Abstract
The expression of the Na+,K+-ATPase α and β subunit isoforms in rat skeletal muscle and its age-associated changes have been shown to be muscle-type dependent. The cellular basis underlying these findings is not completely understood. In this study, we examined the expression of Na+,K+-ATPase isoforms in individual fiber types and tested the hypothesis that, with age, the changes in the expression of the isoforms differ among individual fibers. We utilized immunohistochemical techniques to examine the expression of the subunit isoforms at the individual fiber levels. Immunofluorescence staining of the subunit isoforms in both white gastrocnemius (GW) and red gastrocnemius (GR) revealed a predominance of staining on the sarcolemmal membrane. Compared to the skeletal muscle of 6-month old rats, there were substantial increases in the levels of α1, β1, and β3 subunit isoforms, and decreases in the levels of α2 and β2 in 30-month old rats. In addition, we found distinct patterns of staining for the α1, α2, β1, and β2 isoforms in tissue sections from young and aged rats. Muscle fiber-typing was performed to correlate the pattern of staining with specific fiber types. Staining for α1 and α2 isoforms in the skeletal muscle of young rats was generally evenly distributed among the fibers of GW and GR, with the exception of higher α1 levels in slow-twitch oxidative Type I fibers of GR. By contrast, staining for the β1 and β2 isoforms in the mostly oxidative fibers and the mostly glycolytic fibers, respectively, was almost mutually exclusive. With age, there was a fiber-type selective qualitative decrease of α2 and β2 in Type IIB fibers, and increase of β1 in Type IIB fibers and β2 in Type IID fibers of white gastrocnemius. These results provide, at the individual fiber level, a cellular basis for the differential expression of the Na+,K+-ATPase subunit isoforms in the muscle groups. The data further indicate that the aged-associated changes in expression of the subunit isoforms occur in both a fiber-type specific as well as an across fiber-type manner. Because of the differing biochemical properties of the subunit isoforms, these changes add another layer of complexity in our understanding of the adaptation of the Na-pump in skeletal muscle with advancing age.
Keywords: Aging, Na+-K+ pump, subunit isoforms
INTRODUCTION
Na+,K+-ATPase is a ubiquitous transporter of Na+ and K+ ions across the plasma membrane in almost all eukaryotic cells. In skeletal muscle, which comprises one of the largest pools of K+ in the body, Na+,K+-ATPase maintains Na+-K+ homeostasis and modulates muscle contractile function [1-4]. Na+,K+-ATPase consists of a large catalytic transmembrane α-subunit and a small β-subunit and multiple isoforms of the α- and β-subunit have been identified [5-9]. Skeletal muscle of mature rats expresses the α1-and α2-subunit isoforms and the three β-subunit isoforms (β1, β2, and β3) [10-14].
Expression of the specific subunit isoforms of the Na+,K+-ATPase is muscle type-specific. Muscles rich in fast- and slow-twitch oxidative fibers express more α1- and β1-isoforms than fast-twitch glycolytic muscles, whereas the opposite is true for the β2-isoform [15-17]. By contrast, the β3-isoform is abundantly expressed in both oxidative and glycolytic muscles [18]. We previously have demonstrated that the expression of the Na+,K+-ATPase isoforms is differentially altered with age and by exercise training in a muscle-type specific manner [18,19]. Because the subunit isoforms possess differing biochemical properties [13,20-24], these alternations suggest an adaptation of Na+,K+-ATPase to the different demands of the muscle groups during the aging process.
Despite these earlier findings, the underlying cellular basis for the muscle-type differential expression is not completely understood. It is worth noting that no muscle type of the hindlimb is comprised of a pure fiber type [25]. Thus, for example, it is unclear whether the higher expression of β1 in the more oxidative muscle, such as the red gastrocnemius, is the result of a high level of expression in just a few selective fibers, or due to a general high level of expression in a broad range of fibers in that muscle. Similarly, it is unclear whether the changes in the expression of the subunit isoforms in aged skeletal muscle are due to an overall change across different fiber-types or select changes in specific fibers. This fundamental information is important for a greater understanding of the adaptation of the skeletal muscle Na-pump during the aging process.
Alterations in the activity of the Na+,K+-ATPase in skeletal muscle during aging could have important physiological and pathological consequences. Because the Na+,K+-ATPase indirectly modulates contractile function of the skeletal muscle, changes in Na+,K+-ATPase activity could play a role in age-associated early muscle fatigue, a pathological condition with complex etiology [26-28]. Furthermore, a reduction in Na+,K+-ATPase activity in skeletal muscle could expose the myocardium to higher extracellular K+ levels, and thus could affect the electrophysiology of the myocardium [29].
Therefore, in the present study we examined the expression of Na+,K+-ATPase isoforms in individual fiber types, and tested the hypothesis that the pattern of expression among the fiber types changes with advancing age. Having a detailed knowledge regarding the distribution of the subunit isoforms in the specific muscle fibers will facilitate our understanding of the functional roles of the isoforms in the muscle groups. The expression of the isoforms at the individual fiber level, in red gastrocnemius (GR) and white gastrocnemius (GW) muscle of the rat, which comprise fibers from slow oxidative to fast glycolytic types (Type I, IIA, IID, IIBD, and IIB fibers) [25], was correlated with fiber typing data. Parts of this paper were published previously in abstract form [30].
MATERIALS AND METHODS
Animals
Male 6-month old and 30-month old Fischer 344 x Brown Norway rats were anesthetized with pentobarbital sodium (50 mg/kg i.p.) at least 15 min following heparin injection (1000 IU/kg). After the hearts were removed, skeletal muscles were dissected, cryoprotected in liquid nitrogen cooled isopentane, and stored at -80°C until use. All animal use protocols were approved by the institutional animal care committee.
Immunofluorescence staining
Cryostat sections (8 μm) of red and white gastrocnemius from both age groups were collected on the same slides so that they underwent the exact same treatments. The sections were fixed in a 1:1 acetone and methanol solution, incubated in 0.5% NaBH4 to decrease auto-fluorescence, and permeabilized in PBS with 0.5% Triton X-100 for 10 min at room temperature.
Immunofluorescence labeling was performed using the following antibodies: monoclonal anti-α1 (α6F, 1:20) (Developmental Studies Hybridoma Bank, University of Iowa), monoclonal anti-α2 (MCB2, 1:10) (kindly provided by K. Sweadner, Harvard University), polyclonal anti-α2 subunit (1:10) (Upstate Biotechnology, Lake Placid, NY), monoclonal anti-β1 (SpEtB1, 1:10) (kindly provided by P. Martin-Vasallo, Tenerife, Spain), monoclonal anti-β2 (GP50, without dilution) (kindly provided by P. Beesley, Royal Holloway and Bedform New College, Egham, Surrey, UK), and polyclonal anti-β3 subunit antibody (1:100) (Upstate Biotechnology, Lake Placid, NY). The tissue sections were incubated with the primary antibodies overnight at 4°C. Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 546 goat anti-mouse IgG (1:200) (Molecular Probes, Molecular Probes, Eugene, OR) were used as secondary antibodies to detect the bound primary antibodies. A negative control, i.e. immunostaining without the respective primary antibody, was included in each set of experiments and the staining was always negligible. Fluorescence images were taken using a Nikon (Melville, NY) fluorescence microscope with the MagnaFIRE SP software (Optronics Inc, Goleta, CA). In every cross-section, we carefully surveyed all the fibers and captured 3-4 images that are representative of the whole cross-section. An image that appears to be close to the “average” of the images is presented in our data and/or used for the semi-quantitative analysis. Images were processed using Photoshop 7 software (Adobe, USA). The data presented are representative of observations from tissue sections obtained from 2-3 animals, one tissue sample per animal.
