Abstract
Previous gene targeting studies have implicated an indispensable role of vascular endothelial growth factor (VEGF) in tumor angiogenesis, particularly in tumors of embryonal or endocrine origin. In contrast, we report here that transformation of VEGF-deficient adult fibroblasts (MDF528) with ras or neu oncogenes gives rise to highly tumorigenic and angiogenic fibrosarcomas. These aggressive VEGF-null tumors (528ras, 528neu) originated from VEGF–/– embryonic stem cells, which themselves were tumorigenically deficient. We also report that VEGF production by tumor stroma has a modest role in oncogene-driven tumor angiogenesis. Both ras and neu oncogenes down-regulated at least two endogenous inhibitors of angiogenesis [pigment epithelium derived factor (PEDF) and thrombospondin 1 (TSP-1)]. This is functionally important as administration of an antiangiogenic TSP-1 peptide (ABT-526) markedly inhibited growth of VEGF–/– tumors, with some ingress of pericytes. These results provide the first definitive genetic demonstration of the dispensability of tumor cell-derived VEGF in certain cases of ‘adult’ tumor angiogenesis, and thus highlight the importance of considering VEGF-independent as well as VEGF-dependent pathways when attempting to block this process pharmacologically.
Keywords: neu/PEDF/ras/TSP-1/VEGF
Introduction
Angiogenesis is triggered by a change in balance between different pro- and anti-angiogenic activities that regulate the behavior of capillary endothelial cells (Hanahan and Folkman, 1996). In this regard, a pre-eminent role has been established for the vascular endothelial growth factor (VEGF)-related family of angiogenic and lymphangiogenic growth factors, which currently includes VEGF-A, -B, -C, -D, -E, and placenta growth factor (PlGF). VEGF-A is believed to play an indispensable role in angiogenesis. Indeed, targeting of the VEGF-A gene in mice resulted in early embryonic lethality due to severe structural and functional abnormalities in the developing vasculature, even when only a single VEGF-A allele was inactivated (Carmeliet et al., 1996; Ferrara et al., 1996). Embryonic lethality is also induced by targeted disruption of either of the two main VEGF receptors expressed by endothelial cells, namely VEGFR-2 (Flk-1/KDR) and VEGFR-1 (Flt-1), the former regarded as the main transducer of positive pro-angiogenic signals (Carmeliet, 2000). The profound influence of the VEGF/VEGF receptor axis on vascular development and angiogenesis is likely linked to its role as a stimulator of endothelial cell survival, mitogenesis, migration, differentiation and self-assembly, as well as vascular permeability and mobilization of endothelial progenitor cells (EPCs) from the bone marrow into the peripheral circulation (Ferrara and Gerber, 2001).
There are numerous reasons to suggest that VEGF also plays an important role in ‘pathological’ forms of angiogenesis, including tumor neovascularization (Ferrara and Gerber, 2001). For instance, VEGF expression is elevated in the majority of human cancers, and in many transformed cell lines in culture (Dvorak et al., 1995). Furthermore, transforming genetic lesions such as activated oncogenes (ras, neu/HER-2 and at least 20 others) (Rak and Kerbel, 2003) and inactivated tumor suppressor genes (e.g. p53, VHL, PTEN, INK4a/p16) have a direct stimulating impact on VEGF expression in cancer cells (Bouck et al., 1996). A causative role for VEGF in tumor angiogenesis has been implicated by numerous studies (Claffey et al., 1996; Ferrara and Gerber, 2001). In this regard, particularly striking are the results involving wild-type (Ferrara et al., 1996) or oncogenically transformed embryonic stem (ES) cells (Shi and Ferrara, 1999), embryonic fibroblasts (Grunstein et al., 1999) or endocrine pancreatic cells (Inoue et al., 2002), in all of which total inactivation of the VEGF gene was found to result in severe suppression of tumorigenic and angiogenic phenotypes. These studies suggest implicitly that not only is VEGF essential and indispensable for tumor growth and neovascularization, but also that tumor cells themselves constitute the major source of VEGF.
There are, however, experimental findings that seem to be at variance with the notion that VEGF is a non-redundant tumor angiogenesis factor that is predominantly produced by cancer cells. For example, appreciable VEGF expression has been detected in tumor-associated fibroblasts (Hlatky et al., 1994; Fukumura et al., 1998; Kishimoto et al., 2000) and inflammatory cells (Coussens et al., 1999). This suggests that the host stromal compartment may constitute a major source of VEGF in tumors (Fukumura et al., 1998). It has also been observed that reduction of VEGF expression in cancer cells may be functionally insignificant in certain advanced experimental tumors (Yoshiji et al., 1997), and that tumor progression is associated with expression of an increasing number of different pro-angiogenic growth factors, in addition to VEGF (Relf et al., 1997). Moreover, while the anti-tumor effects of neutralizing antibodies and pharmacological antagonists of VEGF or its receptors have been promising in many cases (Kim et al., 1992; Ferrara and Gerber, 2001), in other instances the responses have been variable, transient and sometimes rather modest in magnitude, especially in advanced tumors (Garber, 2002). Indeed, a direct analysis has been lacking as to how critical the role of tumor cell-derived versus stromal VEGF is in various tumor angiogenesis settings.
The purpose of our study was to address this question by investigating two fundamentally different forms of tumorigenesis (and associated angiogenesis) occurring in either the presence or absence of the functional VEGF gene and protein expression in cancer cells. Our data indicate that teratomas derived from totipotent (genetically intact) ES cells were absolutely VEGF dependent. In contrast, adult mouse dermal fibroblasts expressing mutant ras or neu oncogenes remained tumorigenic even if rendered VEGF-null. Such tumors recruited VEGF-expressing host cells and down-regulated at least two potent angiogenesis inhibitors, such as pigment epithelium derived factor (PEDF) and thrombospondin 1 (TSP-1). Thus, VEGF production by cancer cells may be non-essential in the context of oncogene-driven tumorigenesis.
Results
Tumorigenic properties of VEGF-deficient ES cells
We decided to test the limits of VEGF involvement in tumor angiogenesis by comparing the impact of VEGF deletion on the tumor forming capacity of ES cell-derived teratomas or their related, but adult, cell descendants transformed with mutant oncogenes.
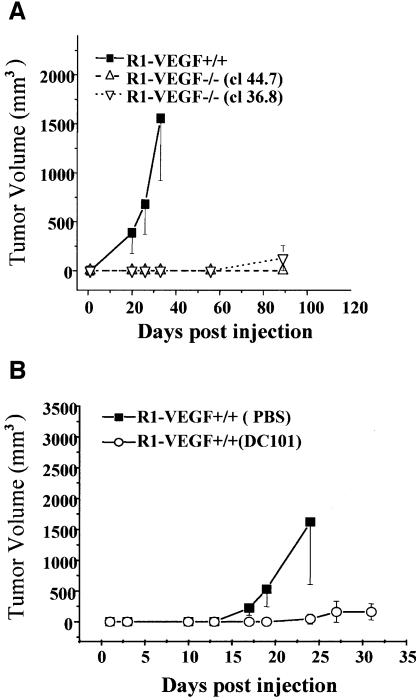
First, we employed the R1 strain of ES cells (Nagy et al., 1993). Both wild-type R1 cells (wtR1) and their VEGF-deficient counterparts (clones 44.7 and 36.8) were injected subcutaneously (s.c.) into SCID mice. As expected, inoculation of wtR1 cells resulted in the rapid growth of aggressive and highly vascularized teratomas (Ferrara et al., 1996) (Figure 1). Such tumors display a complex morphology and contain a rich network of CD31-positive host blood vessels (Yu et al., 2001). In marked contrast, R1 cells in which the VEGF gene was disrupted were unable to form tumors for at least 50 days after inoculation of as many as 7 × 106 cells (Figure 1A). As growth in vitro of both types of ES cells is not influenced by their VEGF status (data not shown), we attributed these in vivo properties of teratomas to VEGF-dependent angiogenesis. Indeed, treatment of mice harboring wild-type R1 tumors with a neutralizing antibody (DC101) directed against VEGFR-2/flk-1 resulted in nearly complete inhibition of tumor growth (Figure 1B). Collectively, these observations are in keeping with the notion that the endogenous production of VEGF and its interaction with endothelial VEGFR-2 are essential events during formation and vascularization of murine R1 teratoma.
