Abstract
Objective
Matrix metalloproteinase-9 (MMP-9) has been widely described to play a critical role in aneurysm development. The goal of this study was to determine the spatiotemporal changes in MMP-9 expression and abundance in the early stages of aortic dilatation during the course of thoracic aortic aneurysm (TAA) formation in a mouse model.
Methods
In this study, TAAs were surgically induced in a transgenic reporter mouse strain expressing the β-galactosidase (β-gal) gene under control of the MMP-9 promoter. Terminal studies were performed during the early stages of TAA development at 1-wk (n=6), 2-wk (n=6), and 4-wk (n=6) post-TAA induction surgery. Changes in aortic outer diameter were determined in vivo by video micrometry. MMP-9 transcriptional activity (β-gal staining) and protein content (immunohistochemistry) were quantified at each time point and expressed as a percentage of unoperated reference control mice (n=6).
Results
Aortic dilatation was evident at 1-wk and reached maximal size at 2-wk (21±6% increase from baseline, p<0.05). MMP-9 transcriptional activity was detected at 1-wk post-TAA induction (722±323%, p=0.19), reached a maximum within the adventitia at 2-wk (1770±505%, p<0.05), and returned to baseline by 4-wk (167±47%, p=0.21). MMP-9 transcription at 2-wk co-localized with fibroblasts and smooth muscle cells. MMP-9 protein content within the aortic adventitia was increased at 2-wk post-TAA induction (413±124%, p<0.05), and remained elevated at 4-wk (222±41%, p<0.05). MMP-9 staining was most intense at the adventitial:medial border and could be detected throughout the elastic media.
Conclusions
These findings demonstrate a unique spatiotemporal pattern of MMP-9 transcriptional activation and protein content in the developing TAA. Colocalization studies suggest that early dilatation may be driven in part by MMP-9 produced by endogenous cells residing within the aortic vascular wall.
Clinical Relevance
The detection of TAA formation and progression remains clinically difficult to manage. TAA development is a multifactorial process influenced by both cellular and extracellular mechanisms that converge on common maladaptive signaling pathways that alter the vascular environment. Active remodeling of the vascular extracellular matrix has been directly implicated in aortic dilatation and aneurysm development, and MMP-9 has been shown in multiple studies to play a critical role in this process. Thus, the goal of this study was to define the spatiotemporal relationship between MMP-9 expression/abundance and the initiation of aortic dilatation in the developing TAA. Understanding when and where MMP-9 is expressed locally defines a therapeutic window during which disruption of MMP-9 activity may aid in attenuating TAA progression.
Keywords: mouse, aneurysm, aorta, thorax, MMP-9, reporter construct, β-galactosidase
INTRODUCTION
Thoracic aortic aneurysms (TAAs) are a serious and potentially deadly disorder associated with high morbidity and mortality.1–4 It has become increasingly appreciated that TAA development proceeds by a multifactorial process driving aberrant events initiated by intra- and extra-cellular signaling pathways. These aberrant events result in the remodeling of the vascular extracellular matrix, an invariant feature of TAA formation, and are the primary reason for the loss of vascular integrity resulting in aortic dilatation. As vital mediators of extracellular matrix remodeling, matrix metalloproteinases (MMPs) have been directly implicated in aneurysm formation.5–10
MMP-9 is a secreted pro-gelatinase capable of degrading many of the extracellular matrix proteins including collagen and elastin. As such, it has been identified as a critical mediator of vascular remodeling and has been described as the predominant MMP involved in the pathogenesis of abdominal aortic aneurysms (AAA).11–13 Studies by Thompson and coworkers colocalized MMP-9 mRNA with CD-68 positive macrophages in human AAA tissue taken at time of resection and suggested that the vascular remodeling associated with AAA may be dependent on infiltrating macrophages.12 This was further substantiated by Pyo et al. who demonstrated that AAA formation could be restored in MMP-9 knockout mice, which failed to produce AAA following elastase perfusion, with wild-type bone marrow transplantation.14 Macrophages were later confirmed as the primary mediator of AAA development by Longo and coworkers when it was demonstrated that CaCl2-induced AAA formation in MMP-9 knockout mice could be restored by infusing wild-type macrophages alone.15 Taken together these data suggest that macrophages provide a significant source of MMP-9 and play a dominant role in the development of AAA.
Studies from our laboratory have previously demonstrated that TAA formation was enhanced in mice carrying a targeted deletion of the TIMP-1 gene.16 This coincided with an increase in gelatinase activity, suggesting that simply disrupting the stoichiometric balance between active MMPs and their inhibitors may be sufficient to effect aneurysm formation. These results were supported by a reciprocal study which demonstrated a 50% reduction in aortic dilatation in MMP-9 knockout mice, thus confirming the importance of MMP-9 in TAA formation.17 To date, however, the source of MMP-9 in the developing TAA has not been determined. Therefore to advance our previous observations, we tested the hypothesis that inflammatory infiltrate was critical for MMP-9 production during TAA development. Through the use of a unique MMP-9 reporter mouse strain and immunohistochemical staining techniques, this study provides coordinated insight into the time-dependent expression and localization of MMP-9 within the aortic wall during TAA formation.
METHODS
Experimental Design
TAAs were surgically induced in approximately equal numbers of male and female transgenic 3445[MMP-9:β-gal]/CD-1 reporter mice (MMP-9 reporter mice). This mouse strain, originally developed by Fini and coworkers,18 expresses the β-galactosidase gene under the control of the MMP-9 promoter in all cells. All mice were 8 to 12 weeks of age at the time of initial surgery. Descending thoracic aortas were harvested at 1-wk (n=6), 2-wk (n=6), and 4-wk (n=6) post-TAA induction. For all studies presented in this report, age-matched unoperated mice (no TAA induction) were used as reference controls (n=6); they were euthanized and harvested in identical fashion to all TAA-induced animals. All animals were treated and cared for in accordance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals” (National Research Council, Washington, DC, 1996) and this animal protocol was approved by the MUSC Institutional Animal Care and Use Committee (ARC# 2146).
