Abstract
Classical models of grooming predict that subordinate primates will direct grooming towards dominants to receive coalitionary support from them. In contrast, recent reviews suggest that grooming asymmetries can change with social system and ecological conditions and should reflect asymmetries in services provided by different members of the dyad. We studied grooming patterns between females in six wild groups of common marmosets, Callithrix jacchus, to investigate the relation between social structure and grooming between females in a cooperatively breeding species. We observed grooming frequently and consistently in all study groups. Breeding females groomed nonbreeding females more than vice versa, and grooming between breeding and nonbreeding females was not related to agonistic behaviour. Our results provide some support to the hypothesis that grooming asymmetries are related to differences in services provided by different group members. We suggest that, in cooperatively breeding systems, breeding females may use grooming as an incentive for helper females to stay in the group.
Cleaning other individuals’ fur with hands or mouth (allogrooming, hereafter grooming) has been described for a number of mammalian species, and two main functions have been traditionally related to this behaviour: (1) removal of ectoparasites from body areas that an animal is not able to reach by itself and (2) maintenance or establishment of social relationships. The social function has been emphasized in primate studies. Primate researchers studying Old World species have used grooming as the main quantitative measure of the strength of social relationships within dyads, and many attempts have been made to explain the distribution of grooming in primate groups in relation to social structure (Seyfarth 1977; Cheney 1992; Hemelrijk & Luteijn 1998). In contrast, the study of grooming patterns in New World primates has received much less attention. In fact, based on a comparative study of grooming rates versus body size and group size in 44 primate species, Dunbar (1991; see also Dunbar 1993) concluded that the lack of relationship between grooming rates and group size found in New World species indicated that grooming never evolved (or lost) its social function in platyrrhine primates.
Contrary to Dunbar’s view, several studies have shown that grooming can be a useful measure of social relationships in some New World primates (spider monkeys, Ateles geoffroyi: Ahumada 1992; wedge-capped capuchins, Cebus olivaceus: O’Brien 1993; tufted capuchins, C. apella: Linn et al. 1995; Di Bitetti 1997; Parr et al. 1997; red howlers, Alouatta seniculus: Sanchez-Viagra et al. 1998; white-faced capuchins, C. capucinus: Manson et al. 1999; see also Schino 2001 for a meta-analysis of grooming distribution in primates that showed few differences between Old World and New World species). Furthermore, although Seyfarth’s (1977) classical model, in which subordinate females exchange grooming for coalitionary support from higher-ranking females, predicted that grooming should be directed mainly up the hierarchy (i.e. subordinates groom dominants more than vice versa), capuchin monkey females frequently direct grooming down the hierarchy, i.e. dominants groom subordinates more often than they are groomed by them (O’Brien 1993; Linn et al. 1995; Di Bitetti 1997; Parr et al. 1997; see also Jones 1979 for a similar result in mantled howler monkeys, Alouatta palliata). These differences in the relationship between the direction of grooming and the dominance hierarchy emphasize the need to expand the range of taxa used in grooming studies if we are to find a widely applicable model.
Seyfarth’s (1977) influential model on the interchange of grooming for coalitionary support between female primates was developed for a particular group, Cercopithecine primates, with a female-bonded social structure. Henzi & Barrett (1999; see also Barrett et al. 1999), using biological market theory (Noë & Hammerstein 1995), proposed a new approach to the study of the social function of grooming. Grooming can provide direct benefits to the individual being groomed through the removal of ectoparasites and the increase of psychological and physiological well-being, including the release of β-endorphine (Meller et al. 1980; Keverne et al. 1989), decreases in heart rate (Boccia et al. 1989; Aureli et al. 1999) and rewarding effect (Taira & Rolls 1996). Henzi & Barrett (1999) therefore suggested that grooming is a currency exchanged between group members, predicting that grooming should be reciprocated within a dyad when no other services are being exchanged, or when the exchange of other services is equal between the members of that dyad. However, when different partners within the dyad offer different services, the amount of grooming given should decrease as the other services offered increase. These services include not only support during aggression, but also tolerance at feeding sites or direct access to resources (e.g. de Waal 1997a). Therefore, the model should apply to a variety of primate groups with different social systems, as long as there is some information about the kind of services that are exchanged between dominants and subordinates. Seyfarth’s model could be considered as a particular case in which coalitionary support is the main service being exchanged and dominant individuals are the ones with the ability to provide it.
Barrett et al. (1999) tested their approach in two populations of chacma baboons, Papio cynocephalus ursinus, with different patterns of dominance relationships. When dominants had ‘less to offer’ to subordinates (or subordinates had ‘less to gain’ from their association with dominants) because of low levels of resource competition, grooming asymmetries between dominants and subordinates decreased. A different test of this model, which would contribute to its generalizability, would be to evaluate its predictions in cooperative breeders with dominant–subordinate relationships that are markedly different from those present in most female-bonded species. Barrett et al. (1999) emphasized the importance of immediate reciprocation of grooming to avoid cheating, but did not consider the timing of grooming with respect to other potential services.
In this study, we first investigated the relation between grooming and other measures of social structure (such as proximity and agonistic behaviour) to show the relevance and social function of this behaviour in a New World primate. Then we expanded on Henzi & Barrett’s (1999) model by analysing grooming patterns in wild groups of cooperatively breeding common marmosets, examining the patterns of female–female grooming and timing of grooming with respect to the main services offered by different group members.
