Abstract
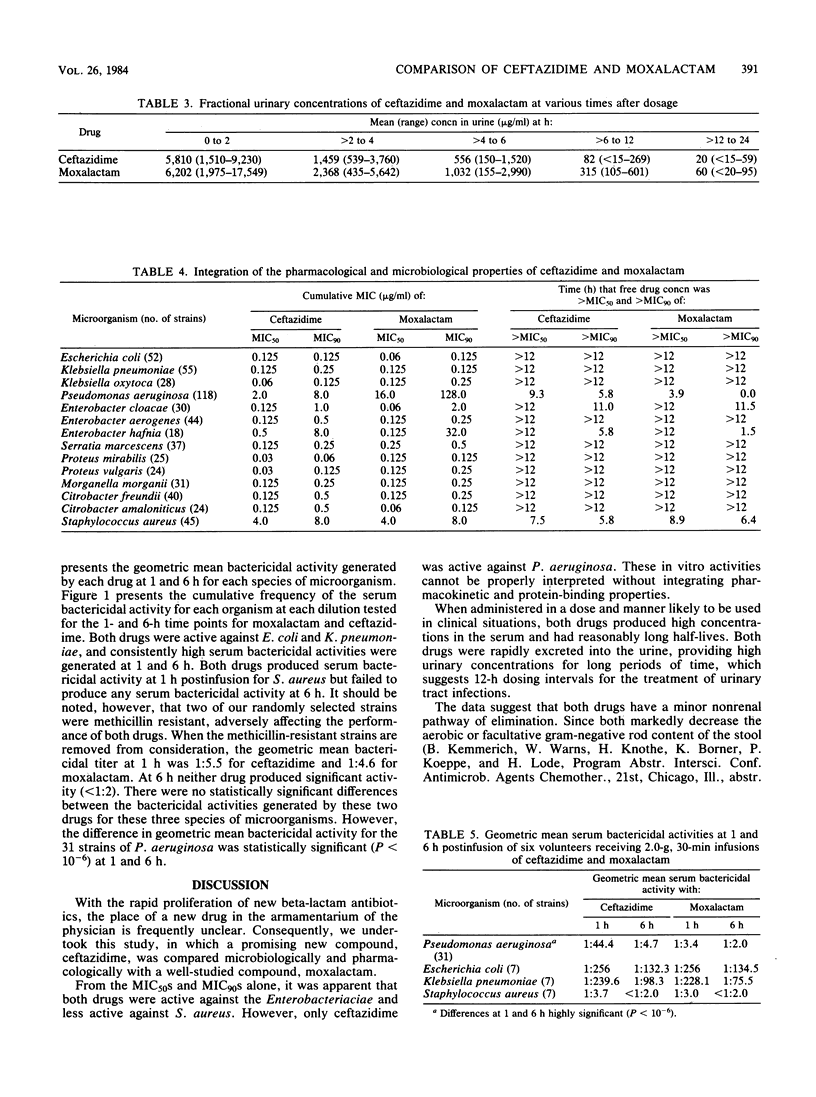
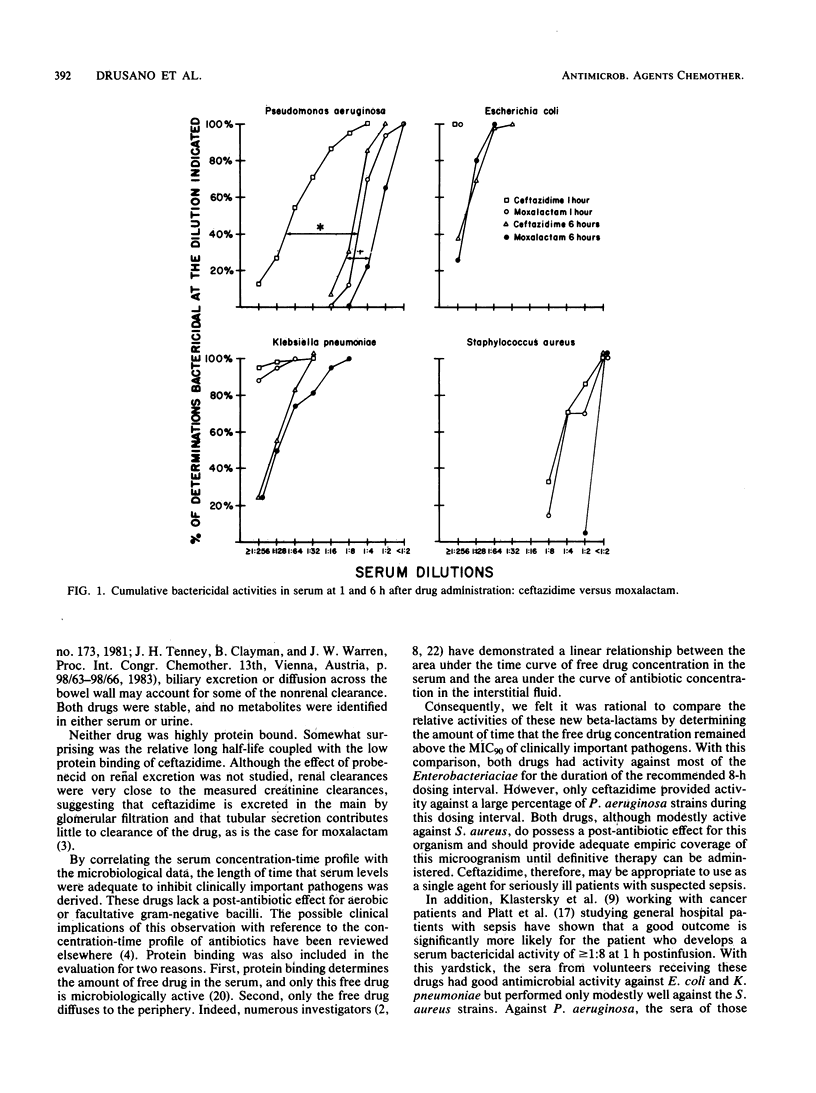
We compared ceftazidime with moxalactam, a commonly utilized, currently available drug. The microbiological activities of ceftazidime and moxalactam were studied. In addition, single-dose pharmacokinetics and serum bactericidal activity 1 and 6 h after a 2.0-g, 30-min infusion of each drug were determined in a crossover study in human volunteers. In vitro, both drugs had MICs for 90% of the isolates of less than 1.0 microgram/ml against the common members of the family Enterobacteriaceae and of 8.0 micrograms/ml against Staphylococcus aureus. Against Pseudomonas aeruginosa ceftazidime was more active than moxalactam, the respective MICs for 90% of the isolates being 8 and 128 micrograms/ml. Mean half-lives were 1.75 (+/- 0.21) h for ceftazidime and 2.5 (+/- 0.38) h for moxalactam. The serum bactericidal titers for both compounds against Escherichia coli and Klebsiella pneumoniae were high. Titers against S. aureus 6 h after infusion were negative. The mean (geometric) serum bactericidal titer of ceftazidime against 31 strains of P. aeruginosa (1:44) was higher than that of moxalactam (1:3.4).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- DeSante K. A., Israel K. S., Brier G. L., Wolny J. D., Hatcher B. L. Effect of probenecid on the pharmacokinetics of moxalactam. Antimicrob Agents Chemother. 1982 Jan;21(1):58–61. doi: 10.1128/aac.21.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drusano G. L., Ryan P. A., Standiford H. C., Moody M. R., Schimpff S. C. Integration of selected pharmacologic and microbiologic properties of three new beta-lactam antibiotics: a hypothesis for rational comparison. Rev Infect Dis. 1984 May-Jun;6(3):357–363. doi: 10.1093/clinids/6.3.357. [DOI] [PubMed] [Google Scholar]
- Hall W. H., Opfer B. J., Gerding D. N. Comparative activities of the oxa-beta-lactam LY127935, cefotaxime, cefoperazone, cefamandole, and ticarcillin against multiply resistant gram-negative bacilli. Antimicrob Agents Chemother. 1980 Feb;17(2):273–279. doi: 10.1128/aac.17.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstedt B., Walder M. Influence of serum protein binding and mode of administration on penetration of five cephalosporins into subcutaneous tissue fluid in humans. Antimicrob Agents Chemother. 