Abstract
The existence of sphingolipid- and sterol-enriched microdomains, known as lipid rafts, in the plasma membrane (PM) of eukaryotic cells is well documented. To obtain more insight into the lipid molecular species required for the formation of microdomains in plants, we have isolated detergent (Triton X-100)-resistant membranes (DRMs) from the PM of Arabidopsis (Arabidopsis thaliana) and leek (Allium porrum) seedlings as well as from Arabidopsis cell cultures. Here, we show that all DRM preparations are enriched in sterols, sterylglucosides, and glucosylceramides (GluCer) and depleted in glycerophospholipids. The GluCer of DRMs from leek seedlings contain hydroxypalmitic acid. We investigated the role of sterols in DRM formation along the secretory pathway in leek seedlings. We present evidence for the presence of DRMs in both the PM and the Golgi apparatus but not in the endoplasmic reticulum. In leek seedlings treated with fenpropimorph, a sterol biosynthesis inhibitor, the usual Δ5-sterols are replaced by 9β,19-cyclopropylsterols. In these plants, sterols and hydroxypalmitic acid-containing GluCer do not reach the PM, and most DRMs are recovered from the Golgi apparatus, indicating that Δ5-sterols and GluCer play a crucial role in lipid microdomain formation and delivery to the PM. In addition, DRM formation in Arabidopsis cells is shown to depend on the unsaturation degree of fatty acyl chains as evidenced by the dramatic decrease in the amount of DRMs prepared from the Arabidopsis mutants, fad2 and Fad3+, affected in their fatty acid desaturases.
Despite the ongoing debate on the size, lifespan, and dynamics of lipid microdomains (Munro, 2003; Pike, 2004; Nichols, 2005; Hancock, 2006), the existence of sterol- and sphingolipid-enriched membrane microdomains in the plasma membrane (PM) of eukaryotic cells is now well recognized (Simons and Ikonen, 1997; Brown and London, 2000; Simons and Toomre, 2000; Bagnat and Simons, 2002; Simons and Vaz, 2004; Hancock, 2006). Evidence for microdomains comes in part from examination of membrane constituents that are resistant to solubilization by nonionic detergents at low temperature, giving rise to the concept of lipid rafts (Simons and Ikonen, 1997; Brown and London, 1998; Röper et al., 2000; Hancock, 2006). Such sterol- and sphingolipid-enriched microdomains can be isolated as detergent-resistant membranes (DRMs). In the following, the terms of DRMs and microdomains will be assigned to respectively indicate the isolated entities and the corresponding membrane entities. Lipid properties of microdomains are similar to liquid-ordered domains, which are characterized by tightly packed hydrocarbon tails but with a high degree of lateral mobility (Brown and London, 2000; Simons and Vaz, 2004). Cholesterol is thought to contribute to the tight packing of lipids in liquid-ordered domains by filling interstitial spaces between lipid molecules (Brown, 1998). Thus, a new model for the organization of the PM of animal cells has emerged, in which cholesterol and sphingolipid-rich microdomains (rafts) coexist with more fluid domains containing phospholipids with unsaturated hydrocarbon chains (Simons and Ikonen, 1997). It has been suggested that these microdomains, to which specific classes of proteins would be associated, play a role in a wide range of biological processes, including cell polarity, protein trafficking, and signal transduction (Simons and Ikonen, 1997; Brown and London, 1998; Keller and Simons, 1998; Simons and Toomre, 2000; Simons and Vaz, 2004; Hancock, 2006). Similar microdomains in the PM of yeast (Saccharomyces cerevisiae) cells, enriched in ergosterol, have also been reported (Bagnat et al., 2000, 2001). Recent reports clearly indicate that microdomains enriched in sterols and sphingolipids also exist in the PM of plant cells (Peskan et al., 2000; Mongrand et al., 2004; Bhat and Panstruga, 2005; Borner et al., 2005). However, the much greater structural diversity of plant sterols and sphingolipids, compared to their animal and fungal counterparts (Hartmann, 1998; Sperling and Heinz, 2003), suggests that plant DRMs might exhibit some specific structural features. In particular, plant DRMs have been shown to contain several sterol molecules, as represented by 24-methylcholesterol, sitosterol, and stigmasterol, instead of one major sterol (cholesterol or ergosterol) and two distinct classes of sphingolipids, inositolphosphorylceramides and glucosylceramides (GluCer; Mongrand et al., 2004; Markham et al., 2006).
To obtain more insight into the lipid molecular species required for the formation of microdomains in plant cells, we isolated and characterized DRMs from different plant materials.
The first objective of this article was to investigate the distribution of microdomains along the secretory pathway of plant cells and the involvement of sterols in this process. In addition to their role at the cell surface, lipid microdomains might indeed govern protein and lipid sorting and trafficking toward the PM and other membrane compartments. In that context, the occurrence of lipid microdomains in the Golgi apparatus (GA; Hansen et al., 2000) as well as in the endoplasmic reticulum (ER; Bagnat and Simons, 2002) has been reported in animal and yeast cells, but the intracellular distribution of such microdomains in plant cells remains an unsolved question. Plant sterols are synthesized in the ER membranes (Hartmann and Benveniste, 1987) and are transported from the ER to the PM via a membrane-mediated process, which likely involves the GA (Moreau et al., 1998b). This transport is blocked by low temperatures and compounds such as monensin or brefeldin A, as also reported for different phospholipid species, especially phosphatidyl-Ser (Moreau et al., 1998a, 1998b; Sturbois-Balcerzak et al., 1999; Vincent et al., 1999).
