Abstract
Extracts of Chinese red yeast rice (RYR, a traditional dietary seasoning of Monascus purpureus) contains several active ingredients including lovastatin, and several trials of its possible lipid-lowering effects have been conducted. This meta-analysis assesses the effectiveness and safety of RYR preparations on lipid modification in primary hyperlipidemia. We included randomized controlled trials testing RYR preparation, compared with placebo, no treatment, statins, or other active lipid-lowering agents in people with hyperlipidemia through searching PubMed, CBMdisk, TCMLARS, the Cochrane Library, and AMED up to December 2004. Ninety-three randomized trials (9625 participants) were included and three RYR preparations (Cholestin, Xuezhikang and Zhibituo) were tested. The methodological quality of trial reports was generally low in terms of generation of the allocation sequence, allocation concealment, blinding, and intention-to-treat. The combined results showed significant reduction of serum total cholesterol levels (weighted mean difference -0.91 mmol/L, 95% confidence interval -1.12 to -0.71), triglycerides levels (-0.41 mmol/L, -0.6 to -0.22), and LDL-cholesterol levels (-0.73 mmol/L, -1.02 to -0.043), and increase of HDL-cholesterol levels (0.15 mmol/L, 0.09 to 0.22) by RYR treatment compared with placebo. The lipid modification effects appeared to be similar to pravastatin, simvastatin, lovastatin, atorvastatin, or fluvastatin. Compared with non-statin lipid lowering agents, RYR preparations appeared superior to nicotinate and fish oils, but equal to or less effective than fenofibrate and gemfibrozil. No significant difference in lipid profile was found between Xuezhikang and Zhibituo. RYR preparations were associated with non-serious adverse effects such as dizziness and gastrointestinal discomfort. Current evidence shows short-term beneficial effects of RYR preparations on lipid modification. More rigorous trials are needed, and long-term effects and safety should be investigated if RYR preparations are to be recommended as one of the alternative treatments for primary hyperlipidemia.
Background
Red yeast rice (RYR) is a traditional Chinese cuisine and medicinal agent prepared by using Monascus purpureus fermented with rice, which has been recorded in ancient Chinese pharmacopoeia Ben Cao Gang Mu-Dan Shi Bu Yi during the Ming Dynasty (1368–1644)[1]. The extracts from RYR contain starch, sterols, isoflavones, and monounsaturated fatty acids, and other compounds [2,3]; depending on Monascus strains used and fermentation conditions, it may contain polyketides called monacolins [4]. Monacolin K is lovastatin, which is a commonly prescribed lipid-lowering drug. Several randomized clinical trials have indicated beneficial effects of the RYR preparations including Xuezhikang and Zhibituo in the treatment of hyperlipidemia [5-9]. Xuezhikang has been in clinical use as a Chinese proprietary medicine in China and has recently been marketed in several European countries including Norway and Italy. As these preparations contain different compositions and concentration of lovastatin, evaluation of their effectiveness and safety from clinical trials is warranted.
People with hyperlipidemia have responded well to the lipid-lowering agents including HMG-CoA reductase inhibitors (statins), fibrates, nicotinic acids, and n-3 fatty acids [10]. However, long-term safety and potential drug interaction between statins and other hypolipidemic agents may become problematic [11-13]. Nowadays, many people would like to use naturaceuticals instead of chemical drugs. A previous systematic review identified four randomized trials of the lipid-lowering effects of RYR and concluded a lack of sufficient clinical research to support their efficacy [14]. The objective of this review is to assess the beneficial effects of lipid modification and safety of RYR preparations for their use in people with primary hyperlipidemia.
Methods
Search strategy
To identify relevant studies, we searched the following databases up to December 2004: The Cochrane Library, PubMed, Chinese Biomedical Database (CBMdisk), Traditional Chinese Medical Literature Analysis and Retrieval System (TCMLARS), and the Allied and Complementary Medicine Database (AMED). We used the search terms 'red yeast rice, Monascus purpureus, Xuezhikang, Cholestin, Hypochol, Hypocol, Lipascor', combined with 'hyperlipidemia, hypercholesterolemia, dyslipidemia, hypertriglyceridemia, hyperlipoproteinemia', and limited our search to clinical trials. Depending on the database, various combinations of both MeSH terms and the free text terms were used, but no limitation with regard to language and report type. We also screened the reference lists of identified papers and review articles, and contacted pharmaceutical companies.
Inclusion criteria
We included randomized clinical trials comparing RYR vs. placebo, no intervention, or established lipid-lowering agents in individuals with primary hyperlipidemia on outcomes of lipid profile and adverse effects. Eligible trials had to include adult participants meeting the National Cholesterol Education Programme diagnostic criteria of hyperlipidemia [15] and excluded secondary causes such as hypothyroidism, familial hypercholesterolemia, diabetes mellitus, liver or kidney diseases. Trials comparing different RYR preparations were included, but trials comparing different dosage of RYR preparations or comparing RYR with other herbal medicines were excluded.
Validity assessment
The methodological quality of trials was assessed using the generation of the allocation sequence, the allocation concealment, double blinding, and withdrawals/dropouts [16-19].
Data abstraction
One author (JL) extracted data and another author (JZ) cross-checked the data, and any disagreement was resolved by consensus. The following study characteristics were abstracted from the trials: design, participants and diagnosis, intervention regimen, and outcome measures.
Data synthesis
We used the statistical package (RevMan 4.2) provided by the Cochrane Collaboration for data analyses. Dichotomous data were presented as relative risk (RR) and continuous outcomes as weighted mean difference (WMD), both with 95% confidence interval (CI). We assessed data by both random effects and fixed effect analyses, but reported the random effect analysis if the heterogeneity was significant, which was assessed by the I2 statistic and used P < 0.10 as significance limit [20].
Results
Included trials
We identified 647 records on RYR preparations from electronic and manual searches. By reading titles and abstracts, we excluded 275 citations that were clearly duplicates, review articles, or non-clinical studies. A total of 372 articles published in Chinese or English were retrieved for further assessment. Of these, 279 articles were excluded because they were non-controlled clinical studies or randomized trials with different research objectives. Two of these were ongoing placebo-controlled trials testing 'Hypocol' in Norway and 'Lipolysar' in Italy [21], but data were not available while writing this report. In total, 93 randomized clinical trials [6-8,22-111] were identified and they reported to allocate participants with primary hyperlipidemia (n = 9625) randomly to RYR preparation or no treatment (2 trials), placebo (8 trials), statins (37 trials), other lipid-lowering agents (42 trials), or to a different RYR preparation (7 trials), in which three trials had more than two arms. The 93 trials were parallel group trials, and 91 were published in Chinese and two published in English [7,49]. Three RYR preparations were tested in the included trials: The RYR dietary supplement (Cholestin), and the Chinese proprietary medicines Xuezhikang and Zhibituo. Their constituents, dosages, and treatment regimens are listed in Table 1. All trials reported lipid profile outcome and 77 trials also reported adverse effects.
Table 1.
Composition and treatment regimens of red yeast rice preparations
| Preparation | Composition | Dosage | Administration |
| Red yeast rice dietary supplement (Cholestin) capsules | Extracts of fermented Monascus purpureus rice | 2.4 g/day (containing 5 mg lovastatin) | 0.6 g/cap 2 caps, twice daily |
| Xuezhikang capsule | Extracts from fermented Monascus purpureus rice, Fructus Crataegi, Radix Salviae miltiorrhizae, Rhizoma Curcumae longae, Radix Rhizoma rhei, etc. | 1.2 g/day (containing 10 mg lovastatin) | 0.3 g/cap 2 caps, twice daily |
| Zhibituo tablet | Extracts from fermented Monascus purpureus rice, Fructus Crataegi, Rhizoma Atractylodis macrocephalae, Rhizoma Alismatis orientalis, etc. | 3.15 g/day (containing lovastatin 9 mg) | 0.35 g/tab 3 tabs, thrice daily |
Methodological quality of included trials
Of the 93 trials, only three trials reported the methods to generate the allocation sequence (random number table or permuted blocks) [7,32,70], and two trials were assessed as having adequate concealment [7,29]. Five trials applied double-blinding [7,25-27,30], and three trials blinded the outcome assessors [29,51,106]. One trial reported prior sample size estimation and information on withdrawal/dropout [7], but no trial mentioned intention-to-treat analysis. Accordingly, the included trials had generally low methodological quality. All trials provided baseline data for the comparability among groups. The average sample size of the randomized trials was 103, ranging from 28 to 450 participants per trial.
Total cholesterol (TC) levels (Tables 2 and 3)
Table 2.
