observed for SP14, SPH42, SPH42, SPH55, serpin-1 and serpin-2 transcripts, whereas SP1, SP3, SP7, SPH19 and serpin-5 mRNA levels decreased. Perhaps, this pathogen evades the host defense (e.g. melanization) system by modulating the SP gene transcription.
Conclusion
In this work, we explored the sequences and possible physiological functions of honey bee SPs/SPHs and serpins. Compared with D. melanogaster and An. gambiae, A. mellifera has much smaller families of SP, SPH, serpin, proPO and other immune proteins (Evans et al., 2006). Perhaps, defense strategies at the colony level largely alleviate the pressure on the immune system in individual insects, resulting in requirement for fewer genes functioning in defense against infection. Sequence, size, specificity and domain structure analyses of SPs provided useful clues to potential components of A. mellifera SP cascades. Quantitative RT-PCR indicated that many SPs and their regulators/substrates are immune responsive. Such information will be useful for elucidating the composition and function of SP-related protein systems in this social insect.
Experimental procedures
Database searching and sequence retrieving
M. sexta proPO-activating protease-1 (PAP-1) (Jiang et al., 1998) was used as a query to perform a BLASTP search of Official Gene Set-1 (Honey Bee Genome Sequencing Consortium, 2006) in the honey bee genome database, BeeBase (http://racerx00.tamu.edu/). Every tenth sequence from the primary list was retrieved and used as the query for another round of searching. The amino acid sequences encoded by predicted genes with significant Blast scores (E-value < 0.1) were retrieved and numbered in the order in which they were identified. Similarly, M. sexta serpin-1, serpin-3, serpin-6, proPO-1 and D. melanogaster spätzle sequences were used to search the database for homologous genes in A. mellifera.
Sequence properties of A. mellifera SPs and SPHs
Sequences were categorized as SPs and SPHs by locating the conserved His, Asp, and Ser residues in the catalytic triad. If all three of these residues were present in the conserved TAAHC, DIAL and GDSGGP regions, the sequences were considered to be SPs. Sequences lacking one or more of these key residues were labelled SPHs. Protein sizes were calculated based on the entire predicted sequences.
Identification of clip domains in SPs and SPHs
The retrieved A. mellifera SP and SPH sequences were reviewed manually to search for clip domains (Ross et al., 2003). SPs and SPHs containing regions N-terminal to the catalytic domain with six cysteine residues with Cys5 and Cys6 at adjacent positions were designated cSPs and cSPHs, respectively. For other SP-like proteins, domain organization and comparison were analysed by CDART at http://www.ncbi.nlm.nih.gov/, PROSITE at http://us.expasy.org/prosite, and SMART at http://smart.embl-heidelberg.de/smart. The chromosomal location and predicted exon-intron boundaries for each annotated sequence were acquired from BeeBase (Glean_3.gff).
Multiple sequence alignment and phylogenetic analysis
SP catalytic domains and SPH protease-like domains were aligned using ClustalX (ftp://ftp-igbmc.u-strasbg.fr/pub/ClustalX/), and cladograms were constructed by the neighbour-joining method and displayed using Treeview (http://taxonomy.zoology.gla.ac.uk/rod/treeview.htm). A Blosum 30 matrix, with a gap penalty of 10 and an extension gap penalty of 0.1 were used in the multiple sequence alignment. In order to compare equivalent regions, 20 sequences lacking a significant portion of the protease-like domain were excluded from the analysis. SP catalytic domains from ∼50 residues upstream of the conserved His to ∼50 residues downstream of the reactive site Ser were compared. The corresponding region in SPHs was also included in the alignment. To compare the clip domain sequences, the region from one residue before Cys1 to one after Cys6 was analysed.
Gene expression analysis
To screen for immune-related transcript changes, adult worker bees from a single local A. mellifera ligustica colony were injected with either phosphate-buffered saline or saline containing 103 live E. coli cells or 103 vegetative spores of P. larvae (Evans, 2004). These bees, along with the uninjected ones, were maintained at 34 °C and high humidity. To assess immune responses following a natural infection, eight 1st instar larvae from the same stock were given per os challenges of P. larvae in their diet (5 spores/ml), and then maintained at 34 °C and high humidity. Control larvae were fed on the same diet but without the spores. Following an incubation period, the adults and larvae were instantly frozen at −80 °C prior to RNA extraction. Total RNA was extracted from whole abdomens of the adults using Trizol (Invitrogen, Carlsbad, CA), whereas the larvae were extracted using the RNAqueous kit (Ambion, Austin, TX). After DNA removal, first-strand cDNA was synthesized as previously described (Evans, 2004).