Cooperatively breeding species are often used as examples of despotic societies in which ‘the winner takes all’; only dominant individuals within a group reproduce (Vehrencamp 1983). However, this extreme reproductive dominance is not accompanied by dominants offering services to lower-ranked individuals. Intragroup aggression is usually rare, coalitions have not been documented in most species, and breeding animals rarely share food resources with subordinates other than infants or juveniles (reviews in Stacey & Koenig 1990; Solomon & French 1997; for a review of aggressive behaviour in marmosets and tamarins, see Schaffner & Caine 2000). In contrast, subordinates assist breeding animals by providing alloparental care and cooperation in territorial defence and antipredator vigilance, and therefore they are valuable assets for dominant animals (reviewed in Solomon & French 1997). As Schaffner & French (1997, page 171) suggested, it is expected that these species ‘express behavioural and social strategies to maintain or increase group size’.
We predicted that, if grooming asymmetries in female–female dyads reflect asymmetries in the services provided by different group members, grooming by marmosets (1) should be directed primarily from dominants to subordinates and (2) should be affected by group size, with more asymmetry being expected in smaller groups. If timing of grooming is influenced by the timing of services provided, dominant females should also increase their investment in a grooming relationship when helper females have ‘more to offer’, i.e. (3) there are dependent infants in the group and (4) on days with intergroup encounters.
METHODS
Study Animal and Study Population
Common marmosets, Callithrix jacchus, are small New World primates that live in groups of 5–15 individuals in which usually only one female reproduces per group (Stevenson & Rylands 1988; but see Digby & Ferrari 1994). Male and female offspring generally stay in their natal group past puberty and cooperate in parental care (reviewed in Tardif 1997), antipredator behaviour (C. Lazaro-Perea, unpublished data) and territorial defence (Lazaro-Perea 2001). Although most subordinate animals within a group are the offspring of the reproductive pair, unrelated individuals or those related to only one parent can also be members of the group (Nievergelt et al. 2000).
This study was carried out at the Nisia Floresta Forest Experimental Station, EFLEX-IBAMA, Brazil (Santee & Arruda 1994). Data were collected between September 1996 and December 1997 from six social groups in which all but two individuals were individually marked (Table 1; Lazaro-Perea et al. 2000).
Table 1.
Summary characteristics of study groups
| Group | Group composition | Observation hours | Median (range) of focal samples per individual | Agonistic interactions/h |
|---|---|---|---|---|
| E | F, M, m, 2f, sm, sf, jm, jf | 233 | 28 (2–41) | 0.15 |
| E2 | F, M, m, f | 154 | 56.5 (53–58) | 0.13 |
| PB | F, M, 4f, 2sf, jf, jm, in | 94 | 11.5 (6–14) | 0.13 |
| Pl | F, M, 2m, 2f | 117 | 20.5 (19–25) | 0.09 |
| Q | F, M, m, 5f, 2sf, 2in | 391 | 65 (18–73) | 0.03 |
| J | F, M, f, 2sf, 2in | 97 | 16 (12–17) | 0.18 |
F: breeding female; M: putative breeding male; f: nonbreeding adult female; m: nonbreeding adult male; sf: subadult female; sm: subadult male; jf: juvenile female; jm: juvenile male; in: infant. For more details about classification of animals, see Lazaro-Perea (2001).
The research was approved by the Animal Care and Use Committee of the University of Wisconsin and by Conselho Nacional de Desenvolvimento Cientifico e Tecnologico.
All subjects were trapped with manually activated traps, following a habituation period to the traps of 2–3 days. No animal was injured during the trapping procedure. To minimize stress, once the animals were within the trap, it was covered by a sheet so that they did not see the observers while being carried to the measuring facilities. Once in the measuring facilities, the animals were anaesthetized with Ketamine (0.12 ml/kg) while still within the trap. When the effects of the drug started to be visible, we removed the animal from the trap and measured it. For marking, we used neck collars, each with three coloured beads, or picric acid to mark different body parts (and tattooed those animals that did not have a tattoo mark, for continuous identification). In the 10 years that these methods have been used at the Nisia Floresta Research Station, we have no evidence that they have negatively affected the animals. After the measuring and marking procedures, the animal was returned to the trap, which was again covered by a sheet. The animals were returned to their home ranges only when we concluded that the effects of the drug had worn off as determined by the animal moving and reacting normally.
Data Collection
All-day or half-day follows were carried out by C.L.-P. and two trained observers for each group in rotation, so that each group was observed usually at least 1 day every week (Table 1). Interobserver reliability scores were calculated following Martin & Bateson (1993: r = 0.95). Ten-minute focal animal samples (Altmann 1974) were carried out for all animals older than 12 months in each group. The order of observation of focal animals was randomly determined before going into the field each morning. If an animal could not be found within 10 min of the end of the previous focal sample, we chose the next animal on the list, but with at least 1 h between two consecutive focal samples of the same animal. No more than three focal samples of an animal were conducted on a single day. We recorded as ‘grooming’ any interaction that involved the focal animal in which one animal picked through the hair of another with the hands or the mouth. We dictated into a tape recorder the initiator and the recipient of the grooming bout and when each bout started and finished. The duration of each bout was later determined during transcription of the tapes using a stopwatch. Grooming bouts were recorded as continuous unless interrupted by resting or another activity for more than 5 s. When animals changed roles (from groomer to groomee or vice versa) a new grooming bout was started. If the focal animal was involved in a grooming interaction with more than one animal at the same time, we recorded one grooming bout for each dyad that included the focal animal. Grooming bouts that had already started at the beginning of one focal sample (12.6% of total number of bouts) were considered to start at the beginning of the focal sample, and grooming bouts that continued after the end of the focal samples (11.2% of total number of bouts) were considered to have ended at the end of the focal samples. We also recorded continuously all approaches and departures to and from the focal animal by any other animal, and by the focal animal to any other animal of the group.
Instantaneous samples (Altmann 1974) were also collected during each minute of the focal sample: at the sound of a beeper, we recorded the activity of the focal animal using nine categories (Table 2), the identity of any animal within one body length, 1 m, and in the same tree, and, if no animal was closer than 1 m, we recorded the identity and distance of the nearest neighbour within a 5-m radius.