Operative Procedure
Aortic diameters were measured at baseline and time of terminal surgery. Murine TAAs were induced as previously described in detail.19 Briefly, mice were orotracheally intubated and maintained under a surgical plane of anesthesia with 2% isoflurane. The mice were then subjected to fifth intercostal space thoracotomy with exposure of the descending thoracic aorta. A sponge soaked in 0.5 M CaCl2 was then placed in direct contact with the distal half of the descending thoracic aorta for 15 min. Following exposure, the sponge was removed, the chest was irrigated, and the lung was re-expanded. The incisions were closed and the mice were allowed to survive for the indicated time periods.
Aortic Harvest and Tissue Preparation
At time of terminal surgery, the animals were re-anesthetized and the initial incisions were re-opened and extended to beneath the xiphoid process. The mice were then euthanized and the circulatory system perfused with normal saline. For spatiotemporal localization of MMP-9 transcriptional activity and protein abundance, the entire aorta was carefully harvested from the aortic root to the abdominal bifurcation and placed directly into 10% formalin solution for 15 min. The samples were then washed 3 x 5 min in phosphate-buffered saline and incubated in X-gal solution (5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside) for 24 hr at 40°C. At the end of the incubation, the samples were washed again as above, fixed in Dent’s solution (80:20, dimethylsulfoxide: methanol), and stored at 4°C until they were paraffin embedded and sectioned (8-μm longitudinal sections).
Aortic Diameter Measurements and Architecture
Digital images of the descending thoracic aorta were obtained using a color CCD camera (KP DZ0B; Hitachi Kokusai Electric Inc., Tokyo, Japan) linked to a laptop computer with digital imaging software (WinTV2000; Hauppauge Computer Works, Inc., Hauppauge, NY). Aortic outer diameter measurements were made via a digital video caliper (DMZR; Techni-Quip, Danville, CA). The architecture of the thoracic aorta was assessed by microscopy of 3 μm cross-sections of aortic tissue counter stained with light green under low (10X) and high (63X) magnification.
Spatiotemporal Localization of MMP-9
In situ MMP-9 transcriptional activation was quantified by measuring the amount of blue precipitate formed upon X-gal cleavage by β-gal. Paraffin embedded TAA tissue was sectioned longitudinally and 8 μm thick sections were counter-stained with eosin and mounted on glass slides for analysis.
Similarly, in situ MMP-9 protein abundance was determined by immunohistochemistry using an antibody specific for both active and latent MMP-9. Eight μm thick paraffin embedded longitudinal sections were mounted on glass slides, blocked with 3% bovine serum albumin, and reacted with a 1:250 dilution of rabbit anti-mouse MMP-9 antibody (Chemicon, #Ab19047). The slides were then washed and reacted with a 1:200 dilution of goat-anti-rabbit-peroxidase conjugated secondary antibody (Vectastain ABC kit, Vector Labs, Burlingame, CA). Positive MMP-9 protein staining was visualized by incubation with 3,3′-diaminobenzidine (DAB), which formed a brown precipitate upon reaction with the peroxidase-conjugate secondary antibody. Tissues sections processed in the same fashion without the addition of primary antisera were used as negative controls.
Localization of MMP-9 Transcriptional Activity with Cell-Specific Markers
For colocalization studies, 2-wk TAAs were surgically induced in three additional MMP-9 reporter mice as described above, and compared to three age-matched unoperated reference control mice. At time of terminal surgery, animals were euthanized and perfused with normal saline as before. This was followed by perfusing the aorta with 10% formalin for 5 minutes, then incubating the whole animal in 10% formalin for 48 hours at 4°C. The treated region of the descending thoracic aorta was then excised from the mouse, incubated in X-gal as indicated above, and stored at 4°C until they were paraffin embedded and sectioned (3 μm cross sections). The aortic tissue sections were then mounted on glass slides, blocked with 3% bovine serum albumin, and reacted with antisera specific for α-smooth muscle actin (αSMA) (anti-αSMA (rabbit), 1:250, Abcam, #Ab5694), discoidin domain receptor 2 (DDR2) (anti-DDR2 (goat), 1:250, Santa Cruz, #SC7555), macrophages (anti-MAC3 (rat), 1:50, BD Pharmingen, #550292), or neutrophils (anti-CD11b (Rat), 1:50, BD Pharmingen, #550282). The slides were washed and reacted with a 1:200 dilution of primary antibody species-specific peroxidase conjugated secondary antibody (Vectastain ABC kit, Vector Labs, Burlingame, CA). Staining was visualized by incubation with 3,3′-diaminobenzidine (DAB), which formed a brown precipitate upon reaction with the peroxidase-conjugated secondary antibody. Tissues sections processed in the same fashion without the addition of primary antisera were used as negative controls.
Microscopy
Microscopic images of the stained tissue sections were captured under 10X (light green stain), 40X (β-gal and MMP-9 imaging), or 63X (light green, cell specific markers) magnification on a Zeiss Axioscop 2 microscope (Carl Zeiss MicroImaging Inc, Thornwood, NY) equipped with an Axiocam MRc cooled color CCD camera connected to a computer running Axiovision Software (v4.2).
Quantitation of MMP-9 mRNA by Quantitative Real-time PCR
MMP-9 mRNA levels were determined in a two separate cohorts of mice. Two-week TAAs were surgically induced in wild-type CD-1 mice (n=6) and transgenic MMP-9 reporter mice (n=6). Relative levels of MMP-9 mRNA were quantitated and compared to unoperated control groups of wild-type CD-1 (n=6) and transgenic MMP-9 reporter mice (n=6). The descending thoracic aortas were harvested, rinsed in phosphate buffered saline, and placed immediately into RNAlater solution (Ambion, Inc. Austin, TX) for 24-hr at 4°C. The tissue was homogenized and total RNA was extracted using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s instructions. The RNA was then assessed for quality and quantity using the Experion Automated Electrophoresis System. High quality RNA was reverse transcribed using an iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, CA). MMP-9 mRNA levels were then determined by quantitative real-time PCR (QPCR) using a Taqman protocol. Primer and probes were supplied in the Taqman Gene Expression Assays on Demand for MMP-9 and 18S rRNA (Applied Biosystems, Foster City, CA). Equal amounts of cDNA (2.5 μl) generated from each aorta were used in each reaction and MMP-9 levels were determined and normalized to 18S rRNA levels from each respective aorta.