Net benefit of RYR preparations in lipid profile in placebo-controlled trials
| No. of subjects | Baseline | Post-treatment | Difference | P value | ||
| Mean (SD) | Mean (SD) | Mean (95% CI) | % Change | |||
| TC levels (mmol/L) | ||||||
| Cholestin | 42 | 6.47 (0.78) | 5.43 (0.80) | -1.04 (-1.38 to -0.70) | -16% | < 0.00001 |
| Placebo | 41 | 6.59 (0.75) | 6.47 (0.93) | -0.12 (-0.49 to 0.25) | -2% | 0.52 |
| 30 | 6.68 (0.98) | 5.31 (0.84) | -1.37 (-1.83 to -0.91) | -21% | ||
| 33 | 5.81 (0.63) | 4.26 (0.63) | -1.55 (-1.85 to -1.25) | -27% | ||
| Xuezhikang | 101 | 7.30 (1.40) | 5.90 (1.40) | -1.40 (-1.79 to -1.01) | -19% | |
| 30 | 5.65 (1.31) | 3.16 (1.31) | -2.49 (-3.15 to -1.83) | -44% | ||
| Subtotal: 194 | -1.63 (-2.00 to -1.26) | < 0.00001 | ||||
| 28 | 6.72 (0.97) | 6.43 (0.93) | -0.29 (-0.79 to 0.21) | -4% | ||
| 30 | 5.84 (0.67) | 4.94 (0.67) | -0.90 (-1.24 to -0.56) | -15% | ||
| Placebo | 51 | 6.80 (1.40) | 6.70 (1.40) | -0.10 (-0.64 to 0.44) | -1% | |
| 20 | 5.55 (1.02) | 4.95 (1.02) | -0.60 (-1.23 to 0.03) | -11% | ||
| Subtotal: 129 | -0.50 (-0.90 to -0.11) | 0.01 | ||||
| 9 | 5.90 (0.95) | 4.94 (0.65) | -0.96 (-1.71 to -0.21) | -16% | ||
| Zhibituo | 30 | 6.70 (1.10) | 5.80 (0.90) | -0.90 (-1.41 to -0.39) | -13% | |
| 104 | 7.10 (1.70) | 5.60 (1.30) | -1.50 (-1.91 to -1.09) | -21% | ||
| Subtotal: 143 | -1.17 (-1.59 to -0.74) | < 0.00001 | ||||
| 9 | 5.83 (0.57) | 5.56 (0.53) | -0.27 (-0.78 to 0.24) | -5% | ||
| Placebo | 30 | 6.70 (1.50) | 6.40 (1.30) | -0.30 (-1.01 to 0.41) | -4% | |
| 101 | 6.00 (1.80) | 6.50 (0.70) | 0.50 (0.12 to 0.88) | 8% | ||
| Subtotal: 140 | 0.02 (-0.56 to 0.60) | 0.95 | ||||
| TG levels (mmol/L) | ||||||
| Cholestin | 42 | 1.50 (0.54) | 1.40 (0.50) | -0.10 (-0.32 to 0.12) | -7% | 0.38 |
| Placebo | 41 | 1.61 (0.52) | 1.65 (0.53) | 0.04 (-0.19 to 0.27) | 2% | |
| 30 | 2.84 (0.57) | 2.38 (0.62) | -0.46 (-0.76 to -0.16) | -16% | ||
| 33 | 2.10 (0.92) | 1.82 (0.92) | -0.28 (-0.72 to 0.16) | -13% | ||
| Xuezhikang | 101 | 3.60 (2.40) | 2.30 (1.60) | -1.30 (-1.86 to -0.74) | -36% | |
| 30 | 2.62 (0.58) | 1.47 (0.58) | -1.15 (-1.44 to -0.86) | -44% | ||
| Subtotal: 194 | -0.78 (-1.26 to -0.31) | 0.001 | ||||
| 28 | 2.74 (0.73) | 2.57 (0.69) | -0.17 (-0.54 to 0.20) | -6% | ||
| Placebo | 30 | 2.14 (0.94) | 1.91 (0.94) 3.00 (1.60) | -0.23 (-0.71 to 0.25) -0.30 (-0.92 to 0.32) | -11% | |
| 51 | 3.30 (1.60) | 3.00 (1.60) | -0.30 (-0.92 to 0.32) | -9% | ||
| 20 | 2.50 (0.50) | 2.11 (0.50) | -0.39 (-0.70 to -0.08) | -16% | ||
| Subtotal: 129 | -0.29 (-0.49 to -0.09) | 0.005 | ||||
| 13 | 2.46 (1.23) | 1.79 (0.57) | -0.67 (-1.41 to 0.07) | -27% | ||
| Zhibituo | 30 | 3.40 (0.90) | 2.10 (1.10) | -1.30 (-1.81 to -0.79) | -38% | |
| 104 | 3.40 (1.50) | 2.30 (1.30) | -1.10 (-1.48 to -0.72) | -32% | ||
| Subtotal: 147 | -1.10 (-1.38 to -0.82) | < 0.00001 | ||||
| 9 | 2.56 (0.88) | 2.36 (0.64) | -0.20 (-0.91 to 0.51) | -8% | ||
| 30 | 3.40 (1.10) | 3.10 (1.20) | -0.30 (-0.88 to 0.28) | -9% | ||
| Placebo | 101 | 3.10 (0.40) | 2.50 (1.40) | -0.60 (-0.88 to -0.32) | -19% | |
| Subtotal: 140 | -0.50 (-0.74 to -0.26) | < 0.0001 | ||||
| LDL-C levels (mmol/L) | ||||||
| Cholestin | 42 | 4.47 (0.70) | 3.49 (0.70) | -0.98 (-1.28 to -0.68) | -22% | < 0.00001 |
| Placebo | 41 | 4.65 (0.78) | 4.53 (0.85) | -0.12 (-0.47 to 0.23) | -3% | 0.51 |
| 30 | 3.94 (0.82) | 2.83 (0.88) | -1.11 (-1.54 to -0.68) | -28% | ||
| 33 | 4.13 (0.52) | 2.80 (0.52) | -1.33 (-1.58 to -1.08) | -32% | ||
| Xuezhikang | 101 | 4.80 (1.60) | 3.50 (1.40) | -1.30 (-1.71 to -0.89) | -27% | |
| 30 | 3.00 (1.03) | 2.10 (1.03) | -0.90 (-1.42 to -0.38) | -30% | ||
| Subtotal: 194 | -1.23 (-1.41 to -1.05) | <0.00001 | ||||
| 28 | 4.13 (0.94) | 4.01 (0.86) | -0.12 (-0.59 to 0.35) | -3% | ||
| 30 | 4.24 (0.53) | 3.41 (0.53) | -0.83 (-1.10 to -0.56) | -20% | ||
| Placebo | 51 | 4.20 (1.60) | 4.30 (1.60) | 0.10 (-0.52 to 0.72) | 2% | |
| 20 | 3.19 (0.87) | 2.70 (0.87) | -0.49 (-1.03 to 0.05) | -15% | ||
| Subtotal: 129 | -0.38 (-0.83 to 0.07) | 0.10 | ||||
| Zhibituo | 104 | 3.70 (1.60) | 3.50 (1.40) | -0.20 (-0.61 to 0.21) | -5% | 0.34 |
| Placebo | 101 | 3.70 (1.20) | 3.70 (1.00) | 0.00 (-0.30 to 0.30) | 0% | 1.00 |
| HDL-C levels (mmol/L) | ||||||
| Cholestin | 42 | 1.29 (0.34) | 1.29 (0.36) | 0.00 (-0.15 to 0.15) | 0% | 1.00 |
| Placebo | 41 | 1.19 (0.26) | 1.19 (0.28) | 0.00 (-0.12 to 0.12) | 0% | 1.00 |
| 30 | 1.50 (0.46) | 1.67 (0.54) | 0.17 (-0.08 to 0.42) | 11% | ||
| 33 | 1.37 (0.21) | 1.16 (0.21) | -0.21 (-0.31 to -0.11) | -15% | ||
| Xuezhikang | 101 | 1.20 (0.40) | 1.40 (0.30) | 0.20 (0.10 to 0.30) | 17% | |
| 30 | 1.20 (0.21) | 1.22 (0.21) | 0.02 (-0.09 to 0.13) | 2% | ||
| Subtotal: 194 | 0.04 (-0.17 to 0.24) | 0.73 | ||||
| 28 | 1.57 (0.69) | 1.53 (0.32) | -0.04 (-0.32 to 0.24) | -3% | ||
| 30 | 1.36 (0.24) | 1.13 (0.24) | -0.23 (-0.35 to -0.11) | -17% | ||
| 51 | 1.30 (0.40) | 1.30 (0.30) | 0.00 (-0.14 to 0.14) | 0% | ||
| Placebo | 20 | 1.22 (0.25) | 1.00 (0.25) | -0.22 (-0.37 to -0.07) | -18% | |
| Subtotal: 129 | -0.13 (-0.26 to -0.01) | 0.04 | ||||
| 13 | 0.89 (0.41) | 1.09 (0.41) | 0.20 (-0.12 to 0.52) | 22% | ||
| 30 | 1.08 (0.11) | 1.31 (0.17) | 0.23 (0.16 to 0.30) | 21% | ||
| Zhibituo | 104 | 0.85 (0.14) | 0.98 (0.26) | 0.13 (0.07 to 0.19) | 15% | |
| Subtotal: 147 | 0.18 (0.10 to 0.26) | < 0.0001 | ||||
| 13 | 0.92 (0.19) | 0.90 (0.17) | -0.02 (-0.16 to 0.12) | -2% | ||
| 30 | 0.99 (0.20) | 1.02 (0.28) | 0.03 (-0.09 to 0.15) | 3% | ||
| Placebo | 101 | 0.89 (0.13) | 0.80 (0.30) | -0.09 (-0.15 to -0.03) | -10% | |
| Subtotal: 144 | -0.04 (-0.12 to 0.03) | 0.26 | ||||
Table 3.
