Abstract
In order to see clearly when a target is moving slowly, primates with high acuity foveae use smooth-pursuit and vergence eye movements. The former rotates both eyes in the same direction to track target motion in frontal planes, while the latter rotates left and right eyes in opposite directions to track target motion in depth. Together, these two systems pursue targets precisely and maintain their images on the foveae of both eyes. During head movements, both systems must interact with the vestibular system to minimize slip of the retinal images. The primate frontal cortex contains two pursuit-related areas; the caudal part of the frontal eye fields (FEF) and supplementary eye fields (SEF). Evoked potential studies have demonstrated vestibular projections to both areas and pursuit neurons in both areas respond to vestibular stimulation. The majority of FEF pursuit neurons code parameters of pursuit such as pursuit and vergence eye velocity, gaze velocity, and retinal image motion for target velocity in frontal and depth planes. Moreover, vestibular inputs contribute to the predictive pursuit responses of FEF neurons. In contrast, the majority of SEF pursuit neurons do not code pursuit metrics and many SEF neurons are reported to be active in more complex tasks. These results suggest that FEF- and SEF-pursuit neurons are involved in different aspects of vestibular-pursuit interactions and that eye velocity coding of SEF pursuit neurons is specialized for the task condition.
Keywords: Smooth pursuit, vergence, gaze velocity, vestibulo-ocular reflex, semi-circular canal, otolith, frontal eye fields, supplementary eye fields
1. Introduction
Classically, vestibular signals are thought to be present in three distinct regions of the cerebral cortex: restricted portions of the parietal and somatosensory cortices, and the parietoinsular vestibular cortex (PIVC; see ref. 29 for a review). Recent studies, however, have shown a more extensive vestibular-cortical projection. The vestibular nuclei send axons to various regions of the thalamus in rats, cats and monkeys. These thalamic areas, in turn, project directly to various cortical areas [59,100]. These various projections raise the question of what are the different functional roles of the vestibular signals represented in various cortical areas.
Vestibular information is necessary for virtually every aspect of our daily life. It is indispensable for the control of eyes, head or whole body through various vestibular reflexes. In addition, many cognitive functions rely on vestibular input for appropriate behavior in three-dimensional space such as perception of self-movement, spatial perception and memory, visual spatial constancy, and visual object motion perception. Vestibular receptors decompose the motion of the head and its orientation in the Earth’s gravitational field into elementary components, each of which is conveyed to the brainstem by specific channels from the three semicircular canals and two otolith organs [60,116]. These distinct channels provide different pathways for various vestibular reflexes such as the vestibulo-ocular reflex (VOR) and vestibulo-collic reflex (VCR). In addition, 2nd order vestibular nuclear neurons project to the thalamus; the latter, in turn, sends projections to various regions of the cerebral cortex [29].
Individual vestibular signals must be integrated to reconstruct the motion of the head and its orientation in space (e.g., ref. 9). In addition, precise reconstruction requires exteroceptive inputs especially vision and pro-prioceptive inputs from the neck. For example, vestibular receptors respond to rotational or translational acceleration, but are unable to respond to constant velocity motion. Thus, constant velocity motion detection in the light must depend on visual inputs. Likewise, vestibular signals alone cannot distinguish whether the head is moving by itself or if the whole body is moving. Neck inputs can provide information for the brain to distinguish between the two. Neck proprioception can also provide static trunk angle signals with respect to the head while the head is stationary in space. However, in this condition, vestibular receptors should not be activated. Processing of vestibular signals requires interactions with visual, somatosensory and even motor command signals for precise reconstruction of head movement in space. Moreover, during these processes, coordinate transformations are necessary because motion detected by the vestibular end organs and visual information derived from the retina are encoded in different coordinate systems and because final motor output signals are coded in the effector coordinates. Thus, signals in various brain areas are thought to be represented in intermediate coordinates, such as head-centered or body-centered coordinates [6,7,16,95]. Vestibular signals represented in various cortical areas may be used for different aspects of processing vestibular information for each of these separate functions.
In this review, we explore the roles of vestibular signals in the frontal cortex related to smooth pursuit eye movements [29]. Smooth-pursuit eye movements are voluntary movements in primates who possess high-acuity foveae. They evolved in order to ensure clear vision while the animal pursued a small object (target) moving slowly and smoothly. Retinal image motion signals are used to drive the pursuit system and the smooth-pursuit rotates left and right eyes in the same direction (Fig. 1A). Because the foveal field (Fig. 1A) covers only about 2° of the visual fields of each eye, precise eye movements are needed. The primate frontal cortex contains two areas related to smooth-pursuit; the caudal part of the frontal eye fields (FEF) in the fundus of the arcuate sulcus and the supplementary eye fields (SEF) in the dorso-medial cortex. In both areas, many smooth-pursuit related neurons (pursuit neurons) are found (e.g., FEF: refs. 17,66, SEF: refs. 92,44,45).
Fig. 1.
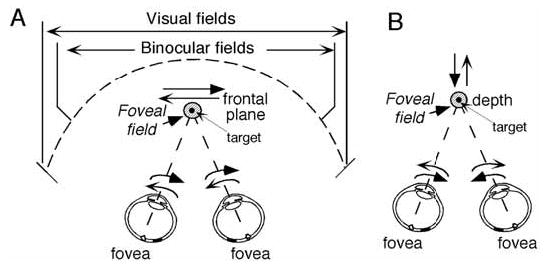
Foveal field and frontal pursuit (A) and vergence pursuit (B). Schematic top views of visual fields and foveal field projection (shaded circles) in 3D space and target (small dot within the foveal field). For frontal target motion (A), left and right eyes rotate in the same directions. For target motion in depth (B), both eyes rotate in the opposite directions.
2. The close functional relationship between pursuit and vestibular systems; requirements for efficient performance of smooth pursuit eye movements
To understand the roles of vestibular signals in the smooth-pursuit areas, it is necessary to know what is required for appropriate execution of pursuit eye movements in the presence of head motion. The goal of the pursuit system is to keep the retinal target image on the fovea by matching the eye velocity in space (i.e., gaze velocity) to target velocity (Fig. 1A). Efficient pursuit performance must meet several criteria. First, if the head and/or whole body move during pursuit, pursuit must compensate for that motion so that the fovea tracks the target-motion-in-space. Head and/or whole body rotation is detected by semi-circular canals and induces the rotational VOR whereas head and/or whole body translation is detected by otolith receptors and induces the translational VOR. The magnitude of the rotational VOR is augmented if the target is very close to the eye, because the axis for eye rotation is a few centimeters in front of the axis for head rotation (the inter-aural midpoint). For the translational (e.g., left-right or fore-aft) VOR, the target distance is critical. The required magnitude can be calculated trigonometrically as the angle between the target distance and the translation distance. If the stationary target is close to the observer (for example, within arm’s reach), the required magnitude is similar to that of the rotational VOR. However, if the target is far away, virtually no compensatory eye movement is required for identical vestibular inputs induced by the same translation [80, 112].
Second, if the target moves in space with the observer (i.e., in the same direction with the same magnitude), the VOR must be cancelled in order to keep the retinal target image on the fovea. How this is done is still controversial, but the pursuit system contributes [60]. Because the required magnitude for the cancellation of the VOR depends on the target distance, the pursuit system must adjust the magnitude of the cancellation signal so that the resulting VOR can be attenuated or augmented.
Third, in daily life, targets move not only in fronto-parallel planes (Fig. 1A) but also in depth. The vergence system is important when the target is close to the observer. This system uses retinal disparity- (i.e., position error signals generated by disparate target images on the retina of each eye) and/or blur-signals as adequate stimuli [60], and rotates left and right eyes in opposite directions (i.e., disconjugately) to pursue targets moving in-depth towards or away from the observer (Fig. 1B). Vergence pursuit during whole body movements also requires interaction with the vestibular system to match the foveal field to the moving target during head rotation as well as head translation. Moreover, both frontal pursuit and vergence systems have the common function of maintaining target images near the foveae to insure online processing of visual signals during target movement. Because targets could move in any direction in three-dimensional space, signals for both systems must be synthesized for pursuit-in-three-dimensions (3D; ref. 30).
Fourth, compared to the short latencies of VOR (about 10 ms), pursuit eye movements have long latencies (about 100 ms for frontal pursuit and about 150 ms for pursuit-in-depth) between changes in target movement and the initiation of changes in pursuit movements [60]. Prediction must be used to compensate for these delays between processing visual motion and/or disparity/disparity-velocity information and the eye-velocity commands that maintain target images near the foveae during pursuit (e.g., ref. 10). Especially during head and/or whole body movement such as sports, predictive pursuit is necessary and is acquired by training. These considerations suggest close functional relationships between pursuit (in the frontal and/or depth planes) and the vestibular systems.
The importance of vestibular inputs to the pursuit system is also demonstrated by the observations that virtually all brain areas known to be related to smooth-pursuit (Fig. 2) respond also to whole-body rotation that activates mainly semicircular canals. These areas include the cerebellar floccular region [11,12,32,58,61, 63,68,69,97], dorsal vermis [88,99], caudal fastigial nucleus [26], medial superior temporal (MST) cortical area [3,7,24,62,79,86,110], caudal FEF [17,34,41,42, 66,105–107], SEF [28,44,45], dorsolateral pontine nucleus (DLPN) and nucleus reticularis tegmenti pontis (NRTP) [78]. Discharge induced by translation, which activates otolith receptors, is also observed in MST neurons [24,78] and caudal FEF [39].
Fig. 2.
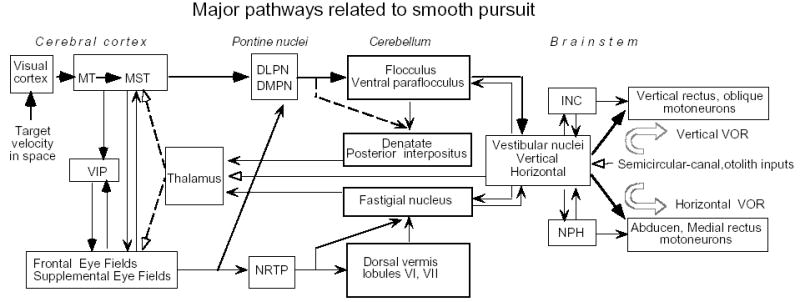
Major pathways related to frontal pursuit and vestibular inputs. Thick lines are proposed main smooth-pursuit pathways [55]. Dashed lines from the thalamus to the cortex indicate that the nature of thalamic pursuit signals are still unknown. Modified from ref. 30.
Figure 3 is an example of vestibular evoked potentials recorded in the frontal cortex following electrical stimulation of the contralateral vestibular nerve; the evoked potentials are localized in the arcuate area including the FEF and post-arcuate area around the spur of the arcuate sulcus [25]. Vestibular projections to the FEF and the immediate vicinity are probably trisynaptic via a relay in the thalamus, as schematically illustrated in Fig. 2 [25].
Fig. 3.
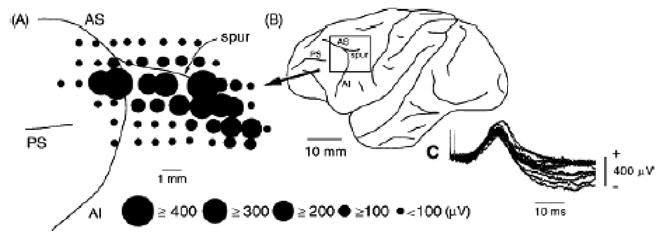
Vestibular-evoked potentials recorded on the surface of the periarcuate cortex of the monkey. A, distribution of vestibular-evoked positive potentials on the periarcuate cortex. The diameter of the circle is proportional to the amplitude of the positive peak of a vestibular-evoked potential at the center of the circle. AI, inferior ramus of the arcuate sulcus; AS, superior ramus of the arcuate sulcus; PS, principal sulcus. B, schematic drawing of the left cerebral cortex showing the recorded periarcuate area in A (boxed area). C, typical surface potentials evoked by stimulation of the contralateral labyrinth with two stimuli at 500 mA. Reproduced from ref. 25 with permission.
