Abstract
The ability to envision specific future episodes is a ubiquitous mental phenomenon that has seldom been discussed in the neuroscience literature. In this study, subjects underwent functional MRI while using event cues (e.g., Birthday) as a guide to vividly envision a personal future event, remember a personal memory, or imagine an event involving a familiar individual. Two basic patterns of data emerged. One set of regions (e.g., within left lateral premotor cortex; left precuneus; right posterior cerebellum) was more active while envisioning the future than while recollecting the past (and more active in both of these conditions than in the task involving imagining another person). These regions appear similar to those emerging from the literature on imagined (simulated) bodily movements. A second set of regions (e.g., bilateral posterior cingulate; bilateral parahippocampal gyrus; left occipital cortex) demonstrated indistinguishable activity during the future and past tasks (but greater activity in both tasks than the imagery control task); similar regions have been shown to be important for remembering previously encountered visual-spatial contexts. Hence, differences between the future and past tasks are attributed to differences in the demands placed on regions that underlie motor imagery of bodily movements, and similarities in activity for these two tasks are attributed to the reactivation of previously experienced visual–spatial contexts. That is, subjects appear to place their future scenarios in well known visual–spatial contexts. Our results offer insight into the fundamental and little-studied capacity of vivid mental projection of oneself in the future.
Keywords: autonoetic consciousness, episodic future thought, episodic memory, functional MRI
Perhaps one of the most adaptive capacities of the human mind is the ability to fashion behavior in anticipation of future consequences (1, 2). Much of this capacity relies on the engagement of executive operations such as anticipation, planning, and monitoring (3–5). These aspects of future-oriented thought have been the focus of much neuroscientific research, and there is strong evidence from lesion (3, 5) and neuroimaging (6–8) research suggesting that regions within frontal cortex are vital for the ability to contemplate the future in relation to oneself.
A separate but related component of future-oriented thought involves envisioning oneself participating in a specific future event (9), a process that might spur the initiation of executive processes to structure behavior. Much of our everyday thought depends on our ability to see ourselves partaking in future events. This ability to envision oneself in a specific future scenario, termed “episodic future thought” (10), has received much less empirical attention than the executive planning processes it might foster. Discussion related to episodic future thought has appeared in the neuropsychological literature in relation to brain-damaged patients who seem to lack this ability (11, 12), but the extent of lesions within such patients is rather diffuse, which makes it difficult to pinpoint which brain regions underlie this set of processes. This article, together with two other recent empirical studies (8, 13), represents an initial step in explicating the neural underpinnings of this important mental capacity through the use of functional neuroimaging techniques (for early reviews see refs. 14 and 15).
One important consideration to be made when using functional neuroimaging to investigate episodic future thought is deciding on an appropriate comparison task, such that regions particularly important for envisioning the future may be isolated. One possibility would be to simply contrast a task requiring the envisioning of future events with a silent resting baseline. However, when subjects are asked to lie still in the scanner they tend to think about their own lives, often in relation to future events (9, 16–18). That is, the default mode of thinking appears to be focused on oneself and one's internal state either in the past, present, or future (19).
Because asking subjects to envision themselves in specific future situations requires the construction of mental images of life-like scenarios, an appropriate comparison task should require subjects to similarly construct mental images of life-like scenarios, but not in relation to their personal future. One task that captures these component processes is recollection of autobiographical memories. Like envisioning the future, this task requires subjects to construct a vivid mental image of themselves participating in an event at a time temporally distinct from the present. The Galton–Crovitz word-cueing technique (20), long used in the study of autobiographical memory, was adapted for both past and future orientation such that subjects received cues (e.g., Birthday) and were asked either to envision themselves in a related future event or to mentally relive (i.e., remember) a related past event. Any cortical regions that exhibit more activity in relation to envisioning the future compared with recollecting the past may be taken as candidates for regions that might be particularly important for episodic future thought.
A potential issue in employing autobiographical memory as the sole comparison task is that both recollection and episodic future thought might similarly activate a set of regions that are critical for both tasks (but would, of course, be overlooked in a direct contrast). Such regions may be just as important for the ability to mentally envision the future as regions showing greater activity for future than past. Indeed, one influential theory of memory suggests that the ability to mentally represent the personal future may go hand in hand with the ability to mentally represent the personal past (2, 12). That is, episodic memory may, at its heart, be the ability to vividly represent oneself in time, in both the past and the future. Evidence from clinical psychology, developmental psychology, and neuropsychology is consistent with this claim. Specifically, individuals, be they depressive patients (21), young children (22), or amnesics (11, 12, 23, 24), who are unable to vividly recollect their past, also seem to be unable to form specific mental images of the future.