Data Analysis
Terminal aortic size was expressed as a percentage increase from baseline size in each mouse. The mean percent change at each time point was then determined and compared to the unoperated reference control group. This study analyzed aortic diameters in 58.3% male and 41.7% female mice. Analysis of variance was performed on aortic diameter measurements using gender as a covariate. The results revealed no significant interaction with respect to gender (F = 0.01 and p = 0.9424). Therefore, the data derived from both male and female mice were pooled for all subsequent analyses.
MMP-9 transcriptional activation and protein content were determined as a percentage of positive staining to total tissue present. From each mouse aorta (n=6), at each time point (unoperated reference control, 1-wk, 2-wk, and 4-wk), three independent sections were analyzed and images were captured from three separate fields, generating nine independent images from each aorta. The area corresponding to active MMP-9 transcriptional activity (blue staining) or MMP-9 protein content (brown staining) was determined by digital planimetry (SigmaScan, v4.0, Systat Software, Point Richmond, CA). Results from each of the nine images were averaged to provide a single value for each aorta. The mean value for each TAA group was then used to determine a percent change from the unoperated reference control group.
Relative levels of MMP-9 mRNA (normalized to 18S rRNA) were calculated by the standard curve method and expressed as a percent change from wild-type CD-1 control mice.
Statistical calculations were performed using the Stata statistical software package (v.8, StataCorp LP, College Station, TX). Aortic diameter results, amount of β-gal staining, MMP-9 protein abundance, and QPCR results were expressed as a percentage of baseline or unoperated reference control values, and were compared using a one-sample, two-tailed, t-test versus a fixed value of 100. Pairwise comparisons between groups were performed by one-way ANOVA with post-hoc Tukey’s wsd calculations. All data are presented as a mean ± SEM and values of p<0.05 were considered to be statistically significant.
RESULTS
Aortic diameter measurements revealed that aortic dilatation, consistent with the early stages of TAA formation, was evident at 1-wk post-CaCl2 exposure, reached a maximum at 2-wk (21±6% increase from baseline, p<0.05), and remained dilated at 4-wk (18±4% increase from baseline, p<0.05) without a further increase in size (Figure 1A). Flattening and thinning of the medial elastic lamellae was observed, as were the early stages of elastin degradation (Figure 1B).
Figure 1. Aortic dilatation and architecture.
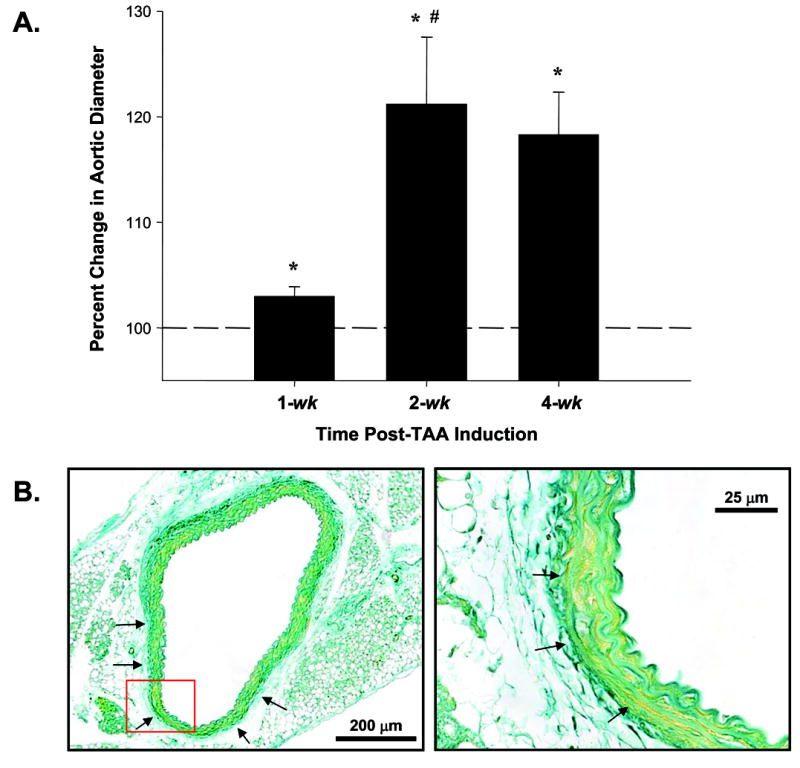
A. Aortic diameters were measured in each mouse at baseline and terminal surgery. Aortic diameters increased at 1-wk, reached a maximum at 2 wks, and remained dilated at 4-wk post-TAA induction. (n=6 at all time points, * = p<0.05 vs. baseline, # = p<0.05 vs. 1-wk post-TAA); B. Cross-section of aorta at 2-wk post-TAA induction counterstained with light green. Low magnification image reveals thinning and flattening of medial elastic lamellae (left, arrows). Red box denotes area of high magnification which reveals flattening and early disruption of elastic lamellae (right, arrows). Scale bars are indicated on each image.
Time-dependent localization of MMP-9 transcriptional activation during TAA formation was determined by staining for β-gal in MMP-9 reporter mice (Figure 2). MMP-9 transcriptional activation was evident 1-wk post-TAA induction within the peri-adventitial adipose layer. Transcriptional activity was increased at 2-wk post-TAA induction, demonstrating significant adventitial β-gal staining, and was diminished at 4-wk post-TAA induction. Quantitation of total β-gal staining within multiple tissue sections was performed as described using digital planimetry. The data revealed an increase in β-gal staining at 1-wk post-TAA induction, which reached a maximum at 2-wk, and returned to control values at 4-wk (Figure 3).
Figure 2. Localization of β-galactosidase.

MMP-9 transcriptional activity was localized by staining for β-galactosidase activity at the indicated time points post-TAA induction. Representative images from 8 μm longitudinal sections are shown. Diffuse β-galactosidase staining was observed within the peri-adventitial adipose layer at all time points post-TAA induction. β-galactosidase staining was elevated within the adventitia 2-wk post-TAA induction. (AL = aortic lumen, bar = 50 μm)
Figure 3. Quantitation of total β-galactosidase activity.