To quantify the relative staining intensity on the sarcolemmal membrane of the fibers, the images first were edited in Adobe Photoshop (San Jose, California), using a Wacom Graphire tablet + stylus and Photoshop”s lasso and fill tools, in order to remove all the non-fiber wall areas from the 256 grey images. These areas were drawn and filled with black. By using the MetaMorph v6.2.6 Premiere image analysis package (Molecular Devices Corporation, Sunnyvale, CA), the edited images subsequently were thresholded by adjusting the inclusion range to include all the fiber wall regions only, and the area measured and logged. The images were then re-thresholded, always set at 90 to 255 (where 0 = darkest black and 255 = brightest white), to only include the bright regions within the fiber walls. The thresholded area was analysed using MetaMorph”s object and region measurements. The staining intensity of the image was represented by the ‘brightest region” thresholded area, expressed as a percentage of the whole image. Relative staining intensity was calculated by normalizing data from 30-month old rats by the corresponding data from 6-month old rats.
Fiber typing by histochemical staining
To identify the different fiber types, myofibrillar actomyosin ATPase activity was histochemically determined at two different pH, as described by Hamalainen and Pette [31]. Based on the pH- and formaldehyde-sensitive enzyme activity of the different fibers, the fibers identified are the Types I, IIA, IID, IIBD, and IIB fibers, in the order from slow oxidative to fast glycolytic fiber types (Table 1).
Table 1.
Staining pattern of the different muscle fibers by the two fiber typing methods (modified from Hamalainen and Pette [31])
| ◀oxidative | glycolytic▶ | ||||
|---|---|---|---|---|---|
| I | IIA | IID | IIBD | IIB | |
| pH10.4 | +++++ | +++++ | ++++ | +++ | |
| pH 7.2 | ++ | +++++ | +++ | ++ | + |
The ‘+’s signifies the relative intensities of the staining; the more the ‘+’s, the darker the fiber was stained
In the first staining protocol, based on the procedure of Guth and Samaha [32], as described by Hamalainen and Pette [31], tissue sections were pre-incubated at pH 10.4. Sections were then fixed for 8 min at 4°C in a solution containing: formaldehyde (1.85% (w/v)), 145 mM sodium cacodylate, 68 mM CaCl2 , and 325 mM sucrose at pH 7.6. They were washed in 100 mM Tris-HCl (pH 7.8), 18 mM CaCl2 buffer, and pre-incubated for 10-14 min in an alkaline solution containing: 34 mM 2-amino-3-methyl-1-propanol, 120 mM CaCl2, 50 mM KCl (pH 10.4). The sections were washed and incubated for 25 min at 37°C in an ATP solution containing: 2.5 mM ATP, 100 mM 2-amino-3-methyl-1-propanol, 18 mM CaCl2, 50 mM KCl (pH 9.4). After three 30 sec washes in 11 mM CaCl2, they were incubated for 3 min in 2% CoCl2. Following four 30 sec washes in 115 mM 2-animo-3-methyl-1-propanol (pH 9.4), the sections were incubated for 3 minutes in 1.5% (v/v) (NH4)2S, washed with water, and mounted.
The second staining protocol pre-incubates tissue sections at pH 7.2 and is similar to that described by Hamalainen and Pette [31] and based on the method of Huges [33]. Sections were fixed in a solution containing 4% (w/v) formaldehyde, 0.44 M sucrose, and 50 mM Tris-maleate (pH 7.20) for 8 min at 4°C, and then transferred to the incubation solution containing: 50 mM Trismaleate, 100 mM KCl, 5 mM MgCl2, 2.6 mM ATP, 4 mM PbN2O6 (pH 7.20 with KOH), and incubated for 60 min at 37°C. The sections were washed in distilled water, incubated for 2 min in 4% (v/v) (NH4)2S, washed with water, and mounted.