To circumscribe this potential issue, an additional comparison task was used that involved many of the same component processes of episodic future thought and remembering life events but that did not involve envisioning oneself at a time other than the present. In this task, subjects imagined a familiar individual participating in life-like events with no explicit temporal reference. Specifically, former President Bill Clinton was chosen because pretesting showed that he is easy to visualize in a variety of situations. This condition had neither the aspect of self nor the element of mental time travel; it allowed the identification of activity that was common to remembering and episodic future thought but different from this imagery control task.†
An event-related functional MRI design was used; subjects were asked to envision themselves (for 10 sec) in specific future [Self-Future (SF)] and past [Self-Remember (SR)] episodes in response to a series of event cues (e.g., Birthday), or to imagine a familiar individual [Clinton-Imagine (CI)] participating in similar events (also 10 sec). A two-step analysis approach was adopted. The first step involved a contrast of activation magnitudes for the two tasks that involved thinking about oneself in time (SF, SR) against the magnitude for the baseline imagery task that did not involve these processes (CI). This contrast (0.5, 0.5, and −1 for SF, SR, and CI, respectively) identified regions that might be particularly important for thinking about oneself in time. The resulting regions of interest (ROIs) were then more fully characterized by a condition (SF, SR) × timepoint (temporal pattern of the blood-oxygen-level-dependent response) analysis of variance (ANOVA). Of particular interest were ROIs showing an interaction such that the timecourse of activation differed for episodic future thought and recollection.
A second, converging analysis stream, which is not reported fully, began with a 3 (orienting cue: SF, SR, CI) × 10 (timepoint) ANOVA (step 1) and follow-up analyses on all regions showing a cue by timepoint interaction (step 2). The resulting regions and timecourses emerging from the two approaches are highly similar. Additional analyses also covaried out factors obtained from the postexperiment questionnaire (vividness, valence, and emotional intensity), and again the basic patterns of results did not change.
Results
The whole-brain voxelwise analysis revealed 23 ROIs that were more active when subjects envisioned personal future and past events, as compared with the imagery control task ([SF + SR] − CI). These ROIs were taken to be candidates for those that are particularly important for episodic future thought. These regions were then classified into three sets: those showing greatest activity for episodic future thought, those showing greatest activity for recollection, and those showing statistically equivalent activity for episodic future thought and recollection (but greater activation for these tasks than the imagery control task). To accomplish this goal, each of the 23 ROIs was submitted to a two-way ANOVA, with condition (SF and SR only) and time (temporal pattern of the blood-oxygen-level-dependent response) being factors. Here, the effects associated with each ROI were calculated by averaging across all voxels within the region, and the resulting values were entered into the 2 × 10 ANOVA. This approach isolated regions in which envisioning the future and past were functionally separated and those in which they showed statistically indistinguishable patterns of activity.
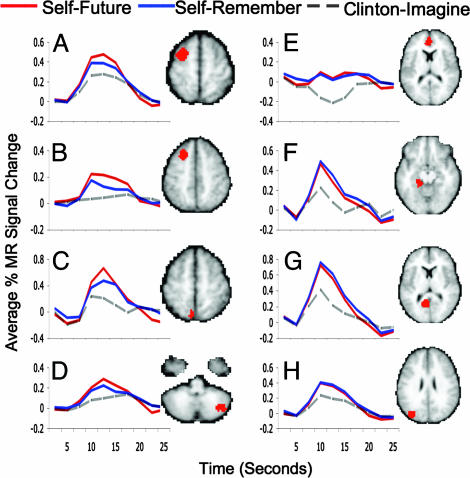
Episodic future thought and recollection showed differential activity in 8 of the 23 ROIs. These ROIs appear within (Table 1 and Fig. 1 A–D) middle frontal gyrus [approximate Brodmann's Area (BA) 6/8], medial posterior parietal cortex (BA 7), and posterior cerebellum (right lateralized). As can be seen in Fig. 1 A–D, each of these ROIs revealed a pattern of activity in which envisioning oneself in the future led to greater activity than envisioning oneself in the past. None of the ROIs showed greatest activity for the SR condition.
Table 1.
Regions identified by the two-way ANOVA as showing a significant condition by time interaction, where SF and SR interact
| Fig. 1 | Region | BA | x | y | z | F ratio (9, 180) | Voxels |
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| A | L middle | 6 | −28 | 3 | 55 | 4.74 | 149 |
| B | L middle | 8 | −24 | 22 | 45 | 3.38 | 119 |
| Parietal | |||||||
| C | L precuneus | 7 | −8 | −66 | 53 | 5.21 | 40 |
| Cerebellum | |||||||
| D | R | 34 | −68 | −29 | 2.99 | 45 | |
| R | 38 | −67 | −42 | 2.88 | 78 | ||
| R | 25 | −78 | −22 | 3.56 | 35 | ||
| R | 12 | −81 | −29 | 1.97 | 70 | ||
| R | 42 | −55 | −34 | 2.50 | 38 |
Fig. 1.