Fig. 1. Dependence of ES-cell-derived mouse teratomas on VEGF/VEGFR-2-driven angiogenesis. (A) Impaired tumor formation by VEGF–/– ES cells (R1, clones 36.8 and 44.7) in comparison to their wild-type (VEGF+/+) counterparts. (B) Inhibition of R1-derived teratoma growth by anti-VEGFR-2 antibody (DC101; 800 µg every 3 days), control mice received the vehicle (PBS).
Generation of VEGF-deficient oncogene-transformed fibrosarcoma cell lines
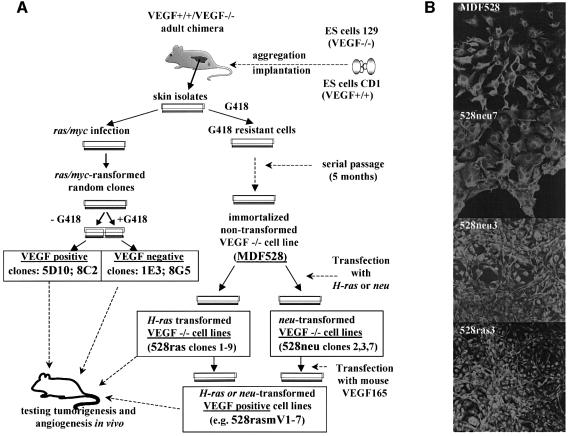
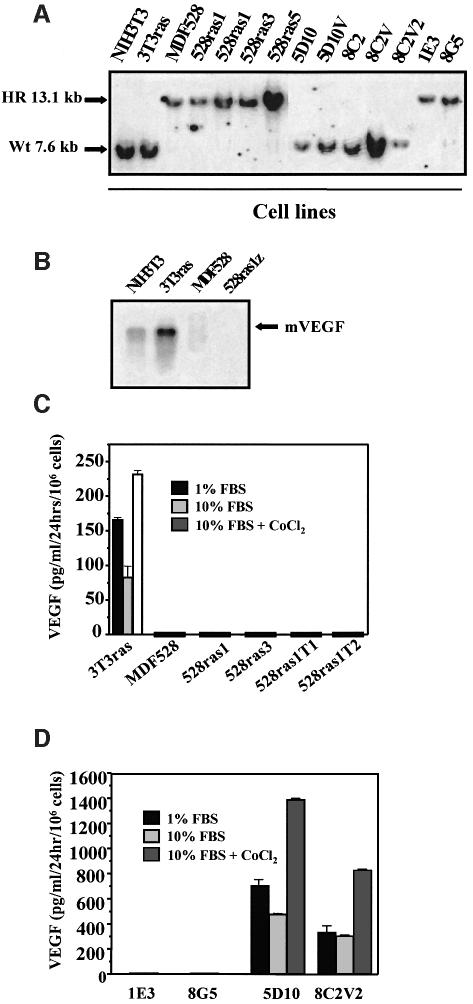
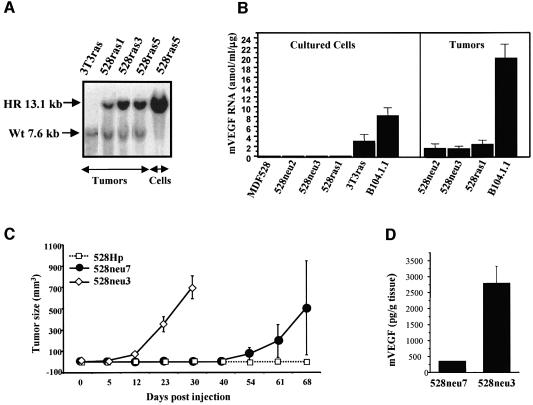
In contrast to the epigenetic nature of ES cell-derived teratomas, the majority of human tumors harbor various transforming genetic alterations. In order to assess the role of VEGF in the latter type, i.e oncogene-driven tumor angiogenesis, we generated a series of oncogene-transformed VEGF-proficient (VEGF+/+) or VEGF-deficient (VEGF–/–) fibrosarcoma cell lines. As summarized in Figure 2A, 4- to 5-month-old chimeric (VEGF–/–,VEGF+/+) mice were used as donors of skin explants. Primary cultures of dermal fibroblasts were subsequently established and infected with a retrovirus expressing both ras and myc oncogenes (Soloway et al., 1996). Alternatively, VEGF–/– fibroblasts were spontaneously immortalized and the resulting cell line (MDF528) was transfected with expression vectors encoding either an activated H-ras or neu oncogene (Figure 2A). All cell lines harboring mutant oncogenes (but not the MDF528 immortalized parental cells or their 528Hp hygromycin-resistant vector transfectants) exhibited the usual features of morphological trasformation (Figure 2B), formed foci in monolayer culture and grew as colonies in semi-solid media (data not shown). VEGF status in all transformed and non-transformed cell lines was verified by testing for G418 resistance (conferred by the VEGF targeting vector), Southern analysis and direct determination of VEGF mRNA and protein expression (Figure 3A–D).
Fig. 2. Derivation of adult oncogene transformed VEGF-deficient fibrosarcoma cell lines. (A) Derivation of VEGF–/– and VEGF+/+ ras- and neu- transformed cell lines (see text). (B) Morphology of fibrosarcoma cells harboring ras or neu oncogenes and their non-transformed parental MDF528 counterparts; immunostaining for vimentin, a fibroblast-specific cytoskeletal marker.
Fig. 3. Characterization of the VEGF status in fibrosarcoma cell lines. (A) Southern analysis of the VEGF gene in tumorigenic and non- tumorigenic fibroblastic cell lines: EcoRI digest (see text and Figure 2). HR, recombined/targeted VEGF gene in VEGF–/– cell lines; Wt, wild-type VEGF gene. (B) Absence of the typical 3.7–4.5 VEGF mRNA in cell lines with targeted VEGF gene. In some cases a larger transcript was detectable as described previously (Ferrara et al., 1996). (C) Absence of VEGF in conditioned medium of MDF528-derived cell lines (VEGF ELISA) treated with VEGF inducers (various FBS concentrations and/or CoCl2). 528ras1T1 and 528ras1T2 are cell lines re-established in culture from tumors that arose after s.c. injection of 528ras1 cells. (D) VEGF levels in ras/myc-transformed clones of primary dermal fibroblasts isolated from chimeric VEGF-positive/negative mice. Transformed clones with a targeted VEGF gene were negative for VEGF protein expression, whereas their G418 sensitive (non- targeted VEGF) counterparts from the same culture produced an abundance of VEGF, particularly in the presence of CoCl2. High serum levels caused a decrease in VEGF production by VEGF+/+ transformed cell lines.