Post-treatment total cholesterol levels (mmol/L) in randomized controlled trials
| Interventions | No. of trials [references] | No. of participants | Weighted mean difference (95% confidence interval) | P value |
| RYR vs. no intervention/placebo | ||||
| Zhibituo vs. no intervention | 2 [22, 23] | 112 | -1.27 (-1.50 to -1.05) | < 0.00001 |
| RYR supplement vs. placebo | 1 [7] | 83 | -1.04 (-1.41 to -0.67) | < 0.00001 |
| Xuezhikang vs. placebo | 4 [8, 24–26] | 323 | -1.04 (-1.46 to -0.62)* | < 0.00001 |
| Zhibituo vs. placebo | 3 [27–29] | 283 | -0.80 (-1.03 to -0.57) | < 0.00001 |
| RYR vs. statins | ||||
| Xuezhikang vs. simvastatin | 15 [6, 30–43] | 1455 | 0.05 (-0.27 to 0.37)* | 0.76 |
| Xuezhikang vs. pravastatin | 7 [44–50] | 594 | - 0.20 (- 0.47 to 0.06)* | 0.14 |
| Xuezhikang vs. lovastatin | 3 [51–53] | 174 | -0.05 (-0.27 to 0.18) | 0.69 |
| Xuezhikang vs. atorvastatin | 1 [54] | 60 | -0.16 (-0.58 to 0.26) | 0.46 |
| Xuezhikang vs. fluvastatin | 1 [55] | 118 | 0.48 (0.24 to 0.72) | 0.0001 |
| Zhibituo vs. simvastatin | 9 [32, 56–63] | 728 | 0.11 (-0.03 to 0.25) | 0.14 |
| Zhibituo vs. provastatin | 1 [22] | 62 | 0.05 (-0.20 to 0.30) | 0.70 |
| Zhibituo vs. lovastatin | 1 [57] | 45 | -0.11 (-0.48 to 0.26) | 0.56 |
| RYR vs. non-statin drugs | ||||
| Xuezhikang vs. inositol nicotinate | 7 [65–71] | 624 | -0.56 (-0.81 to -0.31)* | < 0.0001 |
| Xuezhikang vs. fenofibrate | 5 [32, 73–76] | 337 | -0.13 (-0.46 to 0.20)* | 0.44 |
| Xuezhikang vs. gemfibrozil | 3 [77–79] | 156 | -0.43 (-1.52 to 0.65)* | 0.43 |
| Xuezhikang vs. fish oils | 2 [80, 81] | 116 | -0.81 (-1.11 to -0.50) | < 0.00001 |
| Xuezhikang vs. alginic sodium diester | 1 [84] | 60 | -1.08 (-1.38 to -0.78) | < 0.00001 |
| Xuezhikang vs. conjugated estrogens | 1 [85] | 44 | -0.87 (-1.20 to -0.54) | < 0.00001 |
| Xuezhikang vs. elastase | 1 [86] | 107 | -0.10 (-0.49 to 0.29) | 0.61 |
| Xuezhikang vs. biphenalbid | 1 [87] | 64 | 0.12 (-0.31 to 0.55) | 0.59 |
| Zhibituo vs. inositol nicotinate | 8 [88–95] | 608 | -0.73 (-1.13 to -0.33)* | 0.0004 |
| Zhibituo vs. fish oils | 6 [97–102] | 491 | -0.76 (-1.04 to -0.49)* | < 0.00001 |
| Zhibituo vs. fenofibrate | 2 [32, 104] | 248 | 0.31 (0.04 to 0.59) | 0.02 |
| RYR versus RYR | ||||
| Xuezhikang vs. Zhibituo | 7 [32, 106–111] | 701 | -0.03 (-0.25 to 0.20)* | 0.82 |
* Random effects model
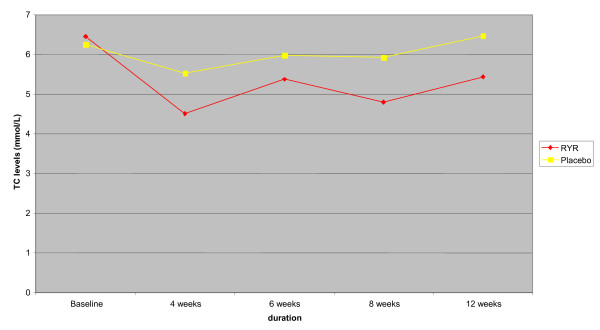
The three RYR preparations significantly reduced serum TC levels and the effect was reached at four weeks after the treatment and remained stable until 12 weeks (Figure 1). The percentage of TC level reduction was 16% for cholestin, 19%–44% for Xuezhikang, and 13%–21% for Zhibituo (Table 2). Compared with no treatment, Zhibituo showed a reduction of serum TC levels (WMD -1.27 mmol/L; 95% CI -1.50 to -1.05; 2 trials, n = 112) [22,23]. Compared with placebo, significant reduction of serum TC levels was found for Cholestin (WMD -1.04 mmol/L; 95% CI -1.41 to -0.67; 1 trial, n = 83) [7], Xuezhikang (WMD -1.04 mmol/L; 95% CI -1.46 to -0.62; 4 trials, n = 323) [8,24-26], and Zhibituo (WMD -0.80 mmol/L; 95% CI -1.03 to -0.57; 3 trials, n = 283) [27-29]. There was no significant heterogeneity among the trials (I2 statistic test) (Table 3). Different treatment duration showed similar effect of RYR preparations in reducing TC levels compared with placebo by 4 weeks (WMD -0.96 mmol/L; 95% CI -1.49 to -0.43; 2 trials, n = 113) [25,26], 6 weeks (WMD -0.61; 95% CI -1.0 to -0.22; 2 trials, n = 78) [27,28], 8 weeks (WMD -1.06; 95% CI -1.39 to -0.73; 5 trials, n = 406) [7,8,24-26], and 12 weeks (WMD -1.04; 95% CI -1.41 to -0.67; 1 trial, n = 83) [7].
Figure 1.
Total cholesterol levels during the treatment in 8 randomized placebo-controlled trials.
Xuezhikang and Zhibituo were compared with simvastatin, pravastatin, lovastatin, atorvastatin, or fluvastatin in 37 trials. There was no statistically significant difference in TC levels between RYR preparation and statins except for one trial, in which Xuezhikang was less effective than fluvastatin (WMD 0.48 mmol/L; 95% CI 0.24 to 0.72; n = 118) [55]. One trial presented data as number of subjects with at least 10% reduction of TC levels, and it showed no difference between Xuezhikang and lovastatin (64/69 vs. 68/76; RR 1.04; 95% CI 0.94 to 1.15) [64]. In these trials, Xuezhikang was used at dosage of 1.2 g/day (containing 10 mg of lovastatin), Zhibituo at 3.15 g/day (containing 9 mg of lovastatin), simvastatin at 10–20 mg/day, pravastatin at 10 mg/day, lovastatin at 20 mg/day, atorvastatin 10 mg/day, and fluvastatin 20 mg/day.
Compared with non-statin lipid-lowering agents, Xuezhikang was more effective in lowering TC levels than inositol nicotinate (WMD -0.56 mmol/L; 95% CI -0.81 to -0.31; 7 trials, n = 624) [65-71], fish oils (WMD -0.81 mmol/L; 95% CI -1.11 to -0.50; 2 trials, n = 116) [80,81]], alginic sodium diester (WMD -1.08 mmol/L; 95% CI -1.38 to -0.78; 1 trial, n = 60) [84], and conjugated estrogens (WMD -0.87 mmol/L; 95% CI -1.20 to -0.54; 1 trial, n = 44) in postmenopausal women[85]. More participants had 10% reduction of TC levels after treatment of Xuezhikang against inositol nicotinate (16/18 vs. 7/17; RR 2.16; 95% CI 1.20 to 3.90) [72]. Xuezhikang was better than fish oils in terms of more participants with 10% reduction of TC levels (WMD 1.36; 95% CI 1.14 to 1.63; 2 trials, n = 146) [82,83]. No significant difference was found between Xuezhikang and fenofibrate or gemfibrozil, Xuezhikang and elastase or biphenalbid in lowering TC levels. However, Zhibituo was less effective than fenofibrate (WMD 0.31 mmol/L; 95% CI 0.04 to 0.59; 2 trials, n = 248) [32,104], but more effective than inositol nicotinate (WMD -0.73 mmol/L; 95% CI -1.13 to -0.33; 8 trials, n = 608) [88-95], and fish oils (WMD -0.76 mmol/L; 95% CI -1.04 to -0.49; 6 trials, n = 491) [97-102] by random effect model due to significant heterogeneity (Table 3). More participants treated by Zhibituo had 10% reduction of TC levels compared with those treated by inositol nicotinate (23/30 vs. 13/30; RR 1.77; 95% CI 1.12 to 2.79) [96] or those treated by fish oils (18/25 vs. 7/25; RR 2.57; 95% CI 1.31 to 5.05) [103]. Zhibituo appeared superior to alginic sodium diester for the number of participants with 10% reduction of TC levels (105/121 vs. 67/89; RR 1.15; 95% CI 1.00 to 1.32) [105].
Xuezhikang did not differ from Zhibituo in TC-lowering effect (WMD -0.03 mmol/L; 95% CI -0.25 to 0.20; 7 trials, n = 701) [32,106-111] (Table 3).
Triglycerides (TG) levels (Tables 2 and 4)
Table 4.