The amount of blue precipitate formed from the conversion of X–gal by β-galactosidase was quantitated by digital planimetry at the indicated time points. β-galactosidase staining was evident at 1-wk post-TAA, reached a maximum at 2-wk post-TAA, and returned to baseline at 4-wk post-TAA. (n=6 at all time points, * = p<0.05 vs. 100, + = p<0.05 vs. 4-wk post-TAA)
Time-dependent localization of MMP-9 protein was likewise assessed during TAA formation by immunohistochemical staining (Figure 4). Increased MMP-9 protein content was evident within the aortic adventitia at 2-wk post-TAA induction, while at 4-wk enhanced MMP-9 staining was observed at the medial:adventitial border and within the elastic medial layers. Quantitation of total endogenous MMP-9 protein from multiple tissue sections was performed as described using digital planimetry. A significant increase in MMP-9 content was demonstrated at 2-wk and 4-wk post-TAA induction, with minimal staining quantitated in the control and 1-wk samples (Figure 5).
Figure 4. Localization of MMP-9.
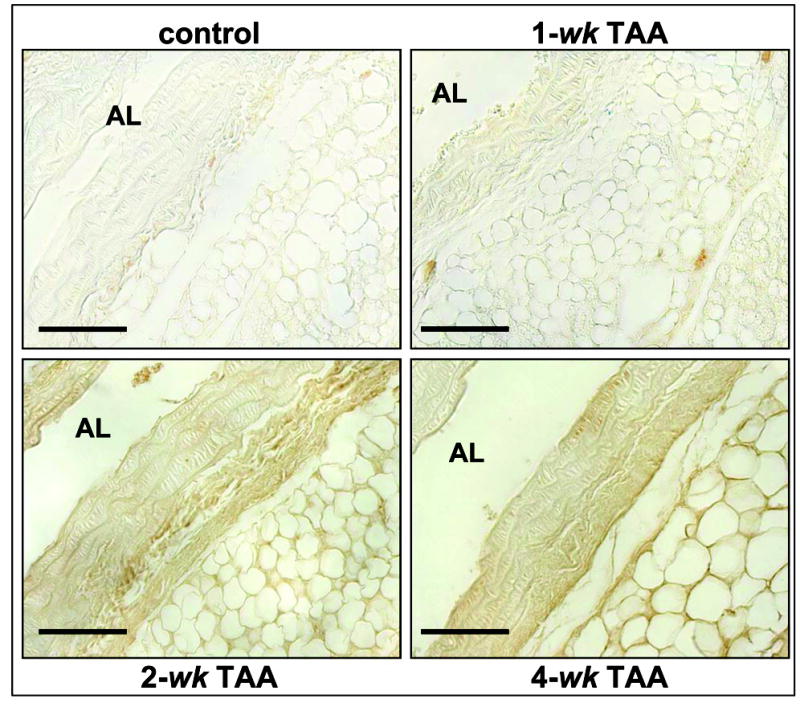
MMP-9 protein was immunolocalized at the indicate time points post-TAA induction. Representative images from 8 μm longitudinal sections are shown. Diffuse MMP-9 staining was observed throughout the tissue specimens at all time points. Enhanced MMP-9 protein levels were observed at 2-wk post-TAA induction within the adventitia. At 4-wk post-TAA induction, MMP-9 protein content was elevated at the medial:adventitial border, as well as throughout the elastic layers of the media. (AL = aortic lumen, bar = 50 μm)
Figure 5. Quantitation of MMP-9 protein content.
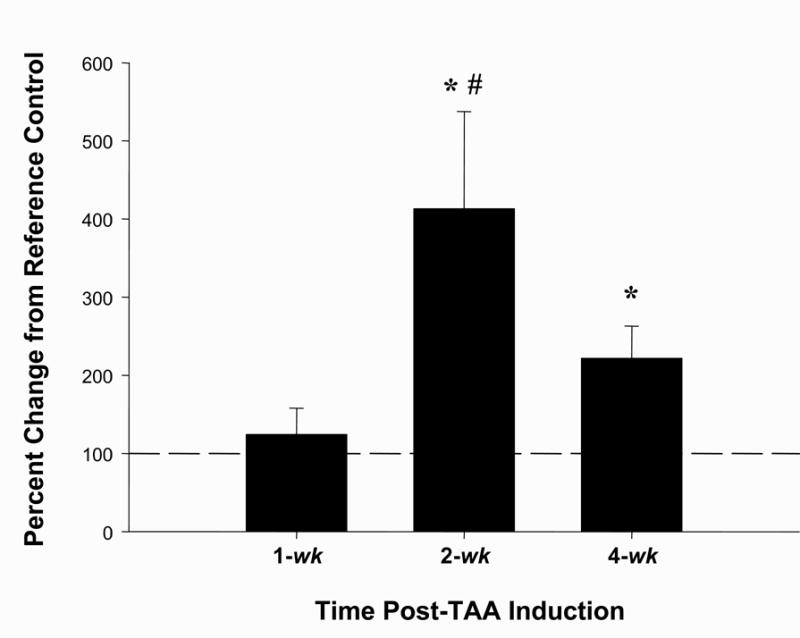
The amount of brown staining, corresponding to amount MMP-9 protein, was quantitated by digital planimetry at the indicated time points. A significant increase in MMP-9 protein abundance was observed at 2-wk post-TAA and remained elevated from reference control at 4-wk post-TAA. (n=6 at all time points, * = p<0.05 vs. 100, # = p<0.05 vs. 1-wk post-TAA)
Colocalization of MMP-9 transcriptional activity with cell-type specific markers 2-wk post-TAA revealed that MMP-9 transcriptional activity, as determined by X-gal staining, colocalized with αSMA (smooth muscle cells/myofibroblasts) and discoidin domain receptor 2 (DDR2) (fibroblasts). No colocalization was observed with MAC3 (macrophages) or CD11b (neutrophils) (Figure 6).
Figure 6. Localization of β-galactosidase activity with cell-type specific markers.