Cortical regions exhibiting activity differences and similarities during past and future thought. (A–D) Percent signal change for representative regions from Table 1 showing a significant interaction such that imagining of future events (SF) led to greater activation over the 10-modeled timepoints than did recollecting oneself in the past (SR). Both self-related tasks also led to greater activity than a control task involving imagery of another person participating in similar events (CI). Regions listed in Table 1 but not shown here demonstrate patterns similar to those shown in the figure. (E–H) Percent signal change for selected regions from Table 2 showing a statistically indistinguishable pattern of activity across time while subjects envisioned their personal future (SF) and recollected the past (SR) in response to a series of event cues (e.g., Birthday). Imagining a familiar individual in similar scenarios (CI) resulted in a pattern of activity different from both the past and future tasks. Regions listed in Table 2 but not shown here demonstrate patterns similar to those shown in the figure.
In the remaining 15 regions, the acts of envisioning the future and remembering the past revealed nearly identical timecourses of activation. As can be seen in Table 2 and Fig. 1 E–H, this pattern was found in regions within medial prefrontal cortex (BA 10), posterior cingulate cortex (BA 30), medial temporal cortex (bilateral parahippocampal gyrus, BAs 28, 35, 36, 37), and occipital cortex (BAs 17 and 19).
Table 2.
Regions identified by the two-way ANOVA as showing a significant condition by time interaction, where SF and SR do not interact
| Fig. 1 | Region | BA | x | y | z | F ratio (9, 180) | Voxels |
|---|---|---|---|---|---|---|---|
| Frontal | |||||||
| E | Medial | 10 | −3 | 40 | 12 | 0.85 | 110 |
| ACC | 32/9/10 | 6 | 26 | 31 | 0.89 | 65 | |
| Medial | 6 | −4 | 30 | 37 | 0.71 | 106 | |
| Medial | 6 | −6 | −16 | 47 | 1.19 | 130 | |
| Temporal | |||||||
| L PHG | 37 | −27 | −40 | −11 | 1.70 | 94 | |
| F | L PHG | 28/35 | −17 | −37 | −9 | 1.13 | 88 |
| R PHG | 36 | 24 | −37 | −8 | 1.63 | 76 | |
| R PHG | 30 | 14 | −46 | 1 | 0.85 | 57 | |
| R PHG | 36 | 28 | −26 | −16 | 0.82 | 33 | |
| L cingulate | 31 | −3 | −40 | 38 | 1.56 | 76 | |
| G | L PCC | 30 | −8 | −60 | 11 | 0.88 | 138 |
| R PCC | 30 | 9 | −55 | 10 | 0.33 | 119 | |
| Occipital | |||||||
| H | L superior | 19 | −34 | −77 | 29 | 0.62 | 88 |
| L cuneus | 18 | −18 | −93 | −11 | 1.47 | 20 | |
| Cerebellum | |||||||
| R | 11 | −55 | −41 | 1.17 | 52 |
Discussion
To summarize, the data shown here can be classified as showing one of two patterns. One set of regions showed greater activity for envisioning oneself in the future than recollecting one's past (and greater activity for both these tasks than the baseline condition requiring vivid imagination of another person). A second set of regions demonstrated no difference in activation as a function of past or future orientation (but greater activity for both tasks than the baseline imagery task involving imagining another). In addition, covarying out vividness, valence, and emotional intensity did not affect the overall pattern of results.
The implications of these results are now considered. Much of the following interpretation relies on reverse inference, and such comparisons are often difficult to defend; nonetheless, the nature of exploratory work often requires such tentative links to the literature until more follow-up empirical work can test the initial hypotheses (25, 26).
Regions Preferentially Engaged During Future Orientation.
Considered first are regions in which self-referential imagery led to greater activity than a nonpersonal control condition ([SF + SR] > CI), and envisioning oneself in the future led to greater activity than recollecting oneself in the past (SF > SR). Because the future and past-oriented tasks were comparable in many ways (e.g., they were both self-relevant, had distinct elements of temporality, and required vivid mental imagery), these regions might, at least at first glance, be considered as particularly important to forming mental images of the future. As will be seen, the interpretation favored here suggests that they are not solely important for future thought.
Each of these regions, most notably those within lateral premotor cortex (27), medial posterior parietal cortex (28), and posterior cerebellum (29), have been implicated in a variety of tasks requiring imagined (simulated) movements of one's body (30, 31). These tasks have ranged from simple motor imagery (e.g., finger tapping) to complex interactions of one's body in mental space. Some of the more complex tasks are very similar to those presented here and include mental navigation (32), perspective taking (33), and autobiographical imagery (34).