Specific primer pairs (Table 3) with calculated annealing temperatures of 59.5–60.5 °C and expected product sizes of 150–200 bp were designed using Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). A total of 28 cDNAs for SP-related proteins were examined by real-time PCR. Each 25 µl reaction contained Taq DNA polymerase (1 U), 1 × buffer (Roche Applied Sciences), 1 mm dNTP mix, 2 mm MgCl2, 0.2 µm primers, 1 × SYBR-Green I dye (Applied Biosystems Foster City, CA), and 10 nm fluorescein. The thermal cycling conditions were 95 °C for 5 min and 40 cycles of 94 °C for 20 s, 60 °C for 30 s, 72 °C for 60 s and 78 °C for 20 s. Amplification was monitored on an iCycler (Bio-Rad, Hercules, CA). Primer pairs that caused dimer formation or other artifacts in no-template controls were excluded. The remaining pairs were arrayed randomly, in duplicate, across a 96-well plate, and all expression data were collected in parallel for each cDNA template. Thresholds were individually calculated for each target gene on the array. For adult bee samples, data were combined for the three replicates in each single-bee injection treatment (or control). The larval RNA samples were pooled before cDNA synthesis, and the cDNA was run in duplicate on the RT-PCR plate. Proper dissociation curves and correct product sizes were examined by melting curve analysis and agarose gel electrophoresis. The transcripts were normalized relative to the levels of ribosomal protein S5 (Evans, 2004; Evans & Wheeler, 2000). Transcript abundance values (Ctcontrol– Cttarget) for each gene were median-normalized across each panel of genes, clustered by average linkage clustering, and presented as relative grey-scale values using Eisen Cluster 3.0 and Eisen TreeView (http://rana.lbl.gov/EisenSoftware.htm).
Table 3.
Oligonucleotides used in real time PCR of Apis mellifera SP-related genes
| Locus | Forward Primer | Reverse primer | Gene ID |
|---|---|---|---|
| SP1 | TGCTCATTGCGTTACATCGT | TTGTCAGCGCAAACAACTTC | GB16147 |
| SP2 | GCGTTTAGAAAGCGTTCGTC | TCCGCGCAAAGTAAGCTATT | GB14247 |
| SP3 | ATGGACCCTTGTTACCACCA | GTTGCGAAGGGTTCAAAGAA | GB11698 |
| SP6 | CGATGACGATGACATTCCTG | TGTGTCCACCCACGATTCTA | GB14077 |
| SP7 | GGCTGGGTTCTTGGTGTTTA | GCTCGACTGTGGTGTAACGA | GB17145 |
| SP8 | GTTTGGTCGACGGAAGAAAA | CCGTCGACTCGAAATCGTAT | GB18767 |
| SP9 | GAGATGTTGAATGGCACGAA | CCACCACTATCTCCCTGACAA | GB18732 |
| SP10N | CCGGTGAACTTGGAAAAGAT | CTTCGCCAGGAATAATGGAA | GB17927 |
| SP10C | GAGATGTTGAATGGCACGAA | CCACCACTATCTCCCTGACAA | GB17927 |
| SP13 | CGGAGCTTAAATGCGAAGAA | TTGTTCCTAGAGCAACCATGTG | GB15640 |
| SP14 | GATTACCCAATGGCATCGAC | GCTGGTGAACCGCAAGTATT | GB14044 |
| SPH19 | ACCATCGAGAAAACCACGAC | GTACACGCTTTCCGTTGGAT | GB17345 |
| SP21 | GCCGGAAACTTACACGGTTA | CGATAATGTGCTTGCGGTAA | GB16220 |
| SP23 | AACGGAAACGAAATGGACAG | GAGCACATGCTTGAACGAAA | GB12538 |
| SP30 | CACCAGAAGGCACTCTCACA | CCTGAGCGAAGCCTAAATTG | GB19649 |
| SPH39 | GCGCCAGGAAACTCTGTTAG | ACGAAGCTTCCCCGTTTATT | GB14366 |
| SPH41 | ACCGGCACAAGCAAAATTAC | GCGAACTCTTCGTGTTGTCA | GB10943 |
| SPH42 | GAAGTCCCCTTGTTTGTCCA | TCGATCCAATCACGAACAGA | GB11298 |
| SP49 | TGTGATGGCATAGCAGATTGT | CAGGCACCATAATCACAACG | GB15317 |
| SPH50 | GCAAATCGAAAGGGAAATGA | CTGATGGAAAGCTGGTGGTT | GB14001 |
| SPH55 | GTCAACGACGTGGAAGGAAT | CGTTGGAAGACATCCCGTAT | GB13397 |
| serpin-1 | CATGGTGACATGCCAATGTT | CGAGTTGTATTTGCAAGCATTT | GB17012 |
| serpin-2 | TCCATGGAGGCAGCAAATA | CCATTGGCCTTTAAAATAAACTG | GB16472 |
| serpin-3 | CGGGAGACGAAACTGATGAT | TTCACCTTGAGCTCCTTCGT | GB12279 |
| serpin-4 | CTGGGCCACGTGTAGATTTT | ATGTCCATTGCTGCTTTTCC | GB13578 |
| serpin-5 | ACTCAGCGAACCGATTATGG | GGACAGCATTTGGATTCGTT | GB19582 |
| Spz-1 | TGCACAAATTGTTTTTCCTGA | GTCGTCCATGAAATCGATCC | GB15688 |
| Spz-2 | AATCGAAGGTTTCGCTGAAG | TTCCGGTATTATGGAACCATTT | GB13503 |
| PPO | AGATGGCATGCATTTGTTGA | CCACGCTCGTCTTCTTTAGG | GB18313 |
Acknowledgments