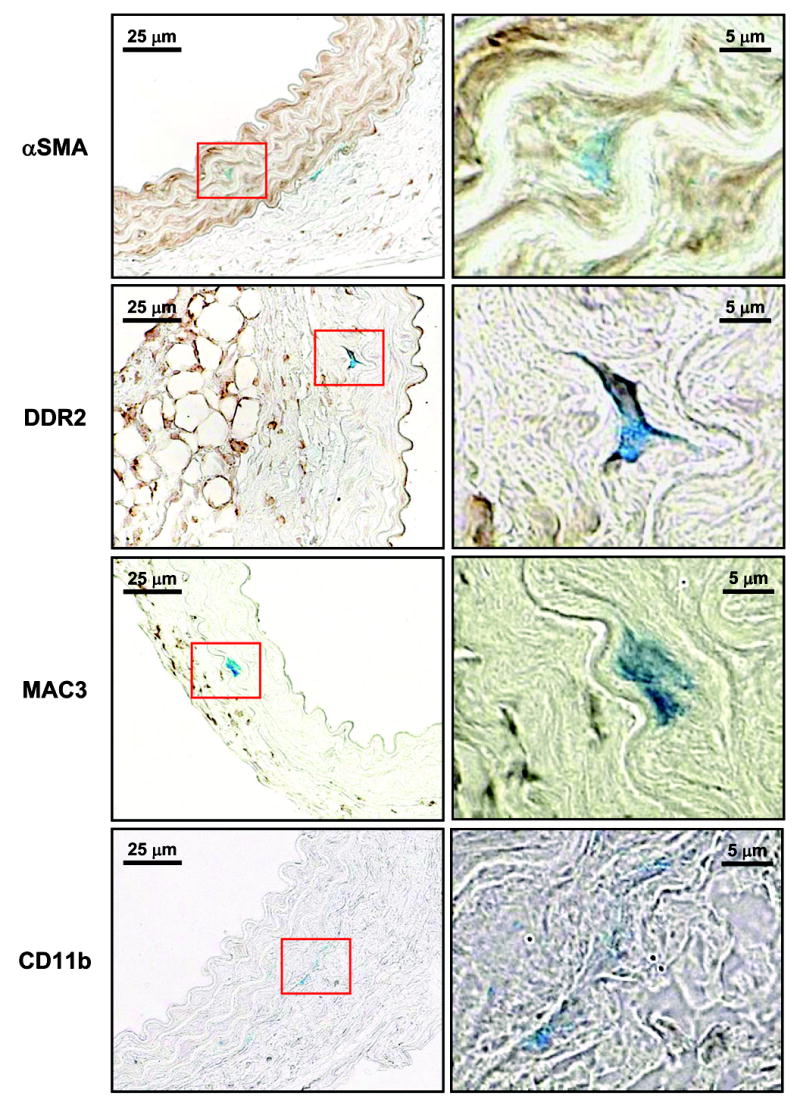
Areas with increased β-galactosidase activity (indicated by blue X-gal staining) were colocalized with cell-type specific markers on 3 μm cross-sections of aortic tissue harvested 2-wk post-TAA induction. The cell-type markers were immunolocalized and visualized by brown DAB staining (alpha-smooth muscle actin (αSMA), fibroblasts (DDR2), macrophages (MAC3) and neutrophils (CD11b)). Images were taken with 63X objective under oil (Plan-NEOFLUAR, 1.25 NA). Representative images of three independent sections are shown. Red box denotes area of high magnification image located to the right. Scale bars are indicated on each image.
Quantitative real-time PCR was performed to measure MMP-9 mRNA in descending aortas of unoperated and 2-wk TAA mice in the MMP-9 reporter strain and its non-transgenic CD-1 background strain. MMP-9 mRNA levels were elevated at 2-wk post-TAA induction in both the CD-1 (464.9%+90.1, p<0.05 vs. 100) and MMP-9 reporter mice (291.8%+57.0, p<0.05 vs. 100).
DISCUSSION
Degenerative changes occurring within the vascular wall are a common feature of all aortic aneurysms.8 These changes include remodeling of the vascular extracellular matrix (ECM), smooth muscle cell apoptosis, and are often associated with the activation of resident and circulating inflammatory cells.20 As vital mediators of ECM remodeling, the matrix metalloproteinases (MMPs) have been implicated in the pathogenesis of aneurysm formation, and MMP-9 has been identified in both the abdominal and thoracic aorta as a vital participant in this process.12, 14, 15, 17, 21 Accordingly, the present study builds upon these past observations by revealing the spatiotemporal localization of MMP-9 transcriptional activity and protein content in situ in an experimental murine model of thoracic aortic aneurysms (TAAs). The unique findings from this study demonstrated increased MMP-9 transcriptional activity 2-wk post-TAA induction within the adventitia and at the border of the adventitial and medial layers, which localized to endogenous cells within the vascular wall. This was accompanied by a concomitant increase in MMP-9 protein content, also within the aortic adventitia. At 4-wk post-TAA induction, in spite of the return of MMP-9 transcriptional activation to baseline, increased MMP-9 protein content was observed at the medial:adventitial border and throughout the medial elastic lamellae. Thus, these findings suggest that MMP-9 is produced by native smooth muscle cells and/or fibroblasts in the early stages of aortic dilatation. This early response may then provide a means by which activated circulating and resident inflammatory cells may further infiltrate the vascular wall and drive aneurysm formation.
In an effort to localize MMP-9 transcriptional activity within the thoracic aorta in the early stages of aneurysm formation, TAAs were surgically induced in transgenic MMP-9 reporter mice18, and followed during the initial 4 weeks of aneurysm formation. These mice carry, in addition to the endogenous MMP-9 gene, a transgene that drives β-gal production under the control of the MMP-9 promoter. The presence of this transgene did not affect normal mouse development or the inducibility of the endogenous MMP-9 transcript, as determined in the present study by quantitative PCR. Aortic outer diameter increased significantly within 1-wk following TAA induction surgery and reached a maximal diameter at 2-wk. Changes in aortic architecture at 2-wk were evident and characterized by early signs of medial disruption indicated by flattening and thinning of the elastic lamellae. These observations were consistent with previous reports of CaCl2-induced elastic lamellar disruption in AAAs.22 At 4-wk post TAA-induction the aortic diameter was not significantly different from the 2-wk time point, suggesting that dilatation reached a plateau.