Anatomical studies in rats have reported that vestibulo-thalamic fibers that originate from the superior vestibular nucleus and rostral-to-middle parts of the medial vestibular nucleus project to the lateral part of the parafascicular nucleus (corresponding to the centromedian nucleus in primates), the transitional zone between the ventrolateral nucleus (VL) and the ventral posterolateral nucleus, the lateral part of the centrolateral nucleus and the dorsal part of the caudal VL. These thalamic areas project to the frontal cortex including FEF [100]. Vestibular evoked potentials have also been reported in the anterior portion of the supplementary motor area with latencies of ~ 6 ms induced by electrical stimulation of the vestibular nerve in patients [23]. This area seems to correspond to the SEF.
3. General methods to examine vestibular-pursuit interactions
Japanese monkeys (Macaca fuscata) were used. Monkeys’ heads were firmly restrained in the primate chair in the stereotaxic plane, and they were rewarded to pursue a laser spot that was back-projected onto a vertical screen during chair rotation (Fig. 4B) [28,34, 36,37]. The inter-aural midpoint of the animals’ head was brought close to the axis of vertical and horizontal rotation (Fig. 4B). The target and chair moved either sinusoidally or stepwise. Vertical and horizontal components of eye movements were recorded using the scleral search coil method. Extracellular recordings of pursuit neurons were made mostly in the fundus of the arcuate sulcus for FEF and dorsomedial frontal cortex for SEF (Fig. 4C) while the monkeys pursued a moving target. Neurons that showed discharge modulation during pursuit were further tested for their responses during sinusoidal whole body rotation.
Fig. 4.
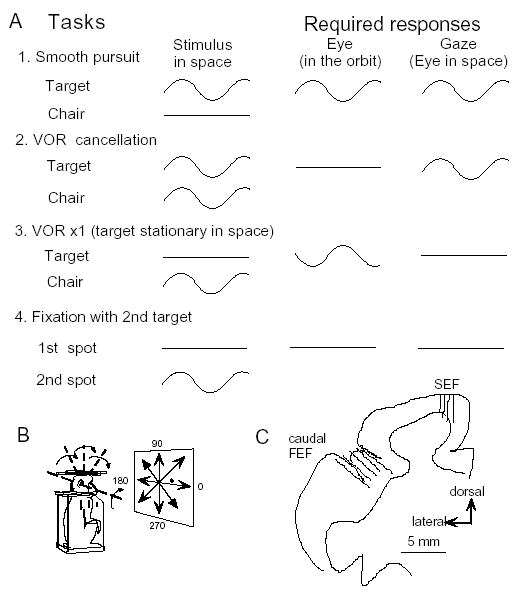
Behavioral tasks used to dissociate eye movements in the orbit from those in space (A,B) and recording locations (C). A and B, Each task (rows) shows the idealized, intended movement of the target and chair-fixed head (first column), the eye (second column) and gaze (third column). C, Recording locations in caudal FEF and SEF [28,34].
Because smooth-pursuit with the head restrained cannot dissociate eye movement in the orbit from eye movement in space (i.e., gaze, Fig. 4A1), two other tasks have routinely been used to distinguish eye movement per se from gaze movement (e.g., ref. 57). In the VOR cancellation task (Fig. 4A2), the monkeys tracked a target that moved in space with the same amplitude, direction and phase as the chair rotation. This condition required the monkeys to cancel the VOR so that the eyes remained virtually motionless in the orbit while gaze moved with the target/chair. In the VOR x1 (Fig. 4A3), the target stayed stationary in space during chair rotation and the monkeys were required to fixate the stationary spot, which required a perfect VOR and gaze remained stationary in space. In addition, to examine visual responses to target motion, a probe stimulus was presented and moved in various directions (2nd spot, 0.6° diameter) while the monkeys fixated a 0.2° stationary spot (1st spot, Fig. 4A4).
4. Comparison of discharge characteristics of FEF and SEF pursuit neurons during passive whole body rotation
To begin to understand the differences between FEF and SEF pursuit neuron activity, we examined their discharge using identical tasks. Basic discharge characteristics of FEF pursuit neurons are illustrated in Fig. 5A1–A4 during smooth pursuit [34,36,38,39,66, 105,107]. The great majority of FEF pursuit neurons have a preferred direction (Fig. 5A2) and the preferred directions of individual FEF neurons are distributed evenly for all directions (Fig. 5A3). For target motion in the preferred direction, the great majority of neurons exhibit discharge modulation that is linearly correlated with peak eye velocity (Fig. 5A4), indicating that FEF pursuit neurons code direction and velocity of pursuit eye movements. About half of FEF pursuit neurons also exhibit visual responses to test-spot motion during fixation of a stationary spot (Fig. 4A4, Table 1). The preferred direction of visual response is similar to the pursuit-preferred direction for each FEF neuron [34, 36]. Moreover, most FEF pursuit neurons respond to vestibular stimulation.
Fig. 5.
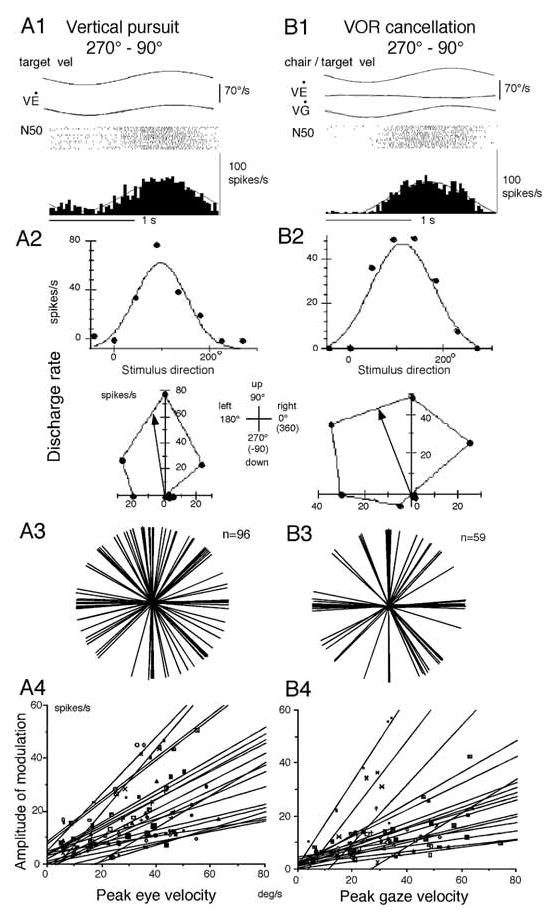
Discharge characteristics of FEF pursuit neurons. A1–A2, discharge of a single neuron during vertical pursuit (A1) and pursuit during different directions (A2). B1 and B2, discharge during VOR cancellation in the pitch plane (B1) and VOR cancellation along different directions (B2). A3 and B3, polar plots of preferred directions of FEF neurons during frontal pursuit (A3) and rotational VOR cancellation (B3). A4 and B4, amplitude of discharge modulation plotted against peak eye (A4) and gaze (B4) velocity for individual neurons (Reproduced and modified from ref. 34 with permission).
Table 1.
Comparison of discharge of SEF and caudal FEF pursuit neurons in different task conditions
| Characteristic | Caudal FEF | SEF |
|---|---|---|
| Frontal-pursuit responding neurons | ||
| Eye velocity coding neurons (among pursuit-related neurons) | ||
| 83% (19/23) | 43% (17/40) | |
| Visual motion (spot) responding neurons (among pursuit-related neurons) | ||
| 53% (21/40) | 20% (7/34) | |
| Vestibular responding neurons | ||
| among pursuit-related neuron | 92% (92/100) | 92% (30/33) |
| Gaze velocity neurons | 66% (66/100) | 17% (4/30) |
| Chair velocity sensitivity (median) | 0.30 sp/s/°/s | 0.35 sp/s/°/s |
| Tracking in 3D space | actual target* stereo target** | stereo target** |
| a. frontal-pursuit + Vergence neurons | 66% (80/122) 63% (106/169) | 27% (15/56) |
| b. frontal-pursuit only neurons | 25% (30/122) 21% (35/169) | 62% (35/56) |
| c. Vergence only neurons | 9% (12/122) 17% (28/169) | 11% (6/56) |
Data for FEF were taken from refs. 2,32,35. Data for SEF were taken from ref. 26. Because the search task conditions were different in the Actual target* [35] and Stereo target** [2,28] tasks, the data are summarized separately. In the former, vertical and horizontal screens were used to present frontal and depth target motion. In the latter, a stereo-target was presented on a computer display (produced by dichoptic presentation of targets to left and right eyes in alternation using shuttered glasses). Vestibular responding neurons indicate the neurons that responded during VOR cancellation and/or VOR in complete darkness.
The great majority of pursuit neurons in SEF also respond to vestibular stimulation and have a preferred direction [28,44,45](see Table 1). However, in contrast to FEF pursuit neurons, over half of SEF pursuit neurons do not code eye velocity, and the majority of SEF pursuit neurons do not exhibit visual motion responses to test-spot motion (Table 1).
4.1. Gaze velocity and eye/head velocity neurons in FEF during passive whole body rotation
During passive whole body rotation, the majority of caudal FEF pursuit neurons exhibit a discharge pattern similar to the gaze velocity response of Purkinje cells in the cerebellar floccular region [61,68]. We have called such neurons gaze-velocity neurons [34]. As illustrated in Fig. 5 (B1–B2) for a representative neuron, such neurons (66/100 = 66%) respond during VOR cancellation and have preferred directions similar to the pursuit-preferred direction (Fig. 5A2, B2, Table 1). Preferred directions for VOR cancellation directions for FEF pursuit neurons are distributed virtually evenly for all directions (Fig. 5B3), and discharge modulation of individual neurons during VOR cancellation along the preferred direction is linearly correlated with peak gaze velocity (Fig. 5B4). These results indicate that signals carried by the majority of FEF pursuit neurons during passive whole body rotation are gaze velocity. This is shown for a representative neuron illustrated in Fig. 6 [32]. Preferred directions and discharge modulation of this neuron during pursuit and VOR cancellation are similar (Fig. 6A, B), but modulation is minimal during VOR x1 that eliminates gaze movement (Fig. 6C, also see Fig. 7A, B, open squares). Therefore, the activity of this neuron is not related to eye movement per se but is related to gaze movement during passive whole body rotation. It also responds, albeit weakly, during VOR in complete darkness with the same preferred direction (Fig. 6D), suggesting that vestibular inputs contribute to the VOR cancellation responses.
Fig. 6.
Discharge characteristics of a single FEF pursuit neuron during different task conditions. A–C, Responses during frontal pursuit, VOR cancellation, and VOR x1, respectively. D, Response during chair rotation in complete darkness. Eye-velocity and gaze-velocity are clipped.
Fig. 7.
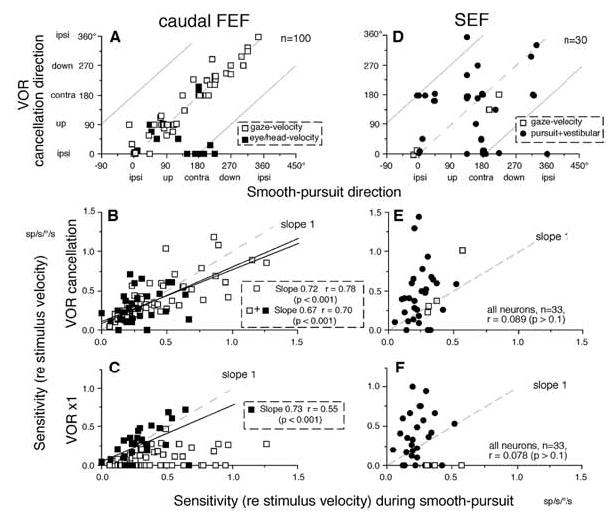
Discharge modulation of FEF and SEF pursuit neurons during frontal-pursuit, VOR cancellation and VOR x1. A and D compare preferred directions during smooth-pursuit and VOR cancellation for FEF and SEF neurons, respectively. Dashed and straight line slopes in A and D = one. B and E compares sensitivity (re stimulus velocity) during smooth-pursuit and VOR cancellation. C and F compares sensitivity (re stimulus velocity) during smooth-pursuit and VOR x1. Open and filled squares in A, B, C are gaze-velocity and eye/head velocity FEF neurons. Open squares and dots in D, E, F are gaze-velocity and pursuit+vestibular SEF neurons, respectively. Reproduced and modified from refs. 28,34 with permission.