Table 2.
Behavioural definitions
| Category | Definition | Classification |
|---|---|---|
| Instantaneous samples | ||
| Resting | Focal animal still, with eyes opened or closed but without scanning surroundings | |
| Scanning | Focal animal still, moving head side to side as if monitoring surroundings | |
| Grooming | Focal animal picking through fur of another animal with hands or mouth | |
| Being groomed | Animal picking through focal animal’s fur with hands or mouth | |
| Autogrooming | Focal animal picking through its own fur with hands or mouth | |
| Eating gum | Focal animal gouging holes or licking at gum holes or moving from hole to hole at a gum tree | |
| Foraging | Focal animal looking for, pursuing or eating animal food (insects, arachnids or vertebrates) or eating or looking for fruit or other plant parts (except gum) | |
| Travelling | Focal animal moving | |
| Other | None of the above categories | |
| Agonistic behaviour | ||
| Chase/lunge | Throwing the body towards another individual/pursuing partner aggressively, without contact aggression | A |
| Hit | Cuff or grab the fur of another individual with hand | A |
| Bite | Normal usage | A |
| Ehr-ehr vocalizations | Low-pitched, staccato chattering (Epple 1968) | A |
| Cower | Sinking down to substrate and moving the body away from another individual (Digby 1995) | S |
| Submissive vocalizations | String of vocalizations that included nga-nga (low-pitched, atonal, infantile squeal; Epple 1968) | S |
| Avoid | Quick movement around or under a branch or trunk in response to another animal’s approach (Digby 1995) | S |
A: aggressive; S: submissive.
Agonistic data were collected using an all-occurrences method (Altmann 1974) whenever an observer accompanied the group (Table 2). Whenever we observed an agonistic interaction, we recorded the identity of the actor and the receiver and the behavioural context in which the interaction occurred. For tabulation of data for each interaction, we determined who the aggressive animal was (the one that performed either chases, lunges, hits, bites or aggressive vocalizations, or received cowers or submissive vocalizations). In those interactions in which one or more aggressive patterns were responded to by one or more submissive patterns, we recorded a single interaction in which the ‘aggressive’ animal was the one that performed the aggression, and considered consecutive interactions separated by less than 1 min as being within the same episode. If one aggressive behaviour was responded to by the recipient with another aggressive behaviour, we registered two agonistic interactions.
Data Analyses
Linear mixed-effects ANOVAs on the raw or transformed data were used to evaluate differences in grooming and proximity patterns as a function of breeding status. In these models, group was included as a random effect to take into account the variability between groups. Data were transformed when examination of residual plots indicated problems with normality or heterogeneity of variances. In these cases, we used the arcsine square-root transformation, as suggested by Martin & Bateson (1993) for data representing proportions. When there was an overall significant effect of breeding status (P<0.05), we used Fisher’s LSD approach to examine differences between pairs of status categories.
Matrix correlations were used to analyse grooming reciprocity at group levels. For each group, we created an ‘actor matrix’, in which rows represented groomers and columns represented groomees. The value in each cell corresponded to the total time in which the row animal was observed grooming the column animal during focal samples of any of the two individuals, corrected by the minutes of focal sampling for each animal of the dyad (i.e. time that A grooms B/(observation time A + observation time B)). A ‘receiver matrix’ (one in which rows are groomees and columns are groomers) was created by transposing the actor matrix. We used Matsquare software (Hemelrijk 1990) to calculate the Kr statistics to test for relative reciprocity in grooming patterns (animals groom more those animals from whom they receive more grooming; Hemelrijk 1990) by correlating the actor matrix with the receiver matrix. The value of this test statistic depends on the sample size and the relative concordant change between the two matrices and tests only within rows, to account for individual differences. To assess the strength of the correlations, the Kr statistics must be corrected for sample size and ties, which is done by calculating the τKr statistics (similar to the Kendall rank correlation coefficient, τ). We used the same test statistic to correlate the total time that each dyad spent grooming with time spent within one body length of each other. We used 10 000 permutations to calculate P values.
Reciprocity of grooming was also studied at a dyadic level. We considered two types of reciprocity, quantitative (i.e. there was reciprocity in the amount of grooming given and received in a particular grooming bout) and qualitative (i.e. both members of the dyad groomed during a particular grooming bout). For the quantitative approach, we calculated the degree of asymmetry in grooming patterns for each dyad involving the breeding female and any nonbreeding female by subtracting grooming received from grooming given by the breeding female during any focal sample. We analysed those samples in which grooming was reciprocated within the focal sample (i.e. those focal samples in which both breeding and nonbreeding female were observed grooming) with linear mixed models, with breeding female, nonbreeding female and focal sample as random effects, and grooming given/grooming received as the fixed effect. To test for qualitative reciprocity, we used generalized linear mixed models (logistic regression with random effects) using a 1/0 response (1 if the animal groomed during the focal sample, 0 if it did not groom during that sample), for all focal animal samples in which we observed grooming (reciprocated and unreciprocated) between the breeding and a nonbreeding female. These analyses take into account that dyads including the same individuals are not independent data points (Diggle et al. 1994).
Wilcoxon signed-ranks tests were used to evaluate the differences in asymmetry in grooming in breeding–nonbreeding female dyads that depended on the presence or absence of dependent infants (<2 months old) in the group and on the occurrence of intergroup encounters. We also used Wilcoxon signed-ranks tests to evaluate differences in asymmetry in grooming in breeding–nonbreeding female dyads that depended on which member of the dyad was responsible for initiating proximity or responsible for terminating proximity. We used the V statistic (sum of ranks that correspond to positive differences), instead of the more common T statistic (sum of either the positive or negative ranks, whichever is smaller in absolute value), as V is the statistic used by the R statistical package for obtaining exact P values for Wilcoxon signed-ranks tests.