Post-treatment triglycerides levels (mmol/L) in randomized controlled trials
| Interventions | No. of trials [references] | No. of participants | Weighted mean difference (95% confidence interval) | P value |
| RYR vs. no intervention/placebo | ||||
| Zhibituo vs. no intervention | 2 [22, 23] | 112 | -0.54 (-0.77 to -0.32) | < 0.00001 |
| RYR supplement vs. placebo | 1 [7] | 83 | -0.25 (-0.47 to -0.03) | 0.03 |
| Xuezhikang vs. placebo | 4 [8, 24–26] | 323 | -0.40 (-0.70 to -0.10)* | 0.008 |
| Zhibituo vs. placebo | 3 [27–29] | 291 | -0.55 (-0.99 to -0.10) | 0.02 |
| RYR vs. statins | ||||
| Xuezhikang vs. simvastatin | 14 [6, 30–42] | 1251 | -0.08 (-0.25 to 0.10)* | 0.39 |
| Xuezhikang vs. pravastatin | 7 [44–50] | 592 | 0.04 (- 0.29 to 0.38)* | 0.79 |
| Xuezhikang vs. lovastatin | 3 [51–53] | 168 | -0.07 (-0.16 to 0.01) | 0.09 |
| Xuezhikang vs. atorvastatin | 1 [54] | 60 | -0.02 (-0.16 to 0.12) | 0.78 |
| Xuezhikang vs. fluvastatin | 1 [55] | 118 | 0.09 (-0.1 4 to 0.32) | 0.44 |
| Zhibituo vs. simvastatin | 9 [32, 56–63] | 732 | 0.05 (-0.17 to 0.26)* | 0.67 |
| Zhibituo vs. provastatin | 1 [22] | 62 | -0.02 (-0.19 to 0.15) | 0.81 |
| Zhibituo vs. lovastatin | 1 [57] | 45 | -0.21 (- 0.61 to 0.19) | 0.30 |
| RYR vs. non-statin drugs | ||||
| Xuezhikang vs. inositol nicotinate | 7 [65–71] | 636 | -0.06 (-0.20 to 0.08) | 0.38 |
| Xuezhikang vs. fenofibrate | 5 [32, 73–76] | 337 | 0.42 (-0.17 to 1.01)* | 0.16 |
| Xuezhikang vs. gemfibrozil | 3 [77–79] | 160 | 0.41 (0.30 to 0.51) | < 0.00001 |
| Xuezhikang vs. fish oils | 2 [80, 81] | 112 | -0.71 (-0.97 to -0.44) | < 0.00001 |
| Xuezhikang vs. alginic sodium diester | 1 [84] | 60 | 0.04 (-0.21 to 0.29) | 0.75 |
| Xuezhikang vs.conjugated estrogens | 1 [85] | 44 | -0.82 (-1.31 to -0.33) | 0.001 |
| Xuezhikang vs. elastase | 1 [86] | 107 | 0.00 (-0.31 to 0.31) | 1.00 |
| Xuezhikang vs. biphenalbid | 1 [87] | 64 | -0.43 (-0.8 1 to -0.05) | 0.03 |
| Zhibituo vs. inositol nicotinate | 7 [88–93, 95] | 598 | -0.39 (-0.62 to -0.16)* | 0.0008 |
| Zhibituo vs. fish oils | 5 [97–100, 102] | 394 | -0.12 (-0.29 to 0.05) | 0.17 |
| Zhibituo vs. fenofibrate | 2 [32, 104] | 248 | 0.33 (-0.12 to 0.78) | 0.15 |
| RYR vs. RTR | ||||
| Xuezhikang vs. Zhibituo | 7 [32, 106–111] | 727 | 0.05 (-0.17 to 0.27)* | 0.66 |
* Random effects model
There was a 13%–44% reduction of TG levels after treatment by Xuezhikang, 27%–38% by Zhibituo, and 7% by Cholestin. Compared with no treatment, Zhibituo showed a significant effect on reducing TG levels (WMD -0.54 mmol/L; 95% CI -0.77 to -0.32; 2 trials, n = 112) [22,23]. Compared with placebo, all three RYR preparations significantly reduced TG levels (Cholestin: WMD -0.25 mmol/L; 95% CI -0.47 to -0.03; 1 trial, n = 83 [7]; Xuezhikang: WMD -0.40 mmol/L; 95% CI -0.70 to -0.10; 4 trials, n = 323 [8,24-26]; and Zhibituo: WMD -0.55 mmol/L; 95% CI -0.99 to -0.10; 3 trials, n = 283) [27-29] (Table 4). Different treatment duration showed similar effect of RYR preparations in reducing TG levels compared with placebo by 4 weeks (WMD -0.32 mmol/L; 95% CI -0.58 to -0.07; 2 trials, n = 113) [25,26], 6 weeks (WMD -0.74; 95% CI -1.10 to -0.37; 2 trials, n = 86) [27,28], 8 weeks (WMD -0.35; 95% CI -0.5 to -0.21; 5 trials, n = 406) [7,8,24-26], and 12 weeks (WMD -0.25; 95% CI -0.47 to -0.03; 1 trial, n = 83) [7].
There was no statistically significant difference in TG levels after treatment between Xuezhikang or Zhibituo and simvastatin, pravastatin, lovastatin, atorvastatin, or fluvastatin. One trial presented data as number of subjects with at least 20% reduction of TG levels, and it showed no difference between Xuezhikang and lovastatin (47/60 vs. 59/77; RR 1.02; 95% CI 0.85 to 1.23) [64].
Compared with non-statin lipid lowering agents, there was no significant difference between Xuezhikang and inositol nicotinate, fenofibrate, alginic sodium diester, or elastase for TG levels. There was no difference between Xuezhikang and inositol nicotinate in number of participants with over 20% reduction of TG levels (11/16 vs. 10/16; RR 1.10; 95% CI 0.67 to 1.82) [72], and between Xuezhikang and fish oils (58/78 vs. 44/70; RR 1.15; 95% CI 0.8 to 1.64) [82,83]. However, Xuezhikang was less effective than gemfibrozil (WMD 0.41 mmol/L; 95% CI 0.30 to 0.51; 3 trials, n = 160) [77-79], but better than fish oils (WMD -0.71 mmol/L; 95% CI -0.97 to -0.44; 2 trials, n = 112) [80,81], conjugated estrogens (WMD -0.82 mmol/L; 95% CI -1.31 to -0.33; 1 trial, n = 44) [85] in postmenopausal women, and biphenalbid (WMD -0.43 mmol/L; 95% CI -0.81 to -0.05; 1 trial, n = 64) [87]. Zhibituo showed a significant better TG-lowering effect (WMD -0.39 mmol/L; 95% CI -0.62 to -0.16; 7 trials, n = 598) [88-93,95] compared with inositol nicotinate. However, there was no significant difference between Zhibituo and inositol nicotinate in the number of participants with over 20% reduction of TG levels (9/30 vs. 4/30) in one trial [96]. Zhibituo did not differ from fish oils (WMD -0.12 mmol/L; 95% CI -0.29 to 0.05; 5 trials, n = 394) [97-100,102] or fenofibrate (WMD 0.33 mmol/L; 95% CI -0.12 to 0.78; 2 trials, n = 248) [32,104] (Table 4). In a small trial more participants appeared to have a 20% reduction of TG levels by Zhibituo than by fish oils (20/23 vs. 9/19; RR 1.84; 95% CI 1.11 to 3.03) [103]. There was a marginal effect of Zhibituo compared with alginic sodium diester for the number of participants with over 20% reduction of TG levels (69/121 vs. 38/89; RR 1.34; 95% CI 1.00 to 1.78) [105].
There was no significant difference between Xuezhikang and Zhibituo in reducing TG levels (WMD 0.05 mmol/L; 95% CI -0.17 to 0.27; 7 trials, n = 727) (Table 4).
Low density lipoprotein cholesterol (LDL-C) levels (Tables 2 and 5)
Table 5.
Post-treatment low-density lipoprotein cholesterol levels (mmol/L) in randomized controlled trials
| Interventions | No. of trials [references] | No. of participants | Weighted mean difference (95% confidence interval) | P value |
| RYR vs. no intervention/placebo | ||||
| Zhibituo vs. no intervention | 1 [22] | 62 | -0.16 (-0.71 to 0.39) | 0.57 |
| RYR supplement vs. placebo | 1 [7] | 83 | -1.04 (-1.38 to -0.70) | < 0.00001 |
| Xuezhikang vs. placebo | 4 [8, 24–26] | 323 | -0.74 (-0.93 to -0.55) | < 0.00001 |
| Zhibituo vs. placebo | 1 [29] | 205 | -0.20 (-0.53 to 0.13) | 0.24 |
| RYR vs. statins | ||||
| Xuezhikang vs. simvastatin | 13 [6, 30–34, 36–38, 40–43] | 1238 | 0.14 (-0.05 to 0.33)* | 0.14 |
| Xuezhikang vs. pravastatin | 7 [44–50] | 587 | -0.09 (- 0.20 to 0.02)* | 0.11 |
| Xuezhikang vs. lovastatin | 3 [51–53] | 191 | 0.00 (- 0.26 to 0.27) | 0.98 |
| Xuezhikang vs. atorvastatin | 1 [54] | 60 | 0.20 (-0.10 to 0.50) | 0.19 |
| Xuezhikang vs. fluvastatin | 1 [55] | 118 | 0.14 (-0.10 to 0.38) | 0.26 |
| Zhibituo vs. simvastatin | 8 [32, 56–62] | 601 | 0.22 (0.04 to 0.39) | 0.02 |
| Zhibituo vs. provastatin | 1 [22] | 62 | -0.11 (-0.60 to 0.38) | 0.66 |
| Zhibituo vs. lovastatin | 1 [57] | 45 | 0.03 (-0.30 to 0.36) | 0.86 |
| RYR vs. non-statin drugs | ||||
| Xuezhikang vs. inositol nicotinate | 4 [66–68, 70] | 299 | -0.63 (-0.96 to -0.30) | 0.0002 |
| Xuezhikang vs. fenofibrate | 3 [32, 74, 76] | 220 | -0.10 (-1.05 to 0.85)* | 0.84 |
| Xuezhikang vs. gemfibrozil | 3 [77–79] | 152 | -0.34 (-0.58 to -0.10) | 0.005 |
| Xuezhikang vs. fish oils | 1 [81] | 95 | -0.89 (-1.41 to -0.37) | 0.0008 |
| Xuezhikang vs. conjugated estrogens | 1 [85] | 44 | -0.10 (-0.43 to 0.23) | 0.55 |
| Xuezhikang vs. biphenalbid | 1 [87] | 64 | -0.06 (-0.32 to 0.20) | 0.65 |
| Zhibituo vs. fish oils | 5 [97–101] | 489 | -0.57 (-0.70 to -0.45) | < 0.00001 |
| Zhibituo vs. fenofibrate | 1 [32] | 90 | 0.3 1 (0.04 to 0.58) | 0.02 |
| RYR vs. RYR | ||||
| Xuezhikang vs. Zhibituo | 5 [32, 106, 108, 109, 111] | 628 | -0.08 (-0.18 to 0.02) | 0.12 |
* Random effects model
There was a 22% reduction of LDL-C levels by Cholestin and 27%–32% by Xuezhikang, but 5% by Zhibituo. Zhibituo appeared to have no effect on reducing LDL-C levels compared with no treatment [22] or placebo [28]. The relative benefit of reducing LDL-C levels by Cholestin against placebo was WMD -1.04 mmol/L (95% CI -1.38 to -0.70; 1 trial, n = 83) [7], and by Xuezhikang against placebo (WMD) -0.74 mmol/L; 95% CI -0.93 to -0.55; 4 trials, n = 323) [8,23-25] (Table 5). Different treatment duration showed similar effect of RYR preparations in reducing LDL-C levels compared with placebo by 4 weeks (WMD -0.77 mmol/L; 95% CI -1.0 to -0.54; 2 trials, n = 113) [25,26], 8 weeks (WMD -0.87; 95% CI -1.15 to -0.60; 5 trials, n = 406) [7,8,24-26], and 12 weeks (WMD -1.04; 95% CI -1.38 to -0.70; 1 trial, n = 83) [7].