RESULTS
Immunostaining of the subunit isoforms in young and aged skeletal muscle
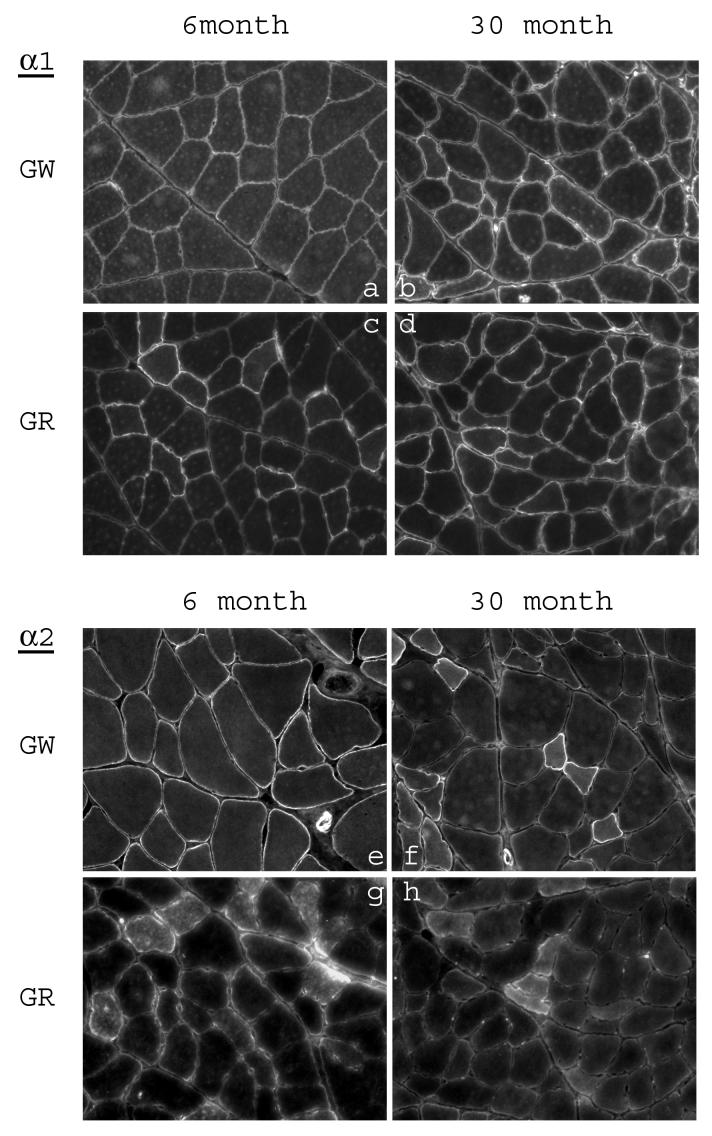
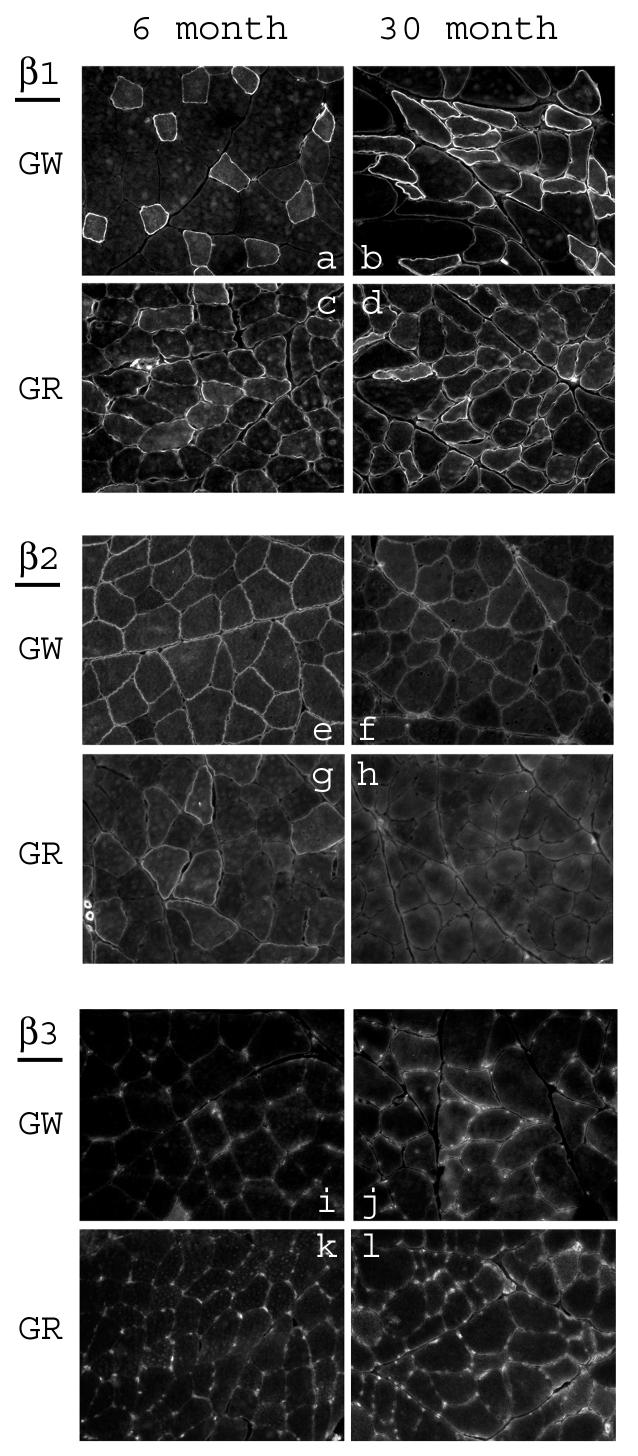
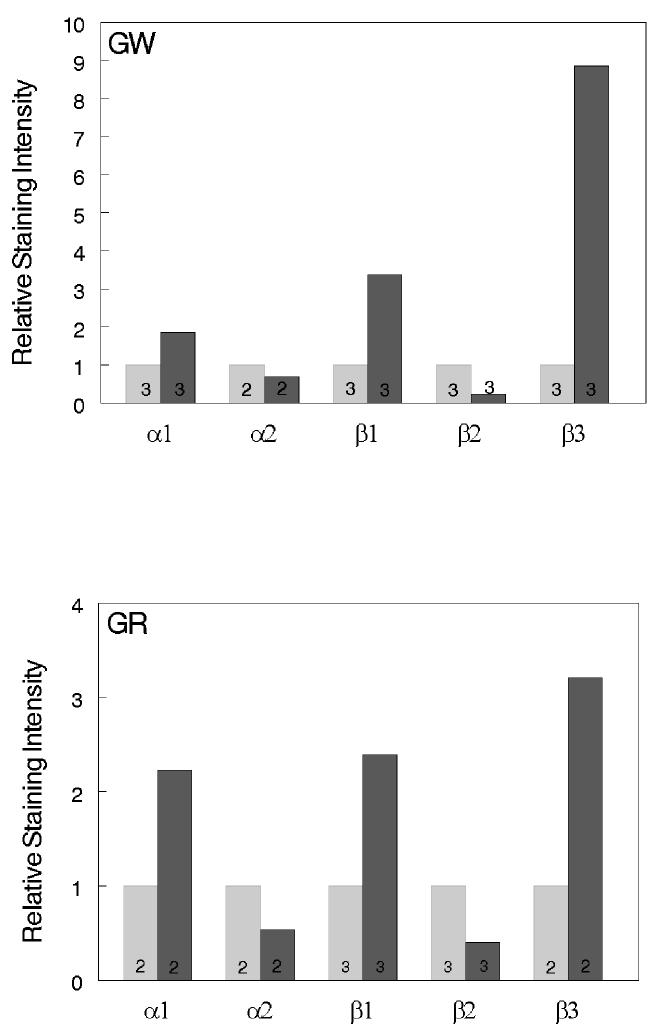
In our previous studies we have shown muscle-type specific alterations of the Na+,K+-ATPase subunit isoforms in skeletal muscle with advancing age. To elucidate the expression at the muscle fiber level, the distribution of the subunit isoforms in the white and red gastrocnemius of young and aged rats were examined by immunostaining (Fig. 1 and 2). The isoform-specific antibodies stained predominantly the sarcolemmal membrane of the fibers. In order to quantify the relative changes in the levels of the subunits between young and aged skeletal muscle, immunostaining images for each isoform subunit from 2-3 pairs of cryosections (6-month old vs. 30-month old) were analyzed for relative staining intensity by the digital imaging method as described in Methods. The results are summarized in Fig. 3. Compared to the skeletal muscle from 6-month old rats, there was a substantial increase in α1, β1, and β3 in both white and red gastrocnemius of 30-month old rats. Furthermore, there was a substantial decrease in α2 and β2 in white and red gastrocnemius of 30-month old rats compared to 6-month old rats. In certain fibers, staining for α2 and β1 showed a relatively strong signal within the fiber. However, these are inconsistent findings in that they were either not reproducible in every tissue section, or were absent when alternative antibody was used (data not shown, see Discussion).
Fig. 1.
Immunofluorescence staining of white and red gastrocnemius from 6-month and 30-month old rats with the anti-α-subunit antibodies. Cross sections of each type of muscle from young and aged rats were placed on the same slide and immunostained as described in methods. a-d: sections stained with anti-α1 antibody; e-h: sections stained with anti-α2 antibody.
Fig. 2.
Immunofluorescence staining of white and red gastrocnemius from 6-month and 30-month old rats with the anti-β subunit antibodies. Cross sections of each type of muscle from young and aged rats were placed on the same slide and immunostained as described in methods. a-d: sections stained with anti-β1 antibody; e-h: sections stained with anti-β2 antibody; i-l: sections stained with anti-β3 antibody.
Staining for α2 in the white gastrocnemius of 30-month old rats was particularly intense in some fibers and very weak in others (Fig. 1e,f), and staining for β1 was apparent only in certain fibers of the white gastrocnemius in both 6- and 30-month old rats (Fig. 2a,b). Similarly, in the red gastrocnemius of 6-month old rats, only a select number of fibers were labeled by the β2 antibody, and the staining was almost completely absent in the skeletal muscle of 30-month old rats (Fig. 2g,h). Thus, in skeletal muscle the age-associated changes in expression of the Na+,K+-ATPase subunit isoforms appear to be fiber-type specific.