In contrast, a minority (32%) of FEF pursuit neurons are called eye/head velocity neurons [34]. Although these neurons also respond during VOR cancellation (Fig. 7A–B, filled squares), they respond clearly during VOR x1 with the magnitude comparable to their response during pursuit (Fig. 7C, filled squares), and more robustly than gaze-velocity neurons during VOR x1 (Fig. 7C, open squares). Moreover, preferred directions of some of this group of neurons during VOR cancellation are opposite to pursuit-preferred directions (Fig. 7A, filled squares on abscissa) but similar to the direction during VOR in complete darkness. These response characteristics are not consistent with the gaze-velocity response [34].
4.2. Pursuit plus vestibular neurons in SEF
Although a similar percentage of SEF pursuit neurons also responded to vestibular stimulation, gaze-velocity signals were rarely represented in SEF pursuit neuron discharge during passive whole body rotation (Table 1). A typical example of SEF discharge is illustrated in Fig. 8 for a single neuron that responds during horizontal pursuit and has a leftward preferred direction (Fig. 8A). During VOR cancellation, its modulation is rightward and almost two times larger than that during horizontal pursuit (Fig. 8B vs 8A). Its activity during VOR x1, which suppresses gaze movement, is even larger than that during VOR cancellation (Fig. 8C). Thus, this neuron does not code gaze velocity [28]. As described earlier, FEF pursuit neurons could be classified either as gaze-velocity or eye/head velocity neurons during whole body rotation (Table 1) [34]. However, the neuron shown in Fig. 8 cannot be classified simply as an eye/head velocity neuron either, because its activity during VOR x1 is almost two times larger than the response during pursuit despite only a small difference in the accompanying eye velocity (eye velocity gains 1.0 vs 0.88 during VOR x1 and pursuit, respectively, Fig. 8A vs C). The clear discharge modulation during VOR in complete darkness further corroborates the existence of vestibular inputs (Fig. 8D). We have therefore, called these SEF neurons pursuit plus vestibular neurons [28].
Fig. 8.
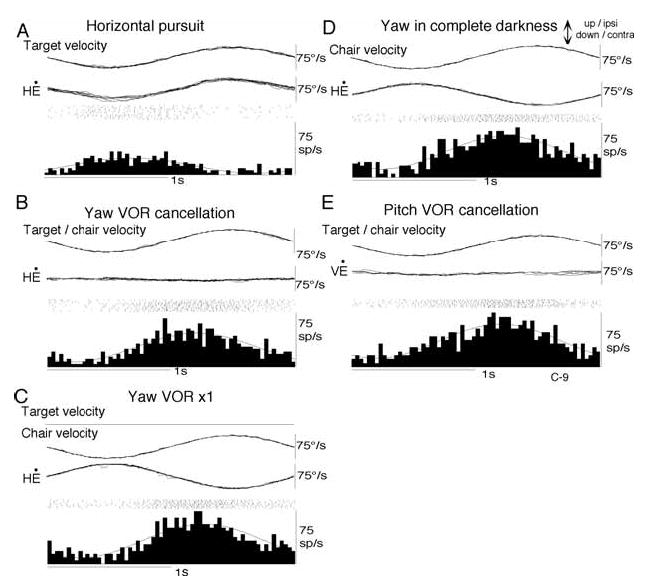
Discharge of a representative pursuit plus vestibular SEF neuron. Discharge during horizontal smooth-pursuit (A), yaw VOR cancellation (B), yaw VOR x1 (C), yaw rotation in complete darkness (D), and pitch VOR cancellation (E). Each section shows stimulus velocity, “de-saccaded” and superimposed horizontal eye-velocity (H Ė or vertical eye velocity (V Ė), spikes rasters, and histograms of neuron discharge with superimposed fitted sine waves. Reproduced from ref. 28 with permission.
Table 1 summarizes the percentage of gaze velocity neurons among neurons that respond to both smooth pursuit and VOR cancellation; only 17% (4/30) of SEF pursuit neurons could be classified as gaze velocity. This percentage is significantly smaller than that of gaze velocity neurons in the caudal FEF (66/100 = 66%, χ2 square test, p < 0.01).
Compared to the vestibular responses of FEF gaze velocity neurons that exhibit clear preferred directions similar to their pursuit-preferred directions (Fig. 5), vestibular preferred directions could not be clearly determined in the majority of SEF pursuit neurons. For example, the neuron shown in Fig. 8 had a leftward preferred direction during smooth-pursuit, and was modulated during VOR cancellation in yaw (Fig. 8B), but was also similarly modulated during pitch (Fig. 8E) and oblique planes (not shown), and during whole body rotation in complete darkness (Fig. 8D). These results suggest that the functional relationship between pursuit and vestibular systems may be different between FEF and SEF.
Figure 7 compares discharge characteristics of SEF and FEF pursuit neurons during smooth pursuit, VOR cancellation and VOR x1. By definition, the gaze velocity response requires similar preferred directions and similar response magnitudes during smooth pursuit and VOR cancellation. Although the majority of caudal FEF pursuit neurons show such responses as described earlier (Fig. 7A,B; points cluster near the dashed line of slope = 1.0), the great majority of SEF pursuit neurons do not (Fig. 7D,E). If neurons coded eye velocity irrespective of vestibular inputs, the modulation during frontal pursuit should have been correlated with modulation during VOR x1, because both require eye movements of identical magnitude. In caudal FEF eye/head velocity neurons (filled squares), significant correlation is observed between the two (Fig. 7C), but there is no clear correlation between the two for SEF pursuit plus vestibular neurons (Fig. 7F, filled circles). These comparisons suggest that the majority of SEF neurons do not code parameters of eye or gaze movement during passive whole body rotation [28].
At present, the role of vestibular signals in SEF is unknown. However, the absence of preferred vestibular directions in SEF pursuit neurons may suggest a role other than signalling gaze velocity. For example, vestibular signals in SEF may play a role in coordinate transformations from eye-centered to head- and/or body- centered coordinates as suggested recently by Park et al. [83, cf. 4; also ref. 67]. Also, as described later (section 8), vestibular information in SEF is necessary for self-centered representation of space during the memory-guided saccade tasks [74,81,82]. Vestibular signals might also be used in learning-related activity (see Section 8.1.).
5. Comparison of FEF and SEF pursuit neurons during pursuit in three dimensions (3D)
Because targets can move in any direction in 3D space, signals for frontal pursuit and pursuit-in-depth must be integrated for pursuit-in-3D to insure efficient performance [30]. Table 1 compares the percentage of pursuit neurons that code pursuit-in-3D in FEF and SEF. Target motion in depth was presented using two different methods for FEF neurons. In earlier experiments, a laser spot was back-projected onto a vertical screen with a horizontal screen at the level of the monkeys’ nose to present another target moving in depth projected from above (Table 1, actual target*, ref. 37). In more recent experiments, a stereo target was presented on a computer display (produced by dichoptic presentation of targets to left and right eyes in alternation using shuttered glasses) (Table 1, stereo target**, refs. 2,28). Frontal pursuit eye movements of our monkeys and neuronal responses of FEF pursuit neurons using the two target presentation conditions were similar [2,28]. The majority of FEF pursuit neurons discharge not only for frontal pursuit but also for vergence eye movements (66% – 63%, Table 1), and their activity during pursuit of a target in 3D space can be approximated by linear addition of their sensitivities to each component [2,37]. About half of FEF neurons responding to vergence pursuit also exhibit visual responses to test-spot-motion-in-depth during fixation of a stationary spot, and the preferred directions of such visual responses are similar to vergence-preferred directions [2]. Moreover, the majority of FEF pursuit neurons discharge before pursuit in frontal and depth planes with typical lead times of 20–40 ms even after subtraction of the visual components of the response. These results suggest that visual signals are appropriate to be converted into pursuit commands carried by FEF pursuit neurons [2].
Using the same task condition (i.e., stereo target presentation, Table 1), the majority of SEF pursuit neurons code either frontal pursuit only (35/56 = 62%) or vergence only (6/56 = 11%), and SEF neurons that respond to both frontal pursuit and vergence (15/56 = 27%) are in the minority (p < 0.05, ref. 28). Table 1 also shows that, in contrast to the majority of caudal FEF neurons that code parameters of frontal pursuit such as eye velocity, gaze velocity, or target velocity, the majority of SEF pursuit neurons do not. These results together with the different responses during passive whole body rotation reviewed in the preceding section, suggest that the SEF and caudal FEF are involved in different aspects of pursuit-vestibular interactions and that eye velocity coding of SEF pursuit neurons is specific to the task condition (see below).
Table 1 indicates that pursuit-in-3D signals are represented in FEF but rarely in SEF. To understand how pursuit-in-3D signals are generated in the caudal FEF, we also compared pursuit signals in MST, because both SEF and FEF are known to receive major projections from MST [60,62]. In particular, many MST pursuit neurons project directly to the FEF [60,111]. Like SEF pursuit neurons, the majority of MST pursuit neurons code either frontal pursuit only (134/219 = 61%) or vergence only (40/219 = 18%), and MST neurons that respond to both frontal pursuit and vergence (45/219 = 21%) are in the minority [3]. These results suggest that pursuit-in-3D signals are generated primarily in the FEF by combining separate MST signals for frontal and pursuit-in-depth signals [2,3].
6. Effects of lesions on pursuit eye movements and VOR cancellation
The close functional relationship between frontal pursuit on the one hand, and VOR cancellation during passive whole body rotation on the other, is also shown during impairment of both functions by inactivation of the FEF pursuit area (Fig. 9E, ref. 33). Injection of a GABA agonist (muscimol, 10–15 μg) into the region where many gaze-velocity neurons are recorded decreases eye velocity during frontal pursuit to nearly half and increases the number of catch-up saccades (Fig. 9A vs C) [96]. VOR cancellation is severely impaired, the monkeys are unable to cancel the VOR, and corrective saccades appear frequently to compensate for impaired VOR cancellation (Fib. 9B vs D). In contrast, the monkeys’ performance of VOR x1, which does not require gaze movement, is not clearly affected by muscimol infusion (not illustrated). Muscimol infusion into the FEF pursuit area also impairs vergence pursuit [37]. These results indicate that gaze pursuit is specifically impaired after caudal FEF lesions, consistent with the loss of signals carried by the majority of pursuit neurons in this region as summarized above (Table 1).
Fig. 9.
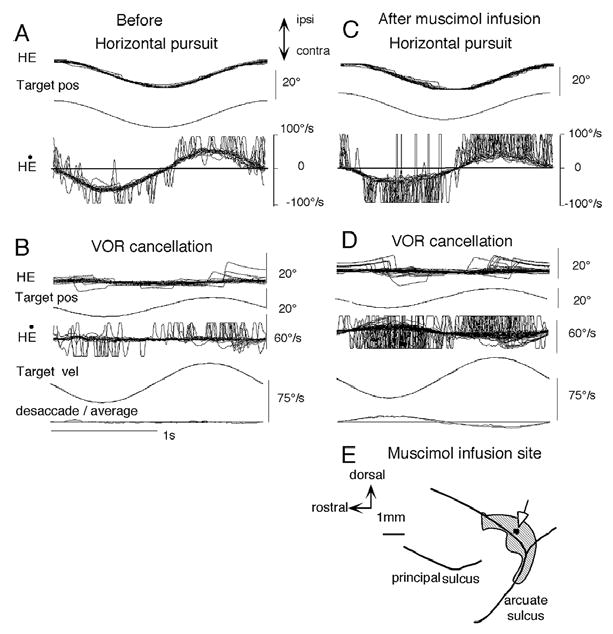
Effects of muscimol injection into the caudal FEF on pursuit. Horizontal pursuit eye movements before (A) and one hour after (C) muscimol injection (15 μg) into the caudal FEF (E). Yaw VOR cancellation before (B) and after (D) muscimol infusion. Abbreviations: HE and H Ė, horizontal eye position and velocity, respectively. Pos and vel, position and velocity, respectively. Reproduced from refs. 33, 39 with permission.