In order to determine the localization of active MMP-9 transcription, β-gal activity in explanted aortas was identified in situ by reaction with the colorimetric substrate X-gal. Because the β-gal enzyme has a very short half-life upon translation (estimated at 13h in fibroblasts23), X-gal staining reveals recent transcriptional activity within the tissue samples, thus allowing quantitation and localization simultaneously. The results demonstrated that β-gal was clearly elevated within the aortic adventitia at 2-wk post-TAA induction. Furthermore, when MMP-9 was immunolocalized within the tissue specimen, increased MMP-9 protein abundance was also observed within the adventitial layer at 2-wk following surgery.
Because MMP-9 can be synthesized by a number of different cell types found within the aortic vascular wall,20, 24 and because of the direct implication of MMP-9 production by infiltrating-macrophages in AAA development,14, 15 it became important to identify the cell types actively transcribing MMP-9 during TAA formation. In order to accomplish this, 2-wk TAAs were induced in MMP-9 reporter mice and the aortas were harvested and reacted with X-gal. Three micron cross-sections of aortic tissue were used to colocalize β-gal activity with specific cell-type markers; smooth muscle cells/myofibroblasts (αSMA25), fibroblasts (DDR226), macrophages (MAC327), and neutrophils (CD11b28). Interestingly, β-gal activity colocalized with αSMA and DDR2 cell markers, indicating that MMP-9 was being produced by smooth muscle cells and/or fibroblasts present in the aortic adventitia. These results advance, and are in direct agreement with, the previous observation by Koullias et al., which suggested that MMP-1 and MMP-9 were produced by endogenous smooth muscle cells within the aortic vascular wall in samples collected from patients with TAA or thoracic aortic dissections.29 Surprisingly, no colocalization of X-gal staining was observed with MAC3 or CD11b however, suggesting that either resident/circulating inflammatory cells do not participate in early aortic dilatation events or simply that these cells types were not actively transcribing MMP-9.
At 4-wk post-TAA induction, immunolocalization of MMP-9 revealed increased MMP-9 protein content at the medial:adventitial border and throughout the elastic medial layer. Importantly, this occurred in the absence of significant β-gal production, suggesting that the MMP-9 present was not the result of recent transcriptional activation.
Several possibilities exist that may explain the presence of MMP-9 in the absence of MMP-9 transcriptional activity. First, the mouse model used for these studies relies on X-gal staining of active β-gal to identify regions of active MMP-9 transcription. Because the half-life of the β-gal protein is very short and the half-life of MMP-9 protein remains unknown, it is possible that β-gal staining preceded the increase in MMP-9, but was missed due to the timing of tissue processing. Second, as both neutrophils and macrophages are known to contain preformed stores of pro-MMP-9, it is possible that release of intracellular stores of MMP-9 could occur in the absence of active MMP-9 transcription. Thus, local MMP-9 levels would be elevated as the result of macrophage/neutrophil degranulation. Third, the increased MMP-9 protein content in the absence of new transcription could be the result of an increase in MMP-9 mRNA stability. A study by Liu et al. previously demonstrated that the use of doxycycline, a broad spectrum MMP inhibitor, reduced MMP-2 production and abundance by a mechanism that resulted in decreased MMP-2 mRNA stability.30 In contrast, recent evidence by Huwiler et al. demonstrated that MMP-9 expression was amplified by extracellular ATP in response to IL-1β by a mechanism involving increased MMP-9 mRNA stability.31 Likewise, Iyer and coworkers demonstrated that MMP-9 mRNA stability was enhanced in response to activation of the mitogen-activated protein kinase pathway and that the α3β1 integrin was required post-transcriptionally to maintain mRNA levels.32 The net outcome of increased steady-state mRNA levels in both cases was a net increase in MMP-9 translation in the absence of new MMP-9 transcription. Lastly, the peri-adventitial adipose layer may function as a storehouse for latent MMPs capable of diffusing into the aortic media. Bouloumié et al. established that MMP-2 and MMP-9 could be produced in adipocytes and that expression of each was enhanced during adipocyte differentiation.33 Interestingly, at all time points post-TAA induction diffuse β-gal staining was observed within the adventitial adipose tissue, suggesting that the adipose layer may function as a source for MMP-9 during aneurysm formation. Additional studies are required to address these possibilities.
Together these data bring into question the hypothesis that infiltrating inflammatory cells are the critical source of MMP-9 in early dilatation events during TAA development. These data clearly show that endogenous fibroblasts and possibly smooth muscle cells at least contribute to MMP-9 production during TAA formation and suggest that further study of these specific cell types may allow the identification of specific signaling pathways that stimulate MMP-9 production. Previous studies have identified that degraded elastin fragments are chemotactic for macrophages and neutrophils.34 Thus, it is conceivable that early signaling events stimulate MMP production in native cells within the aorta, and as a result of early remodeling events (ie. elastin degradation), an inflammatory response ensues. The role of resident/circulating macrophages and neutrophils cannot, however, be discounted. Previous evidence in murine models of AAA demonstrate a requisite role for macrophage-derived MMP-9 in aneurysm formation.15 Additionally, recently published data from our laboratory has identified neutrophil collagenase (MMP-8) and macrophage metalloelastase (MMP-12) in homogenates of murine TAAs at early time points following TAA induction.35 Hence, a strong case exists suggesting that inflammatory infiltrate may contribute to aneurysm formation in the thoracic aorta, as well in the abdomen. Interestingly, the possibility that adipocyte-derived MMP-9 may contribute to TAA formation is intriguing. Additional studies will be required to address these questions more specifically.