Fiber type-specific staining of the subunit isoforms in the white gastrocnemius of young and aged rats
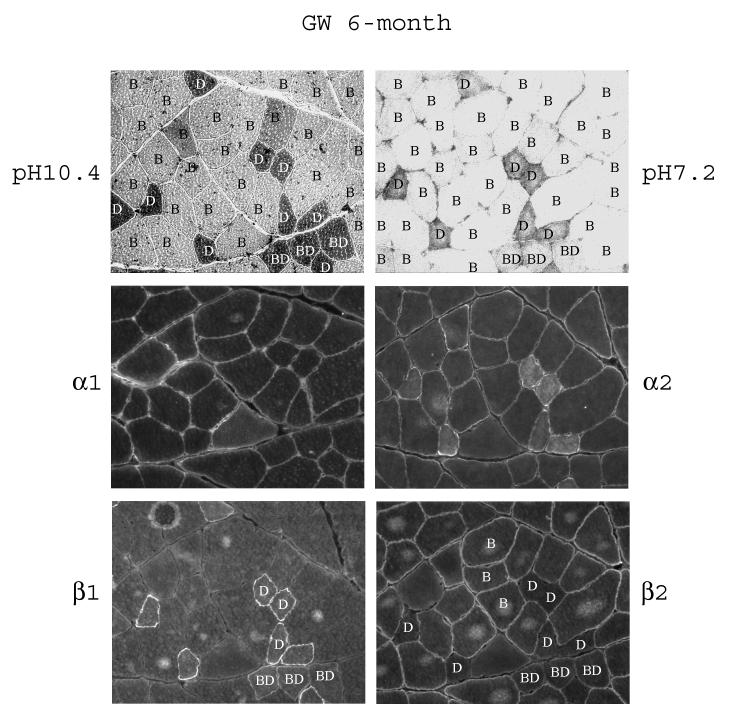
Serial sections of the white gastrocnemius were used to correlate the immunostaining patterns with the specific fibers of the skeletal muscles. By studying the pH sensitive actomyosin ATPase activity, we identified in the white gastrocnemius three major fiber types, they are, in the order from more oxidative to more glycolytic, the fast-twitch oxidative/glycolytic Type IID and IIBD fibers, and the fast-twitch glycolytic Type IIB fibers (Fig. 4). Qualitatively, staining for both α1 and α2 was fairly even among the different fiber types in the white gastrocnemius of 6-month old rats (Fig. 4 and Table 2). The Type IID fibers showed significant intracellular staining for α2, however, as mentioned above, the nature of this staining is unclear. Staining for β1 showed a distinct fiber-type dependent pattern, with Type IID fibers stained at the highest levels, Type IIBD fibes stained at a medium level, and Type IIB fibers showed almost undetectable levels. Interestingly, the staining pattern for β2 was opposite to that of β1; qualitatively, the highest level of staining was in the Type IIB fibers, a lower level in Type IIBD fibers, and almost undetectable levels in Type IID fibers (Fig. 4 and Table 2). Since β3 staining was quite uniformly distributed across different fibers, fiber-typing was not determined for this subunit isoform.
Fig. 4.
Fiber-type specific distribution of the α- and β-subunit isoforms in white gastrocnemius of 6-month old rats. Serial sections of the muscle were subjected to fiber typing at pH 10.4 and pH 7.2 as described in Methods. The letters in the fibers denote the fiber types: B, Type IIB; D, Type IID; BD, Type IIBD. The serial sections were immunostained with anti-α1, anti-α2, anti-β1, and anti-β2 antibodies.
Table 2.
Summary of the qualitative staining intensity of the fibers in white gastrocnemius muscle
| IID | IIBD | IIB | |
|---|---|---|---|
| α1 | high | high | high |
| high | high | high | |
| α2 | high | high | high |
| high | high | low | |
| β1 | high | med | UD |
| high | med | low | |
| β2 | UD | med | high |
| low | med | low |
Qualitative comparisons can be made in the staining intensity of a specific subunit isoform between the different fiber types, and not the staining intensity of the different subunit isoforms in a specific fiber type. Results in the shaded rows are those from aged skeletal muscle. The fibers are arranged, from left to right, from the more oxidative type to the more glycolytic type. med: medium; UD: undetectable
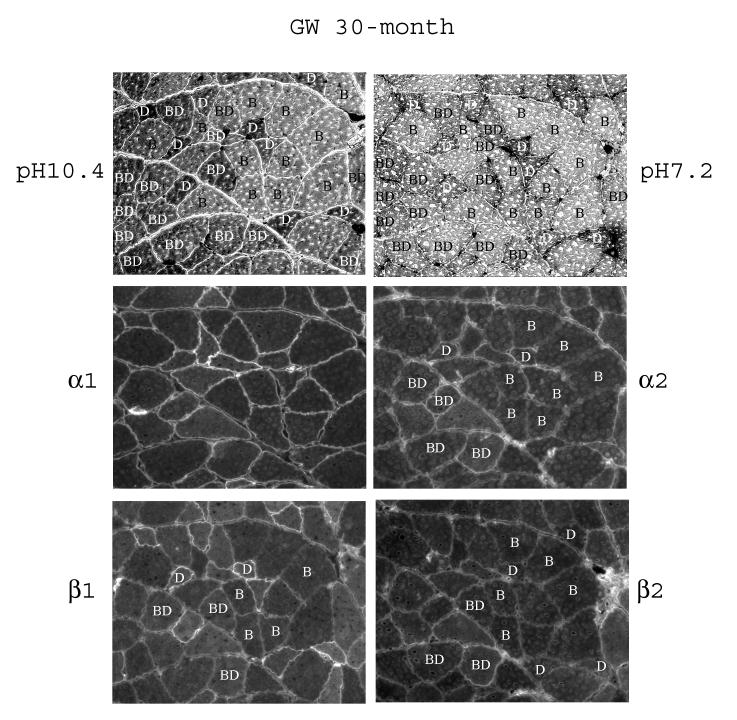
There appears to be age related alterations in the abundance of some of the fiber types and in the expression the subunit isoforms (Fig. 5 and Table 2). The abundance of the Type IIBD fibers appears to have increased in the white gastrocnemius of 30-month old rats compared to 6-month old rats, (compare Fig. 5 with Fig. 4). Staining for α1 across the fibers appears quite uniform in aged rats. α2 staining was present in Type IID and IIBD fibers of aged rats; however, unlike the young skeletal muscle, staining of α2 in Type IIB fibers was very low in the white gastrocnemius of 30-month old rats.
Fig. 5.
Fiber-type specific distribution of the α- and β-subunit isoforms in white gastrocnemius of 30-month old rats. Serial sections of the muscle were subjected to fiber typing at pH 10.4 and pH 7.2 as described in Methods. The letters in the fibers denote the fiber types: B, Type IIB; D, Type IID; BD, Type IIBD. The serial sections were immunostained with anti-α1, anti-α2, anti-β1, and anti-β2 antibodies.
Regarding the β-subunit isoforms, similar to young skeletal muscle, qualitatively, the staining for β1 in the white gastrocnemius of 30-month old rats remained the highest in Type IID fibers and at a medium level in Type IIBD fibers (Fig. 5 and Table 2). Interestingly, unlike young skeletal muscle, β1 staining in Type IIB fibers in aged skeletal muscle became readily detectable. In aged white gastrocnemius, the β2 staining in Type IID fibers became quite visible, as evident by the appearance of the double lines of staining around some of the Type IID fibers. At the same time, staining of β2 in Type IIB fibers became less than that of the IIBD fibers, indicating a relative decrease of β2 in the Type IIB fiber. The above-described qualitative changes in white gastrocnemius have been summarized in Table 2.