Although electrical stimulation of the SEF has been shown to facilitate smooth eye movements [70], SEF lesions are known to have minimum effects on pursuit (see review by ref. 109). Consistent with these results, muscimol injection into the SEF pursuit area failed to induce clear effects on frontal pursuit and VOR cancellation in the same task conditions (see Section 8.2.). These results are in striking contrast to the deficits in pursuit and VOR cancellation induced by caudal FEF lesions or chemical inactivation [33,39,56,57,65,66,91, 96], suggesting that, with simple ocular pursuit tasks a specific role of the SEF can not be detected. Nonetheless, SEF lesions, in fact, impair pursuit eye movements in special conditions as described below (see Sections 8.2).
7. Further properties of pursuit neurons in FEF and SEF
7.1. Responses of FEF pursuit neurons during linear vestibular stimulation
Maintenance of target images on the foveae is also required during translation of the head or whole body that activates otolith organs. The majority of FEF pursuit neurons (41/70 = 58%) respond to translation [39]. To examine the preferred linear motion directions, the horizontal orientation of the animal was selected by positioning the chair (and hence the monkey) at different orientations as schematically illustrated in Fig. 10A. The monkeys were then oscillated along the same earth-horizontal direction (indicated by arrows) so that linear motion was applied along different directions with respect to the monkeys’ body in complete darkness. An example is shown in Fig. 10B for a representative neuron. This neuron responded during convergence (vergence, Fig. 10B) but minimally during horizontal pursuit (not shown). The preferred linear motion direction in complete darkness was near 0° (Fig. 10B, L0, inset). In contrast, neurons that responded vigorously during horizontal pursuit but minimally during vergence exhibited clear modulation during right/left translation but minimal modulation during fore/aft translation [39].
Fig. 10.
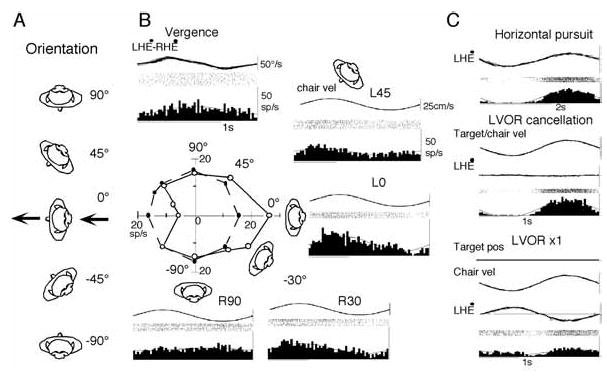
Preferred direction of an FEF pursuit neuron during passive whole body translation in complete darkness (A, B) and discharge modulation of another neuron during right/left translation with a target (C). Linear motion (0.3 Hz, ±10 cm) was given along the same earth-horizontal direction in complete darkness while the orientation of the monkeys’ whole body was changed as indicated in A. B, preferred linear motion direction of a single FEF pursuit neuron. In C, linear motion was applied along left/right direction while the target moved with the monkey (LVOR cancellation) and while the target stayed stationary in space during translation (LVOR x1). Reproduced from ref. 39 with permission.
To understand how otolith inputs interact with pursuit signals, we examined neuronal responses during right/left translation (Fig. 10A, 90°) in two conditions. In one, the target moved with the monkeys and in the other, the target stayed stationary in space. The former required the monkeys to cancel the linear VOR (LVOR) so that the eyes remained stationary in the orbit and gaze moved with the target/chair, whereas the latter required compensatory eye movements and no gaze movement during translation (LVOR x1). These two conditions were tested to compare neuronal responses with gaze velocity responses during the rotational vestibular stimulation (Fig. 6). Representative responses are shown in Fig. 10C for another neuron that discharged during rightward pursuit. This neuron exhibited a gaze velocity response during rotational vestibular stimulation in yaw plane (similar to the responses shown in Fig. 6), and also exhibited robust discharge modulation during LVOR cancellation with modulation and phase similar to that during horizontal pursuit (Fig. 10C). In contrast, the modulation was much weaker when the target stayed stationary in space during right/left translation during LVOR x1 (Fig. 10C). Half of the neurons tested during right/left translation (n = 18) showed similar responses, although some of the remaining neurons responded either only to rotation or to translation [39]. These results suggest that many FEF pursuit neurons carry gaze velocity signals not only during passive whole body rotation which activates mainly semi-circular canals but also during right/left translation which activates otolith organs. Similar analysis has not been done for SEF pursuit neurons.
7.2. Response delay compensation during pursuit and predictive visual responses of FEF and SEF pursuit neurons
Prediction is necessary for efficient pursuit eye movements in order to maintain target images near the foveae. Prediction should occur not only on the motor side as preparation of ongoing movements, but also on the sensory and/or perception side. An example is a visual response that anticipates the eventually renewed direction and speed of a temporarily occluded target movement [115]. Such a mechanism may use memory. Caudal FEF pursuit neurons are involved in prediction [36,66]. Once a predictable target trajectory has been established, changes in motion of a target are compensated in the majority of FEF pursuit neurons, not only for target motion in frontal planes but also in depth. We tested response delay compensation during vergence pursuit for a total of 43 FEF pursuit neurons [2]. As illustrated in Fig. 11, phase shifts (re target velocity) of the majority of FEF neurons (25/43 = 58%) during pursuit-in-depth remain virtually constant up to 1.5 Hz (Fig. 11A, C: open squares, group A neurons); only a minority of FEF pursuit neurons (18/43 = 42%) exhibit clear phase lag as target frequencies increase (Fig. 11C, filled squares, group B neurons).
Fig. 11.
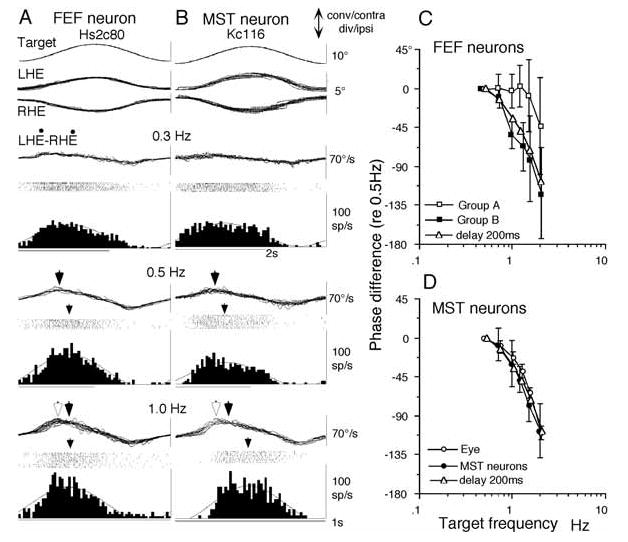
Comparison of vergence pursuit modulation of FEF and MST neurons. A and B, representative discharge modulation of FEF (A) and MST (B) pursuit neurons during sinusoidal vergence pursuit at different frequencies (±5°) as indicated. C compares mean (±SD) phase differences of group A and B FEF pursuit neurons at different frequencies relative to the values at 0.5 Hz. D is a similar plot for MST pursuit neurons and simultaneously recorded eye movement responses. Phase shifts in C and D were calculated by fitting a sinusoid using a least-squared error algorithm in all traces. Notice a distortion manifested in vergence eye velocity at 1.0 Hz (bottom traces in A and B indicated by open and filled arrowheads). Open arrowheads indicate actual peak convergence eye velocity. Filled arrowheads indicate the peak of the fitted function. They are clearly different at 1.0 Hz but virtually identical at 0.5 Hz (also ref. 87). Actual peak convergence eye velocity exhibited phase lag (relative to the value at 0.5 Hz) of less than 10° in A and B at 1.0 Hz that are within the error bars of FEF group A neurons in C. Open triangles in C and D indicate a model that contains a delay of only 200 ms. Reproduced from refs. 2,3 with permission.
Heinen and Liu [45] reported that SEF pursuit neurons exhibit prediction-related activity before initiation of frontal pursuit. Consistent with this observation, many SEF pursuit neurons exhibit phase leads to eye movements during sinusoidal target motion and phase shifts (re target velocity) remain virtually constant during frontal pursuit and pursuit-in-depth, suggesting that SEF pursuit neurons also exhibit delay compensation during sinusoidal pursuit [28].
About half of FEF pursuit neurons receive visual information about target motion in frontal or depth planes [2,34,36]. Visual responses to sinusoidal target motion in depth of many FEF pursuit neurons also exhibit minimum phase lags at higher frequencies [2], suggesting response delay compensation in the visual responses of FEF pursuit neurons. Response delay compensation may be achieved by prediction. Predictive “visual” responses are illustrated in Fig. 12 for a representative FEF pursuit neuron. The monkey fixated a stationary spot while a second spot moved in various directions. Responses to target motion were induced even when it was visible only for a half of the trajectory (Fig. 12A–B, lower panels). Preferred directions were similar with and without a visible target (Fig. 12C, open vs filled circles). Similar responses were evoked even if the second spot was flashed (thus minimizing retinal slip of the second spot image) as it moved while the monkeys fixated the stationary spot [36]. These results suggest that the predictive discharge of FEF pursuit neurons contains visual components that reflect the direction and speed of the reconstructed target image. These signals are sufficient to estimate target motion. Furthermore, because the majority of these neurons receive vestibular inputs with preferred directions similar to the pursuit/visual motion preferred directions as described above (Section 4), FEF pursuit neurons can provide signals for predictive target motion in space during head movement [34].
Fig. 12.
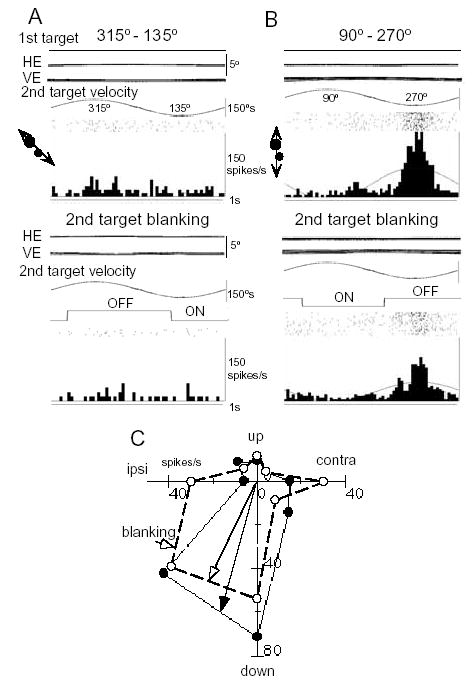
Visual responses of a representative FEF pursuit neuron. For all traces, the monkey fixated a stationary spot while the second test spot moved sinusoidally along different directions (C). Upper panels for A-B are 1st target, horizontal and vertical eye position (HE, VE), second target velocity, and rasters and histograms of neuron responses when the second target was continuously visible. In the lower panels, the second spot was extinguished for more than half of each cycle as indicated (OFF). C shows directional tuning of this neuron with (open circles) and without (filled circles) blanking the second target. Reproduced from ref. 36 with permission.
To understand how response delay compensation is achieved in FEF pursuit neurons during sinusoidal pursuit in depth (Fig. 11), discharge modulation of MST pursuit neurons was examined using the identical task conditions [3]. In contrast to the majority of FEF neurons, MST pursuit neurons exhibit a clear phase lag during vergence pursuit as target frequencies increase (Fig. 11B, D, n = 21) [2,3]. The phase lags of MST neurons (Fig. 11D) and a minority of FEF neurons (group B, Fig. 11C) can be explained by a simple delay of 200 ms (Fig. 11C, D, open triangles) [1]. Thus, response delay during sinusoidal pursuit is compensated in FEF (but not MST) pursuit neurons in our task conditions. How this compensation is accomplished in FEF neurons is unknown. Although SEF may play some role in this process, SEF pursuit neurons rarely exhibit visual responses to target motion in the identical task condition (Table 1). Therefore, the role of the SEF in predictive visual response is not clear.