This work was supported by the National Institutes of Health Grants GM58634 (to H.J.), GM41247 (to M.K), and USDA-NRI 2002–02546 (to J.D.E.). This article was approved for publication by the Director of Oklahoma Agricultural Experimental Station and supported in part under project OKLO2450.
Abbreviations
- SP and cSP
serine protease and clip-domain serine protease
- cSPH and SPH
serine protease homolog with and without clip domain
- PO and proPO
phenoloxidase and its precursor
- PAP
proPO-activating protease
- HP
haemolymph protease
- RT-PCR
reverse transcriptase-polymerase chain reaction
References
- Ashida M, Brey PT. Recent advances on the research of the insect prophenoloxidase cascade. In: Brey PT, Hultmark D, editors. Molecular Mechanisms of Immune Responses in Insects. Chapman & Hall; 1998. pp. 135–172. [Google Scholar]
- Bayer CA, Halsell SR, Fristrom JW, Kiehart DP, von Kalm L. Genetic interactions between the RhoA and Stubble-stubbloid loci suggest a role for a type II transmembrane serine protease in intracellular signaling during Drosophila imaginal disc morphogenesis. Genetics. 2003;165:1417–1432. doi: 10.1093/genetics/165.3.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belvin M, Anderson K. A conserved signaling pathway: the Drosophila Toll-dorsal pathway. Ann Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Danielli A, Loukeris TG, Lagueux M, Muller HM, Richman A, Kafatos FC. A modular chitin-binding protease associated with hemocytes and hemolymph in the mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2000;97:7136–7141. doi: 10.1073/pnas.97.13.7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD. Transcriptional immune responses by honey bee larvae during invasion by the bacterial pathogen, Paenibacillus larvae. J Invert Pathol. 2004;85:105–111. doi: 10.1016/j.jip.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Evans JD, Aronstein K, Chen YP, Hetru C, Imler J-L, Jiang H, Kanost M, Thompson G, Zou Z, Hultmark D. Immune pathways and defense mechanisms in honey bees, Apis mellifera. Insect Mol Biol. 2006;15:645–656. doi: 10.1111/j.1365-2583.2006.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. Expression profiles during honeybee caste determination. Genome Biol. 2000;2:0001.1–0001.6. doi: 10.1186/gb-2000-2-1-research0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MJ, Andreeva OV, Paskewitz SM. Sp22D: a multidomain serine protease with a putative role in insect immunity. Gene. 2000;251:9–17. doi: 10.1016/s0378-1119(00)00181-5. [DOI] [PubMed] [Google Scholar]
- Gupta S, Wang Y, Jiang H. Manduca sexta prophenoloxidase (proPO) activation requires proPO-activating proteinase (PAP) and serine proteinase homologs (SPHs) simultaneously. Insect Biochem Mol Biol. 2004;35:241–248. doi: 10.1016/j.ibmb.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey Bee Genome Sequencing Consortium. Insights into social insects from the genome of the honeybee Apis mellifera. Nature. 2006 doi: 10.1038/nature05260. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T, Wang H, Lee SY, Johansson MW, Söderhäll K, Cerenius L. A cell adhesion protein from the crayfish Pacifastacus leniusculus, a serine proteinase homologue similar to Drosophila masquerade. J Biol Chem. 2000;275:9996–10001. doi: 10.1074/jbc.275.14.9996. [DOI] [PubMed] [Google Scholar]
- Iwanaga S, Kawabata S, Muta T. New types of clotting factors and defense molecules found in horseshoe crab hemolymph: their structures and functions. J Biochem. 1998;123:1–15. doi: 10.1093/oxfordjournals.jbchem.a021894. [DOI] [PubMed] [Google Scholar]