Why would actively constructing a mental image of a scenario involving oneself in the future result in greater activity in these regions than remembering events that have already taken place? It is important to note that previously executed actions (e.g., swinging one's arm) are believed to be reactivated during the imagination of various scenarios involving bodily movements (e.g., hitting a baseball) (35). Perhaps one key is that envisioning the future requires one to simulate a novel sequence of stored action representations. That is, one must anticipate a series of actions that has not occurred before. In envisioning the past, the same store of action representations is accessed, although the precise sequence of actions has been previously experienced. In essence, the representation of the past sequence of actions may be primed, and less neural activity may be needed to access the representation (36). If this speculation is correct, it would suggest that these regions are not special for future thought in any specific way but rather demonstrate the observed patterns as a result of the general task demands. Of note, each of these regions was active, although to a lesser degree, while subjects imagined Bill Clinton. Previous research has shown that neural regions important to simulating one's own behavior may be engaged during observation or simulation of the behavior of others (30).
Alternatively, each of the regions within this network (i.e., premotor, medial parietal, posterior cerebellum; also BA 8, as observed in the present study) have also been implicated in aspects of spatial working memory and attention (37). Clearly, definitive conclusions await future research. Regardless, we find it particularly interesting that many regions outside of the frontal lobes, particularly outside prefrontal cortex (38), appear to be preferentially engaged in envisioning the personal future.
Functional Equivalence Between the Future and Past.
Considered next are regions exhibiting identical patterns of neural activity while subjects envisioned the personal future and recollected the personal past. One challenge in evaluating this pattern of data (aside from those mentioned above) is that a statistical null effect is not the same as a demonstration of equivalence. Nonetheless, in the case of the present data, the null effects seem particularly compelling. As can be seen in Fig. 1 E–H, the activity for future thought and recollecting the past is quite similar at each timepoint for each region. Further, the null effect occurs against the backdrop of a robust effect in that the SF and SR conditions differed from the CI condition; it was not the case that the measure was simply insensitive.
The finding that there was more activation, actually less deactivation (39), in the medial prefrontal cortex when subjects envisioned their personal future or recollected their past, as compared with a control task that involved imagery of another person, is consistent with findings that this region is preferentially engaged during self-referential mental activity (19, 40). Further, the region showed the characteristic timecourse seen in the literature: It de-activates robustly for thoughts of another person and shows little-to-no temporal modulation for self-related thought.
Consider now the other regions showing functional equivalence (Table 2 and Fig. 1 F–H), which were all in posterior cortex. They exhibited quite a different pattern than did medial prefrontal cortex, whereby there was activity in the positive direction in all conditions, but the past and future conditions showed equivalent activation.
How might these results be conceptualized? There are several possibilities. Perhaps the most obvious one is that there is a type II error (and SF and SR really do differ from one another). As addressed above, this explanation seems unlikely. A second possibility is that these regions are especially engaged during self-referential processing. Although this conceptualization fits well for the region of medial prefrontal cortex discussed above, the extent to which self-reference may be mediated by these posterior cortical regions remains uncertain (41). A third possibility is that these regions (or a subset of them) might underlie the construct of “autonoetic consciousness,” a term used by Tulving and colleagues (2, 12, 38) to refer to the uniquely human ability to recollect the past and envision the future. It is even conceivable that these regions could underlie a key process linked to autonoetic consciousness, namely the idea of “mental time travel.” That is, one core feature of autonoetic (self-knowing) consciousness is the ability to take one's conscious thoughts and project them forward or backward in time. Although such a finding would be enormously interesting, the fourth possibility, considered next, seems more plausible.
Our preferred conceptualization of these regions emerges from considering other tasks in the literature that tend to elicit activity in these (or similar) regions. Specifically, autobiographical memory tasks (42) and mental navigation of familiar routes (32, 43, 44) elicit activity in regions of posterior cingulate cortex, medial temporal cortex, and occipital cortex similar (or identical) to the regions presently of interest. Both these tasks encourage subjects to reactivate a previously experienced visual–spatial context.
This view helps to illuminate the finding that the SR task would activate these regions (and more than an imagery control task). However, why would envisioning the future lead to near identical patterns of activation in each of these regions? We propose, as others have (8, 9), that to effectively generate a plausible image of the future, subjects reactivate images (e.g., visual–spatial context) stored in posterior cortical regions. Converging evidence for this hypothesis comes from subject's reports. Postexperiment questionnaires indicate that while envisioning the future, subjects tended to place those images in the context of familiar places (e.g., home, school) and familiar people (e.g., friends), which would require the reactivation of those images from the appropriate neural structures. Conversely, subjects tended to rely more on semantic knowledge when imagining Bill Clinton (e.g., “I see Bill Clinton at a party in the White House, alongside several faceless senators”).