Fiber type-specific staining of the subunit isoforms in the red gastrocnemius of young and aged rats
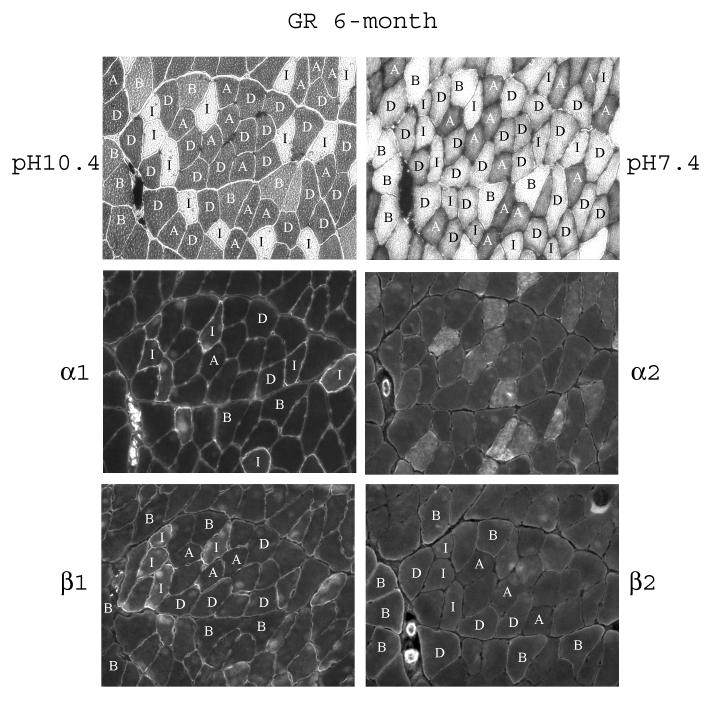
In the red gastrocnemius muscle of 6-month old rats, we were able to identify, in the order of the more oxidative to the more glycolytic fiber types, the slow-twitch Type I fiber, the fast-twitch oxidative/glycolytic Type IIA and IID fibers, and fast-twitch Type IIB fibers (Fig. 6). Qualitatively, staining for α1 was higher in Type I fibers, and quite even among the Type IIA, IIB, and IID fibers (Fig. 6 and Table 3). Staining of α2 was quite even among the different fibers. Again, there was intracellular staining in some of the fibers. Staining of β1 was highest in Type I fibers, intermediate in Type IIA and IID fibers, and was low in Type IIB fibers (Fig. 6 and Table 3). As for β2, staining was highest in Type IIB fibers, visible in Type IID fibers, but almost undetectable in Type I and IIA fibers, a staining pattern opposite to that of the β1.
Fig. 6.
Fiber-type specific distribution of the α- and β-subunit isoforms in red gastrocnemius of 6-month old rats. Serial sections of the muscle were subjected to fiber typing at pH 10.4 and pH 7.2 as described in Methods. The letters in the fibers denote the fiber types: I, Type I; A, Type IIA; D, Type IID; B, Type IIB. The serial sections were immunostained with anti-α1, anti-α2, anti-β1, and anti-β2 antibodies.
Table 3.
Summary of the qualitative staining intensity of the fibers in red gastrocnemius muscle
| I | IIA | IID | IIBDc | IIB | |
|---|---|---|---|---|---|
| α1 | v high | high | high | high | |
| v high | high | high | high | high | |
| α2 | med | med | med | med | |
| med | med | med | med | med | |
| β1 | high | med | med | low | |
| v high | high | high | med | med | |
| β2 | UD | UD | low | med | |
| UD | UD | low | low | med |
: the fiber type detected only in aged skeletal muscle. Qualitative comparisons can be made in the staining intensity of a specific subunit isoform between the different fiber types, and not the staining intensity of the different subunit isoforms in a specific fiber type. Results in shaded rows are those from aged skeletal muscle. The fibers are arranged, from left to right, from the more oxidative type to the more glycolytic type. v high: very high; med: medium; UD: undetectable
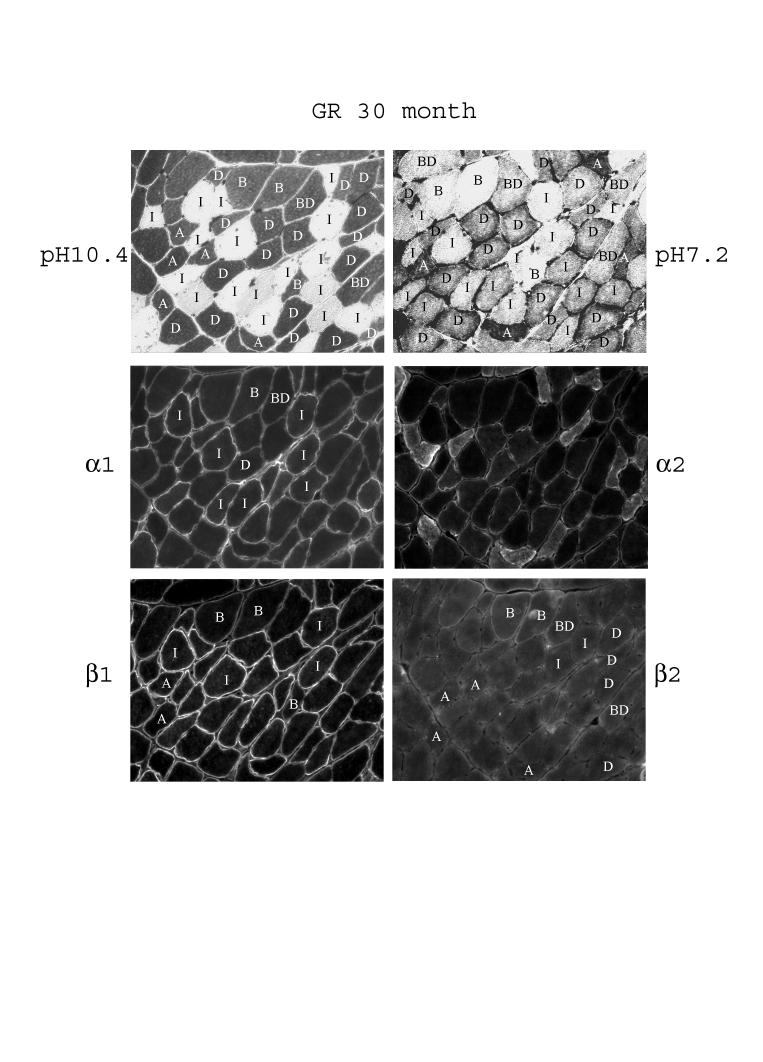
With age, in the red gastrocnemius of 30-month old rats we were able to identify the Type IIBD fibers in addition to the Type I, IIA, IID, and IIB fibers detected in young skeletal muscle (Fig. 7 and Table 3). Qualitatively, staining for α1 remained higher in Type I fibers and quite even between Type IIA, ID, IIBD, and IIB fibers. Staining of α2 was fairly evenly distributed among the fibers, except there was substantial intracellular staining in the Type IID and IIA fibers, a finding that was not reproduced by another α2-specific antibody. Staining of β1 remained the highest in Type I fibers, high in Type IIA, and IID fibers, and at a lower level in Type IIBD, and IIB fibers. By contrast, staining for β2 remained the highest, even though still weak, in Type IIB fibers, low to undetectable levels in Type IIBD and IID fibers, and almost undetectable in Type I and IIA fibers (Fig. 7 and Table 3).
Fig. 7.
Fiber-type specific distribution of the α- and β-subunit isoforms in red gastrocnemius of 30-month old rats. Serial sections of the muscle were subjected to fiber typing at pH 10.4 and pH 7.2 as described in Methods. The letters in the fibers denote the fiber types (see figure legend to Fig. 4 and 5). The serial sections were immunostained with anti-α1, anti-α2, anti-β1, and anti-β2 antibodies.