Because this study focused on in situ methodology for identification and localization of MMP-9 transcriptional activity and protein abundance, this study is not without limitations. First, digital planimetry was used to quantitate the histochemical and immunological staining of aortic tissue sections. It is recognized that this methodology is only semi-quantitative hence care must be taken to not over interpret the ability to quantitate MMP-9 in these tissues. All the same, the strength of this technique is the ability to localize transcriptional activity and protein content. Second, the antibody used in this study recognized both pro and active forms of MMP-9, thus care must be taken to not directly equate MMP-9 protein abundance with MMP-9 activity. Subsequent studies are warranted to directly assess the spatiotemporal localization of MMP proteolytic activity with the aortic wall during aneurysm formation. Moreover, the use of MMP-reporter mice combined with the use of MMP inhibitors, like doxycycline, could substantially advance our understanding of how MMP inhibitors mechanistically affect aortic dilatation during aneurysm development. Third, it must also be noted that antibodies that recognize αSMA cannot differentiate between smooth muscle cells and myofibroblasts; hence αSMA positive cells may be comprised of both cell types.25 Fourth, the present study revealed the spatiotemporal localization of MMP-9 transcriptional activity and abundance within the aortic wall during the early dilatation events occurring with TAA formation. In this study, a significant gender interaction with respect to aortic dilatation could not be demonstrated, thus all data were pooled prior to analysis. Whether gender dependent differences in aortic dilatation exist and could be associated with different patterns of MMP-9 transcriptional activity or abundance remains to be established. Last, although this murine model of TAA recapitulates many of the hallmarks of human aneurysmal disease, care should be taken in the extrapolation of these results to human TAAs.
In spite of study limitations, the data clearly demonstrate time-dependent changes in the localization of MMP-9 transcriptional activity and protein abundance early in the course of aortic dilatation during TAA formation. Furthermore, these results demonstrate, for the first time, that MMP-9 is produced by smooth muscle cells and/or fibroblasts resident within the aortic vascular wall. Thus, early aortic dilatation may be driven by activation of the endogenous cells to produce MMP-9 which may provide a means for subsequent inflammatory cells to infiltrate and invade the adventitial and medial layers leading to greater aortic expansion and aneurysm formation.
Footnotes
This work was supported by NIH/NHLBI R01 Grants HL 075488-01
References
- 1.Chiesa R, Melissano G, Civilini E, de Moura ML, Carozzo A, Zangrillo A. Ten years experience of thoracic and thoracoabdominal aortic aneurysm surgical repair: lessons learned. Ann Vasc Surg. 2004 Sep;18(5):514–520. doi: 10.1007/s10016-004-0072-z. [DOI] [PubMed] [Google Scholar]
- 2.Fleck TM, Koinig H, Czerny M, Hutschala D, Wolner E, Ehrlich M, Grabenwoger M. Impact of surgical era on outcomes of patients undergoing elective atherosclerotic ascending aortic aneurysm operations. Eur J Cardiothorac Surg. 2004 Aug;26(2):342–347. doi: 10.1016/j.ejcts.2004.04.025. [DOI] [PubMed] [Google Scholar]
- 3.Ghansah JN, Murphy JT. Complications of major aortic and lower extremity vascular surgery. Semin Cardiothorac Vasc Anesth. 2004 Dec;8(4):335–361. doi: 10.1177/108925320400800406. [DOI] [PubMed] [Google Scholar]
- 4.Kawaharada N, Morishita K, Fukada J, Hachiro Y, Fujisawa Y, Saito T, Kurimoto Y, Abe T. Stroke in surgery of the arteriosclerotic descending thoracic aortic aneurysms: influence of cross-clamping technique of the aorta. Eur J Cardiothorac Surg. 2005 Apr;27(4):622–625. doi: 10.1016/j.ejcts.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Alexander JJ. The pathobiology of aortic aneurysms. J Surg Res. 2004 Mar;117(1):163–175. doi: 10.1016/j.jss.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Curci JA, Liao S, Huffman MD, Shapiro SD, Thompson RW. Expression and localization of macrophage elastase (matrix metalloproteinase-12) in abdominal aortic aneurysms. J Clin Invest. 1998 Dec 1;102(11):1900–1910. doi: 10.1172/JCI2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002 Feb 22;90(3):251–262. [PubMed] [Google Scholar]
- 8.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005 Feb 15;111(6):816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 9.Longo GM, Buda SJ, Fiotta N, Xiong W, Griener T, Shapiro S, Baxter BT. MMP-12 has a role in abdominal aortic aneurysms in mice. Surgery. 2005 Apr;137(4):457–462. doi: 10.1016/j.surg.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Mao D, Lee JK, VanVickle SJ, Thompson RW. Expression of collagenase-3 (MMP-13) in human abdominal aortic aneurysms and vascular smooth muscle cells in culture. Biochem Biophys Res Commun. 1999 Aug 11;261(3):904–910. doi: 10.1006/bbrc.1999.1142. [DOI] [PubMed] [Google Scholar]
- 11.Tamarina NA, McMillan WD, Shively VP, Pearce WH. Expression of matrix metalloproteinases and their inhibitors in aneurysms and normal aorta. Surgery. 1997 Aug;122(2):264–271. doi: 10.1016/s0039-6060(97)90017-9. discussion 271–262. [DOI] [PubMed] [Google Scholar]
- 12.Thompson RW, Holmes DR, Mertens RA, Liao S, Botney MD, Mecham RP, Welgus HG, Parks WC. Production and localization of 92-kilodalton gelatinase in abdominal aortic aneurysms. An elastolytic metalloproteinase expressed by aneurysm-infiltrating macrophages. J Clin Invest. 1995 Jul;96(1):318–326. doi: 10.1172/JCI118037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamashita A, Noma T, Nakazawa A, Saito S, Fujioka K, Zempo N, Esato K. Enhanced expression of matrix metalloproteinase-9 in abdominal aortic aneurysms. World J Surg. 2001 Mar;25(3):259–265. doi: 10.1007/s002680020062. [DOI] [PubMed] [Google Scholar]