Although it makes intuitive sense to suspect that a mental image of one's future is based on representations reactivated from the past, the data are open to alternative interpretations. For instance, although differences in vividness, valence, and intensity of emotional experience cannot account for these findings, both the future imagery and the memory tasks included an element of contiguity of self in time (1, 11, 38) not present in the nonpersonal control task. An intact representation of oneself is known to be integral for the ability to remember episodes from one's past (45) and to envision specific future scenarios (46). Definitive statements regarding the neural relationship between the personal future and past await the effective decomposition of these components (11).
Conclusions
This article represents an initial attempt to directly investigate the neural correlates underlying the ability to envisage specific future events. The data are notable in two respects. First, a distributed set of cortical regions has been identified that appears to be important for episodic future thought and that is not isolated to regions within frontal cortex. Second, these regions neatly break apart into two sets of regions, each characterized by their pattern of activity across tasks. One set of regions, previously implicated in the simulation of bodily movements, was characterized by greater activity during episodic future thought than recollection of autobiographical memories (and by more activity for both these tasks than imagining a familiar individual). A second set of regions was characterized by equivalent patterns of activity during episodic future thought and recollection (and greater activity for both these tasks than imagining a familiar individual). This latter set of regions is commonly engaged during tasks that require subjects to reinstate visual–spatial context. Accordingly, it is suggested that simulation of bodily actions and reinstatement of visual–spatial context may be particularly relevant to the understanding of the ability to mentally represent a future event. The data offered here may be taken as an empirical starting point regarding the understanding of the neural substrates involved in episodic future thought.
Methods
Subjects.
Subjects (n = 21, 12 females, 9 males, mean age 22.52, range 18–32 years) were recruited from the Washington University community and were paid for their participation. All were right-handed native English speakers who had normal or corrected-to-normal vision and reported no history of significant neurological problems. Subjects provided informed consent in accordance with the guidelines set by the Washington University Human Studies Committee.
Materials.
Event cues (n = 72) representing common life experiences (e.g., Birthday, Getting Lost, Barbecue) were compiled. Counterbalancing was achieved by dividing the event cues into three sets. For each subject, each set of 24 event cues was paired with one of three orienting cues (SF, SR, or CI). Hence, sets were rotated across orienting cue type and subjects, which ensured that each event cue was paired with each orienting cue equally often across subjects.
Procedure.
Scans were obtained on a 1.5-T Siemens Vision System (Erlangen, Germany) with a standard circularly polarized head coil. A Power Macintosh computer (Apple, Cupertino, CA) and Psyscope software (47) displayed the visual stimuli. An Ampro LCD-150 projector (San Jose, CA), shielded with copper wire, displayed stimuli on a screen placed at the head of the bore. Subjects viewed the screen through a mirror fastened to the head coil. A pillow and surgical tape were used to minimize head movement. Headphones dampened scanner noise and enabled communication with subjects.
Structural images were acquired by using a high-resolution sagittal MPRAGE sequence (1.25 × 1 × 1-mm voxels). Functional images were collected with an asymmetric spin-echo-planar sequence sensitive to blood oxygen level-dependent contrast (48, 49). In each functional run, 154 sets of 18 contiguous, 7-mm-thick axial images (repetition time = 2.5 sec, 3.75 × 3.75-mm in-plane resolution) were acquired parallel to the anterior–posterior commissure plane; this procedure offered whole-brain coverage at a high signal-to-noise ratio.‡ At the beginning of each run, four images were acquired to permit longitudinal magnetization to stabilize; these images were not included in the functional analyses but were used to facilitate alignment of the functional data to the structural images. Each run lasted ≈6 min, and ≈3 min elapsed between runs, during which time instructions were given to subjects over their headphones.
Each subject participated in three functional runs. In each run, subjects were presented with a series of 24 event cues, each of which was accompanied by one of the three task-defining orienting cues; subjects were told that the orienting cues should serve to direct their mode of thought regarding each specific event. Specifically, during each run subjects were required to think about eight events in relation to their future (SF), eight events in relation to their past (SR), and eight events in relation to a familiar individual (CI). For instance, if the orienting cue Self-Future appeared above the event cue Birthday, subjects were asked to imagine a future event involving themselves. Subjects were instructed that they should attempt to imagine a specific event that might reasonably happen to them in the future. When an event cue was prefaced by the orienting cue Self-Remember, subjects were required to use the event to help them evoke a specific memory. It was explained in detail that their memories did not have to be directly related to the event cues. That is, subjects were free to think of any specific memory that came to mind, using the event cue as a starting point. Some examples were provided to illustrate what would or would not be considered a specific memory (i.e., specific time, place, etc.). When an event cue was prefaced by the orienting cue Clinton-Imagine, subjects were to vividly imagine Bill Clinton participating in a scenario evoked by the event cue. Subjects were told that Bill Clinton was chosen because most people know what he looks like and because he is easy to visualize. They were further instructed that this task could be performed without considering the event's relation to time (i.e., whether it was occurring in the past or future).