Tumorigenic properties of VEGF–/– cell lines expressing activated oncogenes
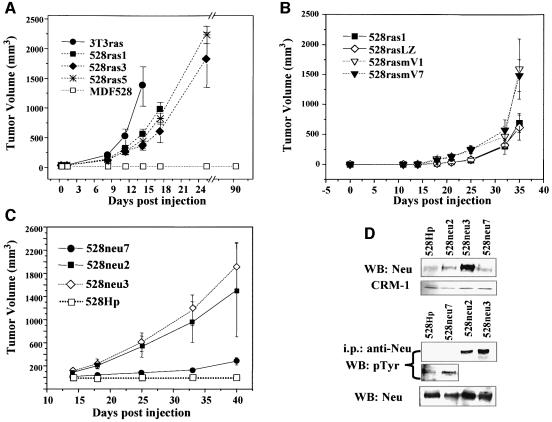
In marked contrast to ES cell-induced teratomas, 100% tumor take occurred in the case of all oncogene-driven fibrosarcomas regardless of their VEGF status, and this was followed by aggressive growth (Figure 4A–C; Table I) of highly vascular tumors (see Figure 7A–C). However, the overall growth kinetics of VEGF–/– ras- or neu-dependent fibrosarcomas was somewhat slower than that of their VEGF+/+ 3T3ras counterparts (Figure 4A) or 528rasmV1-7 cells engineered to re-express mouse VEGF164 (Figure 4B). The relative aggressiveness in vivo of various fibrosarcomas also correlated with their apparent degree of cellular transformation. Thus, 528neu7 cells, with lower expression of activated NEU (Figures 2B and 4D), were considerably less aggressive in vivo (Figures 4C and 6C) than more transformed 528neu3 and 528neu2 cell lines. Collectively, these results suggest that in the case of oncogene-driven tumors, malignant growth capacity in vivo is not dependent on VEGF production by cancer cells (unlike in teratomas).
Fig. 4. In vivo growth characteristics of VEGF–/– fibrosarcoma cell lines. (A) Three independent clones of H-ras-transformed VEGF–/– cells (528ras1, -3 and -5) form aggressive tumors in SCID mice upon s.c. inoculation in a manner comparable to that of NIH 3T3-derived tumor cell line (3T3ras, VEGFwt). Differences in tumorigenic potential of various 528ras clones correlated with their degree of morphological transformation (not shown), while injected non-transformed parental MDF528 cells were non-tumorigenic (up to 7 months). (B) Overexpression of mouse VEGF164 in 528ras1 cells (two independent clones 528rasmV1 and 528rasmV7) results in a moderately increased tumorigenic potential of these cells (106 cells injected s.c) as compared to their 528ras1 parental cells and control transfectant (528rasLZ). (C) Differential tumorigenicity of MDF528-derived cell lines overexpressing neu oncogene versus control polyclonal cell line (528Hp) transfected with the hygromycin expression vector. (D) Correlation between the level/activity (phosphorylation) of the NEU oncoprotein and the tumorigenic potential of MDF528-derived VEGF–/– fibrosarcoma cell lines [compare (C) and (D)]. Blot was exposed for longer time (WB: anti-pTyr, second strip) in order to visualize phosphorylated form of NEU in both control transfectants (528HP) and tumorigenic 528neu7 cells.
Table I. Summary of tumorigenic properties of various VEGF–/– and VEGF+/+ fibroblastic cell lines.
| Cell linea | VEGF genotype | VEGF production | Tumor take |
|---|---|---|---|
| 3T3ras | +/+ | + | 4/4 |
| 5D10 | +/+ | + | 4/4 |
| 8C2V2 | +/+ | + | 4/4 |
| 1E3 | –/– | – | 4/4 |
| 8G5 | –/– | – | 4/4 |
| MDF528 | –/– | – | 0/10 |
| 528ras1z | –/– | – | 4/4 |
| 528ras3z | –/– | – | 4/4 |
| 528ras5z | –/– | – | 4/4 |
| 3T3neu (B104.1.1) | +/+ | + | 5/5 |
| MDF528 hygro (528Hp) | –/– | – | 0/10 |
| 528neu2 | –/– | – | 5/5 |
| 528neu3 | –/– | – | 5/5 |
| 528neu7 | –/– | – | 5/5 |
aSee Materials and methods and Figure 2 for details. 3T3ras are NIH 3T3 cells transfected with mutant H-ras; 5D10, 8G5 fibroblasts infected with ras/myc retrovirus; MDF528 and MDF528 hygro/528Hp, non-transformed immortalized adult dermal fibroblasts; 528ras or 528neu, MDF528 cells transfected with H-ras or neu oncogenes, respectively.
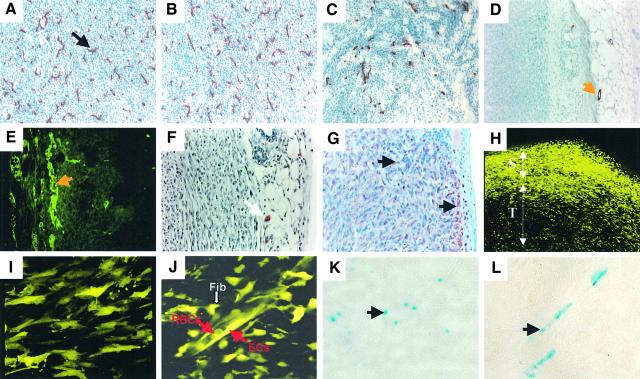
Fig. 7. Cellular composition of VEGF-deficient fibrosarcomas. Both (A) ras- and (B and C) neu-dependent tumors are highly vascularized (528ras1, 528neu3 and 528neu7, respectively) and contain dense networks of capillaries positive for endothelial markers (CD31). (C) Lower vascular density was detected in less aggressive 528neu7 tumors (CD31) than in their 528neu3 counterparts. (D) Capillaries inside tumor masses are mostly devoid of αSMA-positive (528ras1 tumor) or (E) desmin-positive (528neu3) pericytes. Such mural cells are detectable in peri-tumoral connective tissue [(D) and (E), orange arrows]. (F) Single mast cells present at the tumor periphery (CAE staining, white arrow) but not within the tumor. (G) Diffuse staining for vimentin within the 528ras1 tumor. Stroma and connective tissue stains slightly more intensively (black arrows). (H) Confocal image of YFP positive host cells associated with tumors (528ras1 cells non-fluorescent); T, tumor interior; S, skin. (I) Fibroblastoid stromal cells in tumors growing in YFP/SCID mice. (J) YFP-positive tumor blood vessel and surrounding stroma within 528ras1 tumor; Fib, fibroblast-like stromal cells; EC, endothelial cells; RBC, red blood cells. (K and L) LacZ staining of VEGF-producing stromal cells within 528ras1 tumors growing in mice harbouring a LacZ-tagged VEGF allele. Magnifications: (I), (J) and (L), 40×; (A)–(C), (E), (F) and (K), 20×; (D) and (H), 10×.
Fig. 6. Stromal VEGF in oncogene-driven fibrosarcomas. (A) Southern analysis of 528ras tumors and cultured cells. Both targeted (13.1 kb) and wild-type (7.6 kb) VEGF alleles were present in DNA isolated from several tumors but not that from cultured cells (compare with Figure 3). (B) Quantification of VEGF mRNA in cultured cells and tumors (mRNA ELISA). VEGF mRNA was detected only in VEGF+/+ cells (3T3ras, B104.1.1) in culture and not in MDF528-derived transformed and non-transformed VEGF–/– cell lines. In contrast, tumor masses of both VEGF–/– (528ras1, 528neu2, 528neu3) and VEGF+/+ (B104.1.1) fibrosarcomas contained measurable quantities of VEGF mRNA (see text). (C) Differential latency of VEGF–/– tumors expressing different levels of activated neu oncogene (528neu3, high neu expressors; 528neu7, low neu expressors). (D) Degree of malignant transformation (with neu) coincides with the ability of tumor cells to trigger stromal VEGF expression (highly transformed 528neu3 cells, high stromal VEGF; moderately transformed 528neu7 cells, low levels of stromal VEGF; compare with Figure 4D). Only tumors of equivalent size (700 mm3) were compared in this assay.