Xuezhikang did not differ from simvastatin, pravastatin, lovastatin, atorvastatin or fluvastatin for post-treatment LDL-C levels. Zhibituo appeared to have the same effect as pravastatin or lovastatin, but was less effective than simvastatin (WMD 0.22 mmol/L; 95% CI 0.04 to 0.39; 8 trials, n = 601) [32,56-62]. Compared with non-statin lipid-lowering agents, Xuezhikang was similar to fenofibrate, conjugated estrogens or biphenalbid, but significantly better in reducing LDL-C levels than inositol nicotinate (WMD -0.63 mmol/L; 95% CI -0.96 to -0.30; 4 trials, n = 299) [66-68,70], gemfibrozil (WMD -0.34 mmol/L; 95% CI -0.58 to -0.10; 3 trials, n = 152) [77-79], and fish oils (WMD -0.89 mmol/L; 95% CI -1.41 to -0.37, 1 trial, n = 95) [81]. Zhibituo was better than fish oils in reducing LDL-C levels (WMD -0.57 mmol/L; 95% CI -0.70 to -0.45; 5 trials, n = 489) [97-101], but less effective than fenofibrate (WMD 0.31 mmol/L; 95% CI 0.04 to 0.58; 1 trial, n = 90) [32] (Table 5).
No significant difference was found between Xuezhikang and Zhibituo in LDL-C levels (-0.08 mmol/L;-0.18 to 0.02; 5 trials, n = 628) [32,106,108,109,111] (Table 5).
High density lipoprotein cholesterol (HDL-C) levels (Tables 2 and 6)
Table 6.
Post-treatment high-density lipoprotein cholesterol levels (mmol/L) in randomized controlled trials
| Interventions | No. of trials [references] | No. of participants | Weighted mean difference its (95% confidence interval) | P value |
| RYR vs. no intervention/placebo | ||||
| Zhibituo vs. no intervention | 1 [22] | 62 | 0.21 (0.04 to 0.38) | 0.02 |
| RYR supplement vs. placebo | 1 [7] | 83 | 0.10 (-0.04 to 0.24) | 0.16 |
| Xuezhikang vs. placebo | 4 [8, 24–26] | 323 | 0.11 (0.05 to 0.17) | 0.0008 |
| Zhibituo vs. placebo | 3 [27–29] | 291 | 0.21 (0.15 to 0.27) | < 0.00001 |
| RYR vs. statins | ||||
| Xuezhikang vs. simvastatin | 14 [6, 30–34, 36–43] | 1277 | 0.06 (-0.11 to 0.22)* | 0.49 |
| Xuezhikang vs. pravastatin | 7 [44–50] | 587 | -0.01 (-0.06 to 0.03) | 0.51 |
| Xuezhikang vs. lovastatin | 3 [51–53] | 152 | 0.06 (0.00 to 0.11) | 0.05 |
| Xuezhikang vs. atorvastatin | 1 [54] | 60 | 0.01 (-0.17 to 0.19) | 0.91 |
| Xuezhikang vs. fluvastatin | 1 [55] | 118 | -0.02 (-0.1 0 to 0.06) | 0.62 |
| Zhibituo vs. simvastatin | 9 [32, 56–63] | 666 | -0.07 (-0.12 to -0.03) | 0.0009 |
| Zhibituo vs. provastatin | 1 [22] | 62 | -0.02 (-0.22 to 0.18) | 0.85 |
| Zhibituo vs. lovastatin | 1 [57] | 45 | -0.07 (-0.23 to 0.09) | 0.39 |
| RYR vs. non-statin drugs | ||||
| Xuezhikang vs. inositol nicotinate | 7 [65–71] | 608 | 0.17 (0.06 to 0.28)* | 0.002 |
| Xuezhikang vs. fenofibrate | 4 [32, 73, 74, 76] | 257 | 0.03 (-0.06 to 0.13) | 0.49 |
| Xuezhikang vs. gemfibrozil | 2 [77, 79] | 108 | -0.03 (-0.3 5 to 0.28) | 0.83 |
| Xuezhikang vs. fish oils | 2 [80, 81] | 70 | 0.17 (0.09 to 0.25) | < 0.0001 |
| Xuezhikang vs. alginic sodium diester | 1 [84] | 60 | 0.86 (0.75 to 0.97) | < 0.00001 |
| Xuezhikang vs. conjugated estrogens | 1 [85] | 44 | 0.00 (-0.09 to 0.09) | 1.00 |
| Xuezhikang vs. elastase | 1 [86] | 107 | 0.20 (0.10 to 0.30) | < 0.0001 |
| Xuezhikang vs. biphenalbid | 1 [87] | 64 | 0.25 (0.11 to 0.39) | 0.0003 |
| Zhibituo vs. inositol nicotinate | 6 [89–91, 93–95] | 422 | 0.18 (0.09 to 0.27)* | < 0.0001 |
| Zhibituo vs. fish oils | 6 [97–102] | 400 | 0.14 (0.06 to 0.23)* | 0.001 |
| Zhibituo vs. fenofibrate | 2 [32, 104] | 248 | -0.13 (-0.37 to 0.11) | 0.28 |
| RYR vs. RYR | ||||
| Xuezhikang vs. Zhibituo | 7 [32, 106–111] | 627 | 0.04 (-0.02 to 0.11)* | 0.20 |
* Random effects model
There was an increase of HDL-C levels between 15% and 22% by Zhibituo. However, the findings for Xuezhikang were not consistent ranging from a 2% to 17% increase and 15% decrease in four trials. Cholestin did not change the HDL-C levels after the treatment [7]. A beneficial effect of increasing HDL-C levels was shown when Xuezhikang was compared with placebo (WMD 0.11 mmol/L; 95%CI 0.05 to 0.17; 4 trials, n = 323) [8,24-26], and when Zhibituo was compared with no treatment (WMD 0.21 mmol/L; 95% CI 0.04 to 0.38; 1 trial, n = 62) [22] and with placebo (WMD 0.21 mmol/L; 95% CI 0.15 to 0.27; 3 trials, n = 291) [27-29] (Table 6). Different treatment durations showed that of RYR preparations increased HDL-C levels compared with placebo by 6 weeks (WMD 0.27; 95% CI 0.17 to 0.38; 2 trials, n = 86) [27,28] and 8 weeks (WMD 0.11; 95% CI 0.05 to 0.16; 5 trials, n = 406) [7,8,24-26]. There was no significant difference between RYR and placebo at 4 weeks and at 12 weeks for HDL-C levels [7,25,26].
Compared with statins, Xuezhikang appeared better than lovastatin in raising HDL-C levels (WMD 0.06 mmol/L; 95% CI 0.00 to 0.11; 3 trials, n = 152) [51,53]. Zhibituo was inferior to simvastatin (WMD -0.07 mmol/L; 95% CI -0.12 to -0.03; 9 trials, n = 666) [32,56-63]. There was no significant difference among other comparisons of RYR preparations and statins. Compared with non-statins, Xuezhikang was superior to inositol nicotinate (WMD 0.17 mmol/L; 95% CI 0.06 to 0.28; 7 trials, n = 608) by random effects model [65-71], fish oils (WMD 0.17 mmol/L; 95% CI 0.09 to 0.25; 2 trials, n = 70) [80,81], alginic sodium diester (WMD 0.86 mmol/L; 95% CI 0.75 to 0.97; 1 trial, n = 60) [84], elastase (WMD 0.20 mmol/L; 95% CI 0.10 to 0.30; 1 trial, n = 107) [86], and to biphenalbid (WMD 0.25 mmol/L; 95% CI 0.11 to 0.39; 1 trial, n = 64) [87]. There was no significant difference between Xuezhikang and fenofibrate, gemfibrozil, or estrogens in affecting HDL-C levels. Zhibituo was superior to inositol nicotinate (WMD 0.18 mmol/L; 95% CI 0.09 to 0.27; 6 trials, n = 422) [89-91,93-95] and to fish oils (WMD 0.14 mmol/L; 95% CI 0.06 to 0.23; 6 trials, n = 400) [97-102] both in random effects model. There was no significant difference between Zhibituo and fenofibrate (WMD -0.13 mmol/L; 95% CI -0.37 to 0.11; 2 trials, n = 248) [32,104].
No significant difference was found between Xuezhikang and Zhibituo in affecting HDL-C levels (WMD 0.04 mmol/L; 95% CI -0.02 to 0.11; 7 trials, n = 627) [32,106-111] (Table 6).
Adverse effects
Seventy-seven trials reported outcomes of adverse effects, and the incidence rate ranged from 1.3% to 36%. The most commonly reported adverse effects were dizziness, low appetite, nausea, stomach-ache, abdominal distension, and diarrhoea. A small proportion of participants suffered from increased serum BUN and ALT levels. The trials did not report serious adverse events.