We used Spearman rank correlation to evaluate the relation between group size and the asymmetry in grooming between breeding and nonbreeding females. After calculating the mean grooming asymmetry for each breeding female–nonbreeding female dyad, we computed the mean of the log-transformed asymmetries for each group (some values were negative, and the largest negative value was −51.5, so we added 51.5 before log transforming; adding other numbers, such as 52, 60 or 65 had no qualitative effect on the results). Next, we performed a Spearman rank correlation test between the mean asymmetry and the group size.
We used the Splus library nlme (Pinheiro & Bates 2000) and SAS’s PROC MIXED (Little et al. 1996) for linear mixed models, and Splus v. 3.3 (Statistical Sciences 1995) and R for the rest of the analyses. All tests except those performed with Matsquare software were two tailed.
RESULTS
Social Relationships: Proximity and Agonistic Behaviour
There were significant differences with status in how much time individuals spent in proximity with other animals during focal animal samples (infants and juveniles excluded; linear mixed-effects ANOVA on arcsine square-root-transformed data, F4.29 = 5.68, P = 0.0016; Fig. 1) and all groups presented similar proximity patterns.
Figure 1.

Back-transformed mean ± SE proportion of instantaneous samples during focal animal samples in which focal animals from the different status categories were within one body length of at least one other adult/subadult animal of the group. *P<0.05; **P<0.01. F: breeding female; M: putative breeding male; f: nonbreeding adult female; m: nonbreeding adult male; sb: subadult (Lazaro-Perea 2001).
Agonistic interactions were rare: we observed only 164 interactions ( ± SE = 0.12 ± 0.02 agonistic interactions/observation hour per group). Most agonistic interactions were related to food resources such as eating gum (34.7%) and foraging for fruit or insects (10.9%), but agonism was also associated with handling of infants (4.3%), grooming (5.5%) and intergroup interactions (6.1%). Agonistic interactions occurred equally within (51.2%) and between sexes (48.8%). In three groups, the distribution of agonistic interactions between males and females was not different from that expected, taking into account the composition of the groups (chi-square tests: group E: χ32 = 5.03; group P: χ32 = 2.18; group J: χ32 = 1.25; all NS). In the other three groups, we found significant deviations from expected values (group E2: χ32 = 6.5, NS; group PB: χ22 = 8.94, P<0.025; group Q: χ32 = 21.61, P<0.001); males were aggressive towards females more often than expected, but these interactions never involved the reproductive female. The breeding female was observed directing aggressive behaviours towards the breeding male in five of the six groups, but breeding males never directed aggressive behaviour towards their breeding female. Agonistic interactions were consistent with the reproductive hierarchy in all groups: we observed nonreproductive females directing aggressive behaviours towards breeding animals only twice (once towards a breeding male and once towards a breeding female), and nonreproductive males directed aggressive behaviour towards the breeding female only once and never towards the breeding male. We never observed the occurrence of coalitions (i.e. one animal providing support to another group member during aggression towards a third member of the group).
Grooming Patterns
Grooming was observed in 421 of the 1263 (33.3%) focal animal samples (38.5 net h of grooming). Grooming patterns were consistent in all the groups and matched the social structure derived from proximity and agonistic patterns. Total time spent grooming (given plus received) varied with status (linear mixed-effects ANOVA on the arcsine square-root-transformed data; F4.30 = 6.40, P< 0.001; Fig. 2a), as did the difference between grooming given and grooming received (linear mixed-effects ANOVA: F4.29 = 10.56, P<0.001; Fig. 2b). The breeding animals spent more time grooming than did the rest of the group, and the reproductive male was the most active groomer in all groups.
Figure 2.
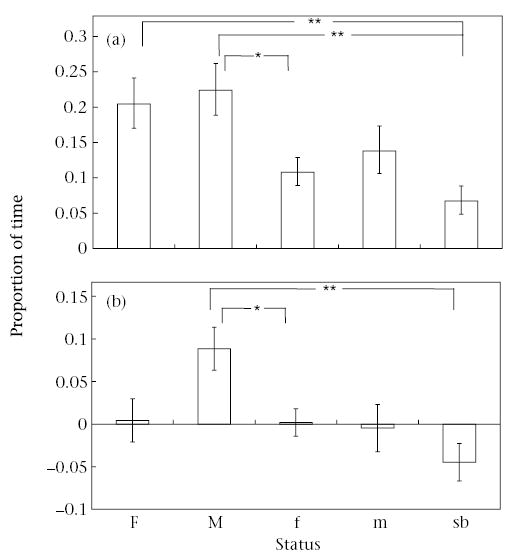
(a) Back-transformed mean ± SE proportion of observation time that animals of the different status categories spent grooming or being groomed. Status categories as in Fig. 1. *P<0.05; **P<0.01. (b) Mean ± SE difference between time spent grooming given and time spent being groomed for animals of each status categories. *P<0.05; **P<0.01.
When males were included in the analyses, animals distributed grooming across group members according to how much grooming they received from them in all but one of the groups (relative reciprocity at group level: E2: τKr = 0.33, N = 4, Pr = 0.25; E: τKr= 0.61, N = 7, Pr = 0.001; J: τKr = 0.697, N = 5, Pr = 0.026; P: τKr = 0.59, N = 6, Pr = 0.003; PB: τKr = 0.34, N = 8, Pr = 0.01; Q: τKr = 0.489, N = 10, Pr <0.001). Analyses were repeated including only females for those groups in which sample size allowed it; only the two largest groups showed statistical significance (E: τKr = 0.23, N = 4, Pr = 0.29; J: τKr = 0.17, N = 4, Pr = 0.46; PB: τKr = 0.32, N = 7, Pr = 0.01; Q: τKr = 0.47, N = 8, Pr <0.001). However, within each dyad, grooming was not reciprocated symmetrically. Grooming between the breeding female and nonbreeding females was reciprocated within the same focal sample in 85 of the 115 (73.9%) focal samples in which grooming occurred. When reciprocity occurred, the breeding female groomed nonbreeding females for significantly longer than she was groomed by them (linear mixed-effects ANOVA: F1.55 = 6.97, P = 0.011). When we analysed all instances of grooming (reciprocated and unreciprocated) between breeding and nonbreeding females considering only (on a 1/0 basis) which member of the dyad performed grooming during any particular focal animal sample, we found similar results: breeding females were more likely to perform grooming than nonbreeding females (generalized linear mixed models: P = 0.001; Fig. 3). We found a marginally significant negative correlation between group size and the asymmetry in grooming between breeding and nonbreeding females (rS = −0.83, N = 6, P = 0.058).