7.3. Prediction in the timing of pursuit eye movement initiation and FEF neuron activity
Vestibular signals are effective in inducing predictive pursuit eye movement initiation [35,114]. This has been shown in the following experiments; monkeys were trained to pursue a spot moving in a trapezoidal trajectory (20°/s, ± 10°) either vertically or horizontally during whole body rotation with the same trajectory but in the orthogonal plane as illustrated in Fig. 13A. When the target moved at the same time as chair rotation (i.e., 0 delay), latencies of initial frontal pursuit eye movements to vertical spot motion during horizontal rotation were shortened adaptively from about 100 ms (i.e., normal pursuit latency) to less than 50 ms (a latency too short for visual feedback) and initial eye velocities increased within 30 min of training. This initial eye movement response was induced even without a target and the latencies depended on the training task conditions, consistent with the interpretation that it was induced predictively.
Fig. 13.

Stimulus trajectory for cross-axis vestibular-pursuit training and vertical pursuit eye movements after training. A, stimulus trajectory. Inter-trial intervals for chair motion were random (top trace). Chair was rotated in the yaw plane at 20°/s for 1 s. Delay between the onset of chair motion and target motion onset is marked by vertical dashed lines. B shows de-saccaded mean ± SD vertical eye velocity. Dashed line in B indicates the onset of chair motion. Vertical bars with rightward arrows in B indicate the onset of actual target motion at different delays. Upward arrows in B indicate onset of vertical smooth eye movements. All traces are aligned on the onset of chair motion. C plots mean ± SD latencies against the delays between the onset of chair and target motion for cross-axis training in two monkeys. Open diamonds and filled circles are values with and without blanking the target, respectively. 7–10 different recording sessions were combined to calculate mean and SD which was smaller than the symbol size in most cases. Only one plus SD is shown for means with blanking and one minus SD is shown for means without blanking. Linear regressions are shown for filled circles (i.e., without blanking). Reproduced from ref. 114 with permission.
The predictive nature of the initial eye movement response is made clear by changing the delay between the onset of target motion and chair rotation from 100 to 700 ms [114]. Pursuit eye movements after training were initiated before the onset of target motion (Fig. 13B, compare upward arrows and vertical bars). The latencies were proportional to (but shorter by 22–36% than) the actual delays used for training in two monkeys (Fig. 13C, D, filled symbols). Even without the presence of the target, the latencies and velocity of pursuit were similar (Fig. 13C, D, open symbols); for this test, the target was briefly (for 500–700 ms) extinguished at 80 ms after the onset of chair rotation.
To examine whether the vestibular signals acted only indirectly by providing a temporal cue for future target motion, we applied auditory stimuli briefly for 0.5 s or 1 s at the onset of the chair rotation combined with vertical target motion. The latencies of vertical pursuit in this condition were shortened suggesting that the auditory signal was an effective pre-cue. However, when the monkey was tested for the effects of the auditory stimuli combined with the vertical target motion but without chair rotation, this training did not induce an initial pursuit response as it had following vestibular-pursuit training [114]. Thus an auditory pre-cue was not sufficient to produce pursuit prediction. These results suggest that vestibular signals specifically contribute to the timing of predictive pursuit eye movement initiation in cross-axis vestibular-pursuit interactions.
Vergence-vestibular interaction training also shortens considerably the latency of vergence eye movements and increases initial vergence eye velocity induced by a vergence target during pitch rotation, suggesting that similar mechanisms are involved in predictive pursuit-in-depth initiation [1,87].
Although the neural substrates for predictive, cross-axis pursuit are still unknown, the requisite signals are found in the caudal FEF. For example, the majority of FEF pursuit neurons there respond to chair rotation during VOR cancellation and in complete darkness (Table 1) and also respond to frontal pursuit and pursuit in depth. Figure 14 shows an example [39]. This neuron had a downward preferred direction, and during vertical pursuit with a spot alone it was activated with a latency of ~100 ms in association with downward eye velocity (Fig. 14B). Before training, it showed only a weak response to yaw rotation in complete darkness (Fig. 14A). After 30 min of cross-axis pursuit training (yaw rotation combined with vertical pursuit, Fig. 14C), latency to the pursuit response (VĖ) clearly shortened to nearly 50 ms (Fig. 14B vs C, downward arrows onV Ė), and response latencies of this neuron also decreased (Fig. 14B vs C, open arrows on spike histograms). This short-latency, eye movement response must have been produced by the interaction training (Fig. 14C) because it cannot be explained by either the vestibular input alone (Fig. 14A) or by pursuit alone (Fig. 14B). We observed similar changes in 5 FEF pursuit neurons in association with predictive pursuit eye movements. Moreover, the latencies of 13 FEF pursuit neurons examined during ramp chair rotation ranged from 20–90 ms with a modal value of 24 ms (Akao et al. unpub. obs.). These short latency vestibular responses are sufficient for FEF pursuit neurons to contribute to vestibular induced short latency eye-movementresponses [35,39,114]. Similar analysis has not been done for SEF pursuit neurons. Therefore, it is unknown whether predictive pursuit signals during cross axis vestibular-pursuit interactions are generated in the FEF or SEF.
Fig. 14.
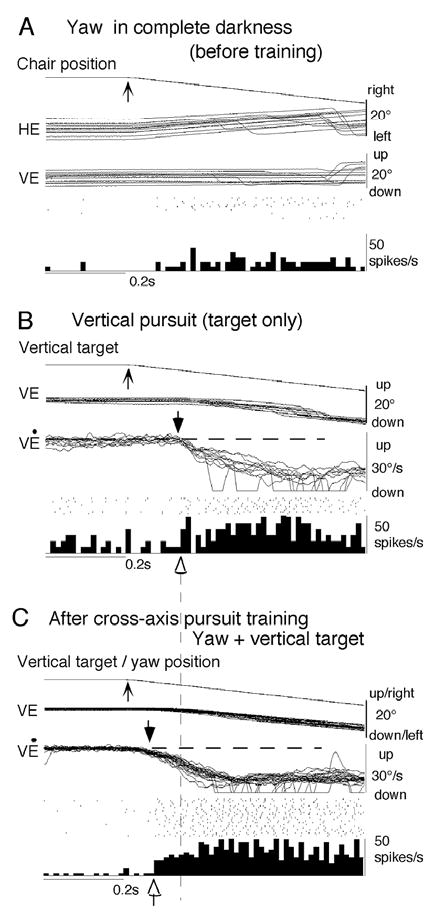
Responses of a caudal FEF pursuit neuron during adaptive pursuit induced by cross-axis vestibular-pursuit training. Superimposed traces of chair/target position and eye position and velocity before training to yaw rotation in complete darkness (A), to downward spot movement (B), and combined presentation of vertical target and yaw rotation after 30 min of similar training (C). Upward arrows on chair/target traces indicate onset of leftward chair rotation (A, C) and downward spot movement (B, C). HE, VE, and VĖ indicate horizontal eye position, vertical eye position and vertical eye velocity, respectively. Reproduced from ref. 39 with permission.
8. Functional differences between FEF and SEF in vestibular-pursuit interactions
To summarize the main results reviewed above, the majority of FEF pursuit neurons receive vestibular (semicircular canal and otolith) inputs and code parameters of pursuit such as eye velocity, gaze velocity, retinal image motion for target velocity, and pursuit-in-3D (Table 1). Response delay compensation is observed in the majority of pursuit neuron activity, and vestibular inputs contribute to predictive pursuit responses of FEF neurons. In contrast, although the majority of SEF pursuit neurons receive vestibular inputs, they do not code pursuit parameters or gaze velocity. These results suggest that the SEF and caudal FEF are involved in different aspects of vestibular-pursuit interactions and that eye velocity coding of SEF pursuit neurons is only manifest under specialized task conditions.
SEF has been implicated as the area that integrates complex visuo-spatial information and controls eye-head gaze shifts (e.g., ref. 67). Single-unit recordings have shown that saccade-related SEF neurons can encode visual targets in both eye-centered and object-centered coordinates in the context of task conditions [76,77,83,93]. The importance of vestibular signals for SEF function has been suggested by clinical studies using the vestibular contingent memory-guided saccade tasks in order to examine spatial perception and memory [13]. Briefly, the subjects fixated a stationary target and then changed fixation to a very low intensity, small light-emitting diode (LED) that was fixed to the head. The subjects were instructed to continue fixating the LED during sudden whole-body rotation in the horizontal plane either to the left or right in complete darkness. After cessation of rotation, the LED was extinguished, and this was the signal that the subject had to make a voluntary saccadic eye movement to the remembered location of the original earth-fixed target. Normal subjects could accurately locate the target position in space in this task [13,14], whereas labyrinthine-defective subjects were unable to do so [74], indicating the necessity of vestibular information for this task. Israël et al. [49,50] and Pierrot-Deseilligny et al. [81, 82] reported that vestibular contingent memory-guided saccades are impaired in patients with SEF lesions, although they did not exhibit abnormalities in memory-guided saccade tasks without vestibular stimulation. These observations suggest that vestibular information is necessary for self-centered representation of space during the memory-guided saccade tasks.
8.1. Task-dependent SEF neuronal activity
In addition to its well-known saccade-related activity [85,90,92],the SEF has learning-related activity [19, 75] and is thought to play an important role in complex behaviors such as, planning of saccades [77], decision-making processes [22], sequential performance of saccades [47,48,64,85], antisaccades [93], and eye-hand reach coordination [72]. Reward-predicting activity has also been reported [5]. These results indicate that, as Tanji [108] clearly states, “the usage of the SEF is more dependent on the behavior or conditional state than the usage of the FEF”. As described earlier (Figs 13–14), vestibular signals specifically contributed to the timing of predictive pursuit eye movement initiation in cross-axis vestibular-pursuit interactions. The possibility that SEF could be involved in this process needs to be tested in future studies.
8.2. Involvement of SEF in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates
For execution of appropriate pursuit eye movements, visual target-motion signals must be processed spatially (i.e., direction) and temporally (i.e., speed), and the strength of this visual-motor transmission for pursuit (i.e., gain) must be appropriately controlled [62, 94]. Young primates (8–11 years old human children, 3–4 years old Japanese monkeys) exhibit asymmetric eye movements during vertical pursuit across a textured backgroundsuch that upward pursuit has low gain and requires many catch-up saccades [104]. This upward pursuit deficit is correlated with inability to cancel the downward VOR during upward pitch rotation when monkeys are required to fixate a target that moves with them. Although several suggestions have been made for the neural mechanisms of VOR cancellation (Fig. 4A2, for review, see ref. 60), the asymmetric eye movements during vertical pursuit are specific for upward, primarily eye pursuit in the orbit [54], suggesting that the inability to generate appropriate upward eye pursuit signals to cancel the downward VOR is the main cause for the poor performance during the VOR cancellation task in young primates.
It is well known that upward and downward pursuit signals are organized asymmetrically in the brainstem (e.g., refs. 15,53; for review, see ref. 60) and the floccular region, which consists of the flocculus and ventral paraflocculus [31,58,63,97,101]. The flocculus projects to the vestibular nuclei, particularly the medial and ventrolateral parts of the medial vestibular nucleus, superior vestibular nucleus and y group, whereas the ventral paraflocculus projects not only to the above vestibular nuclear regions but also to the posterior in-terpositus and dentate nuclei [73]. Preferred directions of majority of pursuit Purkinje cells in the simian floccular region are either ipsiversive or downward [32, 58,69,97,101] (also ref. 99 for dorsal vermis Purkinje cells). It has been assumed that downward floccular Purkinje cells inhibit upward eye- and head- velocity neurons in the vestibular nuclei and y group [20,98, 117]. In contrast, upward floccular Purkinje cells that presumably inhibit downward eye velocity neurons in the vestibular nuclei are reported to be scarce [117]. These observations suggest that floccular control of upward pursuit will be done not only by increased activity of upward Purkinje cells through inhibition of downward eye velocity neurons in the vestibular nuclei but, more importantly, by decreased activity of downward Purkinje cells through dis-inhibition of upward eye-and head- velocity neurons in the vestibular nuclei and y group [117].
