Abstract
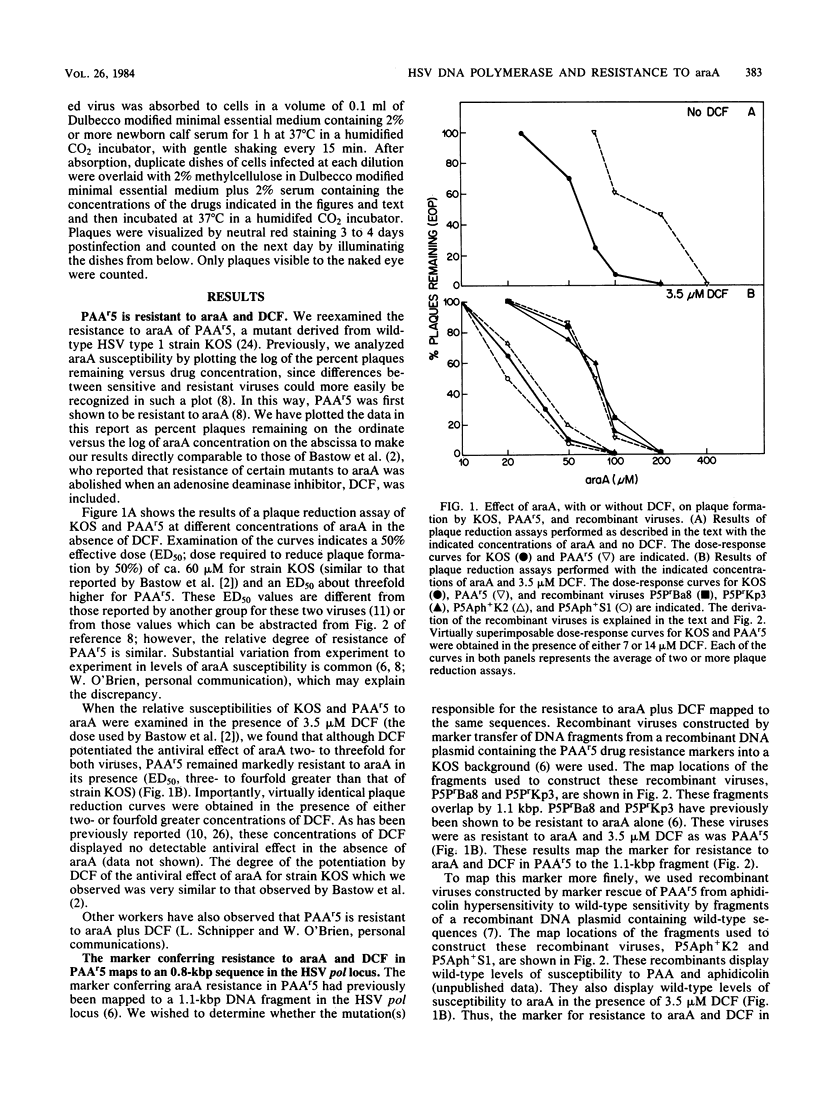
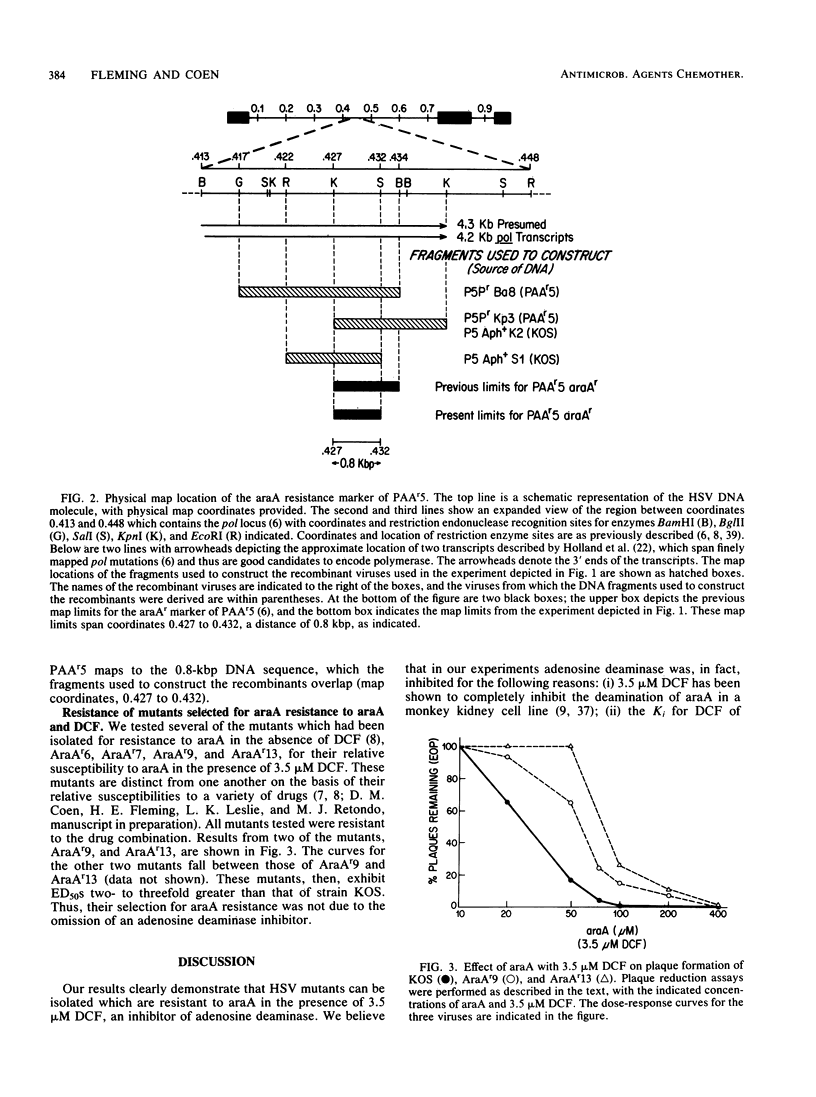
Herpes simplex virus mutants PAAr5, AraAr6, AraAr7, AraAr9, and AraAr13, which previously had been shown to be resistant to arabinosyladenine (araA) alone, were found to be resistant to araA in the presence of high concentrations of the adenosine deaminase inhibitor, deoxycoformycin. The marker conferring resistance to araA and deoxycoformycin in mutant PAAr5 was mapped finely to an 0.8-kilobase-pair region in the herpes simplex virus DNA polymerase locus. These results indicate that the mutants are resistant to araA itself rather than to its deamination product and confirm the importance of the viral polymerase in the antiviral action of araA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Crumpacker C. S., Schaffer P. A., Wilkie N. M. Physical and genetic analysis of the herpes simplex virus DNA polymerase locus. Virology. 1980 Jun;103(2):311–326. doi: 10.1016/0042-6822(80)90190-7. [DOI] [PubMed] [Google Scholar]
- Chartrand P., Stow N. D., Timbury M. C., Wilkie N. M. Physical mapping of paar mutations of herpes simplex virus type 1 and type 2 by intertypic marker rescue. J Virol. 1979 Aug;31(2):265–276. doi: 10.1128/jvi.31.2.265-276.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Aschman D. P., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene conferring hypersensitivity to aphidicolin. Nucleic Acids Res. 1983 Aug 11;11(15):5287–5297. doi: 10.1093/nar/11.15.5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Furman P. A., Gelep P. T., Schaffer P. A. Mutations in the herpes simplex virus DNA polymerase gene can confer resistance to 9-beta-D-arabinofuranosyladenine. J Virol. 1982 Mar;41(3):909–918. doi: 10.1128/jvi.41.3.909-918.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. D., Sweetman L., Carey S., Stuckey M. A., Buchanan R. Effect of adenosine deaminase upon the antiviral activity in vitro of adenine arabinoside for vaccinia virus. Antimicrob Agents Chemother. 1974 Nov;6(5):630–636. doi: 10.1128/aac.6.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Kowalsky P. N., Sherman D. M. Resistance of herpes simplex virus to adenine arabinoside and E-5-(2-bromovinyl)-2'-deoxyuridine: a physical analysis. J Infect Dis. 1982 Aug;146(2):167–172. doi: 10.1093/infdis/146.2.167. [DOI] [PubMed] [Google Scholar]
- Derse D., Bastow K. F., Cheng Y. Characterization of the DNA polymerases induced by a group of herpes simplex virus type I variants selected for growth in the presence of phosphonoformic acid. J Biol Chem. 1982 Sep 10;257(17):10251–10260. [PubMed] [Google Scholar]