Cost-effectiveness
One trial evaluated cost-effectiveness of Xuezhikang vs. pravastatin for treatment of hypercholesterolemia [49]. For a reduction of 1 mmol/L TC level, the cost of Xuezhikang and pravastatin was 57 USD and 78 USD respectively. For a reduction of 1 mmol/L TG level, the cost of Xuezhikang and pravastatin was 242 USD and 820 USD respectively; and for a reduction of 1 mmol/L LDL-C level, the cost was 59 USD and 84 USD respectively.
Discussion
Based on this review and meta-analysis, three different kinds of RYR preparations tested by in randomized trials demonstrate beneficial effects on reducing TC, TG, and LDL-C levels, and on increasing HDL-C levels in individuals with hyperlipidemia. The treatment duration of RYR ranged from 4 to 24 weeks (median of 8 weeks), and the lipid modification effects have been shown at four weeks of the treatment, and the effects remained at 24 weeks of the treatment. Long-term follow-up effects after the treatment have not been reported by the trials. The use of RYR preparations seems safe and well tolerated.
Before accepting the findings of this review to form a basis for clinical practice, we need to consider the following weaknesses in this review. First, the randomized trials in this review had several methodological flaws in terms of insufficient reporting of generation methods of the allocation sequence, allocation concealment, and double blinding. The trials provided limited descriptions of study design, and most trials stated only that patients were randomly assigned; thus the information does not allow a judgement of whether or not it was conducted properly. We therefore state that the differences between RYR preparation and control drugs may be associated with the methodologically less rigorous trials [16-19]. The sample size for trials comparing RYR with statins or other established treatments was not justified and we do not know if the trials were designed as 'equivalence trials'. The limited number of trials with adequate quality prohibits us from performing meaningful sensitivity analyses to illuminate robustness of the results in the review.
Second, Vickers and colleagues [112] found that some countries, including China, publish unusually high proportions of positive results, for which publication bias is a possible explanation. All identified studies for this systematic review originated from China except one trial conducted in the USA and published in an international peer-reviewed journal [7]. Inability to identify unpublished eligible trials from the searching, trials with small samples and positive findings may raise the issue of publication bias.
There are some variations in RYR preparations and treatment regimens including composition, dosage and duration. Cholestin is an extract from RYR containing a special strain of yeast which produces monacolin K (lovastatin) [7]. Xuezhikang and Zhibituo are two Chinese proprietary medicines that contain other herbs in addition to RYR as main components. In some trials, placebo effects are substantial compared with baseline as demonstrated in trials of Xuezhikang where placebo treatment achieved 1% to 15% reduction of TC levels, and 6% to 16% reduction of TG levels (Table 2). Therefore, in non-placebo-controlled and non-double blind trials, placebo effects may add to the complexity of interpreting the present findings of the overall beneficial effects, and the interpretation should be taken with caution.
Given the generally low methodological quality of the randomized trials and potential publication bias, we suggest further rigorously designed trials are still needed before RYR preparation could be recommended for clinical use or as an alternative treatment to statins. The currently ongoing placebo-controlled trials in Europe may provide useful information [21]. In addition to anti-hyperlipidemic effects of RYR preparations, cost-effectiveness and safety should be further investigated in future trials [113].
Conclusion
Current evidence from randomized trials shows short-term beneficial effects of RYR preparations on lipid modification. More rigorous trials are needed, and long-term effects and safety should be investigated if RYR preparations are to be recommended as one of the alternative treatments for primary hyperlipidemia.
Competing interests
The author(s) declare that they have no competing interests.
Authors' contributions
JL conceived, designed, drafted the review, and performed study selection, data extraction, analyses, and interpretation. JZ did the literature search, study selection, and cross-checked the data extraction; YS developed the search strategy, performed electronic searches and retrieved articles; SG, TA, and VF provided methodological perspectives, and revised the review. All authors contributed to the writing of the review.
Acknowledgments
Acknowledgements
We thank Dr Antonio Bianchi for providing information on ongoing study on RYR preparation 'Lipolysar', and Beijing Weixin for unpublished study on Xuezhikang, and Pharmalogica in Norway for 'Hypocol'.
Contributor Information
Jianping Liu, Email: Jianping.liu@fagmed.uit.no.
Jing Zhang, Email: n@fagmed.uit.no.
Yi Shi, Email: a@fagmed.uit.no.
Sameline Grimsgaard, Email: b@fagmed.uit.no.
Terje Alraek, Email: c@fagmed.uit.no.
Vinjar Fønnebø, Email: Vinjar.Fonnebo@fagmed.uit.no.
References
- Monascus purpureus (monograph) Altern Med Rev. 2004;9:208–210. [PubMed] [Google Scholar]
- Ma J, Li Y, Ye Q, Li J, Hua Y, Ju D, Zhang D, Cooper R, Chang M. Constituents of red yeast rice, a traditional Chinese food and medicine. J Agric Food Chem. 2000;48:5220–5. doi: 10.1021/jf000338c. [DOI] [PubMed] [Google Scholar]
- Patrick L, Uzick M. Cardiovascular disease: C-reactive protein and the inflammatory disease paradigm: HMG-CoA reductase inhibitors, alpha-tocopherol, red yeast rice, and olive oil polyphenols. A review of the literature. Altern Med Rev. 2001;6:248–71. [PubMed] [Google Scholar]
- Heber D, Lembertas A, Lu QY, Bowerman S, Go VL. An analysis of nine proprietary Chinese red yeast rice dietary supplements: implications of variability in chemical profile and contents. J Altern Complement Med. 2001;7:133–9. doi: 10.1089/107555301750164181. [DOI] [PubMed] [Google Scholar]
- Wang J, Lu Z, Chi J, Wang W, Su M, Kou W, Yu P, Yu L, Chen L, Zhu JS, Chang J. Multicenter clinical trial of the serum lipid-lowering effects of a Monascus purpureus (red yeast) rice preparation from traditional Chinese medicine. Cur Ther Res. 1997;58:964–978. doi: 10.1016/S0011-393X(97)80063-X. [DOI] [Google Scholar]
- Kou WR, Lu ZL, Guo JX, Li HY, Xue SW, Lin YZ, Wu XS, Chen H. Effect of Xuezhikang on the treatment of primary hyperlipidemia. Zhonghua Neike Zazhi. 1997;36:529–31. [PubMed] [Google Scholar]
- Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol-lowering effects of a proprietary Chinese red-yeast-rice dietary supplement. Am J Chin Nutr. 1999;69:231–6. doi: 10.1093/ajcn/69.2.231. [DOI] [PubMed] [Google Scholar]
- Shen ZW, Yu PL, Sun MZ, Chi JM, Zhou YF, Zhu XS, Yang CY, He CF. Prospective study of Xuezhikang for treatment of primary hyperlipidemia. Natl Med J China. 1996;76:156–7. [Google Scholar]
- Liu SS, Kou MK, Ding H, Li CM, He L, He L, Zhang CM, Li YF, Li ZF, Yang MJ. The clinical observation of hyperlipoproteinemia treated with Zhibituo. Chengdu Zhongyiyao Daxue Xuebao. 1996;19:12–5. [Google Scholar]
- Kreisberg RA, Oberman A. Medical management of hyperlipidemia/dyslipidemia. J Clin Endocrinol Metab. 2003;88:2445–61. doi: 10.1210/jc.2003-030388. [DOI] [PubMed] [Google Scholar]
- Moghadasian MH. A safety look at currently available statins. Expert Opin Drug Saf. 2002;1:269–74. doi: 10.1517/14740338.1.3.269. [DOI] [PubMed] [Google Scholar]
- Alcocer L. Statins for everybody? New evidence on the efficacy and safety of the inhibitors of HMG Co-A reductase. Am J Ther. 2003;10:423–8. doi: 10.1097/00045391-200311000-00008. [DOI] [PubMed] [Google Scholar]
- Bellosta S, Paoletti R, Corsini A. Safety of statins: focus on clinical pharmacokinetics and drug interactions. Circulation. 2004;109:11150–7. doi: 10.1161/01.CIR.0000131519.15067.1f. [DOI] [PubMed] [Google Scholar]