- Jang IH, Chosa N, Kim SH, Nam HJ, Lemaitre B, et al. A spätzle-processing enzyme required for toll signaling activation in Drosophila innate immunity. Dev Cell. 2006;10:45–55. doi: 10.1016/j.devcel.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ji C, Wang Y, Guo X, Hartson S, Jiang H. A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. J Biol Chem. 2004;279:34101–34106. doi: 10.1074/jbc.M404584200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kanost MR. The clip-domain family of serine proteinases in arthropods. Insect Biochem Mol Biol. 2000;30:95–105. doi: 10.1016/s0965-1748(99)00113-7. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu X-Q, Kanost MR. Prophenoloxidase-activating proteinase-2 (PAP-2) from hemolymph of Manduca sexta: a bacteria-inducible serine proteinase containing two clip domains. J Biol Chem. 2003a;278:3552–3561. doi: 10.1074/jbc.M205743200. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Yu X-Q, Zhu Y, Kanost MR. Prophenoloxidase-activating proteinase-3 (PAP-3) from Manduca sexta hemolymph: a clip-domain serine proteinase regulated by serpin-1J and serine proteinase homologs. Insect Biochem Mol Biol. 2003b;33:1049–1060. doi: 10.1016/s0965-1748(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Gu Y, Guo X, Zou Z, Scholz F, Trenczek TE, Kanost MR. Molecular identification of a bevy of serine proteinases in Manduca sexta hemolymph. Insect Biochem Mol Biol. 2005;35:931–943. doi: 10.1016/j.ibmb.2005.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Wang Y, Kanost MR. Pro-phenol oxidase activating proteinase from an insect, Manduca sexta: a bacteria-inducible protein similar to Drosophila easter. Proc Natl Acad Sci USA. 1998;95:12220–12225. doi: 10.1073/pnas.95.21.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol. 1999;23:291–301. doi: 10.1016/s0145-305x(99)00012-9. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Clarke T. Proteases. In: Gilbert LI, Iatrou K, Gill S, editors. Comprehensive Molecular Insect Science. Vol. 4. Oxford: Elsevier; 2005. pp. 247–266. [Google Scholar]
- Kanost MR, Jiang H, Yu X-Q. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kawabata S, Tokunaga F, Kugi Y, Motoyama S, Miura Y, et al. Limulus factor D, a 43-kDa protein isolated from horseshoe crab hemocytes, is a serine protease homologue with antimicrobial activity. FEBS Lett. 1996;398:146–150. doi: 10.1016/s0014-5793(96)01224-0. [DOI] [PubMed] [Google Scholar]
- Kim MS, Baek M, Lee M, Park JW, Lee SY, Söderhäll K, Lee BL. A new easter-type serine protease cleaves a masquerade-like protein during prophenoloxidase activation in Holotrichia diomphalia larvae. J Biol Chem. 2002;277:39999–40004. doi: 10.1074/jbc.M205508200. [DOI] [PubMed] [Google Scholar]
- Krem MM, Di Cera E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem Sci. 2002;27:67–74. doi: 10.1016/s0968-0004(01)02007-2. [DOI] [PubMed] [Google Scholar]
- Kwon TH, Kim MS, Choi HW, Joo CH, Cho MY, Lee BL. A masquerade-like serine proteinase homologue is necessary for phenoloxidase activity in the coleopteran insect, Holotrichia diomphalia larvae. Eur J Biochem. 2000;267:6188–6196. doi: 10.1046/j.1432-1327.2000.01695.x. [DOI] [PubMed] [Google Scholar]
- Lee SY, Kwon TH, Hyun J, Choi J, Kawabata S, Iwanaga S, Lee BL. In vitro activation of prophenoloxidase by two kinds of prophenoloxidase-activating factors isolated from hemolymph of coleopteran, Holotrichia diomphalia larvae. Eur J Biochem. 1998;254:50–57. doi: 10.1046/j.1432-1327.1998.2540050.x. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Langley E, Green C, Gubb D, Ashburner M, Hoffmann JA, Reichhart JM. Constitutive activation of toll-mediated antifungal defense in serpin-deficient Drosophila. Science. 1999;285:1917–1919. doi: 10.1126/science.285.5435.1917. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM. Activation of Drosophila Toll during fungal infection by a blood serine protease. Science. 2002a;297:114–116. doi: 10.1126/science.1072391. [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Ji C, Leclerc V, Duvic B, Belvin M, Jiang H, Hoffmann JA, Reichhart JM. A serpin mutant links Toll activation to melanization in the host defense of Drosophila. EMBO J. 2002b;21:6330–6337. doi: 10.1093/emboj/cdf661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco A, Zufelato M, Bitondi M, Simoes ZL. Molecular characterization of a cDNA encoding prophenoloxidase and its expression in Apis mellifera. Insect Biochem Mol Biol. 2005;35:541–552. doi: 10.1016/j.ibmb.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Munier AI, Medzhitov R, Janeway CA, Jr, Doucet D, Capovilla M, Lagueux M. GRAAL: a Drosophila gene coding for several mosaic serine proteases. Insect Biochem Mol Biol. 2004;34:1025–1035. doi: 10.1016/j.ibmb.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Murugasu-Oei B, Rodrigues V, Yang X, Chia W. Masquerade, a novel secreted serine proteinase-like molecule is required for somatic muscle attachment in Drosophila embryo. Gene Dev. 1995;9:139–154. doi: 10.1101/gad.9.2.139. [DOI] [PubMed] [Google Scholar]