It should be pointed out that downward eye velocity neurons in the vestibular nuclei are activated by inputs from the posterior semi-circular canal (see ref. 60 for a review). The difficulty young primates have in cancelling the downward VOR during upward pitch rotation [104] may reflect the scarcity of upward floccular Purkinje cells that presumably inhibit downward eye velocity vestibular neurons as described above [117]. Asymmetry in floccular inhibition of VOR relay neurons is well known in rabbits and cats [46,52,89]. Asymmetry in low frequency responses of anterior and posterior canal vestibulo-ocular neurons in the vestibular nuclei has also been reported in alert cats when the animals are rotated on their side [15].
In addition, possible non-linearity in discharge modulation of downward Purkinje cells for off-direction upward pursuit in simians may further contribute to the asymmetry [63,97]. Moreover, many floccular, pursuit Purkinje cells discharge for vergence eye movements and the majority of them have vergence eye position sensitivity [69,113]. This suggests that off-direction target distance may further augment non-linearity in the discharge rate-eye velocity relationships of downward Purkinje cells during upward pursuit by affecting their resting discharge rates. These results suggest that the directional asymmetry most probably reflects the difference in the floccular-vestibular organization and the possible non-linearity in discharge rates of pursuit neurons in the component pathways [54].
The directional asymmetry is compensated in adult primates [54,104]. Although neural mechanisms for this compensation are still unknown, chemical inactivation of the SEF in adult monkeys reproduced the directional asymmetry that had been compensated developmentally as illustrated in Fig. 15 (B–E). If the target was moved across a stationary structured background, after muscimol infusion into the SEF pursuit area, our monkeys exhibited impairment of upward pursuit (Fig. 15B) and impairment of downward VOR cancellation during upward pitch (Fig. 15E) despite the fact that the same monkeys did not show impairment in pursuit across a homogeneous background after infusion (Fig. 15C) [27]. This is in contrast to the impairment induced by muscimol infusion into the caudal FEF. FEF inactivation also impaired vertical pursuit across the textured background, but the effects were less selective, because a similar impairment was observed across the homogeneous background. These results suggest that the SEF is involved specifically in the compensation of the directional asymmetry [27].
Fig. 15.
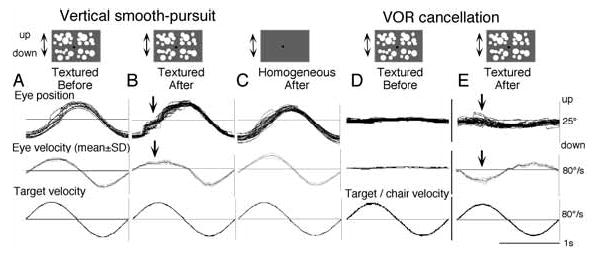
Effects of muscimol infusion into SEF (A–E) for vertical pursuit and pitch VOR cancellation. All eye velocity records in A–E were de-saccaded and averaged. Arrows in B and E indicate impaired smooth eye movements. Reproduced from ref. 27 with permission.
A hint to the neural basis for the compensation of the directional asymmetry might be found by examining how the directional asymmetry reappears during SEF inactivation. Because preferred directions for individual SEF pursuit neurons are distributed virtually evenly for all directions [28,44], it is difficult to explain the reproduced directional asymmetry solely by the loss of SEF output signals. Alternatively, the directional asymmetry may be reproduced by the loss of input signals to the SEF [27,54]. Because the floccular region could furnish ascending pursuit signals through the deep cerebellar and vestibular nuclei as described above, it may well be that the loss of upward eye-velocity-feedback signals from the floccular region to the SEF is responsible for the reappearance of the directional asymmetry induced by SEF inactivation (Fig. 2, Fig. 15B, E). Conversely, the compensation for the low gain of upward visual-motor transmission may well be achieved by a gain increase in the eye-velocity-feedback loop from the floccular region to the SEF [27,54]. The floccular region is well known to be involved in motor learning [51]. The compensation process may involve motor learning through the eye-velocity-feedback loop (Fig. 2) [27,31,51]. It is also well known that eye-velocity-feedback is necessary for the gain control of accurate pursuit [84], although the neural basis for such feedback is still incompletely understood (see ref. 60 for a review). An involvement of the SEF in smooth pursuit gain control has been shown on the basis of electrical stimulation of the SEF [70, 71]. Gain control in pursuit by the FEF has also been demonstrated by electrical stimulation of the FEF pursuit area [106], and this control is primarily to control gaze velocity during passive rotation [18].
Although further studies are needed to critically test these possibilities for the neural mechanisms of the directional asymmetry and of its compensation observed in Japanese macaques, a similar asymmetry was also reported in a rhesus macaque during vertical pursuit across a dimly illuminated stationary background [43]. This observation together with previous results showing an analogous asymmetry in human children [104] suggests that the directional asymmetry between upward and downward pursuit is observed widely in young primates.
9. Unresolved questions and future studies
The list of possibly different roles of the FEF and SEF in vestibular-pursuit interactions is limited by our lack of information. Many unresolved questions have been pointed out in the preceding sections. Two additional points should be added here. First, there remains the question of how pursuit neurons in FEF and SEF respond during active gaze pursuit with the head free to move. In daily life, the head is unrestrained and eye and head movements are coordinated during pursuit. It would be useful to examine whether pursuit signals in SEF and FEF code specifically eye-in-space movements or if they code head movements as well. Moreover, during head pursuit on a stationary trunk, somatosensory neck inputs must be activated. Because vestibular inputs cannot make a distinction whether the head alone is moving or whether the whole body is moving, neck inputs must contribute to that distinction. Second, information about coordinate frames is needed for pursuit signals in FEF and SEF. This must be examined in head-free conditions to dissociate head-centered and body-centered coordinates.
Preliminary studies in monkeys with their head free to rotate about a vertical axis indicate that FEF contains many pursuit neurons that exhibit a gaze velocity response during head free gaze pursuit. Also, the majority of FEF pursuit neurons are modulated not only during eye- and gaze-pursuit but also during head-pursuit to a moving reward (juice feeder) while the monkeys fixate an earth-stationary spot without gaze movement [40]. Moreover, if the trunk alone was rotated while the head remained stationary in space facing a screen during pursuit, discharge of the majority of FEF pursuit neurons was modulated by neck inputs, suggesting that both head-centered and body-centered coordinates are present in FEF for processing eye-, head, and gaze-pursuit signals [4] (also Akao et al. un-pub. obs). Further studies are needed for SEF pursuit neurons to compare the different roles of FEF and SEF pursuit signals.
10. Summary
We have reviewed the differences in pursuit neuron activity between FEF and SEF in primates using identical task conditions with or without whole body rotation. Evoked potential studies indicate vestibular projections to both areas. Pursuit neurons in both areas respond to vestibular stimulation. The majority of FEF pursuit neurons code parameters of pursuit such as eye velocity, gaze velocity, retinal image motion for target velocity, and pursuit in three dimensions. Moreover, vestibular inputs contribute to predictive pursuit responses of FEF neurons, and response delay is compensated in FEF pursuit neuron activity. In contrast, the majority of SEF pursuit neurons do not code pursuit parameters or gaze velocity. These results suggest that the SEF and caudal FEF are involved in different aspects of vestibular-pursuit interactions and that eye velocity coding of SEF pursuit neurons is specific to the task conditions.
Acknowledgments
Supported by Grant-in-Aid for Scientific Research on Priority Areas (System study on higher-order brain functions) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17022001), Marna Cosmetics, Toyota Riken, and National Institutes of Health Grants EY-06558 (NEI) and RR-00166.
References
- 1.Akao T, Kurkin S, Fukushima K. Latency of adaptive vergence eye movements induced by vergence-vestibular interaction training in monkeys. Exp Brain Res. 2004;158:129–132. doi: 10.1007/s00221-004-2002-2. [DOI] [PubMed] [Google Scholar]
- 2.Akao T, Kurkin S, Fukushima J, Fukushima K. Visual and vergence eye movement related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp Brain Res. 2005;164:92–108. doi: 10.1007/s00221-004-2213-6. [DOI] [PubMed] [Google Scholar]
- 3.Akao T, Mustari MJ, Fukushima J, Kurkin S, Fukushima K. Discharge characteristics of pursuit neurons in MST during vergence eye movements. J Neurophysiol. 2005;93:2415–2434. doi: 10.1152/jn.01028.2004. [DOI] [PubMed] [Google Scholar]
- 4.Akao T, Kasahara S, Kurkin S, Fukushima K. Coordinate frames in representing pursuit signals in simian frontal eye fields (FEF) J Physiol Sci. 2006;56(Suppl):S187. (Abstr). [Google Scholar]
- 5.Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol. 2000;84:2166–2170. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- 6.Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- 7.Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- 8.Angelaki DE. Eyes on target: what neurons must do for the vestibuloocular reflex during linear motion. J Neurophysiol. 2004;92:20–35. doi: 10.1152/jn.00047.2004. [DOI] [PubMed] [Google Scholar]
- 9.Angelaki DE, Shaikh AG, Green AM, Dickman JD. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. doi: 10.1038/nature02754. [DOI] [PubMed] [Google Scholar]
- 10.Barnes G. Visual-vestibular interaction in the control of head and eye movement: the role of visual feedback and predictive mechanisms. Prog Neurobiol. 1993;41:435–472. doi: 10.1016/0301-0082(93)90026-o. [DOI] [PubMed] [Google Scholar]
- 11.Belton T, McCrea RA. Contribution of the cerebellar flocculus to gaze control during active head movements. J Neurophysiol. 1999;81:3105–3109. doi: 10.1152/jn.1999.81.6.3105. [DOI] [PubMed] [Google Scholar]
- 12.Belton T, McCrea RA. Role of the cerebellar flocculus region in cancellation of the VOR during passive whole body rotation. J Neurophysiol. 2000;84:1599–1613. doi: 10.1152/jn.2000.84.3.1599. [DOI] [PubMed] [Google Scholar]
- 13.Bloomberg J, Melvill GJ, Segal B, McFarlane S, Soul J. Vestibular-contingent voluntary saccades based on cognitive estimates of remembered vestibular information. Adv Oto-Rhinolaryngol. 1988;41:71–75. doi: 10.1159/000416034. [DOI] [PubMed] [Google Scholar]
- 14.Bloomberg J, Melvill GJ, Segal B. Adaptive modification of vestibularly perceived rotation. Exp Brain Res. 1991;84:47–56. doi: 10.1007/BF00231761. [DOI] [PubMed] [Google Scholar]