- Thompson Coon JS, Ernst E. Herbs for serum cholesterol reduction: a systematic review. J Fam Pract. 2003;52:468–78. [PubMed] [Google Scholar]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes R, Altman D. Empirical evidence of bias. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, Tugwell P, Klassen TP. Does quality of reports of randomized trials affect estimates of intervention efficacy reported in meta-analyses. Lancet. 1998;352:609–13. doi: 10.1016/S0140-6736(98)01085-X. [DOI] [PubMed] [Google Scholar]
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomized trials in meta-analyses. Ann Intern Med. 2001;135:982–9. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- Clarke M, Oxman AD. The Cochrane Library. Oxford: Update Software; 2003. Assessment of study quality. Cochrane Reviewers' Handbook 4.2.0 [updated March 2003] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Personal communication with the trial sponsors in Norway and Italy
- Tang HL. Randomized controlled study of Diao Zhibituo for lipid modulation effect. Sichuan Yixue. 2004;25:78–9. [Google Scholar]
- Zhao YL, Ouyang HB. Observation on therapeutic effects of Zhibituo for treatment of hyperlipidemia. Changchun Yixue Zazhi. 1998;11:21. [Google Scholar]
- Cheng XM, Yu ZM, Luo HD, Qiu YH, Chen MX. Effect of Xuezhikang on endothelial function in patients with hyperlipidemia. Chin J Arterioscler. 2001;9:235–7. [Google Scholar]
- Qin SC, Zhang WQ, Qi P, Zhao ML, Dong ZN, Li YC, Xu XM, Fang X, Fu L. Randomized, double blind, controlled trial for the clinical therapeutic effects of Xuezhikang in the elderly with hyperlipidemia. Zhonghua Neike Zazhi. 1998;37:401–2. [Google Scholar]
- Xiao M, Ye P. Clinical observation of Xuezhikang for treatment of hyperlipidemia. Shuli Yixue Zazhi. 2001;14:244–5. [Google Scholar]
- Huang GZ, Yang S, Wu ZG. Observation on therapuetic effect of Zhibituo for treatment of hyperlipidemia. Dier Junyi Daxue Xuebao. 1998;19:94–5. [Google Scholar]
- Peng DY. Observation on the effect of Zhibituo in treating hyperlipidemia. Zhongguo Yejin Gongye Yixue Zazhi. 1998;15:201–3. [Google Scholar]
- Xu JM, Chen SX, Hu WY, Cai NS, Xu Q, Wu ZG, Sun KX. Zhibituo vs. placebo treatment of hyperlipidemia a double blind randomized and multicenter study. Zhongguo Xinyao Yu Linchuang Zazhi. 1997;16:47–51. [Google Scholar]
- Chen FJ, Ruan Q, Qi HW, Yuan PY. Clinical observation of Xuezhikang in treating middle and old age hyperlipidemia. Shanghai Yufangyixue Zazhi. 2003;15:222–3. [Google Scholar]
- Chen LL, Liu J. Effects of Xuezhikang on hypercholesterolemia. Yiyao Daobao. 2002;21:31–2. [Google Scholar]
- Li QL, Zhang YF. Clinical observation on effect of Taizhi'an capsule in treating 150 patients with hyperlipidemia. Zhongguo Zhongxiyi Jiehe Zazhi. 2003;23:335–7. [PubMed] [Google Scholar]
- Lu GP, Huo SQ, Shen YC, Gong LS. Comparison of the effects of Xuezhikang with simvastatin on lipid profile modification in patients with hypercholesterolemia. Zhonghua Neike Zazhi. 1998;37:371–3. [Google Scholar]
- Quan SL, Wang W, Qu XW, Chen J. Controlled observation on therapeutic effects of Xuezhikang and simvastatin for treatment of hypercholesterolemia. Zhonghua Shiyong Yixue. 2003;3:427. [Google Scholar]
- Shen G, Wang J, Wang JF. Comparison of the effects of the herbal lipid regulator decoction, Xuezhikang and simvastatin on lipid modulation in the middle-aged and elderly subjects with hyperlipidemia. Zhongguo Jiceng Yiyao. 2000;7:280–1. [Google Scholar]
- Wang SH, Sun JL, Liu HQ. Therapeutic observations of Xuezhikang for treatment of 60 cases of elderly with hyperlipidemia. Anhui Linchuang Zhongyi Zazhi. 2003;15:474–5. [Google Scholar]
- Wang XL, Hu XM. Comparison of Xuezhikang and simvastatin in regulating lipids for hypercholesterolemia. Zhongxiyi Jiehe Xinnaoxueguanbing Zazhi. 2004;2:319–20. [Google Scholar]
- Xi BL, Ren JY. Effects of Xuezhikang vs. simvastatin in treating hyperlipidemia. Guowai Yixue: Xin Xueguanbing Fence. 2002;29:233–4. [Google Scholar]
- Zeng TK. Observation of the therapeutic effects of Xuezhikang for treatment of 77 elderly with hyperlipidemia. Shoudu Yiyao. 1999;6:49. [Google Scholar]
- Zhang G, Zhang KX, Xu Z, Guang XF. Comparison of lipid-lowering effects of Xuezhikang and Simvastatin for hyperlipidemia. Shoudu Yiyao. 1998;5:35–6. [Google Scholar]
- Zhao XH, Jiang XM, Ao LJ. Therapeutic observations of Xuezhikang for treatment of hyperlipidemia. Yunnan Yiyao. 1998;19:26. [Google Scholar]
- Zheng Y, Luo XZ, Wang SL, Yang YJ. Clinical controlled study on the therapeutic effects of Xuezhikang and Simvastatin. Zhongguo Yaoshi. 2001;36:715. [Google Scholar]
- Zhu QF, Jiang L, Wang Y. Effects of Xuezhikang and simvastatin on apolipoprotein B and A1 in patients with hyperlipidemia. Guangming Zhongyi. 2003;18:24–5. [Google Scholar]
- Chen L, Qin YW, Guo RB. Clinical efficacy of capsule Xuezhikang in treatment of hypercholesterolemia. Yaoxue Fuwu Yu Yanjiu. 2002;2:39–40. [Google Scholar]
- Jin W, Yang H, Zhang C, Zhang CJ, Xu YH. Therapeutic observations of Xuezhikang for treatment of primary hyperlipidemia. Zhongguo Zhongxiyi Jiehe Zazhi. 1997;17:434–5. [Google Scholar]
- Lei HZ. Effects of Xuezhikang on endothelial function in aged patients with hyperlipidemia. Guangxi Yixue. 2004;26:495–7. [Google Scholar]
- Li YS, Lei HZ, Zhu MJ. Clinical observations of 41 cases of elderly with hyperlipidemia treated with Xuezhikang. Zhongguo Quanke Yixue. 2003;6:163. [Google Scholar]
- Wang DG, Li D, Nie ZY. Application of cost-effectiveness analysis in Xuezhikang and pravastatin for the treatment hypercholesterolemia. Zhongguo Yaoshi. 2003;12:53–5. [Google Scholar]
- Xu CB, Hu DY, Kang LP, Tian YW, Gao MM, Xu ZM, Jin SY, Ma FY, Ma M, Shi XY, Zhang BH, Long NZ, Li L, Xue L, Zhang JH, Chen XL, Dai CX. Comparative study of relatively long-term therapy for dyslipidemia with low-dose Xuezhikang or pravastatin in Chinese patients. Zhongguo Yaoxue. 2000;9:218–22. [Google Scholar]
- Yu CY, Zhang C, Yang H, Jin W. Observations of therapeutic effects of Xuezhikang for treatment of primary hyperlipidemia. Heilongjiang Yixue Zazhi. 2004;17:151–2. [Google Scholar]
- Li BH, Zheng GJ, Zhang WG, Xu M, Ren P. Clinical observations of Ruanmaijianzhi capsule in the treatment of dyslipidemia. Hebei Zhongyi Zazhi. 2004;26:657–9. [Google Scholar]
- Xu SG. Analysis of therapeutic effect of Xuezhikang for treatment of primary hypercholesterolemia. Henon Yixue Xinxi. 2002;10:6–7. [Google Scholar]
- Zheng FS, Long XD, Liu HM, Liu YL, Bao YZ, Yu M. Comparison of lovastatin and Chinese Xuezhikang in lipid modification for primary hyperlipidemia. Yunan Yixue. 2000;21:442–3. [Google Scholar]
- Shen MY. Comparison of the effects of Xuezhikang and atorvastatin for treatment of hyperlipidemia. Zhonghua Shiyong Yiyao Zazhi. 2003;2:439–40. [Google Scholar]
- Wang AH, Zhang GD. Comparison of the therapeutic effects of Xuezhikang and Lescol for treatment of hyperlipidemia. Zhongguo Zonghe Yixue. 2002;3:617–8. [Google Scholar]
- Chen ZM. Comparison of the therapeutic effects of simvastatin and Zhibituo for treatment of hyperlipidemia. Guangxi Yike Daxue Xuebao. 2001;18:543. [Google Scholar]
- Guo WC, Feng WJ. Clinical observations of statin alone or combined with unsaturated fatty acids for the treatment of combined hyperlipidemia in elderly people. Beijing Yixue. 2003;25:25–7. [Google Scholar]
- Guo XM, Tu L, Mi S. Comparison of the therapeutic effects of Zhibituo and simvastatin for regulating dyslipidemia. Zhongyao Yaoli Yu Linchuang. 1999;15:46–8. [Google Scholar]
- Huang YL, Zhou JG, Zhang HF, Shi YX, Wang MS. Comparison of the therapeutic effects of simvastatin and Zhibituo for the elderly with hyperlipidemia. Yixue Yu Gongcheng. 2001;3:24–7. [Google Scholar]
- Yang WJ, Fu XJ. Comparison of the therapeutic effects of Zhibituo and simvastatin for treatment of hyperlipidemia. Zhongguo Xiandai Yiyao Yu Jishu. 2003;3:2–4. [Google Scholar]
- Zhang GR. Comparison of Zhibituo and simvastatin for their effects on hyperlipidemia. Guangxi Yixue. 2002;24:713–4. [Google Scholar]
- Zhang QL. Comparative interventional therapies of dyslipidemia by simvastatin and Zhibituo with 60 cases. Guoji Yiyao Weisheng Daobao. 2004;10:29–30. [Google Scholar]