- Derse D., Cheng Y. C. Herpes simplex virus type I DNA polymerase. Kinetic properties of the associated 3'-5' exonuclease activity and its role in araAMP incorporation. J Biol Chem. 1981 Aug 25;256(16):8525–8530. [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Eriksson B., Oberg B. Characteristics of herpesvirus mutants resistant to phosphonoformate and phosphonoacetate. Antimicrob Agents Chemother. 1979 Jun;15(6):758–762. doi: 10.1128/aac.15.6.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H., McMillan A., Darby G. The sensitivity of acyclovir-resistant mutants of herpes simplex virus to other antiviral drugs. J Infect Dis. 1981 Feb;143(2):281–285. doi: 10.1093/infdis/143.2.281. [DOI] [PubMed] [Google Scholar]
- Furth J. J., Cohen S. S. Inhibition of mammalian DNA polymerase by the 5'-triphosphate of 1-beta-d-arabinofuranosylcytosine and the 5'-triphosphate of 9-beta-d-arabinofuranoxyladenine. Cancer Res. 1968 Oct;28(10):2061–2067. [PubMed] [Google Scholar]
- Hall J. D., Coen D. M., Fisher B. L., Weisslitz M., Randall S., Almy R. E., Gelep P. T., Schaffer P. A. Generation of genetic diversity in herpes simplex virus: an antimutator phenotype maps to the DNA polymerase locus. Virology. 1984 Jan 15;132(1):26–37. doi: 10.1016/0042-6822(84)90088-6. [DOI] [PubMed] [Google Scholar]
- Hay J., Subak-Sharpe J. H. Mutants of herpes simplex virus types 1 and 2 that are resistant to phosphonoacetic acid induce altered DNA polymerase activities in infected cells. J Gen Virol. 1976 Apr;31(1):145–148. doi: 10.1099/0022-1317-31-1-145. [DOI] [PubMed] [Google Scholar]
- Hirsch M. S., Schooley R. T. Drug therapy. Treatment of herpesvirus infections. N Engl J Med. 1983 Oct 27;309(17):1034–1039. doi: 10.1056/NEJM198310273091706. [DOI] [PubMed] [Google Scholar]
- Holland L. E., Sandri-Goldin R. M., Goldin A. L., Glorioso J. C., Levine M. Transcriptional and genetic analyses of the herpes simplex virus type 1 genome: coordinates 0.29 to 0.45. J Virol. 1984 Mar;49(3):947–959. doi: 10.1128/jvi.49.3.947-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Watson D. H. Herpes simplex virus resistance and sensitivity to phosphonoacetic acid. J Virol. 1977 Feb;21(2):584–600. doi: 10.1128/jvi.21.2.584-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jofre J. T., Schaffer P. A., Parris D. S. Genetics of resistance to phosphonoacetic acid in strain KOS of herpes simplex virus type 1. J Virol. 1977 Sep;23(3):833–836. doi: 10.1128/jvi.23.3.833-836.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. E., Zahn R. K., Bittlingmaier K., Falke D. Inhibition of herpesvirus DNA synthesis by 9-beta-D-arabinofuranosyladenine in cellular and cell-free systems. Ann N Y Acad Sci. 1977 Mar 4;284:34–48. doi: 10.1111/j.1749-6632.1977.tb21935.x. [DOI] [PubMed] [Google Scholar]