Discussion
The present study demonstrated for the first time in skeletal muscle fiber-type specific immunostaining of the subunits of Na+,K+-ATPase and their differential changes with advancing age. The important findings of the study are that 1) substantial differences in expression of the subunit isoforms in young and aged skeletal muscle can be demonstrated by semi-quantitative immunofluorescence technique; 2) both the α1 and α2 isoforms were fairly evenly distributed among the different fibers in both white and red gastrocnemius, with the exception of a qualitatively higher level of α1 in Type I fibers of red gastrocnemius; 3) the β1 isoform was expressed preferentially in the more oxidative fibers (Type I, IIA, and IID), the β2 isoform expressed preferentially in the more glycolytic fibers (Type IIB and IIBD), and β3 was evenly distributed among the different fibers; and 4) With age, fiber-type specific differential changes in staining of α2, β1, and β2 were observed in white gastrocnemius but not in red gastrocnemius. Thus, our study extends the previous knowledge regarding the expression of the subunit isoforms in the different skeletal muscle groups and during advancing age.
Previous reports from our laboratory and others have demonstrated the differential expression of the Na+,K+-ATPase subunit isoforms at the muscle-type level. Hundal et al. [16] showed that slow and fast-twitch oxidative muscles expressed mostly the β1 subunit, whereas fast-twitch glycolytic muscle expressed mostly β2. Thompson and McDonough [15] and Fowles et al. [17] similarly showed that slow and fast oxidative muscles expressed much higher levels of α1 and β1, and fast glycolytic muscle expressed the highest levels of β2, which was absent in slow oxidative muscle. Tsakiridis et al. [34] also demonstrated preferential expression of β1 and β2 in the red and white hindlimb muscle of the rat, respectively. Similarly, our previous results also pointed to preferential expression of β1 in oxidative muscle and β2 in glycolytic muscle [18]. However, the underlying cellular basis for the differential expression of the subunits in the muscle groups was not understood and the current study provides some new insight.
Our results show that staining of the two α-subunit isoforms, α1 and α2, in skeletal muscle was evenly distributed among Type IID, IIBD, and IIB fibers of the white gastrocnemius muscle. In red gastrocnemius, while α2 staining appears even among Type I, IIA, IID, and IIB fibers, staining of α1 was higher in Type I fibers (Table 2 and 3). Thus, the previous reports [15,17] of higher expression of α1 in oxidative muscle than in glycolytic muscle may be attributed, at least in part, to a higher number of Type I fibers in the slow and fast oxidative muscle than in glycolytic muscle.
The nature of the intracellular staining for α2 and β1 found in some sections remains unclear because these are not consistent findings. Williams et al. [35] showed that the α2 isoform was detectable in the sarcoplasm and determined that the intracellular staining represents the α2 isoform localized in the t-tubules. However, when we performed immunostaining of the α2 isoform using an alternative anti-α2 antibody (Upstate Biologicals, Charlottesville, VA ) we failed to detect similar intracellular staining pattern. We cannot completely exclude the possibility that some of this ‘intracellularly” localized subunits may have been ‘washed away” during fixation. Thus, the true identify of the immunostaining found intracellularly in our study remains to be elucidated.
Compared to the α-subunit isoforms, staining of the β-subunit isoforms in skeletal muscle showed much greater fiber-type dependency, especially in the white gastrocnemius. There was with very little or undetectable staining of β1 in the glycolytic Type IIBD and IIB fibers, and, conversely, there was very little staining of β2 in the oxidative Type I, IIA, and IID fibers (Table 2 and 3). Thus, there is almost a mutually exclusive expression of the β1 and β2 subunit in the mostly oxidative and mostly glycolytic muscle fibers, respectively. These findings appear to provide a definitive underlying explanation for previous results showing low levels of expression of β1 and β2 in white and red gastrocnemius muscle, respectively [16,18,33,34]. There appears to be areas of concentrated staining on the surface of the fibers. It is possible, although currently without any proof, that these may represent satellite cells on the surface of the fibers.
Our previous results have demonstrated that, with age, expression of the Na+,K+-ATPase subunit isoforms in skeletal muscle, as determined by western blotting, is altered in an isoform-specific and muscle-type dependent manner [19]. By using immunofluorescence technique, results from the current study match very well with our earlier findings. Thus, with age, there were discernible increases in the levels of α1, β1, and β3, and decreases in the levels of α2 and β2. Our current results extend those findings by demonstrating that some of the age-related changes occur in a fiber-type specific fashion. Specifically, with age, there was a marked decrease in α2 in the fast glycolytic Type IIB fibers of white gastrocnemius. This fiber-type selective decrease in α2 probably accounts for, at least in part, the previously reported age-associated decrease in α2 in white gastrocnemius [18,19]. The mechanism underlying this selective downregulation of α2 in fast glycolytic fibers remains to be elucidated. Whether this suggests a decreased demand in Na-pump activity in this select fiber with age remains to be determined. It is of interest to note, we previously demonstrated that endurance exercise training reversed the decreased expression of α2 in aged skeletal muscle [19]. Whether exercise training selectively affects this isoform in the Type IIB fibers will be determined in future studies.
At the same time, our results also suggest an across fiber change in the expression of the α subunit isoforms with age. With respect to the α1 isoform, we did not detect any fiber-type differential changes with age, and yet, our previous immunoblotting data showed a marked increase in α1 with age [18,19]. Thus, taken together, the current and previous results indicate, with age, both a fiber specific as well as an across fiber change in the expression of the α subunit isoforms.
Compared to the α-subunit isoforms, the age-associated changes in staining of the β-subunit isoform were more fiber-type specific, and there were important differences between white and red gastrocnemius. Specifically, in white gastrocnemius, with age, there was a substantial qualitative increase in β1 and a decrease in β2 in the fast glycolytic Type IIB fibers, and there was an increase in β2 in the fast oxidative-glycolytic Type IID fibers, fiber type that expressed little or undetectable levels of these isoforms in young skeletal muscle. Thus, the selective qualitative increase of β1 and decrease of β2 in Type IIB fibers contribute, at least in part, to the previously reported age-associated increase in β1 and decrease in β2 in white gastrocnemius [18]. In red gastrocnemius, by contrast, there were no obvious fiber-type specific differential changes in the staining of the β subunits with age. Nevertheless, a substantial age-associated qualitative decrease of β2 in red gastrocnemius was detected in the present study (Fig. 2 g,h) as well as by immunoblotting as reported previously [18]. Thus, taken together, these data again supported a fiber specific as well as an across fiber change in expression of the β-subunit isoforms with age.
The precise composition of the α-β heterodimers of the Na+,K+-ATPase in the individual fiber types remains to be elucidated, although our results have provided some new insight. Based on muscle-type specific expression of the subunit isoforms, McDonough and co-workers [15] concluded that α2β2 is the predominant heterodimer in fast glycolytic muscle and that both α1β1 and α2β1 heterodimers are expressed in muscles rich in oxidative fibers. Our results are in general agreement with those predictions, but also provide greater details on an individual fiber bases. Thus, it appears that the more oxidative Type I and IIA fibers are likely to express the α1β1 and α2β1 heterodimers, and the more glycolytic Type IIB and IIBD fibers are likely to express the α1β2 and α2β2 heterodimers. The Type IID fiber would be predicted to express both β1 and β2 heterodimers. In addition, the relatively uniform expression of β3 among the different fibers suggests the likely presence of α1β3 and α2β3 heterodimers. Double immunofluorescence labeling and confocal fluorescence microscopy studies will provide greater details in the composition of the heterodimers in individual fibers.