The orienting cue appeared directly above the event cue, centered on the subjects' viewing mirror. It was explained to subjects that the orienting cues would appear in random order and that they should be prepared to switch their mode of thought at any time. On the occasion that subjects may not have been able to fully remember or imagine a certain event in the time allotted, they were instructed to let those events out of mind and to focus all of their attention on the next trial. However, this outcome was not expected to occur (and in fact, it did not occur) because all subjects were able to recollect memories and generate imagined events in response to the event cues. Subjects also encountered eight randomly interleaved fixation trials per run, introducing temporal jitter into the design to allow for event-related analyses (50), during which they were instructed to give themselves a mental break and remain still.
Each of the 32 trials within each run began at the onset of an image acquisition (or frame). Each trial spanned four such frames (2.5 sec each): The stimulus appeared for 9,500 msec, followed by a fixation crosshair for 500 msec. The four types of trials (SF, SR, CI, and fixation) were ordered in a balanced fashion such that each trial type preceded and followed each other trial type approximately the same number of times (to facilitate response modeling with the general linear model).
In all cases, subjects were instructed to do their best to perform each task for the entire 10 sec. It was stressed that they were not to simply generate a quick image and then stop thinking about it. Instead, they were required to come up with as vivid an image as they could for the entire 10-sec period. Subjects were never required to respond; rather, they were required only to think about each event. Prescan practice with a small subset of event cues indicated that all subjects were able to imagine an event to each cue under the specified parameters of the study. Once in the scanner, subjects were presented with novel events. Further, it was also explained to subjects that they should not close their eyes when thinking about each event because to do so might cause them to miss the onset of an upcoming event in the sequence.
Finally, subjects completed a postscan questionnaire, during which they were re-presented with all of the event cues (accompanied by orienting cues) that they had observed while in the scanner. Subjects were asked to briefly describe what they had thought about for each event and to rate the phenomenological qualities of the mental images they had formed. Specifically, they rated each image for vividness (1 = not at all vivid, 7 = extremely vivid), emotional valence (−3 = extremely negative, 3 = extremely positive), and emotional intensity (1 = not at all intense, 7 = extremely intense). The study described in ref. 51 showed that subjects were able to provide reliable ratings to similar mental images in the context of such a postscan questionnaire. Details of these behavioral ratings will be reported elsewhere.
Functional MRI Data Analysis.
Data for each subject were preprocessed to remove noise and artifacts. These steps included correction for intensity differences across odd- and even-numbered slices, interpolation to 3 × 3 × 3-mm voxels, alignment to correct for slice-based within-trial differences in acquisition times, automated movement-correction within and across runs (52), removal of the linear slope on a voxel-by-voxel basis to correct for frequency drift (53), whole-brain normalization to a common mode of 1,000 to facilitate comparisons across subjects, transformation into standardized atlas space (54), and application of a Gaussian smoothing filter (9-mm full-width half-maximum) to further accommodate variations in activation loci across subjects.
The resulting data were analyzed by using an implementation of the general linear model (55). The timecourse of activity for the 10 frames (i.e., time points) immediately after each orienting cue (SF, SR, CI) was modeled in the generalized linear model in an assumption-free manner (for each voxel for each subject).
Generation of ROIs (step 1) was accomplished by cross-correlating the estimated timecourse of the blood-oxygen-level-dependent response corresponding to each orienting cue at each voxel with a reference gamma function (56); a latency parameter of three values (γ = 2 sec, λ = 1.25 sec) at 1-sec steps was used to accommodate slight variations in onset time of the response. The greatest of the three resulting magnitudes (in percent signal change units) was entered into the appropriately weighted contrast [(SR + SF) − CI] to identify voxels that showed more activity for the SF and SR conditions (combined) than the CI baseline task. To achieve a whole-brain P value of 0.05, the resulting image was then thresholded such that only voxels exceeding P < 5 × 10−6 and contiguous with at least three other such voxels were considered. An automated peak-search algorithm smoothed the group-averaged Z statistic map, using a 5-mm-radius sphere and then identified the location, in atlas coordinates (54), of peak activation on the basis of level of statistical significance with the provision that they be separated by at least 10 mm, or else the peaks were consolidated by coordinate averaging. Regions around the peak activations were identified by choosing contiguous voxels that were within 10 mm of the peak activation and that surpassed the statistical threshold.