- Zheng CJ, Wang P. Zhibituo vs. simvastatin in treatment of hyperlipidemia. Zhongguo Yaoshi. 2001;4:447–8. [Google Scholar]
- Wang SX. Comparison of the therapeutic effects of Xuezhikang and lovastatin. Xiandai Zhongxiyi Jiehe Zazhi. 2004;13:2707. [Google Scholar]
- Li DX, Li YF, Lu HY. Comparison of the effects of Xuezhikang with inositol hexanicotinate on lipid profile modification. Henan Zhigong Yixueyuan Xuebao. 2000;12:17–8. [Google Scholar]
- Li YM, Sun RX. Therapeutic observations on Xuezhikang for treatment of hyperlipidemia. Zhongguo Shiyong Xiangcun Yisheng Zazhi. 2004;11:25–6. [Google Scholar]
- Liu L, Li JP, Shen PN. Clinical observations of Xuezhikang for treatment of mixed type of hyperlipidemia. Zhonghua Shiyong Yixue. 2000;16:1047–8. [Google Scholar]
- Qi P, Huang Y, Deng J. Effects of Xuezhikang vs. Inositol niacinate in treating hyperlipidemia. Jiangxi Yixueyuan Xuebao. 2002;42:24–5. [Google Scholar]
- Yang SS. Xuezhikang for treatment of 76 patients with hyperlipidemia. Zhongchengyao. 2002;24:815–6. [Google Scholar]
- Zhang JS. Comparison of Xuezhikang and inositol nicotinate for treatment of hyperlipidemia. Zhiye Yu Jiankang. 2002;18:138–40. [Google Scholar]
- Zheng JR, Wang B. Effects of Xuezhikang on treatment of primary hyperlipidemia. Yixue Luntan Zazhi. 2004;25:21–2. [Google Scholar]
- Zhou ZL, Liu CH. Xuezhikang for treatment of 20 cases of hyperlipidemia. Hunan Zhongyiyao Daobao. 1999;5:25. [Google Scholar]
- Jiang HP. Comparison of the therapeutic effects and compliance of Xuezhikang and Fenofibrate in patients with hyperlipidemia. Xiandai Zhenduan Yu Zhiliao. 2001;12:29. [Google Scholar]
- Li GR, Li JP, Mai WY, Zeng Y. Micronised fenofibrate for treatment of mixed type of hyperlipidemia. Guangdong Yixue. 1999;20:895–6. [Google Scholar]
- Ma F, Ma XH. Clinical observation of Finofibrate and Xuezhikang for treatment of hyperlipidemia. Zhongguo Hangtianye Yiyao. 2003;5:55–6. [Google Scholar]
- Zhu WM, Wu SR. Effects and safety of combined treatment by Xuezhikang and micronized fenofibrate in patients with hyperlipidemia. Zhongguo Linchuang Yixue Zazhi. 2003;4:18–20. [Google Scholar]
- Jiang JB, Hao XY, Deng CQ, Zhou HT, Lin J. Effects of Xuezhikang on serum lipid profile, thromboxane A2 and prostacyclin in patients with hyperlipidemia. Zhonghua Neike Zazhi. 1999;38:517–9. [PubMed] [Google Scholar]
- Wang CW, Gao FM, You L. Clinical study on the therapeutic effects of Xuezhikang for treatment of hyperlipidemia. Mudanjiang Yixueyuan Xuebao. 2000;21:13. [Google Scholar]
- Wang YF, Yang CK, Xu WJ, Sun L, Liu B, Wang GG. Xuezhikang, gemfibrozil for regulation of hyperlipidemia in elderly and insulin sensitivity. Zhongguo Xinyao Zazhi. 1998;7:209–11. [Google Scholar]
- Liu ZB. Xuezhikang for treatment of 40 cases of hyperlipidemia. Linchuang Huicui. 1998;13:367–8. [Google Scholar]
- Xia CH. Comparison of lipid modification of Xuezhikang and Duoxikang in hyperlipidemia. Suzhou Yixueyuan Xuebao. 1999;19:1015–6. [Google Scholar]
- Xu WY, Yan YZ, Tang ZH. Therapeutic observations on Xuezhikang capsules for treatment of hyperlipidemia. Haixia Yaoxue. 2003;15:65–6. [Google Scholar]
- Zheng H, Dan XY, Ning H, Xue B. Observations of Xuezhikang on clinical effects and haemorheology. Hebei Yiyao. 2001;7:46–8. [Google Scholar]
- Zhang WM, Yang JX, Li F, Zhang GW. Observations of Xuezhikang in treatment of hyperlipidemia and abnormal haemorheology. Henan Shiyong Shenjingbing Zazhi. 2000;3:47–8. [Google Scholar]
- Feng JC, Wang JS, Wang CP, Jiang YJ, Tan GZ. Effects of conjugated estrogen and Xuezhikang in low dosage on blood lipid in postmenopausal women. Xiandai Zhongxiyi jiehe Zazhi. 2000;9:2334–5. [Google Scholar]
- Kong YM, Gao H, Liu XL. Clinical observations of the lipid-lowering effects of Xuezhikang and elastase. Zhongguo Yaoshi. 1999;8:56. [Google Scholar]
- Yan HD, Guo JH, Jia ST. Observations of the short-term effects of Probucol in treatment of hyperlipidemia. Shanxi Linchuang Yixue. 1999;8:103–4. [Google Scholar]
- Bi JZ, Ma SZ, Li YQ. Observations on the therapeutic effects of Diao Zhibituo for treatment of hyperlipidemia. Shiyong Zhongxiyi jiehe Zazhi. 1996;9:729. [Google Scholar]
- Cai MX, Deng JX, Lu LF. Clinical observations on Diao Zhibituo for treatment of hyperlipidemia. Fujian Yiyao Zazhi. 1997;19:83–4. [Google Scholar]
- Cong B. Observations on the therapeutic effects of Zhibituo for treatment of hyperlipidemia. Hebei Yiyao. 1999;5:60–1. [Google Scholar]
- Huang LJ, Chen MS. Comparison of Diao Zhibituo and Inositol nicotinate for the lipid lowering effects. Zhongyuan Yikan. 1997;24:8–10. [Google Scholar]
- Li FL, Zeng WH. Comparison of Zhibituo and Inositol nicotinate for treatment of hyperlipidemia. Nongken Yixue. 2002;24:198–9. [Google Scholar]
- Ma L, Gao Y, Li BZ. Observations of the therapeutic effects of Zhibituo for treatment of 50 patients with hyperlipidemia. Xibei Yaoxue Zazhi. 2000;15:126. [Google Scholar]
- Qiu JP, Wang ZJ, Xu XP, Ma SY, Kuang RJ. Observations of Zhibituo on the therapeutic effects in treatment of 60 patients with hyperlipidemia. Shandong Yiyao. 2002;42:45–6. [Google Scholar]
- Yang MJ, Wang RZ. Zhibituo for treatment of 100 cases of hyperlipidemia. Zhongguo Xinyao Yu Linchuang Zazhi. 1997;16:9–10. [Google Scholar]
- Yu HY, Li TH. Observations on the therapeutic effects of Zhibituo for treatment of hyperlipidemia. Xinxueguan Kangfu Yixue Zazhi. 1999;8:30–1. [Google Scholar]
- Chen GY. Observations on the therapeutic effects of Zhibituo for treatment of hyperlipidemia in the elderly. Guizhou Yiyao. 1999;23:307–8. [Google Scholar]
- Chen JF, Yan ZZ, Li PT. Comparison of Zhibituo and Duoxikang for treatment of hyperlipidemia. Zhongguo Xinyao Yu Linchuang Zazhi. 1997;16:15–17. [Google Scholar]
- Fu G, Liu WJ, Wang GT. Comparison of Zhibituo and fish oil capsules for treatment of hyperlipidemia. Heilongjiang Yiyao. 2000;23:93–4. [Google Scholar]
- Jin WQ, Li CW, Xu M, Gao YX, Xu XW. Comparison of Zhibituo and Duoxikang in treating 108 patients with hyperlipidemia. Zhongguo Xinyao Yu Linchuang Zazhi. 1997;16:61–2. [Google Scholar]
- Li Y, Min YB, Fan XJ. Comparison of the therapeutic effects of Zhibituo and fish oils for treatment of 30 cases of hyperlipidemia. Guangdong Yaoxue Zazhi. 2000;10:43–5. [Google Scholar]
- Wang LB, Qiao JJ, Li YM. Clinical evaluation of Zhibituo and concentrated fish oils for treatment of hyperlipidemia. Jiamusi Yixueyuan Xuebao. 1998;21:62–3. [Google Scholar]
- Yang Q, Xue HQ. Observations on the therapeutic effects of Zhibituo for treatment of hyperlipidemia. Zhongguo Jiceng Yiyao. 1999;6:129. [Google Scholar]
- Gu ZY, Lu ZF, Zhu HQ. Observations on the therapeutic effects of Zhibituo for treatment of 158 patients with hyperlipidemia. Nantong Yixueyuan Xuebao. 1998;18:374–5. [Google Scholar]
- Chen ZL. Controlled study of Diao Zhibituo and alginic sodium diester for lipid lowering effect. Henan Shiyong Shenjingbing Zazhi. 1998;1:20. [Google Scholar]
- Chen L, Qin YW, Zheng X. Effects of lipid modification of Diao Zhibituo capsules. Zhongguo Zhongxiyi Jiehe Zazhi. 2003;23:389. [Google Scholar]
- Guo XL, Li Y, Yin GN. Xuezhikang for treatment of 30 cases of hyperlipidemia. Ningxia Yixue Zazhi. 1999;21:418. [Google Scholar]
- Lu WX, Wang JX, Zhu JG, Xu DS, Yang MJ, Wang HW, Wang RZ, Zheng R. Zhibituo capsules in treatment of hyperlipidemia: a multi-centre clinical trial. Zhongguo Xinyao Yu Linchuang Zazhi. 1999;18:365–7. [Google Scholar]
- Lu YS, Gu JS, Zhou WG. Comparison of the therapeutic effects of Xuezhikang and Zhibituo in treatment of adults with hyperlipidemia. Zhongguo Zhongxiyi Jiehe Zazhi. 1998;18:467. [Google Scholar]
- Sun FF, Ding XF, Wang M. Comparison of lipid-lowering effects of Xuezhikang and Zhibituo. Jiceng Yixue Luntan. 2004;8:121–2. [Google Scholar]
- Xiao CL, Yao ZQ, He SM. Comparison of the lipid modification effects of Xuezhikang and Zhibituo for hypercholesterolemia. Guangdong Yixue Zazhi. 2000;21:430–1. [Google Scholar]
- Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Contral Clin Trials. 1998;19:159–66. doi: 10.1016/S0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–90. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]