Figure 3.
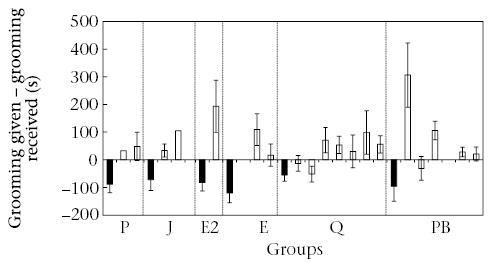
Mean ± SE difference between time spent grooming by the breeding female and time that she received grooming from other females (□; data on breeding female–breeding male dyads are also shown for comparison: ▪). Values were averaged for each focal animal. Positive values indicate that the breeding female gave more grooming than she received from an individual. Negative values indicate that the breeding female received more grooming than she gave to an individual. Within each group, estimated ages of females are in descending order.
Grooming between breeding and nonbreeding females was more likely during periods with no dependent infants in the group (Wilcoxon signed-ranks test: V = 0, N = 12 dyads, P<0.001). For the subset of dyads that were observed grooming in both periods, with and without infants, the asymmetry in grooming was higher in periods with no dependent infants in the group (V = 5, N = 10, P = 0.019). This greater asymmetry was due to increased grooming by breeding females, whereas there was no change in grooming time by nonbreeding females. Breeding females groomed more on days when no intergroup encounters occurred (V = 10, N = 12, P = 0.021), resulting in a marginally significant trend towards more asymmetry on days with no territorial encounters (V = 15, N = 12, P = 0.064). For the subset of dyads that were observed grooming both on days when the non-breeding female had participated in intergroup encounters and on days when she had not, we found no significant differences (grooming by breeding females: V = 25, N = 8, P = 0.383; grooming asymmetry: V = 15, N = 8, P = 0.742).
Grooming between breeding and nonbreeding female marmosets was rarely associated with agonism. Of the 115 focal animal samples in which we observed grooming between a breeding and a nonbreeding female, in only seven cases (6.1%) was there agonism between that dyad during the same day, and in only two of those did the agonism occur during the same focal sample as the grooming interaction. Grooming between breeding and nonbreeding females was preceded by an approach by the breeding female in 54.4% of the cases and by the nonbreeding females in 45.6% of the cases, and was terminated by the dominant female in 63.7% of the cases versus 36.2% by the subordinate. For the nine dominant–subordinate dyads for which we had data on grooming bouts following approaches of both members of the dyad, there were no differences in the amount of asymmetry in grooming depending on who was responsible for the approach (Wilcoxon test: V = 33, N = 9, P = 0.25), and for the 11 dyads for which we had data on grooming bouts followed by departures by both members of the dyad, there were no differences in grooming asymmetry depending on who terminated proximity after the grooming bout (V = 42, N = 11, P = 0.465).
DISCUSSION
There was a close match between social structure and grooming patterns and a consistent relation between grooming asymmetry and reproductive dominance hierarchy. Marmosets spent, on average, 14.7% of the observation time in grooming interactions. Grooming rates per dyad ranged from 0 to 17% per dyad when all dyads are considered, and from 0 to 5% for grooming between the breeding female and other females of the group. These figures are at the upper range of values found in studies of Old World female-bonded species using similar methods (e.g. Altmann 1980; Seyfarth 1980). The rates that we found do not seem unusual for callitrichids. Digby (1995) found almost identical individual rates of grooming (14%) in three groups of common marmosets in the same population, and saddleback tamarins, Saguinus fuscicollis, in Goldizen’s (1989) study groomed for 7–9% of the observation time. Heymann (1996) reported rates of only 2% of focal observation time in two wild groups of moustached tamarins, S. mystax, but he indicated that these rates were probably underestimated, because grooming tended to occur in locations with poor visibility at his site. These results strongly suggest that, contrary to Dunbar’s (1991, 1993) conclusion, grooming does have a social function in these New World monkeys (see also Schino 2001).
Agonistic interactions were infrequent, as reported in other studies of callitrichid groups (Schaffner & Caine 2000), and did not allow the construction of a linear hierarchy among adult animals. Nevertheless, breeding females dominated all other females.
Contrary to the pattern observed in noncooperatively breeding species, and more specifically in female-bonded primate groups, grooming between breeding and nonbreeding female marmosets was less frequent than grooming between the breeding animals; however, when it occurred, breeding females groomed nonbreeding females more than the reverse. Grooming between females has been studied extensively in many primate species, and theoretical models of grooming behaviour have dealt primarily with the distribution of grooming between females (e.g. Seyfarth 1977; Cheney 1992; Henzi & Barrett 1999). However, data on the distribution of grooming between adult female callitrichids are surprisingly scarce. Most captive studies have concentrated on social relationships between the breeding pair. Goldizen’s (1989) study of a polyandrous group of wild saddleback tamarins focused mainly on male–female relationships. In Heymann’s (1996) study of social interactions in two polyandrous groups of wild moustached tamarins, he suggested that grooming occurs down the hierarchy. Nevertheless, his results refer to male–male grooming, because neither of the two groups studied had multiple adult females. Stevenson & Rylands (1988) presented data on grooming rates in two wild groups of common marmosets but gave no information on the direction of grooming between dominant and subordinate females. Similarly, Digby’s study on three groups of common marmosets at the same field site as ours did not provide data on the direction of grooming between females, although she reported a ‘stronger trend for nonbreeding adults to receive grooming from breeding adults’ (Digby 1995, page 371).