1981 Dec;20(6):783–786. doi: 10.1128/aac.20.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klastersky J., Daneau D., Swings G., Weerts D. Antibacterial activity in serum and urine as a therapeutic guide in bacterial infections. J Infect Dis. 1974 Feb;129(2):187–193. doi: 10.1093/infdis/129.2.187. [DOI] [PubMed] [Google Scholar]
- Lang S. D., Edwards D. J., Durack D. T. Comparison of cefoperazone, cefotaxime, and moxalactam (LY127935) against aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1980 Mar;17(3):488–493. doi: 10.1128/aac.17.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Miner D. J., Coleman D. L., Shepherd A. M., Hardin T. C. Determination of moxalactam in human body fluids by liquid chromatographic and microbiological methods. Antimicrob Agents Chemother. 1981 Aug;20(2):252–257. doi: 10.1128/aac.20.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblinger M. J., Bowers J. T., Sande M. A., Mandell G. L. Moxalactam therapy vs. standard antimicrobial therapy for selected serious infections. Rev Infect Dis. 1982 Nov-Dec;4 (Suppl):S639–S649. doi: 10.1093/clinids/4.supplement_3.s639. [DOI] [PubMed] [Google Scholar]
- Platt R., Ehrlich S. L., Afarian J., O'Brien T. F., Pennington J. E., Kass E. H. Moxalactam therapy of infections caused by cephalothin-resistant bacteria: influence of serum inhibitory activity on clinical response and acquisition of antibiotic resistance during therapy. Antimicrob Agents Chemother. 1981 Sep;20(3):351–355. doi: 10.1128/aac.20.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reller L. B., Stratton C. W. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977 Aug;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- Standiford H. C., Drusano G. L., McNamee W. B., Tatem B., Ryan P. A., Schimpff S. C. Comparative pharmacokinetics of moxalactam, cefoperazone, and cefotaxime in normal volunteers. Rev Infect Dis. 1982 Nov-Dec;4 (Suppl):S585–S594. doi: 10.1093/clinids/4.supplement_3.s585. [DOI] [PubMed] [Google Scholar]
- Tompsett R., Shultz S., McDermott W. The Relation of Protein Binding to the Pharmacology and Antibacterial Activity of Penicillins X, G, Dihydro F, and K. J Bacteriol. 1947 May;53(5):581–595. doi: 10.1128/jb.53.5.581-595.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]