- 14.Pyo R, Lee JK, Shipley JM, Curci JA, Mao D, Ziporin SJ, Ennis TL, Shapiro SD, Senior RM, Thompson RW. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000 Jun;105(11):1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002 Sep;110(5):625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikonomidis JS, Gibson WC, Butler JE, McClister DM, Sweterlitsch SE, Thompson RP, Mukherjee R, Spinale FG. Effects of deletion of the tissue inhibitor of matrix metalloproteinases-1 gene on the progression of murine thoracic aortic aneurysms. Circulation. 2004 Sep 14;110(11 Suppl 1):II268–273. doi: 10.1161/01.CIR.0000138384.68947.20. [DOI] [PubMed] [Google Scholar]
- 17.Ikonomidis JS, Barbour JR, Amani Z, Stroud RE, Herron AR, McClister DM, Jr, Camens SE, Lindsey ML, Mukherjee R, Spinale FG. Effects of deletion of the matrix metalloproteinase 9 gene on development of murine thoracic aortic aneurysms. Circulation. 2005 Aug 30;112(9 Suppl):I242–248. doi: 10.1161/CIRCULATIONAHA.104.526152. [DOI] [PubMed] [Google Scholar]
- 18.Mohan R, Rinehart WB, Bargagna-Mohan P, Fini ME. Gelatinase B/lacZ transgenic mice, a model for mapping gelatinase B expression during developmental and injury-related tissue remodeling. J Biol Chem. 1998 Oct 2;273(40):25903–25914. doi: 10.1074/jbc.273.40.25903. [DOI] [PubMed] [Google Scholar]
- 19.Ikonomidis JS, Gibson WC, Gardner J, Sweterlitsch S, Thompson RP, Mukherjee R, Spinale FG. A murine model of thoracic aortic aneurysms. J Surg Res. 2003 Nov;115(1):157–163. doi: 10.1016/s0022-4804(03)00193-8. [DOI] [PubMed] [Google Scholar]
- 20.Thompson RW, Geraghty PJ, Lee JK. Abdominal aortic aneurysms: basic mechanisms and clinical implications. Curr Probl Surg. 2002 Feb;39(2):110–230. doi: 10.1067/msg.2002.121421. [DOI] [PubMed] [Google Scholar]
- 21.Sinha I, Bethi S, Cronin P, Williams DM, Roelofs K, Ailawadi G, Henke PK, Eagleton MJ, Deeb GM, Patel HJ, Berguer R, Stanley JC, Upchurch GR., Jr A biologic basis for asymmetric growth in descending thoracic aortic aneurysms: a role for matrix metalloproteinase 9 and 2. J Vasc Surg. 2006 Feb;43(2):342–348. doi: 10.1016/j.jvs.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Basalyga DM, Simionescu DT, Xiong W, Baxter BT, Starcher BC, Vyavahare NR. Elastin degradation and calcification in an abdominal aorta injury model: role of matrix metalloproteinases. Circulation. 2004 Nov 30;110(22):3480–3487. doi: 10.1161/01.CIR.0000148367.08413.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobsen KD, Willumsen BM. Kinetics of expression of inducible beta-galactosidase in murine fibroblasts: high initial rate compared to steady-state expression. J Mol Biol. 1995 Sep 22;252(3):289–295. doi: 10.1006/jmbi.1995.0496. [DOI] [PubMed] [Google Scholar]
- 24.Patel MI, Melrose J, Ghosh P, Appleberg M. Increased synthesis of matrix metalloproteinases by aortic smooth muscle cells is implicated in the etiopathogenesis of abdominal aortic aneurysms. J Vasc Surg. 1996 Jul;24(1):82–92. doi: 10.1016/s0741-5214(96)70148-9. [DOI] [PubMed] [Google Scholar]
- 25.Skalli O, Schurch W, Seemayer T, Lagace R, Montandon D, Pittet B, Gabbiani G. Myofibroblasts from diverse pathologic settings are heterogeneous in their content of actin isoforms and intermediate filament proteins. Lab Invest. 1989 Feb;60(2):275–285. [PubMed] [Google Scholar]
- 26.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004 Aug;230(4):787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 27.Springer TA. Monoclonal antibody analysis of complex biological systems. Combination of cell hybridization and immunoadsorbents in a novel cascade procedure and its application to the macrophage cell surface. J Biol Chem. 1981 Apr 25;256(8):3833–3839. [PubMed] [Google Scholar]
- 28.Springer T, Galfre G, Secher DS, Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- 29.Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann Thorac Surg. 2004 Dec;78(6):2106–2110. doi: 10.1016/j.athoracsur.2004.05.088. discussion 2110–2101. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Xiong W, Baca-Regen L, Nagase H, Baxter BT. Mechanism of inhibition of matrix metalloproteinase-2 expression by doxycycline in human aortic smooth muscle cells. J Vasc Surg. 2003 Dec;38(6):1376–1383. doi: 10.1016/s0741-5214(03)01022-x. [DOI] [PubMed] [Google Scholar]
- 31.Huwiler A, Akool el S, Aschrafi A, Hamada FM, Pfeilschifter J, Eberhardt W. ATP potentiates interleukin-1 beta-induced MMP-9 expression in mesangial cells via recruitment of the ELAV protein HuR. J Biol Chem. 2003 Dec 19;278(51):51758–51769. doi: 10.1074/jbc.M305722200. [DOI] [PubMed] [Google Scholar]
- 32.Iyer V, Pumiglia K, DiPersio CM. Alpha3beta1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci. 2005 Mar 15;118(Pt 6):1185–1195. doi: 10.1242/jcs.01708. [DOI] [PubMed] [Google Scholar]
- 33.Bouloumie A, Sengenes C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001 Sep;50(9):2080–2086. doi: 10.2337/diabetes.50.9.2080. [DOI] [PubMed] [Google Scholar]
- 34.Senior RM, Griffin GL, Mecham RP. Chemotactic activity of elastin-derived peptides. J Clin Invest. 1980 Oct;66(4):859–862. doi: 10.1172/JCI109926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbour JR, Stroud RE, Lowry AS, Clark LL, Leone AM, Jones JA, Spinale FG, Ikonomidis JS. Temporal disparity in the induction of matrix metalloproteinases and tissue inhibitors following thoracic aortic aneurysm formation. J Thorac Cardiovasc Surg. 2006 doi: 10.1016/j.jtcvs.2006.05.052. Accepted. [DOI] [PubMed] [Google Scholar]