As discussed earlier, grooming asymmetry from dominant to subordinate has been observed in other species. Grooming from mother to offspring has been observed often and has been interpreted as provision of maternal care to offspring. Sociodemographic data suggest that, as expected for cooperatively breeding groups, most nonbreeding females are daughters of the breeding female. Therefore, the grooming that nonbreeding females received from the breeding female may be an extension of maternal care continuing past infancy. Nevertheless, other evidence suggests that grooming from breeding to nonbreeding females was not related only to maternal care. First, in two breeding–nonbreeding female dyads (in groups P and E2), the breeding female was probably not the mother of the nonbreeding female. In group P, the nonbreeding female had immigrated as a subadult, and in group E2 the breeding female had immigrated after the disappearance of the dominant female. However, these two dyads did not differ from the other female–female dyads. Second, if grooming by breeding females was a form of parental care, we would expect younger females to receive more grooming than older ones. Although we could not statistically test for the effect of age on the distribution of grooming between females because of the small sample size, the data do not suggest the existence of this effect (Fig. 3).
Grooming by dominants to subordinates has been interpreted as reducing tension by redirecting or replacing potential aggression in several species (reviews in Harrison 1965; Sparks 1967). That interpretation would agree not only with the direction of grooming but also with the rarity of observing grooming and agonistic interactions during the same day. Nevertheless, in this case, the grooming bouts should be preceded by an approach by the dominant female and terminated by the subordinate (as reported for capuchin monkeys; O’Brien 1993). In our study, grooming between breeding and nonbreeding females was rarely associated with aggression, was not more likely to occur after approaches by the breeding female than after approaches by the nonbreeding female, and was not more likely to be terminated by the breeding than by the nonbreeding female. Therefore, our results do not suggest a relation between grooming patterns and the reduction of aggression between breeding and nonbreeding females, or that grooming is a reconciliation of past conflict.
Our data are more consistent with a view of grooming in which the asymmetry of grooming behaviour within a dyad is related to asymmetries of services offered by each animal (Henzi & Barrett 1999). de Waal (1997b) suggested that grooming of lower-ranking partners by female capuchin monkeys was related to the benefits that dominants might get, such as food sharing or support in coalitions. In our groups, subordinate females were observed carrying and sharing food with infants (C. Lazaro-Perea, unpublished data); they were more active in territorial defence than the breeding females of their groups (Lazaro-Perea 2001), and they participated in alarm calling and mobbing behaviour (C. Lazaro-Perea, unpublished data). Therefore, subordinate females provided valuable services for the breeding pair, and reproductive females should be interested in subordinate females remaining in the group and not dispersing. In a captive study, Schaffner & French (1997) found that Callithrix kuhli’s breeding females modulated their aggressive response to experimental intruders depending on group size. The results were interpreted as being a consequence of the interest of breeding females in regulating group size (in that case by tolerating intruders more in smaller groups, which would need more helpers). We found that breeding females’ grooming behaviour was also affected, although marginally, by group size, with greater asymmetry in grooming in smaller groups. If our interpretation is correct, that is, if grooming can function as an exchange currency to reward nonbreeding females for their staying in the group and cooperating, then this might be an additional mechanism used by breeding females to regulate group size.
However, we found no relation between the timing of parental care or participation in territorial defence and grooming received by nonbreeding females. Therefore, our hypotheses related to the timing of grooming in relation to the services provided were not supported by the results. We cannot exclude that this lack of relationship was caused by the lack of detail that we used to analyse the services provided by the nonbreeding females: we had to use coarse measures to analyse the relation between provision of parental care and grooming (periods with and without dependent infants in the group) and occurrence (or lack) of intergroup encounters during the same day as grooming interaction. Alternatively, it is also possible that time or energy constraints during periods of infant dependency and on days with intergroup interactions may decrease the time available for other activities, thus precluding immediate reciprocation between grooming and services provided. We think, however, that even if there were a lack of immediate reciprocity, this would not invalidate the general model (exchange of grooming by other services) as the need to reinforce relationships may be in advance of needed services.
In summary, grooming has a social function in common marmosets in regulating social interactions between breeding and nonbreeding females. The asymmetries of grooming are consistent with an interpretation of grooming as a currency whose asymmetry rewards the services provided by interacting animals. However, it remains to be shown how the timing of services influences the distribution of grooming.
The variations in grooming patterns observed within and between species are probably related to variation in the type and value of dyadic relationships within groups. Social, demographic and ecological conditions will affect the kind of ‘services’ that group members can offer to each other, so we should expect that grooming patterns will not necessarily follow dominance lines but will instead vary with conditions (e.g. Barrett et al. 1999).
Groups that show similarities in their social system and in the characteristics of the dominance/subordinate relations should share similar grooming patterns. Data on the distribution of grooming among adult female callitrichids are scarce.
In those species of cooperatively breeding birds and mammals for which there are data on grooming (or preening, in the case of birds) relationships, there is a greater tendency for dominants to groom/preen subordinates (e.g. jungle babblers, Turdoides striatus: Gaston 1977; Arabian babblers, Turdoides squamiceps: Zahavi 1990; stripe-backed wrens, Campylorhynchus nuchalis: Rabenold 1990; dwarf mongooses, Helogale parvula: Rasa 1987). Although in birds (Harrison 1965) and viverrid mammals (Rasa 1987), the direction of allogrooming/allopreening from dominants to subordinates has commonly been interpreted as a signal of dominance that replaces aggressive behaviour, we found no relation between grooming and aggressive behaviour in common marmosets, and offer an alternative interpretation that might apply to other cooperatively breeding species. Subordinates in cooperatively breeding systems offer multiple ‘services’ to dominants, and grooming them might be a way in which dominant individuals signal their interest in maintaining that social bond (Zahavi & Zahavi 1997) and offer a possible incentive to stay in the group.