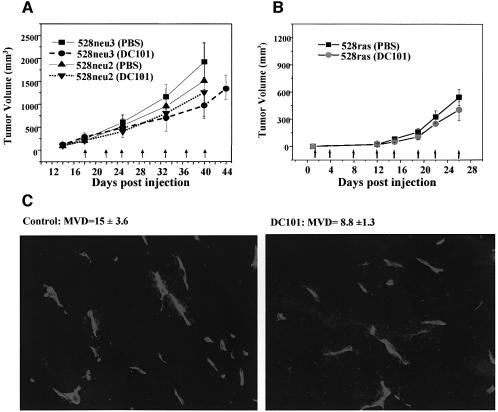
We reasoned that stromal cells could be a source of VEGF in VEGF-deficient tumors, and if so, the administration of VEGFR-2 antagonists could still suppress tumor growth. Therefore, mice harboring 528neu3 tumors were treated with the DC101 antibody (against mouse VEGFR-2), and tumor growth inhibition of up to 50% (Figure 5A), as well as ∼2-fold reduction in microvascular density, were indeed observed (Figure 5C). However, responsiveness to such treatment (DC101, 800 µg twice a week) was negligible in the case of two other fibrosarcomas (i.e. 528neu2 and 528ras1; Figure 5A and B, respectively). This suggests that activation of the VEGF/VEGFR-2 pathway by tumor stroma is not a sine qua non in fibrosarcoma.
Fig. 5. The impact of VEGFR-2 inhibition on growth of VEGF-deficient tumors. (A) Variable responsiveness to anti-VEGFR-2 antibody (DC101; 800 µg every 3–4 days) of neu-driven fibrosarcoma tumors (528neu3 tumors exhibit 50% growth inhibition upon administration of DC101, no such responses were observed in the case of 528neu2 tumors). (B) Ras-dependent tumors (528ras1) responded weakly to treatment with DC101. (C) Reduction in mean blood vessel density (MVD) in 528neu3 tumors upon DC101 treatment (CD31 immunofluorescence).
Expression of VEGF by host stromal cells recruited by oncogene-driven tumors
Regardless of their responsiveness to VEGFR-2 inhibition, ample stromal cell infiltration was observed in all oncogene-driven fibrosarcomas tested. For instance, even in relatively DC101-unresponsive 528ras1 tumors, Southern analysis revealed both the wild-type (host) and the targeted VEGF alleles (the former being absent in cultured cells) (Figures 3A and 6A). Moreover, Figure 6 shows that in contrast to various 528ras and 528neu VEGF-deficient cultured cell lines, the material explanted from the corresponding tumors contained measurable quantities of VEGF mRNA (Figure 6B) and protein (Figure 6D). Although VEGF was detectable in the original tumor masses, cancer cell lines re-established from such tumors (528rasT1, 528rasT2) were still devoid of any VEGF expression, even when exposed to various serum concentrations or to cobalt chloride (Figure 3C). Interesting but presently unexplained differences in the expression of stromal VEGF and microvascular vascular densities (MVD) were noted between fibrosarcomas with different levels of oncogenic transformation (e.g. 528neu7 versus 528neu 3) (Figures 4D, 6D, and 7B and C). Overall, these results can be interpreted as an (indirect) indication that, while (cultured) tumor cells of the 528 series were completely VEGF-negative, VEGF-positive stromal cells were, in fact, relatively abundant in the corresponding in vivo tumors.
To gain a more direct insight into the cellular sources of the stromal-derived VEGF, we injected 528ras or 528neu cells into syngeneic or SCID mice harboring the yellow fluorescent protein (YFP) transgene. Confocal images (Figures 7H–J) of the resulting tumors revealed a rich network of YFP-positive host cells (tumor cells being non-fluorescent) detectable at the boundary between the YFP-negative tumor cell masses and the overlaying YFP-positive skin (Figure 7H). YFP-positive cells were also present within the tumor interior and decorated vascular channels and their surroundings (Figure 7I and J). By immunostaining of the corresponding tumor sections we were able to positively identify numerous endothelial cells (CD31-positive) comprising tumor capillaries (Figure 7A–C). Those were not associated with pericytes, unlike vessels present in the surrounding connective tissues (Figure 7D and E). In addition, CAE-positive single mast cells were detected in the tumor periphery (Figure 7F). Diffused specific staining for fibroblastic markers (vimentin) was detected throughout fibrosarcomas. This staining was somewhat more intense within the connective tissue surrounding and infiltrating the tumors than within the parenchyma (Figure 7G).
Direct evidence for VEGF production by stromal cells infiltrating VEGF-negative tumors came from experiments with mice harbouring the LacZ-tagged VEGF allele (Miquerol et al., 1999). Because the VEGF-LacZ cassette is under control of the endogenous, unmodified VEGF promoter, individual stromal cells expressing VEGF can be detected by LacZ staining in a more reliable manner than in other VEGF reporter systems based on the exogenous VEGF promoter (Kishimoto et al., 2000). As shown in Figure 7K and L, numerous LacZ-positive fibroblast-like stromal cells were detected throughout the tumors and in para-vascular areas. This experiment demonstrates directly that at least some elements of the tumor stroma actively produce VEGF in situ, and that detection of this growth factor in whole tumor lysates was not a result of ‘contamination’ with blood cells and/or platelets.
Pleiotropic effects of ras and neu oncogenes on the angiogenic phenotype
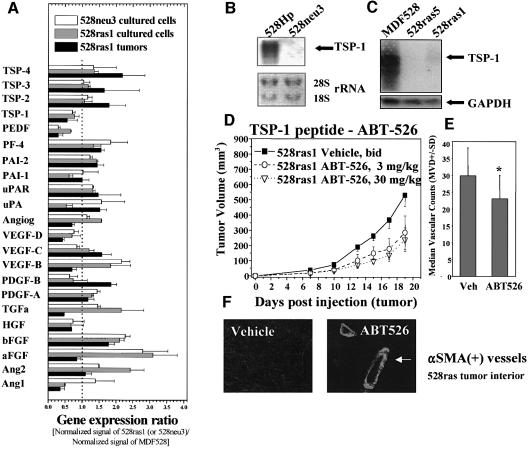
Despite the presence of VEGF-positive stroma, growth of VEGF–/– fibrosarcomas was, for the most part, only partially inhibited by the DC101 antibody, even when administered at doses that obliterated growth of ES-derived teratomas (compare Figure 5A and B with Figure 1). It follows that in oncogene-driven tumors, ‘angiogenic switching’ continues to occur despite the absence of VEGF production by tumor cells and inactivation of the endothelial VEGFR-2. This implies additional/alternative (VEGF-unrelated) pro-angiogenic mechanisms. In order to assess this aspect, we performed a targeted gene expression screen involving 23 known angiogenesis-related transcripts (Figure 8; see Materials and methods). GEArray is a version of a ‘reverse northern’ analysis where specific probes are immobilized on a membrane, which is then exposed to a pool of reverse-transcribed (radiolabeled) cellular cDNA. Such comparison between parental MDF528 cells and either ras- or neu-transformed fibrosarcoma counterparts indicated the existence of several oncogene-induced changes. Thus, acidic (aFGF), basic (bFGF) fibro blast growth factors and VEGF-B were all up-regulated in both ras- and neu-transformed cells as compared to their parental control MDF528 fibroblasts. A moderate increase in expression of transforming growth factor α (TGF-α) and angiopoietin 2 (Ang2) was also observed (mainly in 528ras1 cells), along with several less consistent changes (e.g. affecting PF-4, PAI-1, uPA). However, when tumor-derived RNA was analyzed instead of RNA isolated from the cultured cell lines, many of these changes were no longer detectable, with the exception of a modest increase in expression of bFGF in 528ras1 tumors (Figure 8A). Interestingly, both transformed cell lines (528neu3, 528ras1) and 528ras1 tumors reproducibly displayed transcript down-regulation for at least two different angiogenesis inhibitors: PEDF and TSP-1. In fact, the magnitude of TSP-1 down-regulation by ras and neu oncogenes appears to be underestimated by GEArray analysis, as indicated by near complete suppression of TSP-1 expression in northern blots performed with several tumor cell lines, including 528neu3 (Figure 8B), 528ras5 and 528ras1 (Figure 8C), relative to control cell lines (MDF528 and 528Hp/MDF528hygro). This result was confirmed at the protein level in both cultured cell lines and tumors (data not shown).