- O’Brien D, McVey J. Blood coagulation, inflammation, and defense. In: Sim E, editor. The Natural Immune System. Humoral Factors: IRL Press; 1993. pp. 257–280. [Google Scholar]
- Perona JJ, Craik CS. Structural basis of substrate specificity in the serine proteases. Protein Sci. 1995;4:337–360. doi: 10.1002/pro.5560040301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlings RD, Barrett AJ. Evolutionary families of peptidases. Biochem J. 1993;290:205–218. doi: 10.1042/bj2900205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhart JM. Tip of another iceberg: Drosophila serpins. Trends Cell Biol. 2005;15:659–665. doi: 10.1016/j.tcb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Ross J, Jiang H, Kanost MR, Wang Y. Serine proteases and their homologs in the Drosophila melanogaster genome: an initial analysis of sequence conservation and phylogenetic relationship. Gene. 2003;304:117–131. doi: 10.1016/s0378-1119(02)01187-3. [DOI] [PubMed] [Google Scholar]
- Satoh D, Horii A, Ochiai M, Ashida M. Prophenoloxidase-activating enzyme of the silkworm, Bombyx mori. J Biol Chem. 1999;274:7441–7453. doi: 10.1074/jbc.274.11.7441. [DOI] [PubMed] [Google Scholar]
- Tong Y, Jiang H, Kanost MR. Identification of plasma proteases inhibited by Manduca sexta serpin-4 and serpin−5 and their association with components of the prophenoloxidase activation pathway. J Biol Chem. 2005;280:14932–14942. doi: 10.1074/jbc.M500532200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Prophenoloxidase (PPO) activation in Manduca sexta: an initial analysis of molecular interactions among PPO, PPO-activating proteinase-3 (PAP-3), and a cofactor. Insect Biochem Mol Biol. 2004;34:731–742. doi: 10.1016/j.ibmb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jiang H. Interaction of β-1,3-glucan with its recognition protein activates hemolymph proteinase 14, an initiation enzyme of the prophenoloxidase activation system in Manduca sexta. J Biol Chem. 2006;281:9271–9278. doi: 10.1074/jbc.M513797200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zou Z, Jiang H. An expansion of the dual clip-domain serine proteinase family in Manduca sexta: gene organization, expression, and evolution of prophenoloxidase-activating proteinase-2, hemolymph proteinase 12, and other related proteinases. Genomics. 2006;87:399–409. doi: 10.1016/j.ygeno.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watorek W. Azurocidin – inactive serine proteinase homolog acting as a multifunctional inflammatory mediator. Acta Biochim Pol. 2003;50:743–752. [PubMed] [Google Scholar]
- Whaley K, Lemercier C. The complement system. In: Sim E, editor. The Natural Immune System. New York: Humoral Factors: IRL Press; 1993. pp. 121–150. [Google Scholar]
- Yu X-Q, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in phenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;33:197–208. doi: 10.1016/s0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Gorman MJ, Jiang H, Kanost MR. Manduca sexta serpin-3 regulates prophenoloxidase activation in response to infection by inhibiting prophenoloxidase-activating proteinases. J Biol Chem. 2003;278:46556–46564. doi: 10.1074/jbc.M309682200. [DOI] [PubMed] [Google Scholar]
- Zou Z, Jiang H. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8: cDNA cloning, protein expression, inhibition kinetics, and function elucidation. J Biol Chem. 2005;280:14341–14348. doi: 10.1074/jbc.M500570200. [DOI] [PMC free article] [PubMed] [Google Scholar]