As we have tried to show with this study, this hypothesis is testable in studies of both captive and field populations that consider the kind of services that subordinates offer to dominant females, along with the opportunities for dispersal for subordinate animals. Our results, however, have not shown that grooming is exchanged for specific services. Perhaps experimental studies in which the contribution of different animals to territorial defence and parental care is measured together with their involvement in grooming interactions could provide enough data to test the model more exhaustively.
Acknowledgments
We thank IBAMA and the Departamento de Fisiologia da Universidade Federal do Rio Grande do Norte for permission to carry out this study and for providing logistic support. Fabíola Alburquerque and Edinaldo Nascimento helped with capturing and marking the monkeys. Simone Porfirio de Souza and Maria Carla Lopes de Nascimento provided help in data collection and habituation of one of the groups. Ramón Díaz-Uriarte provided statistical advice and logistic support during all parts of the study. D. H. Abbott, J. Altmann, J. R. Baylis, R. Díaz-Uriarte, C. A. Marler and K. B. Strier provided comments on the manuscript. This research was supported by the National Geographic Society (C.L.-P. and C.T.S.), NIMH (MH 35 215 to C.T.S.), University of Wisconsin Graduate School (C.L.-P.), and the Wisconsin Regional Primate Research Center (RR 00 167). This research was carried out under an agreement of cooperation between the Departamento de Fisiologia, Universidade Federal do Rio Grande do Norte, Brazil, and the Department of Psychology, University of Wisconsin-Madison, U.S.A. This is publication 43-005 of the WRPRC.
References
- Ahumada JA. Grooming behavior of spider monkeys (Ateles geoffroyi) on Barro Colorado Island, Panama. International Journal of Primatology. 1992;13:33–49. [Google Scholar]
- Altmann J. Observational study of behaviour: sampling methods. Behaviour. 1974;49:227–265. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon Mothers and Infants. Cambridge, Massachusetts: Harvard University Press; 1980. [Google Scholar]
- Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. Journal of Comparative Psychology. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proceedings of the Royal Society of London, Series B. 1999;266:663–670. [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the physiology of grooming in a pigtail macaque. Physiology and Behavior. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Cheney DL. Intragroup cohesion and intergroup hostility: the relation between grooming distributions and intergroup competition among female primates. Behavioral Ecology. 1992;3:334–345. [Google Scholar]
- Di Bitetti MS. Evidence for an important social role of allogrooming in a platyrrhine primate. Animal Behaviour. 1997;54:199–211. doi: 10.1006/anbe.1996.0416. [DOI] [PubMed] [Google Scholar]
- Digby LJ. Social organization in a wild population of Callithrix jacchus: II. Intragroup social behavior. Primates. 1995;36:361–375. [Google Scholar]
- Digby LJ, Ferrari SF. Multiple breeding females in free-ranging groups of Callithrix jacchus. International Journal of Primatology. 1994;15:389–397. [Google Scholar]
- Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- Dunbar RIM. Functional significance of social grooming in primates. Folia Primatologica. 1991;57:121–131. [Google Scholar]
- Dunbar RIM. Coevolution of neocortical size, group size and language in humans. Behavioral and Brain Sciences. 1993;16:681–735. [Google Scholar]
- Epple G. Comparative studies on vocalization in marmoset monkeys (Hapalidae) Folia Primatologica. 1968;8:1–40. doi: 10.1159/000155129. [DOI] [PubMed] [Google Scholar]
- Gaston AJ. Social behaviour within groups of jungle babblers (Turdoides striatus) Animal Behaviour. 1977;25:828–848. [Google Scholar]
- Goldizen AW. Social relationships in a cooperatively polyandrous group of tamarins (Saguinus fuscicollis) Behavioral Ecology and Sociobiology. 1989;24:79–89. [Google Scholar]
- Harrison CJO. Allopreening as agonistic behaviour. Behaviour. 1965;24:161–209. [Google Scholar]
- Hemelrijk C. Models of, and tests for, reciprocity, unidirectionality and other social interaction patterns at a group level. Animal Behaviour. 1990;39:1013–1029. [Google Scholar]
- Hemelrijk CK, Luteijn M. Philopatry, male presence and grooming reciprocation among female primates: a comparative perspective. Behavioral Ecology and Sociobiology. 1998;42:207–215. [Google Scholar]
- Henzi SP, Barrett L. The value of grooming to female primates. Primates. 1999;40:47–59. doi: 10.1007/BF02557701. [DOI] [PubMed] [Google Scholar]
- Heymann EW. Social behavior of wild moustached tamarins, Saguinus mystax, at the Estacion Biologica Quebrada Blanco, Peruvian Amazonia. American Journal of Primatology. 1996;38:101–113. doi: 10.1002/(SICI)1098-2345(1996)38:1<101::AID-AJP8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Jones CB. Grooming in the mantled howler monkey, Alouatta palliata Gray. Primates. 1979;20:289–292. [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Lazaro-Perea C. Intergroup interactions in wild groups of common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Animal Behaviour. 2001;62:11–21. [Google Scholar]
- Lazaro-Perea C, Castro CSS, Harrison R, Araujo A, Arruda MF, Snowdon CT. Behavioral and demographic changes following the loss of the breeding female in cooperatively breeding marmosets. Behavioral Ecology and Sociobiology. 2000;48:137–146. [Google Scholar]