Fig. 8. Oncogene-induced angiogenic gene expression profiles. (A) GEArray analysis of changes in expression of angiogenesis-related genes in 528neu3 and 528ras1 cells in culture (or 528ras1 tumors) relative to their parental MDF528 cells (expression ratio; see Materials and methods). (B) Down-regulation of TSP-1 mRNA by mutant neu oncogene in 528neu3 cells. (C) Down-regulation of TSP-1 mRNA by mutant ras oncogene in 528ras1 and 528ras5 cells. (D) Growth suppression of 528ras1 tumors by administration of TSP-1 peptide ABT-526 (twice daily s.c. at doses indicated). (E) Vascular counts (MVD) within 528ras tumor ‘hot spots’ in mice treated with the vehicle (Veh) or ABT-526 peptide (P < 0.05). (F) Appearance of αSMA(+) blood vessels in 528ras tumors exposed to ABT-526.
Because of the magnitude, consistency and apparent generality of TSP-1 down-regulation observed in various oncogene-harboring cell types (Zabrenetzky et al., 1994; Rak et al., 2000; Watnick et al., 2003), we decided to examine the functional significance of this change in the context of VEGF-deficient 528ras1 cells. In order to reconstitute the anti-angiogenic TSP-1 activity in the environment of 528ras1 tumors, we decided to systemically administer TSP-1 peptides derived from the type I (properdin-like) repeats believed to participate in crucial interactions between TSP-1 and its endothelial cell CD36 receptor (Dawson and Bouck, 1999; Reiher et al., 2002). Figure 8D shows that systemic administration of such a TSP-1 peptide (ABT-526) resulted in marked and specific (albeit incomplete) growth inhibition of 528ras1 fibrosarcoma in vivo, even at relatively low doses (3 mg/kg). A much higher dose (30 mg/kg) of ABT-526 induced a somewhat greater (70%) but still incomplete anti-tumor effect. This effect was coupled with a decrease in vascular ‘hot spot’ MVD (Figure 8E). Interestingly, while we did not observe αSMA(+) vessels in untreated fibrosarcoma tumors (Figure 7D), such vessels (albeit only a fraction of all) were detectable in tumors exposed to the TSP-1 peptide (Figure 8F). Overall, these observations support the notion that suppression of TSP-1 activity represents an important aspect of the ras-dependent angiogenic phenotype.
Discussion
While our results overall are consistent with the importance of VEGF in tumor angiogenesis, they challenge the perceived generic nature of this relationship. Indeed, we describe two pathways of tumor-induced angiogenesis, which differ fundamentally in their genesis (epigenetic versus genetic), their absolute dependence on tumor cell-derived VEGF, and on the apparent relative contribution of the VEGF/VEGFR-2 pathway as such (Figure 9). Thus, in the case of epigenetically driven (tumor) angiogenesis exemplified by ES cell-induced teratomas, both VEGF production and biological activity (particularly the aspect mediated by VEGFR-2) are clearly essential and indispensable for tumor (teratoma) formation. This is consistent with other reports, in which obliteration of VEGF production in tumorigenic cell lines of embryonal origin resulted in suppression of their malignant properties (Ferrara et al., 1996; Grunstein et al., 1999, 2000; Shi and Ferrara, 1999; Tsuzuki et al., 2000). Also, development of spontaneous endocrine insulinomas under the influence of the early region of the SV40 large T oncogene (Rip-Tag mice) was recently shown to be inhibited in the context of conditional VEGF-deletion (VEGFfl/fl) (Inoue et al., 2002). It is noteworthy that in this study, the transforming genetic lesion is present in mice throughout embryonic and post-embryonic periods, and malignant disease develops in relatively young animals (10–16 weeks). Moreover, this process affects an organ (pancreatic beta islets), which unlike many non-endocrine tissues is a priori VEGF-positive (Dvorak et al., 1995), and possibly VEGF-dependent for its normal function. These circumstances could conceivably separate the Rip-Tag model of tumorigenesis from cancers occurring in non-endocrine and VEGF-negative adult tissues.
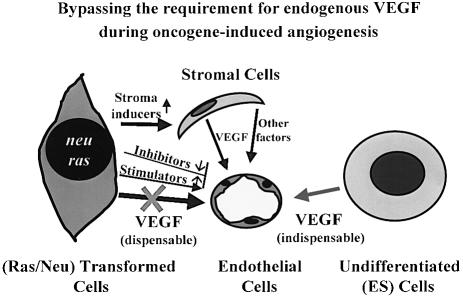
Fig. 9. Bypassing VEGF-dependence during oncogene-induced tumor angiogenesis. Totipotent ES cells form tumors (teratomas) upon s.c. inoculation due to perturbations in their differentiation program, in the apparent absence of stable genetic alterations and in a manner entirely dependent on VEGF-driven tumor angiogenesis. In contrast, tumors cells harbouring mutant oncogenes may develop redundant angiogenic VEGF-independent pathways involving changes in endogenous expression of angiogenesis stimulators and inhibitors, and recruitment of a pro-angiogenic tumor stroma. Consequently, in some cases VEGF production by oncogene-expressing cancer cells could become dispensable for tumor growth and angiogenesis in vivo.
In sharp contrast to the indolent behavior of VEGF–/– teratomas, their genetically related, VEGF–/– oncogene-induced fibrosarcomas exhibited a robust tumorigenic and angiogenic phenotype in vivo. Clearly, in this case, genetically altered (transformed) tumor cells were able to substitute for VEGF deficiency by triggering alternative/additional angiogenic (and tumorigenic) pathways. It is noteworthy that all VEGF–/– fibrosarcoma cells used in our study were derived from dermal connective tissue of mice that were at least 4–5 months of age, a circumstance that sharply separates these cells from their totipotent VEGF–/– ES ancestors and other embryonic cell types often used for tumor studies.
Our in situ analyses revealed numerous, elongated (fibroblastoid) VEGF expressing host stromal cells within 528ras and 528neu tumors. However, the functional significance of such stromal VEGF production in cancer is a subject of considerable controversy. Thus, the absolute abrogation of tumorigenesis by targeting the VEGF gene in various experimental tumor systems (e.g. in ES cell-derived teratomas) implicitly suggests that VEGF-positive host stromal cells make a negligible contribution in these instances (Ferrara et al., 1996; Grunstein et al., 1999, 2000; Shi and Ferrara, 1999; Tsuzuki et al., 2000; Inoue et al., 2002). This is contrary to strong, albeit circumstantial, suggestions that stromal VEGF production plays a critical role in tumor angiogenesis (Hlatky et al., 1994; Fukumura et al., 1998). Ironically, a rigorous inquiry in this regard would require exclusion of tumor cell-derived VEGF (i.e. analysis of tumors that are VEGF-deficient). By using such VEGF–/– oncogene-driven tumors we have demonstrated that the contribution of stromal VEGF to overall angiogenic competence in vivo is variable, modest and mostly non-essential. This is apparent from experiments in which two out of three VEGF–/– fibrosarcomas tested were only slightly growth inhibited by obliteration of VEGFR-2 activity. Our results bear an intriguing resemblance to the outcome of the recent phase III clinical trial in which a specific monoclonal anti-VEGF antibody (bevacizumab/Avastin®) unexpectedly failed to meet the efficacy endpoints when used (in combination with Xeloda® chemotherapy) to treat patients with advanced metastatic breast cancer (Garber, 2002). Our experimental observations suggest that such a failure might be indicative of a decline in the importance of the VEGF/VEGFR-2 pathway that may follow genetic tumor progression.