Functional implications of the differential expression of the subunit isoforms in individual muscle fibers are not completely understood at present. Fowles et al. [17] demonstrated that, compared to α2 and β2, the α1 and β1 content are the better predictor of skeletal muscle Na+,K+-ATPase enzyme activity. Such observations will predict that in the more oxidative Type I, IIA and IID fibers, with higher expression of α1 and β1, the Na+,K+-ATPase enzyme activity in these fibers is expected to be higher, particularly the Type I fiber which has the highest levels of α1 among the fibers. Whether these predictions are correct will require future detailed study. In addition, the isozymes have different affinities for Na+ and K+ ions [20-22], and the α1-isozyme appears to be a better substrate for phosphorylation by kinases [23,36]. Insulin and exercise have been reported to selectively translocate the α2-isozyme from the intracellular site to the plasma membrane [13,24], although a more recent study did not find evidence to support an exercise-induced translocation of the subunits [37]. Thus, the Na+-pump in individual fiber types may be regulated differently and serve distinct functions. In addition, skeletal muscle of transgenic mice lacking one copy of the α2-isoform shows greater contractile force, whereas skeletal muscle of transgenic mice lacking one copy of the α1-isoform shows decreased contractile force [38], providing some evidence for the distinct functional roles of the subunit isoforms in skeletal muscle. However, it also has been suggested that compensatory changes that accumulate during development may play a role in changing the contractile function of the mouse heart lacking one copy of the α1-isoform [39]. With respect to the β-subunit isoforms, it has previously been reported that isozymes with the β2 isoform have a lower apparent affinity for K+ [40] and higher affinity for Na+ than isozymes with the β1 isoform [41,42]. Clausen et al. [43] recently demonstrated a greater K+ efflux and Na+ influx per action potential in the Type II fast-twitch than in the Type I slow-twitch fibers, raising the possibility that the Na+-K+ gradients between oxidative and glycolytic fibers indeed may be different. Thus, an intriguing question is whether the different β-subunit isoforms in these fibers is optimally expressed to regulate the pump activity in the different fibers.
In summary, these results provide, at the individual fiber level, a cellular basis for the differential expression of the Na+,K+-ATPase subunit isoforms in the muscle groups. The data suggest that aged-associated changes in expression of the subunit isoforms occur in both a fiber-type specific as well as an across fiber-type manner. Because of the differing biochemical properties of the subunit isoforms, these changes add another layer of complexity in our understanding of the adaptation of the Na-pump in skeletal muscle, as well its influence on contractile function, with advancing age.
Fig. 3.
Comparisons of the relative immunofluorescence staining intensity between 6-month and 30-month old rats. Staining intensity of the images was quantitated as described in Methods. Mean relative intensity was calculated by normalizing data from 30-month old rats (darker bars) by that of the corresponding 6-month old rats (lighter bars). The numbers within the bars indicate the pairs of sections used in the analysis. Top: white gastrocnemius; Bottom: red gastrocnemius.
Acknowledgments
Acknowledgement-We thank Drs. K. Sweadner, P. Martin-Vasallo, and P. Beesley for kindly providing the antibodies. The monoclonal α6F antibody developed by Dr. D.M. Fambrough was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242. We also thank Linda Chung for careful reading of the manuscript. This work was supported by National Institutes of Health Grant AG-16822.
Reference List
- 1.Fowles JR, Green HJ, Tupling R, O’Brien S, Roy BD. Human neuromuscular fatigue is associated with altered Na+-K+-ATPase activity following isometric exercise. J. Appl. Physiol. 2002;92:1585–1593. doi: 10.1152/japplphysiol.00668.2001. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OB, Clausen T. The Na+/K(+)-pump protects muscle excitability and contractility during exercise. Exercise & Sport Sciences Reviews. 2000;28:159–164. [PubMed] [Google Scholar]
- 3.Overgaard K, Nielsen OB, Flatman JA, Clausen T. Relations between excitability and contractility in rat soleus muscle: role of the Na+-K+ pump and Na+/K+ gradients. Journal of Physiology. 1999;518:215–225. doi: 10.1111/j.1469-7793.1999.0215r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonough AA, Thompson CB, Youn JH. Skeletal muscle regulates extracellular potassium. [Review] [52 refs] Am J Physiol Renal Physiol. 2002;282:F967–F974. doi: 10.1152/ajprenal.00360.2001. [DOI] [PubMed] [Google Scholar]
- 5.Levenson R. isoforms of the Na,K-ATPase: Family members in search of function. Review in Physiology, Biochemistry, and Pharmacology. 1994;123:1–45. doi: 10.1007/BFb0030902. [DOI] [PubMed] [Google Scholar]
- 6.Lingrel JB, Orlowski J, Shull MM, Price EM. Molecular genetics of Na,K-ATPase. Prog. Nucleic Acid Res. Mol. Biol. 1990;38:37–89. doi: 10.1016/s0079-6603(08)60708-4. [DOI] [PubMed] [Google Scholar]
- 7.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim. Biophys. Acta . 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 8.Malik N, Canfield VA, Beckers MC, Gros P, Levenson R. Identification of the mammalian Na,K-ATPase beta 3 subunit. J. Biol. Chem. 1996;271:22754–22758. doi: 10.1074/jbc.271.37.22754. [DOI] [PubMed] [Google Scholar]
- 9.Shamraj OI, Lingrel JB. A putative fourth Na+,K+-ATPase alpha-subunit gene is expressed in testis. PNAS. 1995;91:12952–12956. doi: 10.1073/pnas.91.26.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azuma KK, Hensley CB, Putnam DS, McDonough AA. Hypokalemia decreases Na(+)-K(+)-ATPase alpha 2-but not alpha 1-isoform abundance in heart, muscle, and brain. Am. J. Physiol. 1991;260:C958–C964. doi: 10.1152/ajpcell.1991.260.5.C958. [DOI] [PubMed] [Google Scholar]
- 11.Azuma KK, Hensley CB, Tang M-J, McDonough AA. Thyroid hormone specifically regulates skeletal muscle Na+-K+-ATPase alpha2- and beta2-isoforms. Am. J. Physiol. 1993;265:C680–C687. doi: 10.1152/ajpcell.1993.265.3.C680. [DOI] [PubMed] [Google Scholar]
- 12.Arystarkhova E, Sweadner KJ. Tissue-specific expression of Na,K-ATPase beta-3 subunit - The presence of beta-3 in lung and liver addresses the problem of the missing subunit. J. Biol. Chem. 1997;272:22405–22408. doi: 10.1074/jbc.272.36.22405. [DOI] [PubMed] [Google Scholar]
- 13.Hundal HS, Marette A, Mitsumoto Y, Ramlal T, Blostein R, Klip A. Insulin induces translocation of the alpha2 and beta1 subunits of the Na+/K+-ATPase from intracellular compartments to the plasma membrane in mammalian skeletal muscle. J. Biol. Chem. 1992;267:5040–5043. [PubMed] [Google Scholar]