- Linn GS, Mase D, Lafrancois D, O’Keeffe RT, Lifshitz K. Social and menstrual cycle phase influences on the behavior of group-housed Cebus apella. American Journal of Primatology. 1995;35:41–57. doi: 10.1002/ajp.1350350105. [DOI] [PubMed] [Google Scholar]
- Little RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, North Carolina: SAS Institute; 1996. [Google Scholar]
- Manson JH, Rose LM, Perry S, Gros-Louis J. Dynamics of female–female relationships in wild Cebus capucinus: data from two Costa Rican sites. International Journal of Primatology. 1999;20:679–706. [Google Scholar]
- Martin P, Bateson P. Measuring Behaviour: An Introductory Guide. 2nd edn. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Meller RE, Keverne EB, Herbert J. Behavioural and endocrine effects of naltrexone in male talapoin monkeys. Pharmacology, Biochemistry and Behavior. 1980;13:663–672. doi: 10.1016/0091-3057(80)90010-6. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Digby LJ, Ramakrishnan U, Woodruff DS. Genetic analysis of group composition and breeding system in a wild common marmoset (Callithrix jacchus) population. International Journal of Primatology. 2000;21:1–20. [Google Scholar]
- Noë R, Hammerstein P. Biological markets. Trends in Ecology and Evolution. 1995;10:336–339. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- O’Brien TG. Allogrooming behaviour among adult female wedge-capped capuchin monkeys. Animal Behaviour. 1993;46:499–510. [Google Scholar]
- Parr LA, Matheson MD, Bernstein IS, de Waal FBM. Grooming down the hierarchy: allogrooming in captive brown capuchin monkeys, Cebus apella. Animal Behaviour. 1997;54:361–367. doi: 10.1006/anbe.1996.0419. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed-effects Models in S. Berlin: Springer-Verlag; 2000. [Google Scholar]
- Rabenold KN. Campylorhynchus wrens: The ecology of delayed dispersal and cooperation in the Venezuelan savannah. In: Stacey PB, Koenig WD, editors. Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 1990. pp. 157–196. [Google Scholar]
- Rasa OAE. The dwarf mongoose: a study of behavior and social structure in relation to ecology in a small, social carnivore. Advances in the Study of Behavior. 1987;17:121–161. [Google Scholar]
- Sanchez-Viagra MR, Pope TR, Salas V. Relation of intergroup variation in allogrooming to group social structure and ectoparasite loads in red howlers (Alouatta seniculus) International Journal of Primatology. 1998;19:473–491. [Google Scholar]
- Santee DP, Arruda MF. The Nisia Floresta common marmoset research station. Neotropical Primates. 1994;2:8–11. [Google Scholar]
- Schaffner CM, Caine NG. The peacefulness of cooperatively breeding primates. In: Aureli F, de Waal FBM, editors. Natural Conflict Resolution. Berkeley, California: University of California Press; 2000. pp. 155–169. [Google Scholar]
- Schaffner CM, French JA. Group size and aggression: ‘recruitment incentives’ in a cooperatively breeding primate. Animal Behaviour. 1997;54:171–180. doi: 10.1006/anbe.1996.0413. [DOI] [PubMed] [Google Scholar]
- Schino G. Grooming, competition and social rank among female primates: a meta-analysis. Animal Behaviour. 2001;62:265–271. [Google Scholar]
- Seyfarth RM. A model of social grooming among adult female monkeys. Journal of Theoretical Biology. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Seyfarth RM. The distribution of grooming and related behaviours among adult female vervet monkeys. Animal Behaviour. 1980;28:798–813. [Google Scholar]
- Solomon NG, French JA. Cooperative Breeding in Mammals. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Sparks J. Allogrooming in primates: a review. In: Morris D, editor. Primate Ethology. Chicago: Aldine; 1967. –148.pp. 175 [Google Scholar]
- Statistical Sciences. S-Plus Guide to Statistical and Mathematical Analysis, V. 3.3. Seattle, Washington: StatSci; 1995. [Google Scholar]
- Stacey PB, Koenig WD. Cooperative Breeding in Birds. Long-term Studies of Ecology and Behaviour. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Stevenson MF, Rylands AB. The marmosets, genus Callithrix. In: Mittermeier RA, Rylands AB, Coimbra-Filho da Fonseca AF, AB G, editors. Ecology and Behavior of Neotropical Primates. Washington, D.C.: World Wildlife Fund; 1988. pp. 131–222. [Google Scholar]
- Taira K, Rolls ET. Receiving grooming as a reinforcer for the monkey. Physiology and Behavior. 1996;59:1189–1192. doi: 10.1016/0031-9384(95)02213-9. [DOI] [PubMed] [Google Scholar]
- Tardif SD. The bioenergetics of parental behavior and the evolution of alloparental care in marmosets and tamarins. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge: Cambridge University Press; 1997. –11.pp. 33 [Google Scholar]
- Vehrencamp SL. A model for the evolution of despotic versus egalitarian societies. Animal Behaviour. 1983;31:667–682. [Google Scholar]
- de Waal FBM. The chimpanzee’s service economy: food for grooming. Evolution and Human Behavior. 1997a;18:375–386. [Google Scholar]
- de Waal FBM. Food transfers through the mesh in brown capuchins. Journal of Comparative Psychology. 1997b;111:370–378. doi: 10.1037/0735-7036.111.4.370. [DOI] [PubMed] [Google Scholar]
- Zahavi A. Arabian babblers: the quest for social status in a cooperative breeder. In: Stacey PB, Koenig WD, editors. Cooperative Breeding in Birds. Cambridge: Cambridge University Press; 1990. pp. 103–130. [Google Scholar]
- Zahavi A, Zahavi A. The Handicap Principle: A Missing Piece of Darwin’s Puzzle. Oxford: Oxford University Press; 1997. [Google Scholar]