Oncogenic transformation is often associated with a multitude of molecular alterations (Zuber et al., 2000). In this regard, increasing contribution of pro-angiogenic growth factors other than VEGF has been detected in human tumors (Relf et al., 1997; Yoshiji et al., 1997). Our GEArray profiling also suggests several potentially relevant alternative changes in gene expression including up-regulation of angiogenesis stimulators (aFGF, bFGF, TGF-α, VEGF-B) or down-regulation of inhibitors (TSP-1, PEDF, PAI-1). In some instances these changes were associated with a particular oncogene (e.g. Ang1 down-regulation in ras- but not in neu-transformed cells). At least two different angiogenesis inhibitors, TSP-1 and PEDF, were altered both in vitro and in VEGF-deficient tumors (528ras1) in vivo. Interestingly, the latter changes were detected despite the presence of stromal cell material in tumor preparations. This may suggest an even greater magnitude of change in cancer cells or altered expression of TSP-1 and PEDF in stromal cells in vivo (a ‘field effect’) (Rak and Kerbel, 2003).
TSP-1 is of particular interest as a potent and well-studied angiogenesis inhibitor (Dawson and Bouck, 1999), and a known target of several transforming genes (Dameron et al., 1994; Zabrenetzky et al., 1994; Izumi et al., 2002). A detailed pathway of this regulation by ras has recently been delineated (Watnick et al., 2003). Our results with the anti-angiogenic TSP-1 peptide (ABT-526) suggest that the loss of TSP-1 expression is a significant event during tumor progression driven by oncogenes, but also emphasize the multi-factorial nature of such pro-angiogenic influences. Thus, even in the presence of potent TSP-1 peptides combined with VEGF deficiency (of tumor cells), growth of oncogene-driven fibrosarcomas was not obliterated completely. This may signify a redundancy of angiogenic pathways (e.g. impact of PEDF; Dawson et al., 1999) and/or the possible oncogene-dependent changes in vascular dependence of cancer cells, something that we have recently suggested (Rak et al., 2002). Of note, TSP-1 peptides provoked not only reduction in MVD but also a trend towards the appearance of αSMA(+)/pericyte-covered blood vessels within the interiors of 528ras tumors, which normally express very low levels of Ang-1 and no αSMA(+) vasculature. It remains to be seen whether TSP-1 has any direct impact on the Ang-1/tie-2 pathway. Apart from its anti-angiogenic effects, TSP-1 may also exhibit some direct inhibitory activity against cancer cells (Lawler, 2002), something that we have detected with 528ras cells engineered to overexpress TSP-1 or exposed to high concentrations of ABT-526 peptide (not shown).
Here we describe considerable differences in the role of VEGF during formation of teratomas versus oncogene-induced fibrosarcomas. Naturally occurring human tumors may be closer to one end or the other of this spectrum, depending on the cell/tumor type, and whether genetic or epigenetic influences predominate in expression of their pro-angiogenic characteristics. Carcinomas could obviously also differ from fibrosarcomas. Nevertheless, our study suggests that the functional role of VEGF in cancer is not always indispensable, regardless of its apparent presence.
Materials and methods
Cell lines and culture conditions
R1 ES cells and their VEGF–/– variants were cultured as previously described (Nagy et al., 1993; Carmeliet et al., 1996). ES cells were cultured on 0.5% gelatin (Sigma) in Dulbecco’s modified Eagle’s medium (DMEM), with 20% fetal bovine serum (FBS; Hyclone), 2000 U of leukemia inhibitory factor (LIF), with or without mitomycin C (Sigma)-treated feeders. All other cell lines were maintained in DMEM, 5% FBS (Gibco-BRL) under standard conditions (37°C, 5% CO2). Dr M.-Ch.Hung (M.D. Anderson Cancer Center, Houston, TX) generously provided B104.1.1 cells, while the packaging cell line expressing the ecotropic ras/myc retrovirus was a gift from Dr Paul Soloway (Roswell Park Memorial Institute, Buffalo, NY) (Soloway et al., 1996).
Generation of VEGF-deficient and VEGF-proficient fibroblastic cell lines
Chimeric mice were generated by in vitro aggregation from VEGF–/– R1 ES cells (129X1/Sv × 129S1/SvJ) and eight-cell stage embryos of wild-type (VEGF+/+) mice (CD1), as described previously (Tanaka et al., 1997). The resulting aggregates were implanted into surrogate mothers and embryos were allowed to develop to term. The degree of chimerism (usually <20%) was assessed by the presence of agouti (ES cell-derived, VEGF –/–) or albino (CD1, VEGF+/+) skin patches. Chimera were killed at 4–5 months and skin fragments were explanted, trypsinized overnight at 4°C, stripped of epidermis and digested with collagenase, hyaluronidase and elastase. Dermal fibroblasts were allowed to attach and expand in culture. Some primary cultures were transformed with the retrovirus expressing ras and myc oncogenes and foci subcloned into duplicates. These were grown with and without 400 µg/ml of G418 (Gibco-BRL) such that VEGF–/– or VEGF+/+ cells could be identified based on their G418 resistance. Independent sets of primary fibroblastic cultures were subjected to serial passage in the presence of G418 for 5 months, whereby a polyclonal, spontaneously immortalized, VEGF–/– cell line (MDF528) was established (Figure 2). This cell line was subsequently transfected with expression vectors encoding either mutant (V12) H-ras or activated HER-2/neu oncogenes using a lipofectin protocol (Gibco-BRL) and pcDNA3.1Zeo or pcDNA3.1Hygro (both from Invitrogen) for an additional selectable marker. Multiple transfected MDF528-derived clones were isolated and their transformed phenotype assessed morphologically, by focus and soft agar colony formation assays. The expression of both H-Ras and HER-2/NEU proteins was verified by western blotting with pan-RAS antibody (Oncogene Science), or immunoprecipitation with the anti-NEU antibody SC-284 (Santa Cruz Biotechnologies) followed by staining with phosphotyrosine-specific antibody 05-321 (Upstate Biotechnology). In cell lines expressing ras or neu (designated 528ras or 528neu, respectively; Figure 2) murine VEGF164 or LacZ (control) was expressed by transfection (vector was kindly provided by Dr Kevin Claffey). The resulting cell lines (e.g. 528rasmV and 528rasLZ, respectively) expressed additional selectable markers due to co-transfection with pcDNA3.1Hygro or pcDNA3.1Zeo (Invitrogen). Finally the VEGF status of all cell lines was verified by a combination of Southern (with exon 8 genomic sequence of VEGF), northern (exon 1–5 cDNA obtained from Dr Harold Dvorak), VEGF mRNA enzyme-linked immunosorbent assay (ELISA) (R&D Systems) and mouse VEGF protein ELISA (R&D Systems). The fibroblastic nature of cell lines was confirmed by morphology, growth requirements and staining with anti-vimentin antibody (see below).
Determination of VEGF protein and RNA
VEGF protein levels were determined in either conditioned medium or tumor homogenates using a mVEGF ELISA kit (R&D Systems) in the presence or absence of FBS (1%, 5%, 10%), and/or 100 µM cobalt chloride (CoCl2), a hypoxia mimetic (Minchenko et al., 1994). The medium was conditioned for 24 h, cleared by high-speed centrifugation and stored at –70°C. The cell number/well was used for assay normalization. Tumor fragments (∼0.5 g) were minced into small pieces and homogenized [in phosphate-buffered saline (PBS) on ice] with Virtishear blender (The Virtis Company, Inc.). Tumor homogenates were then cleared by centrifugation and supernatants stored at –70°C. Total RNA was isolated from either cultured cells or tumor tissue using TRIzol reagent (Life Technologies). To quantify expression of the mVEGF transcript, 5–10 µg of total RNA was applied to the Quantikine® mRNA kit (R&D Systems) or 10–50 µg of such RNA was analyzed by northern blotting, as described previously (Rak et al., 2000).