Once the ROIs had been generated, the second step was to characterize the patterns of activity seen in these regions. That is, to identify regions exhibiting activity differences (or similarities) while subjects envisioned the personal future and past, a two-way repeated-measures analysis of variance (ANOVA) was applied to the average timecourse data for each of the ROIs; condition (SF and SR) and time point (at the 10 measured frames) were independent variables in this analysis. Regions showing a significant 2 (orienting cue: SF, SR) × 10 (timepoint) interaction were of most interest.
Glossary
Abbreviations
- SR
Self-Remember
- SF
Self-Future
- CI
Clinton-Imagine
- ROIs
regions of interest
- BA
Brodmann's area.
Footnotes
The authors declare no conflict of interest.
†Another possible comparison task would have involved imagining oneself in the present. However, giving subjects event cues (e.g., Birthday, Circus, Road Trip) and asking them to imagine themselves currently experiencing those events in the immediate present (while lying in the scanner) seemed an unnatural task and one that would likely be interpreted by subjects much like the future task.
‡Conturo, T. E., McKinstry, R. C., Akbudak, E., Snyder, A. Z., Yang, T. Z., Raichle, M. E. (1996) Soc Neurosci Abstr 22:7.
References
- 1.Suddendorf T, Corballis MC. Genet Social Gen Psychol Monogr. 1997;123:133–167. [PubMed] [Google Scholar]
- 2.Tulving E. Stuss DT, Knight RC. Principles of Frontal Lobe Function. New York: Oxford Univ Press; 2002. pp. 311–325. [Google Scholar]
- 3.Fuster JM. The Prefrontal Cortex Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. 2nd Ed. New York: Raven; 1989. [Google Scholar]
- 4.Shallice T. From Neuropsychology to Mental Structure. Cambridge, UK: Cambridge Univ Press; 1988. [Google Scholar]
- 5.Stuss DT, Benson DF. The Frontal Lobes. New York: Raven; 1986. [Google Scholar]
- 6.Baker SC, Rogers RD, Owen AM, Frith CD, Dolan RJ, Frackowiak RSJ, Robbins TW. Neuropsychologia. 1996;34:515–526. doi: 10.1016/0028-3932(95)00133-6. [DOI] [PubMed] [Google Scholar]
- 7.Burgess PW, Quayle A, Frith CD. Neuropsychologia. 2001;39:545–555. doi: 10.1016/s0028-3932(00)00149-4. [DOI] [PubMed] [Google Scholar]
- 8.Okuda J, Fujii T, Ohtake H, Tsukiura T, Tanji K, Suzuki K, Kawashima R, Fukuda H, Itoh M, Yamadori A. NeuroImage. 2003;19:1369–1380. doi: 10.1016/s1053-8119(03)00179-4. [DOI] [PubMed] [Google Scholar]
- 9.Ingvar DH. Hum Neurobiol. 1985;4:127–136. [PubMed] [Google Scholar]
- 10.Atance CM, O'Neill CM. Trends Cognit Sci. 2001;5:533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- 11.Klein SB, Loftus J, Kihlstrom JF. Soc Cognit. 2002;20:353–379. [Google Scholar]
- 12.Tulving E. Can Psychol. 1985;26:1–12. [Google Scholar]
- 13.Addis DR, Wong AT, Schacter DL. Neuropsychologia. 2006 in press. [Google Scholar]
- 14.Schacter DL, Addis DR. Philos Trans R Soc London B. in press. [Google Scholar]
- 15.Buckner RL, Carroll DC. Trends Cogn Sci. 2006 doi: 10.1016/j.tics.2006.11.004. in press. [DOI] [PubMed] [Google Scholar]
- 16.Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Am J Psychiatry. 1995;152:1576–1585. doi: 10.1176/ajp.152.11.1576. [DOI] [PubMed] [Google Scholar]
- 17.Ingvar D. Acta Neurol Scand. 1979;60:12–25. doi: 10.1111/j.1600-0404.1979.tb02947.x. [DOI] [PubMed] [Google Scholar]
- 18.Stark CEL, Squire LR. Proc Natl Acad Sci USA. 2001;98:12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crovitz HF, Schiffman H. Bull Psychonom Soc. 1974;4:517–518. [Google Scholar]
- 21.Williams JMG, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. Mem Cognit. 1996;24:116–125. doi: 10.3758/bf03197278. [DOI] [PubMed] [Google Scholar]
- 22.Busby J, Suddendorf T. Cognit Dev. 2005;20:362–372. [Google Scholar]
- 23.Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, Tulving E, Stuss D. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- 24.Stuss DT. In: Awareness of Deficit After Brain Injury. Prigatano GP, Schacter DL, editors. New York: Oxford Univ Press; 1991. pp. 63–83. [Google Scholar]