- North T. W., Cohen S. S. Erythro-9-(2-hydroxy-3-nonyl)adenine as a specific inhibitor of herpes simplex virus replication in the presence and absence of adenosine analogues. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4684–4688. doi: 10.1073/pnas.75.10.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrander M., Cheng Y. C. Properties of herpes simplex virus type 1 and type 2 DNA polymerase. Biochim Biophys Acta. 1980 Sep 19;609(2):232–245. doi: 10.1016/0005-2787(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Lewis R. B., Powell K. L. Identification of the herpes simplex virus DNA polymerase gene. Nature. 1977 Oct 13;269(5629):621–623. doi: 10.1038/269621a0. [DOI] [PubMed] [Google Scholar]
- Reinke C. M., Drach J. C., Shipman C., Jr, Weissbach A. Differential inhibition of mammalian DNA polymerases alpha, beta and gamma and herpes simplex virus-induced DNA polymerase by the 5'-triphosphates of arabinosyladenine and arabinosylcytosine. IARC Sci Publ. 1978;(24 Pt 2):999–1005. [PubMed] [Google Scholar]
- Schinazi R. F., Peters J., Williams C. C., Chance D., Nahmias A. J. Effect of combinations of acyclovir with vidarabine or its 5'-monophosphate on herpes simplex viruses in cell culture and in mice. Antimicrob Agents Chemother. 1982 Sep;22(3):499–507. doi: 10.1128/aac.22.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz P. M., Shipman C., Jr, Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in the presence of an adenosine deaminase inhibitor. Antimicrob Agents Chemother. 1976 Jul;10(1):64–74. doi: 10.1128/aac.10.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. H., Shipman C., Jr, Drach J. C. Deoxyadenosine antagonism of the antiviral activity of 9-beta-D-arabinofuranosyladenine and 9-beta-D-arabinofuranosylhypoxanthine. Cancer Res. 1978 Jul;38(7):1916–1921. [PubMed] [Google Scholar]
- Spector T. Mammalian adenylosuccinate lyase. Participation in the conversion of 2'-dIMP and beta-D-arabinosyl-IMP to adenine nucleotides. Biochim Biophys Acta. 1977 Apr 12;481(2):741–745. doi: 10.1016/0005-2744(77)90308-4. [DOI] [PubMed] [Google Scholar]
- Spector T., Miller R. L. Mammalian adenylosuccinate synthetase. Nucleotide monophosphate substrates and inhibitors. Biochim Biophys Acta. 1976 Sep 14;445(2):509–517. doi: 10.1016/0005-2744(76)90104-2. [DOI] [PubMed] [Google Scholar]
- Weller S. K., Aschman D. P., Sacks W. R., Coen D. M., Schaffer P. A. Genetic analysis of temperature-sensitive mutants of HSV-1: the combined use of complementation and physical mapping for cistron assignment. Virology. 1983 Oct 30;130(2):290–305. doi: 10.1016/0042-6822(83)90084-3. [DOI] [PubMed] [Google Scholar]