Tumor implantation analysis and in vivo treatments
All cell lines were tested for tumorigenic and angiogenic capacity in SCID mice 6–8 weeks of age (unless indicated otherwise). Briefly, 1–5 × 106 transformed or non-transformed (MDF528) fibroblastic cells were injected orthotopically (s.c.) in 0.2 ml PBS. In the case of R1 (ES) cells and their two independent VEGF–/– clones (36.8 and 44.7) 5–7 × 106 cells/mouse were inoculated (s.c.). Tumor take and growth were followed by measurements with the vernier caliper, followed by tumor volume calculation as a function of time (Rak et al., 2000). Most cell lines and treatment conditions were tested in groups of 5–10 mice (occasionally four mice) and repeated at least twice (sometimes up to five times) with similar results. Neutralizing monoclonal antibody against mouse VEGFR-2 (DC101) was generated by ImClone Systems Inc. (New York, NY). This antibody was reconstituted in sterile PBS and injected intraperitoneally into tumor bearing mice twice a week, starting 1–4 days after tumor cell injection (unless otherwise indicated). An effective dose of DC101 (800 µg per injection) was previously established (Lyden et al., 2001). The nonapeptide (Ac-Sar-Gly-Val-D-Ile-Thr-Nva-Ile-Arg-Pro-ethylamide), which contains a fragment of the second type-I TSP-1 repeat (ABT-526 or DITSP), was generated by Abbott Laboratories. The peptide was dissolved in 5% dextrose and injected s.c. into tumor bearing SCID mice twice daily. Treatment was initiated 1 day after injection of 106 528ras1 cells s.c. All animal experiments were performed in accordance with guidelines of the Canadian Council of Animal Care (CCAC) and McMaster University’s Animal Research Ethics Board (AREB).
Histological analysis and immunohistochemistry
Formalin-fixed, paraffin-embedded specimens of 528neu or 528ras tumors were sectioned (5–7 µm) and processed for gross histological analysis (hematoxylin and eosin), and immunostaining for αSMA, desmin or chloroacetate esterase (CAE) histochemistry, as described elsewhere (Coussens et al., 1999). Anti-αSMA antibody A-2547 (Sigma) was used at 40 µg/ml and desmin was detected with mouse monoclonal antibody D33 (DAKO). Endothelial cells were visualized with anti-CD31 (PECAM) antibody 01951D (PharMingen) at 2.5–10 µg/ml (5–7 µm thick frozen sections). Appropriate horseradish peroxidase-, fluorescein- or Texas Red-conjugated secondary antibodies were used in these experiments. The microvascular density count was carried out by using Northern Eclipse software, version 6. Basic statistical analysis was performed with the GraphPAD InSTAT software, version 1.14 (GraphPAD, Inc.). Tumor stroma was visualized by injecting tumor cells into syngeneic or SCID mice harboring ubiquitously expressed YFP transgene (Hadjantonakis et al., 1998). Tumors 10–15 mm in diameter were removed, sectioned and immediately analyzed by confocal microscopy (Zeiss). Identification of LacZ-expressing (VEGF-producing) stromal cells was performed after s.c. injection of tumor cells into syngeneic mice harboring a LacZ-tagged VEGF allele (VEGF-LacZ-KI mice). Briefly, tumors were fixed in 0.2% glutaraldehyde and 2% paraformaldehyde, embedded in OCT compound (Tissue Tek), frozen, sectioned and stained as described previously (Miquerol et al., 1999).
GEArray analysis
Expression profiling of 23 angiogenesis-related genes in oncogene-transformed cells (or tumors) versus control MDF528 cells was performed by using the Mouse Cancer/Angiogenesis-2 GEArray kit (SuperArray Biosience Corp., Frederick, MD) following the manufacturer’s instructions. Briefly, cells were grown to confluence (in DMEM 5% FBS) or (transformed cells) inoculated s.c. into SCID mice to generate tumors (100–150 mm3). RNA was extracted using TRIzol method, reverse-transcribed and 32P-labeled cDNA was hybridized overnight at 68°C to membranes containing immobilized specific cDNA probes. The membranes were washed and specific bound radioactivity was quantified using PhosphorImager™ and ImageQuant software 5.0 (Molecular Dynamics). VEGF signal was omitted from analysis of VEGF–/– cell lines, as it is known to represent an incorrect transcript. The level of gene expression was calculated according to manufacturer’s recommendations. Local median was applied for background correction and the volume of each gene spot was averaged (for each two spots) and standardized to the expression of β-actin. The ratio between normalized signal intensity of transformed cells (or tumors) versus parental cells (MDF528) was calculated in each experiment separately, and the data were presented as the average of at least three such independent quantifications (± SD).
Acknowledgments
Acknowledgements
We are thankful to Drs Kevin Claffey, Harold Dvorak and Paul Soloway for providing us with valuable reagents, and to Dr Dan Dumont for critical reading of our manuscript. We also thank Drs Dan Hicklin and Peter Bohlen from ImClone Systems, Inc. for providing DC101 antibody and for valuable input in preparation of this manuscript. J.R. is a recipient of a Scientist Award of the National Cancer Institute of Canada. This work was supported by the Terry Fox Grant for new investigators from the National Cancer Institute of Canada (NCIC) and Henderson Research Centre grant to J.R., by a grant from the Canadian Institutes for Health Research (CIHR) to R.S.K. and a grant from NCIC to A.N., who is a senior scientist of the CIHR. We are indebted to our colleagues and our families for their continued support. A.V.-P. is a recipient of a CIHR pre-doctoral fellowship.