- 25.Poldrack RA. Trends Cognit Sci. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Henson R. Q J Exp Psychol A. 2005;58:193–233. doi: 10.1080/02724980443000502. [DOI] [PubMed] [Google Scholar]
- 27.Picard N, Strick PL. Curr Opin Neurobiol. 2001;11:663–672. doi: 10.1016/s0959-4388(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 28.Cavanna AE, Trimble MR. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 29.Irvy RB, Fiez JA. In: The New Cognitive Neurosciences. Gazzaniga MS, editor. Cambridge, MA: MIT Press; 2000. pp. 999–1011. [Google Scholar]
- 30.Decety J, Grezes J. Brain Res. 2006;1079:4–14. doi: 10.1016/j.brainres.2005.12.115. [DOI] [PubMed] [Google Scholar]
- 31.Hesslow G. Trends Cognit Sci. 2002;6:242–247. doi: 10.1016/s1364-6613(02)01913-7. [DOI] [PubMed] [Google Scholar]
- 32.Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, Denis M. NeuroReport. 1997;8:739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- 33.Ruby P, Decety J. Nat Neurosci. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- 34.Fink GR, Markowitsch HJ, Reinkemeier M, Bruckbauer T, Kessler J, Heiss WD. J Neurosci. 1996;16:4275–4282. doi: 10.1523/JNEUROSCI.16-13-04275.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decety J, Ingvar DH. Acta Psychol. 1990;73:13–24. doi: 10.1016/0001-6918(90)90056-l. [DOI] [PubMed] [Google Scholar]
- 36.Schacter DL, Buckner RL. Neuron. 1998;20:185–195. doi: 10.1016/s0896-6273(00)80448-1. [DOI] [PubMed] [Google Scholar]
- 37.Cabeza R, Nyberg L. J Cognit Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 38.Wheeler MA, Stuss DT, Tulving E. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- 39.Cabeza R, Prince SE, Daselaar SM, Greenberg DL, Budde M, Dolcos F, LaBar KS, Rubin DC. J Cognit Neurosci. 2004;16:1583–1594. doi: 10.1162/0898929042568578. [DOI] [PubMed] [Google Scholar]
- 40.Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. J Cognit Neurosci. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- 41.Northoff G, Bermpohl F. Trends Cognit Sci. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Svoboda E, McKinnon MC, Levine B. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellet E, Bricogne S, Tzourio-Mazoyer N, Ghaem O, Petit L, Zago O, Berthoz A, Mazoyer B, Denis M. NeuroImage. 2000;12:588–600. doi: 10.1006/nimg.2000.0648. [DOI] [PubMed] [Google Scholar]
- 44.Rosenbaum SR, Ziegler M, Winocur G, Grady CL, Moscovitch M. Hippocampus. 2004;14:826–835. doi: 10.1002/hipo.10218. [DOI] [PubMed] [Google Scholar]
- 45.Klein SB. In: Individual Self, Relational Self, Collective Self. Sedikides C, Brewer MB, editors. Philadelphia: Psychology Press; 2001. pp. 25–46. [Google Scholar]
- 46.Ackerley SS, Benton AL. Res Publ Assoc Res Nerv Ment Dis. 1947;27:479–504. [PubMed] [Google Scholar]
- 47.Cohen JD, MacWhinney B, Flatt M, Provost J. Behav Res Methods Instrum Comput. 1993;25:257–271. [Google Scholar]
- 48.Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R. Proc Natl Acad Sci USA. 1992;89:5675–5679. doi: 10.1073/pnas.89.12.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ogawa S, Tank DW, Menon R, Ellermann JM, Kim SG, Merkle H, Ugurbil K. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dale AM, Buckner RL. Hum Brain Mapp. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 51.Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- 52.Snyder AZ. In: Qualifications of Brain Function Using PET. Bailey D, Jones T, editors. San Diego: Academic; 1996. pp. 131–137. [Google Scholar]
- 53.Bandettini PA, Jesmanowicz A, Wong EC, Hyde JS. Magn Reson Med. 1993;30:161–173. doi: 10.1002/mrm.1910300204. [DOI] [PubMed] [Google Scholar]
- 54.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. NY: Thieme; 1988. [Google Scholar]
- 55.Worsley KJ, Friston K. NeuroImage. 1995;2:173–182. doi: 10.1006/nimg.1995.1023. [DOI] [PubMed] [Google Scholar]
- 56.Boynton GM, Engel SA, Glover GH, Heeger DJ. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]