- 15.Brettler SC, Baker JF. Timing of low frequency responses of anterior and posterior canal vestibulo-ocular neurons in alert cats. Exp Brain Res. 2003;149:167–173. doi: 10.1007/s00221-002-1348-6. [DOI] [PubMed] [Google Scholar]
- 16.Brotchie PR, Andersen RA, Snyder LH, Goodman SJ. Head position signals used by parietal neurons to encode locations of visual stimuli. Nature. 1995;375:232–235. doi: 10.1038/375232a0. [DOI] [PubMed] [Google Scholar]
- 17.Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- 18.Carey MR, Lisberger SG. Signals that modulate gain control for smooth pursuit eye movements in monkeys. J Neurophysiol. 2004;91:623–631. doi: 10.1152/jn.00525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LL, Wise SP. Neuronal activity in the supplementary eye field during acquisition of conditional oculomotor associations. J Neurophysiol. 1995;73:1101–1121. doi: 10.1152/jn.1995.73.3.1101. [DOI] [PubMed] [Google Scholar]
- 20.Chubb MC, Fuchs AF. Contribution of y group of vestibular nuclei and dentate nucleus of cerebellum to generation of vertical smooth eye movements. J Neurophysiol. 1982;48:75–99. doi: 10.1152/jn.1982.48.1.75. [DOI] [PubMed] [Google Scholar]
- 21.Churchland AK, Lisberger SG. Discharge properties of MST neurons that project to the frontal pursuit area in macaque monkeys. J Neurophysiol. 2005;94:1084–1090. doi: 10.1152/jn.00196.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coe B, Tomihara K, Matsuzawa M, Hikosaka O. Visual and anticipatory bias in three cortical eye fields of the monkey during an adaptive decision-making task. J Neurosci. 2002;22:5081–5090. doi: 10.1523/JNEUROSCI.22-12-05081.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Waele C, Baudonniere PM, Lepecq JC, Huy PTB, Vidal PP. Vestibular projections in the human cortex. Exp Brain Res. 2001;141:541–551. doi: 10.1007/s00221-001-0894-7. [DOI] [PubMed] [Google Scholar]
- 24.Duffy CJ. MST neurons respond to optic flow and translational movement. J Neurophysiol. 1998;80:1816–1827. doi: 10.1152/jn.1998.80.4.1816. [DOI] [PubMed] [Google Scholar]
- 25.Ebata S, Sugiuchi Y, Izawa Y, Shinomiya K, Shinoda Y. Vestibular projection to the periarcuate cortex in the monkey. Neurosci Res. 2004;49:55–68. doi: 10.1016/j.neures.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs AF, Robinson FR, Straube A. Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol. 1994;72:2714–2728. doi: 10.1152/jn.1994.72.6.2714. [DOI] [PubMed] [Google Scholar]
- 27.Fukushima J, Akao T, Takeichi N, Kaneko CRS, Fukushima K. Involvement of the frontal oculomotor areas in developmental compensation for the directional asymmetry in smooth pursuit eye movements in young primates. Ann NY Acad Sci. 2003;1004:451–456. [Google Scholar]
- 28.Fukushima J, Akao T, Takeichi N, Kurkin S, Kaneko CRS, Fukushima K. Pursuit-related neurons in the supplementary eye fields: discharge during pursuit and passive whole body rotation. J Neurophysiol. 2004;91:2809–2825. doi: 10.1152/jn.01128.2003. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima K. Corticovestibular interactions: anatomy, electrophysiology, and functional considerations. Exp Brain Res. 1997;117:1–16. doi: 10.1007/pl00005786. [DOI] [PubMed] [Google Scholar]
- 30.Fukushima K. Frontal cortical control of smooth-pursuit. Curr Opin Neurobiol. 2003;13:647–654. doi: 10.1016/j.conb.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Fukushima K. Roles of the cerebellum in pursuit-vestibular interactions. Cerebellum. 2003;2:223–232. doi: 10.1080/14734220310016178. [DOI] [PubMed] [Google Scholar]
- 32.Fukushima K, Fukushima J, Kaneko CRS, Fuchs AF. Vertical Purkinje cells of the monkey floccular lobe: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol. 1999;82:787–803. doi: 10.1152/jn.1999.82.2.787. [DOI] [PubMed] [Google Scholar]
- 33.Fukushima K, Sato T, Fukushima J. Vestibular-pursuit interactions: gaze-velocity and target-velocity signals in the monkey frontal eye fields. Ann NY Acad Sci. 1999;871:248–259. doi: 10.1111/j.1749-6632.1999.tb09189.x. [DOI] [PubMed] [Google Scholar]
- 34.Fukushima K, Sato T, Fukushima J, Shinmei Y, Kaneko CRS. Activity of smooth pursuit-related neurons in the monkey periarcuate cortex during pursuit and passive whole body rotation. J Neurophysiol. 2000;83:563–587. doi: 10.1152/jn.2000.83.1.563. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima K, Wells SG, Takeichi N, Yamanobe T, Fukushima J. Adaptive changes in smooth pursuit eye movement induced by pursuit-vestibular interaction training in monkeys. Exp Brain Res. 2001;139:473–481. doi: 10.1007/s002210100792. [DOI] [PubMed] [Google Scholar]
- 36.Fukushima K, Yamanobe T, Shinmei Y, Fukushima J. Predictive responses of periarcuate pursuit neurons to visual target motion. Exp Brain Res. 2002;145:104–120. doi: 10.1007/s00221-002-1088-7. [DOI] [PubMed] [Google Scholar]
- 37.Fukushima K, Yamanobe T, Shinmei Y, Fukushima J, Kurkin S, Peterson BW. Coding of smooth eye movements in three-dimensional space by frontal cortex. Nature. 2002;419:157–162. doi: 10.1038/nature00953. [DOI] [PubMed] [Google Scholar]
- 38.Fukushima K, Yamanobe T, Shinmei Y, Fukushima J, Kurkin S. Role of the frontal eye fields in smooth gaze tracking. Prog Brain Res. 2004;143:391–401. doi: 10.1016/S0079-6123(03)43037-9. [DOI] [PubMed] [Google Scholar]
- 39.Fukushima K, Akao T, Kurkin S, Fukushima J. Role of vestibular signals in the caudal part of the frontal eye fields in pursuit eye movements in three-dimensional space. Ann NY Acad Sci. 2005;1039:272–282. doi: 10.1196/annals.1325.026. [DOI] [PubMed] [Google Scholar]
- 40.Fukushima K, Kasashara S, Akao T, Kurkin SA. Discharge of pursuit neurons in frontal eye fields during active head movements, Program No. 166.6. 2005 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; Nov. 12–16, 2005. [Google Scholar]
- 41.Gottlieb JP, Bruce CJ, MacAvoy MG. Smooth eye movements elicited by microstimulation in the primate frontal eye field. J Neurophysiol. 1993;69:786–799. doi: 10.1152/jn.1993.69.3.786. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth pursuit eye movements and their correspondence with electrically elicited slow eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- 43.Grasse KL, Lisberger SG. Analysis of a naturally occurring asymmetry in vertical smooth pursuit eye movements in a monkey. J Neurophysiol. 1992;67:164–179. doi: 10.1152/jn.1992.67.1.164. [DOI] [PubMed] [Google Scholar]
- 44.Heinen SJ. Single neuron activity in the dorsomedial frontal cortex during smooth pursuit eye movements. Exp Brain Res. 1995;104:357–361. doi: 10.1007/BF00242022. [DOI] [PubMed] [Google Scholar]
- 45.Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Visual Neurosci. 1997;14:853–865. doi: 10.1017/s0952523800011597. [DOI] [PubMed] [Google Scholar]
- 46.Hirai N, Uchino Y. Floccular influence on excitatory relay neurones of vestibular reflexes of anterior semicircular canal origin in the cat. Neurosci Res. 1984;1:327–340. doi: 10.1016/0168-0102(84)90037-3. [DOI] [PubMed] [Google Scholar]
- 47.Isoda M, Tanji J. Cellular activity in the supplementary eye field during sequential performance of multiple saccades. J Neurophysiol. 2002;88:3541–3545. doi: 10.1152/jn.00299.2002. [DOI] [PubMed] [Google Scholar]
- 48.Isoda M, Tanji J. Contrasting neuronal activity in the supplementary and frontal eye fields during temporal organization of multiple saccades. J Neurophysiol. 2003;90:3054–3065. doi: 10.1152/jn.00367.2003. [DOI] [PubMed] [Google Scholar]
- 49.Israël I, Rivaud S, Berthoz A, Pierrot-Deseilligny C. Cortical control of vestibular memory-guided saccades. Ann NY Acad Sci. 1992;656:472–484. doi: 10.1111/j.1749-6632.1992.tb25229.x. [DOI] [PubMed] [Google Scholar]
- 50.Israël I, Fetter M, Koenig E. Vestibular perception of passive whole-body rotation about horizontal and vertical axes in humans: goal-directed vestibulo-ocular reflex and vestibular memory-contingent saccades. Exp Brain Res. 1993;96:335–346. doi: 10.1007/BF00227113. [DOI] [PubMed] [Google Scholar]
- 51.Ito M. Movement and thought: identical control mechanisms by the cerebellum. Trends Neurosci. 1993;16:448–450. doi: 10.1016/0166-2236(93)90073-u. [DOI] [PubMed] [Google Scholar]
- 52.Ito M, Nishimaru N, Yamamoto M. Specific patterns of neuronal connections involved in the control of the rabbit’s vestibulo-ocular reflexes by the cerebellar flocculus. J Physiol. 1977;265:833–854. doi: 10.1113/jphysiol.1977.sp011747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaneko CRS, Fukushima K. Discharge characteristics of vestibular saccade neurons in alert monkeys. J Neurophysiol. 1998;79:835–847. doi: 10.1152/jn.1998.79.2.835. [DOI] [PubMed] [Google Scholar]
- 54.Kasahara S, Akao T, Fukushima J, Kurkin S, Fukushima K. Further evidence for selective difficulty of upward eye pursuit in young monkeys: effects of optokinetic stimulation, static roll tilt, and active head movements. Exp Brain Res. 2006;171:306–321. doi: 10.1007/s00221-005-0278-5. [DOI] [PubMed] [Google Scholar]
- 55.Kawano K. Ocular tracking: behavior and neurophysiology. Curr Opin Neurobiol. 1999;9:467–473. doi: 10.1016/S0959-4388(99)80070-1. [DOI] [PubMed] [Google Scholar]
- 56.Keating EG. Frontal eye field lesions impair predictive and visually-guided pursuit eye movements. Exp Brain Res. 1991;86:311–323. doi: 10.1007/BF00228954. [DOI] [PubMed] [Google Scholar]
- 57.Keating EG. Lesions of the frontal eye field impair pursuit eye movements, but preserve the predictions driving them. Behav Brain Res. 1993;53:91–104. doi: 10.1016/s0166-4328(05)80268-2. [DOI] [PubMed] [Google Scholar]
- 58.Krauzlis RJ, Lisberger SG. Directional organization of eye movement and visual signals in the floccular lobe of the monkey cerebellum. Exp Brain Res. 1996;109:289–302. doi: 10.1007/BF00231788. [DOI] [PubMed] [Google Scholar]
- 59.Lai H, Tsumori T, Shiroyama T, Yokota S, Nakano K, Yasui U. Morphological evidence for a vestibulo-thalamo-striatal pathway via the parafascicular nucleus in the rat. Brain Res. 2000;872:208–214. doi: 10.1016/s0006-8993(00)02457-4. [DOI] [PubMed] [Google Scholar]
- 60.Leigh RJ, Zee DS. The neurology of eye movements. 3rd ed. Oxford University Press: New York; 1999. pp. 4–197. [Google Scholar]
- 61.Lisberger SG, Fuchs AF. Role of primate flocculus during rapid behavioral modification of vestibuloocular reflex. I. Purkinje cell activity during visually guided horizontal smooth-pursuit eye movements and passive head rotation. J Neurophysiol. 1978;41:733–763. doi: 10.1152/jn.1978.41.3.733. [DOI] [PubMed] [Google Scholar]
- 62.Lisberger S, Morris E, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- 63.Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994;72:954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- 64.Lu X, Matsuzawa M, Hikosaka O. A neural correlate of oculomotor sequences in supplementary eye field. Neuron. 2002;34:317–325. doi: 10.1016/s0896-6273(02)00657-8. [DOI] [PubMed] [Google Scholar]
- 65.Lynch JC. Frontal eye field lesions in monkeys disrupt visual pursuit. Exp Brain Res. 1987;68:437–441. doi: 10.1007/BF00248811. [DOI] [PubMed] [Google Scholar]