- 14.Lavoie L, Levenson R, Martin-Vasallo P, Klip A. The molar ratios of alpha and beta subunits of the Na-K-ATPase differ in distinct subcellular membranes from rat skeletal muscle. Biochemistry. 1997;36:7726–7732. doi: 10.1021/bi970109s. [DOI] [PubMed] [Google Scholar]
- 15.Thompson CB, McDonough AA. Skeletal muscle Na,K-ATPase alpha and beta subunit protein levels respond to hypokalemic challenge with isoform and muscle type specificity. J. Biol. Chem. 1996;271:32653–32658. doi: 10.1074/jbc.271.51.32653. [DOI] [PubMed] [Google Scholar]
- 16.Hundal HS, Marette A, Ramlal T, Liu Z, Klip A. Expression of beta subunit isoforms of the Na+,K+-ATPase is muscle type-specific. Febs Lett. 1993;328:253–258. doi: 10.1016/0014-5793(93)80938-q. [DOI] [PubMed] [Google Scholar]
- 17.Fowles JR, Green HJ, Ouyang J. Na+-K+-ATPase in rat skeletal muscle: content, isoform, and activity characteristics. J. Appl. Physiol. 2004;96:316–326. doi: 10.1152/japplphysiol.00745.2002. [DOI] [PubMed] [Google Scholar]
- 18.Ng YC, Nagarajan M, Jew KN, Mace LC, Moore RL. Exercise training differentially modifies age-associated alteration in expression of Na+-K+-ATPase subunit isoforms in rat skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 2003;285:R733–R740. doi: 10.1152/ajpregu.00266.2003. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Nagarajan M, Beesley PW, Ng YC. Age-associated differential expression of Na(+)-K(+)-ATPase subunit isoforms in skeletal muscles of F-344/BN rats. J. Appl. Physiol. 1999;87:1132–1140. doi: 10.1152/jappl.1999.87.3.1132. [DOI] [PubMed] [Google Scholar]
- 20.Jewell EA, Lingrel JB. Comparison of the substrate dependence properties of the rat Na, K-ATPase alpha 1, alpha 2, and alpha 3 isoforms expressed in HeLa cells. J. Biol. Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- 21.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. [Review] [221 refs] Am. J. Physiol. 1998;275:F633–F650. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 22.Daly SE, Lane LK, Blostein R. Functional consequences of amino-terminal diversity of the catalytic subunit of the Na,K-ATPase. J. Biol. Chem. 1994;269:23944–23948. [PubMed] [Google Scholar]
- 23.Beguin P, Peitsch MC, Geering K. Alpha-1 but not alpha-2 or alpha-3 isoforms of Na,K-ATPase are efficiently phosphorylated in a novel protein kinase C motif. Biochemistry. 1996;35:14098–14108. doi: 10.1021/bi960516o. [DOI] [PubMed] [Google Scholar]
- 24.Juel C, Nielsen JJ, Bangsbo J. Exercise-induced translocation of Na(+)-K(+) pump subunits to the plasma membrane in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1107–R1110. doi: 10.1152/ajpregu.2000.278.4.R1107. [DOI] [PubMed] [Google Scholar]
- 25.Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. American Journal of Anatomy. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- 26.Chan KM, Doherty TJ, Brown WF. Contractile properties of human motor units in health, aging, and disease. [Review] [97 refs] Muscle & Nerve. 2001;24:1113–1133. doi: 10.1002/mus.1123. [DOI] [PubMed] [Google Scholar]
- 27.Larsson L, Yu F, Hook P, Ramamurthy B, Marx JO, Pircher P. Effects of aging on regulation of muscle contraction at the motor unit, muscle cell, and molecular levels. [Review] [94 refs] Int J Sport Nutrit & Exer Met. 2001;11(Suppl):S28–S43. doi: 10.1123/ijsnem.11.s1.s28. [DOI] [PubMed] [Google Scholar]
- 28.Welle S. Cellular and molecular basis of age-related sarcopenia. [Review] [153 refs] Can J Appl Physiol. 2002;27:19–41. doi: 10.1139/h02-002. [DOI] [PubMed] [Google Scholar]
- 29.Kjeldsen K. Muscle Na,K-pump dysfunction may expose the heart to dangerous K levels during exercise. Can. J. Sport Sci. 1991;16:33–39. [PubMed] [Google Scholar]
- 30.Zhang L, Ng YC. Distribution of Beta3-subunit isoform of Na+,K+-ATPase in skeletal muscle and in cell culture. Faseb Journal. 2004;18:A345. [Google Scholar]
- 31.Hamalainen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. Journal of Histochemistry & Cytochemistry. 1993;41:733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- 32.Guth L, Samaha FJ. Procedure for the histochemical demonstration of actomyosin ATPAse. Experimental Neurology. 1970;28:365. [PubMed] [Google Scholar]
- 33.Hughes VM. A new histochemical method for magnesium actomyosin adenosine triphosphatase at physiological pH. Stain Technol. 1986;61:201. doi: 10.3109/10520298609109938. [DOI] [PubMed] [Google Scholar]
- 34.Tsakiridis T, Wong PPC, Liu Z, Rodgers CD, Vranic M, Klip A. Exercise increases the plasma membrane content of the Na+-K+ pump and its mRNA in rat skeletal muscles. J. Appl. Physiol. 1996;80:699–705. doi: 10.1152/jappl.1996.80.2.699. [DOI] [PubMed] [Google Scholar]
- 35.Williams MW, Resneck WG, Kaysser T, Ursitti JA, Birkenmeier CS, Barker JE, Bloch RJ. Na,K-ATPase in skeletal muscle: two populations of beta-spectrin control localization in the sarcolemma but not partitioning between the sarcolemma and the transverse tubules. Journal of Cell Science. 2001;114:751–762. doi: 10.1242/jcs.114.4.751. [DOI] [PubMed] [Google Scholar]
- 36.Feschenko MS, Sweadner KJ. Phosphorylation of Na,K-ATPase by protein kinase C at Ser(18) occurs in intact cells but does not result in direct inhibition of ATP hydrolysis. J. Biol. Chem. 1997;272:17726–17733. doi: 10.1074/jbc.272.28.17726. [DOI] [PubMed] [Google Scholar]
- 37.McKenna MJ, Gissel H, Clausen T. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J Physiol. 2003;547:567–580. doi: 10.1113/jphysiol.2003.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.He S, Shelly DA, Moseley AE, James PF, James JH, Paul RJ, Lingrel JB. The alpha(1)- and alpha(2)-isoforms of Na-K-ATPase play different roles in skeletal muscle contractility. Am J Physiol Regul Integr Comp Physiol. 2001;281:R917–R925. doi: 10.1152/ajpregu.2001.281.3.R917. [DOI] [PubMed] [Google Scholar]
- 39.Dostanic-Larson I, Lorenz JN, Van Huysse JW, Neumann JC, Moseley AE, Lingrel JB. Physiological role of the alpha1- and alpha2-isoforms fo the Na+-K+-ATPase and biological significance of their cardiac glycoside binding site. Am J Physiol Regul Integr Comp Physiol. 2006;290:R524–R528. doi: 10.1152/ajpregu.00838.2005. [DOI] [PubMed] [Google Scholar]
- 40.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J. Biol. Chem. 2000;275:1976–1986. doi: 10.1074/jbc.275.3.1976. [DOI] [PubMed] [Google Scholar]
- 41.Blanco G, Koster JC, Sanchez G, Mercer RW. Kinetic properties of the alpha2 beta 1 and alpha 2 beta 2 isozymes of the Na,K-ATPase. Biochemistry. 1995;34:319–325. doi: 10.1021/bi00001a039. [DOI] [PubMed] [Google Scholar]
- 42.Blanco G, Sanchez G, Mercer RW. Comparison of the enzymatic properties of the Na,K-ATPase alpha3-beta1 and alpha3-beta2 isozymes. Biochemistry. 1995;34:9897–9903. doi: 10.1021/bi00031a011. [DOI] [PubMed] [Google Scholar]
- 43.Clausen T, Overgaard K, Nielsen OB. Evidence that the Na+-K+ leak/pump ratio contributes to the difference in endurance between fast- and slow-twitch muscles. Acta. Physiol. Scand. 2004;180:209–216. doi: 10.1111/j.0001-6772.2003.01251.x. [DOI] [PubMed] [Google Scholar]