References
- Bouck N., Stellmach,V. and Hsu,S.C. (1996) How tumors become angiogenic. Adv. Cancer Res., 69, 135–174. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. (2000) Mechanisms of angiogenesis and arteriogenesis. Nat. Med., 6, 389–395. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. et al. (1996) Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature, 380, 435–439. [DOI] [PubMed] [Google Scholar]
- Claffey K.P., Brown,L.F., del Aguila,L.F., Tognazzi,K., Yeo,K.-T., Manseau,E.J. and Dvorak,H.F. (1996) Expression of vascular permeability factor/vascular endothelial growth factor melanoma cells increases tumor growth, angiogenesis, and experimental metastasis. Cancer Res., 56, 172–181. [PubMed] [Google Scholar]
- Coussens L.M., Raymond,W.W., Bergers,G., Laig-Webster,M., Behrendtsen,O., Werb,Z., Caughey,G.H. and Hanahan,D. (1999) Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev., 13, 1382–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dameron K.M., Volpert,O.V., Tainsky,M.A. and Bouck,N. (1994) Control of angiogenesis in fibroblasts by p53 regulation of thrombospondin-1. Science, 265, 1582–1584. [DOI] [PubMed] [Google Scholar]
- Dawson D.W. and Bouck,N.P. (1999) Thrombospondin as an inhibitor of angiogenesis. In Teicher,B.A. (ed.), Antiangiogenic Agents in Cancer Therapy. Humana Press, Totowa, NJ, pp. 185–203. [Google Scholar]
- Dawson D.W., Volpert,O.V., Gillis,P., Crawford,S.E., Xu,H., Benedict,W. and Bouck,N.P. (1999) Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science, 285, 245–248. [DOI] [PubMed] [Google Scholar]
- Dvorak H.F., Brown,L.F., Detmar,M. and Dvorak,A.M. (1995) Review: Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol., 146, 1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Ferrara N. and Gerber,H.P. (2001) The role of vascular endothelial growth factor in angiogenesis. Acta Haematol., 106, 148–156. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Carver-Moore,K., Chen,H., Dowd,M., Lu,L., O’Shea,K.S., Powell-Braxton,L., Hillan,K.J. and Moore,M.W. (1996) Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature, 380, 439–442. [DOI] [PubMed] [Google Scholar]
- Fukumura D. et al. (1998) Tumor induction of VEGF promoter activity in stromal cells. Cell, 94, 715–725. [DOI] [PubMed] [Google Scholar]
- Garber K. (2002) Angiogenesis inhibitors suffer new setback. Nat. Biotechnol., 20, 1067–1068. [DOI] [PubMed] [Google Scholar]
- Grunstein J., Roberts,W.G., Mathieu-Costello,O., Hanahan,D. and Johnson,R.S. (1999) Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res., 59, 1592–1598. [PubMed] [Google Scholar]
- Grunstein J., Masbad,J.J., Hickey,R., Giordano,F. and Johnson,R.S. (2000) Isoforms of vascular endothelial growth factor act in a coordinate fashion to recruit and expand tumor vasculature. Mol. Cell. Biol., 20, 7282–7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjantonakis A.K., Gertsenstein,M., Ikawa,M., Okabe,M. and Nagy,A. (1998) Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech. Dev., 76, 79–90. [DOI] [PubMed] [Google Scholar]
- Hanahan D. and Folkman,J. (1996) Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell, 86, 353–364. [DOI] [PubMed] [Google Scholar]
- Hlatky L., Tsionou,C., Hahnfeldt,P. and Coleman,C.N. (1994) Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res., 54, 6083–6086. [PubMed] [Google Scholar]
- Inoue M., Hager,J.H., Ferrara,N., Gerber,H.P. and Hanahan,D. (2002) VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell, 1, 193–202. [DOI] [PubMed] [Google Scholar]
- Izumi Y., Xu,L., diTomaso,E., Fukumura,D. and Jain,R.K. (2002) Herceptin acts as an anti-angiogenic cocktail. Nature, 416, 279–280. [DOI] [PubMed] [Google Scholar]
- Kim K.J., Li,B., Houck,K., Winer,J. and Ferrara,N. (1992) The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors, 7, 53–64. [DOI] [PubMed] [Google Scholar]
- Kishimoto J., Ehama,R., Ge,Y., Kobayashi,T., Nishiyama,T., Detmar,M. and Burgeson,R.E. (2000) In vivo detection of human vascular endothelial growth factor promoter activity in transgenic mouse skin. Am. J. Pathol., 157, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J. (2002) Thrombospondin-1 as an endogenous inhibitor of angiogenesis and tumor growth. J. Cell. Mol. Med., 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D. et al. (2001) Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat. Med., 7, 1194–1201. [DOI] [PubMed] [Google Scholar]
- Minchenko A., Salceda,S., Bauer,T. and Caro,J. (1994) Hypoxia regulatory elements of the human vascular endothelial growth factor gene. Cell. Mol. Biol. Res., 40, 35–39. [PubMed] [Google Scholar]
- Miquerol L., Gertsenstein,M., Harpal,K., Rossant,J. and Nagy,A. (1999) Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev. Biol., 212, 307–322. [DOI] [PubMed] [Google Scholar]
- Nagy A., Rossant,J., Nagy,R., Abramow-Newerly,W. and Roder,J.C. (1993) Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc. Natl Acad. Sci. USA, 90, 8424–8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rak J. and Kerbel,R.S. (2003) Oncogenes and tumor angiogenesis. In Rak,J. (ed.), Oncogene-Directed Therapies. Humana Press, Totowa, NJ, pp. 171–218. [Google Scholar]
- Rak J., Mitsuhashi,Y., Sheehan,C., Tamir,A., Viloria-Petit,A., Filmus,J., Mansour,S.J., Ahn,N.G. and Kerbel,R.S. (2000) Oncogenes and tumor angiogenesis: differential modes of vascular endothelial growth factor up-regulation in ras-transformed epithelial cells and fibroblasts. Cancer Res., 60, 490–498. [PubMed] [Google Scholar]
- Rak J.W., Yu,J.L., Kerbel,R.S. and Coomber,B.L. (2002) What do oncogenic mutations have to do with angiogenesis/vascular dependence of tumors. Cancer Res., 62, 1931–1934. [PubMed] [Google Scholar]
- Reiher F.K., Volpert,O.V., Jimenez,B., Crawford,S.E., Dinney,C.P., Henkin,J., Haviv,F., Bouck,N.P. and Campbell,S.C. (2002) Inhibition of tumor growth by systemic treatment with thrombospondin-1 peptide mimetics. Int. J. Cancer, 98, 682–689. [DOI] [PubMed] [Google Scholar]
- Relf M. et al. (1997) Expression of the angiogenic factors vascular endothelial cell growth factor, acidic and basic fibroblast growth factor, tumor growth factor beta-1, platelet-derived endothelial cell growth factor, placenta growth factor, and pleiotrophin in human primary breast cancer and its relation to angiogenesis. Cancer Res., 57, 963–969. [PubMed] [Google Scholar]
- Shi Y.P. and Ferrara,N. (1999) Oncogenic ras fails to restore an in vivo tumorigenic phenotype in embryonic stem cells lacking vascular endothelial growth factor (VEGF). Biochem. Biophys. Res. Commun., 254, 480–483. [DOI] [PubMed] [Google Scholar]
- Soloway P.D., Alexander,C.M., Werb,Z. and Jaenisch,R. (1996) Targeted mutagenesis of Timp-1 reveals that lung tumor invasion is influenced by Timp-1 genotype of the tumor but not by that of the host. Oncogene, 13, 2307–2314. [PubMed] [Google Scholar]
- Tanaka M., Gertsenstein,M., Rossant,J. and Nagy,A. (1997) Mash2 acts cell autonomously in mouse spongiotrophoblast development. Dev. Biol., 190, 55–65. [DOI] [PubMed] [Google Scholar]
- Tsuzuki Y., Fukumura,D., Oosthuyse,B., Koike,C., Carmeliet,P. and Jain,R.K. (2000) Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha→hypoxia response element→VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res., 60, 6248–6252. [PubMed] [Google Scholar]
- Watnick R.S., Cheng,Y.-N., Rangarajan,A., Ince,T.A. and Weinberg,R.A. (2003) Ras modulates Myc activity to repress thrombospondin-1 expression and increase tumor angiogenesis. Cancer Cell, 3, 219–231. [DOI] [PubMed] [Google Scholar]
- Yoshiji H., Harris,S.R. and Thorgeirsson,U.P. (1997) Vascular endothelial growth factor is essential for initial but not continued in vivo growth of human breast carcinoma cells. Cancer Res., 57, 3924–3928. [PubMed] [Google Scholar]
- Yu J.L., Rak,J.W., Carmeliet,P., Nagy,A., Kerbel,R.S. and Coomber,B.L. (2001) Heterogeneous vascular dependence of tumor cell populations. Am. J. Pathol., 158, 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabrenetzky V., Harris,C.C., Steeg,P.S. and Roberts,D.D. (1994) Expression of the extracellular matrix molecule thrombospondin inversely correlates with malignant progression in melanoma, lung and breast carcinoma cell lines. Int. J. Cancer, 59, 191–195. [DOI] [PubMed] [Google Scholar]
- Zuber J., Tchernitsa,O.I., Hinzmann,B., Schmitz,A.C., Grips,M., Hellriegel,M., Sers,C., Rosenthal,A. and Schafer,R. (2000) A genome-wide survey of RAS transformation targets. Nat. Genet., 24, 144–152. [DOI] [PubMed] [Google Scholar]