- 66.MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth pursuit eye movement representation in the primate frontal eye field. Cerebral Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- 67.Martinez-Trujillo JC, Medendorp WP, Wang H, Crawford JD. Frames of reference for eye-head gaze commands in primate supplementary eye fields. Neuron. 2004;44:1057–1066. doi: 10.1016/j.neuron.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 68.Miles FA, Fuller JH. Visual tracking and the primate flocculus. Science. 1975;189:1000–1003. doi: 10.1126/science.1083068. [DOI] [PubMed] [Google Scholar]
- 69.Miles FA, Fuller JH, Braitman DJ, Dow BM. Long-term adaptive changes in primate vestibuloocular reflex. III. Electrophysiological observations in flocculus of normal monkeys. J Neurophysiol. 1980;43:1437–1476. doi: 10.1152/jn.1980.43.5.1437. [DOI] [PubMed] [Google Scholar]
- 70.Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- 71.Missal M, Heinen SJ. Supplementary eye fields stimulation facilitates anticipatory pursuit. J Neurophysiol. 2004;92:1257–1262. doi: 10.1152/jn.01255.2003. [DOI] [PubMed] [Google Scholar]
- 72.Mushiake H, Fujii N, Tanji J. Visually guided saccade versus eye-hand reach: contrasting neuronal activity in the cortical supplementary and frontal eye fields. J Neurophysiol. 1996;75:2187–2191. doi: 10.1152/jn.1996.75.5.2187. [DOI] [PubMed] [Google Scholar]
- 73.Nagao S, Kitamura T, Nakamura N, Hiramatsu T, Yamada J. Differences of the primate flocculus and ventral paraflocculus in the mossy and climbing fiber input organization. J Comp Neurol. 1997;16:480–498. doi: 10.1002/(sici)1096-9861(19970616)382:4<480::aid-cne5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura T, Bronstein A. The perception of head and neck angular displacement in normal and labyrinthine-defective subjects: A quantitative study using a ‘remembered saccade’ technique. Brain. 1995;118:1157–1168. doi: 10.1093/brain/118.5.1157. [DOI] [PubMed] [Google Scholar]
- 75.Nakamura K, Sakai K, Hikosaka O. Neuronal activity in medial frontal cortex during learning of sequential procedures. J Neurophysiol. 1998;80:2671–2687. doi: 10.1152/jn.1998.80.5.2671. [DOI] [PubMed] [Google Scholar]
- 76.Olson CR, Gettner SN. Macaque SEF neurons encode object-centered directions of eye movements regardless of the visual attributes of instructional cues. J Neurophysiol. 1999;81:2340–2346. doi: 10.1152/jn.1999.81.5.2340. [DOI] [PubMed] [Google Scholar]
- 77.Olson CR, Gettner SN, Ventura V, Carta R, Kass RE. Neuronal activity in macaque supplementary eye field during planning of saccades in response to pattern and spatial cues. J Neurophysiol. 2000;84:1369–1384. doi: 10.1152/jn.2000.84.3.1369. [DOI] [PubMed] [Google Scholar]
- 78.Ono S, Das VE, Mustari MJ. Gaze-related response properties of DLPN and NRTP neurons in the rhesus macaque. J Neurophysiol. 2004;91:2484–2500. doi: 10.1152/jn.01005.2003. [DOI] [PubMed] [Google Scholar]
- 79.Page WK, Duffy CJ. Heading representation in MST: sensory interactions and population encoding. J Neurophysiol. 2003;89:1994–2013. doi: 10.1152/jn.00493.2002. [DOI] [PubMed] [Google Scholar]
- 80.Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. II. Visual-vestibular interactions and kinematic considerations. J Neurophysiol. 1991;6:1183–1196. doi: 10.1152/jn.1991.65.5.1183. [DOI] [PubMed] [Google Scholar]
- 81.Pierrot-Deseilligny C, IsraëlA I, Berthoz Rivaud S, Gaymard B. Role of the different frontal lobe areas in the control of the horizontal component of memory-guided saccades in man. Exp Brain Res. 1993;95:166–171. doi: 10.1007/BF00229665. [DOI] [PubMed] [Google Scholar]
- 82.Pierrot-Deseilligny C, Rivaud S, Gaymond B, Müri R, Vermersch AI. Cortical control of saccades. Ann Neurol. 1995;37:557–567. doi: 10.1002/ana.410370504. [DOI] [PubMed] [Google Scholar]
- 83.Park J, Schlag-Rey M, Schlag J. Frames of reference for saccadic command tested by saccade collision in the supplementary eye field. J Neurophysiol. 2006;95:159–170. doi: 10.1152/jn.00268.2005. [DOI] [PubMed] [Google Scholar]
- 84.Robinson DA. A model of cancellation of the vestibulo-ocular reflex. In: Keller E, editor. Functional basis of ocular motility disorders. Pergamon press; Oxford: 1982. pp. 5–13. [Google Scholar]
- 85.Russo GS, Bruce CJ. Neurons in the supplementary eye field of rhesus monkeys code visual targets and saccadic eye movements in an oculocentric coordinate system. J Neurophysiol. 1996;76:825–848. doi: 10.1152/jn.1996.76.2.825. [DOI] [PubMed] [Google Scholar]
- 86.Sakata H, Shibutani, Kawano K. Functional properties of visual tracking neurons in posterior parietal association cortex of the monkey. J Neurophysiol. 1983;49:1364–1380. doi: 10.1152/jn.1983.49.6.1364. [DOI] [PubMed] [Google Scholar]
- 87.Sato F, Akao T, Kurkin S, Fukushima J, Fukushima K. Adaptive changes in vergence eye movements induced by vergence-vestibular interaction training in monkeys. Exp Brain Res. 2004;156:164–173. doi: 10.1007/s00221-003-1777-x. [DOI] [PubMed] [Google Scholar]
- 88.Sato H, Noda H. Posterior vermal Purkinje cells in macaques responding during saccades, smooth pursuit, chair rotation and/or optokinetic stimulation. Neurosci Res. 1992;12:583–595. doi: 10.1016/0168-0102(92)90065-k. [DOI] [PubMed] [Google Scholar]
- 89.Sato U, Kawasaki T. Operational unit responsible for plane-specific control of eye movement by cerebellar flocculus in cat. J Neurophysiol. 1990;64:551–564. doi: 10.1152/jn.1990.64.2.551. [DOI] [PubMed] [Google Scholar]
- 90.Schall JD. Neuronal activity related to visually guided saccadic eye movements in the supplementary motor area of rhesus monkeys. J Neurophysiol. 1991;66:530–558. doi: 10.1152/jn.1991.66.2.530. [DOI] [PubMed] [Google Scholar]
- 91.Schiller PH, Chou I. The effects of frontal eye field and dorsomedial frontal cortex lesions on visually guided eye movements. Nature Neurosci. 1998;3:248–253. doi: 10.1038/693. [DOI] [PubMed] [Google Scholar]
- 92.Schlag J, Schlag-Rey M. Evidence for a supplementary eye field. J Neurophysiol. 1987;57:179–200. doi: 10.1152/jn.1987.57.1.179. [DOI] [PubMed] [Google Scholar]
- 93.Schlag-Rey M, Amador N, Sanchez H, Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz J, Lisberger S. Initial tracking conditions modulate the gain of visuo-motor transmission for smooth pursuit eye movements in monkeys. Visual Neurosci. 1994;11:411–424. doi: 10.1017/s0952523800002352. [DOI] [PubMed] [Google Scholar]
- 95.Shaikh AG, Meng H, Angelaki DE. Multiple reference frames for motion in the primate cerebellum. J Neurosci. 2004;24:4491–4497. doi: 10.1523/JNEUROSCI.0109-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shi D, Friedman HR, Bruce CJ. Deficits in smooth pursuit eye movements after muscimol inactivation within the primate frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- 97.Shidara M, Kawano K. Role of Purkinje cells in the ventral paraflocculus in short-latency ocular following responses. Exp Brain Res. 1993;93:185–195. doi: 10.1007/BF00228385. [DOI] [PubMed] [Google Scholar]
- 98.Scudder CA, Fuchs AF. Physiological and behavioral identification of vestibular nucleus neurons mediating the horizontal vestibuloocular reflex in trained rhesus monkeys. J Neurophysiol. 1992;94:244–264. doi: 10.1152/jn.1992.68.1.244. [DOI] [PubMed] [Google Scholar]
- 99.Shinmei Y, Yamanobe T, Fukushima J, Fukushima K. Purkinje cells of the cerebellar dorsal vermis in the monkey: simple-spike activity during pursuit and passive whole body rotation. J Neurophysiol. 2002;87:1836–1849. doi: 10.1152/jn.00150.2001. [DOI] [PubMed] [Google Scholar]
- 100.Shiroyama T, Kayahara T, Yasui Y, Nomura J, Nakano K. Projections of the vestibular nuclei to the thalamus in the rat: a Phaseolus vulgaris leucoagglutinin study. J Comp Neurol. 1999;407:318–332. [PubMed] [Google Scholar]
- 101.Stone LS, Lisberger SG. Visual responses of Purkinje cells in the cerebellar flocculus during smooth-pursuit eye movements in monkeys: I. Simple spikes. J Neurophysiol. 1990;63:1241–1261. doi: 10.1152/jn.1990.63.5.1241. [DOI] [PubMed] [Google Scholar]
- 102.Suzuki DA, Keller EL. Vestibular signals in the posterior vermis of the alert monkey cerebellum. Exp Brain Res. 1982;47:145–147. doi: 10.1007/BF00235896. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki DA, Keller EL. The role of the posterior vermis of monkey cerebellum in smooth-pursuit eye movement control. I. Eye and head movement-related activity. J Neurophysiol. 1988;59:1–18. doi: 10.1152/jn.1988.59.1.1. [DOI] [PubMed] [Google Scholar]
- 104.Takeichi N, Fukushima J, Kurkin S, Yamanobe T, Shinmei Y, Fukushima K. Directional asymmetry in smooth ocular tracking in the presence of visual background in young and adult primates. Exp Brain Res. 2003;149:380–390. doi: 10.1007/s00221-002-1367-3. [DOI] [PubMed] [Google Scholar]
- 105.Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- 106.Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- 107.Tanaka M, Lisberger SG. Role of arcuate frontal cortex of monkeys in smooth pursuit eye movements. I. Basic response properties to retinal image motion and position. J Neurophysiol. 2002;87:2684–2699. doi: 10.1152/jn.2002.87.6.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tanji J. New concepts of supplementary motor area. Curr Opin Neurobiol. 1996;6:782–787. doi: 10.1016/s0959-4388(96)80028-6. [DOI] [PubMed] [Google Scholar]
- 109.Tehovnik EJ, Sommer MA, Chou-H I, Slocum WM, Schiller PH. Eye fields in the frontal lobes of primates. Brain Res Rev. 2000;32:413–448. doi: 10.1016/s0165-0173(99)00092-2. [DOI] [PubMed] [Google Scholar]
- 110.Thier P, Erickson R. Responses of visual-tracking neurons from cortical area MST-l to visual, eye and head motion. Eur J Neurosci. 1992;4:539–553. doi: 10.1111/j.1460-9568.1992.tb00904.x. [DOI] [PubMed] [Google Scholar]
- 111.Tian J, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of Cebus monkeys. J Neurophysiol. 1996;76:2740–2771. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- 112.Tomko DL, Paige GD. Linear vestibuloocular reflex during motion along axes between nasooccipital and interaural. Ann N Y Acad Sci. 1992;656:233–241. doi: 10.1111/j.1749-6632.1992.tb25212.x. [DOI] [PubMed] [Google Scholar]
- 113.Tsubuku T, Akao T, McCrea RA, Kurkin S, Fukushima J, Fukushima K. Purkinje cell activity in the cerebellar floccular region during vergence eye movements, Soc. Neurosci. (Abstr.) Program No. 880.3. (2004), Abstract Viewer/Itinerary Planner. San Diego; CA.: [Google Scholar]
- 114.Tsubuku T, Akao T, Kurkin SA, Fukushima K. Prediction in the timing of pursuit eye movement initiation revealed by cross-axis vestibular-pursuit training in monkeys. Exp Brain Res. 2006;168:427–435. doi: 10.1007/s00221-005-0102-2. [DOI] [PubMed] [Google Scholar]
- 115.Umeno MM, Goldberg ME. Spatial processing in the monkey frontal eye field. I. Predictive visual responses. J Neurophysiol. 1997;78:1373–1383. doi: 10.1152/jn.1997.78.3.1373. [DOI] [PubMed] [Google Scholar]
- 116.Wilson VJ, Melvill GJ. Mammalian vestibular physiology. Plenum Press; New York: 1979. [Google Scholar]
- 117.Zhang Y, Partsalis AM, Highstein SM. Properties of superior vestibular nucleus flocculus target neurons in the squirrel monkey. I. General properties in comparison with flocculus projecting neurons. J Neurophysiol. 1995;73:2261–2278. doi: 10.1152/jn.1995.73.6.2261. [DOI] [PubMed] [Google Scholar]