We have determined the intracellular distribution of lipid microdomains in leek (Allium porrum) seedlings by preparing DRMs from ER-, GA-, and PM-enriched fractions and probed the involvement of sterols in microdomain formation by treatment with fenpropimorph (FEN), a sterol biosynthesis inhibitor that inhibits the cycloeucalenol-obtusifoliol isomerase (Rahier et al., 1986) and induces an accumulation of 9β,19-cyclopropylsterols in place of the usual Δ5-sterols (Hartmann et al., 2002). We have shown that most of the DRMs normally originate from the PM but that they are more abundant in the GA instead of the PM in FEN-treated plants.
The second objective of this article was to check the impact of the unsaturation degree of phospholipid fatty acyl chains on the segregation of lipids into DRMs. For this purpose, we took advantage of two Arabidopsis (Arabidopsis thaliana) cultured cell lines, fad2 and Fad3+, which are affected in fatty acid desaturases. Our results indicate that the amount of DRMs recovered from Arabidopsis cells is closely dependent on the unsaturation degree of phospholipid fatty acyl chains.
RESULTS
Characterization of DRMs from the PM of Leek and Arabidopsis Seedlings
PM, ER, and GA fractions from leek seedlings were purified and characterized as previously published (Moreau et al., 1998b; Vincent et al., 1999). Table I shows the enrichment of several marker enzyme activities in these purified fractions. The PM from Arabidopsis seedlings was purified by using a two-phase polyethylene glycol (PEG)-dextran partitioning system as explained in “Materials and Methods.” We found that 85% of the H+-ATPase activity, pH 6.5, of this membrane fraction was vanadate sensitive, confirming its enrichment in PM vesicles.
Table I.
Enrichment factors for several marker enzyme activities in the ER, GA, and PM fractions isolated from etiolated leek seedlings
Enzyme activities were measured and expressed as established in Sturbois-Balcerzak et al. (1999) and Vincent et al. (1999). The enrichment factors correspond to the ratios of the activities determined in the membrane fractions to that of the microsomal fraction from which the different membranes were purified. The percentage of inhibition of the K+-stimulated Mg2+ATPase activity in the PM by vanadate varied from 87% to 95%. Data are the mean values ± sd from three to five independent measurements.
| Marker Enzymes | Enrichment Factors
|
||
|---|---|---|---|
| ER | GA | PM | |
| NADPH cytochrome c reductase | 5.4 ± 1.4 | 0.46 ± 0.16 | 0.11 ± 0.04 |
| CDP-choline phosphotransferase | 4.2 ± 0.7 | 0.16 ± 0.07 | 0.07 ± 0.03 |
| Glucuronyl-transferase | 0.42 ± 0.13 | 3.8 ± 1.10 | 0.45 ± 0.13 |
| Glucane synthetase II | 0.03 ± 0.01 | 0.16 ± 0.07 | 5.5 ± 1.7 |
| K+-stimulated Mg2+ATPase | 0.02 ± 0.01 | 0.06 ± 0.05 | 16.8 ± 2.9 |
To determine the best conditions to isolate DRMs from the membranes of leek and Arabidopsis seedlings, we first tested different Triton X-100-to-protein ratios varying from 2:16, at 1% Triton final concentration. We obtained the maximum yields and the more constant lipid composition of DRMs at a detergent to protein ratio of 8, and this ratio was used in the following experiments.
Under these conditions, the amount of DRMs recovered from the PM of leek seedlings was about 10% to 15% of the PM on a protein basis, whereas DRMs from the PM of Arabidopsis seedlings represented only 2% to 3%, indicating that the PM arising from different plants may not contain similar proportions of microdomains, at least according to the present method of isolation.
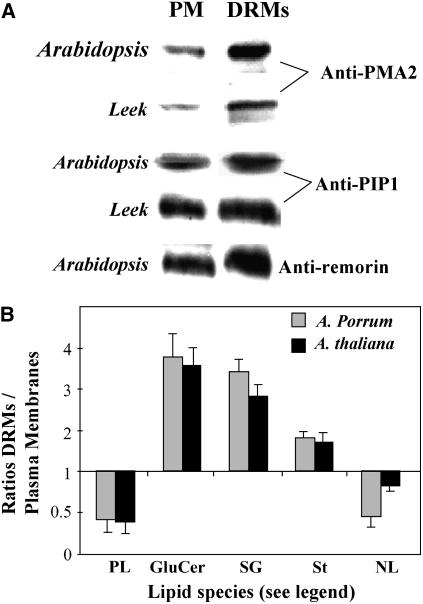
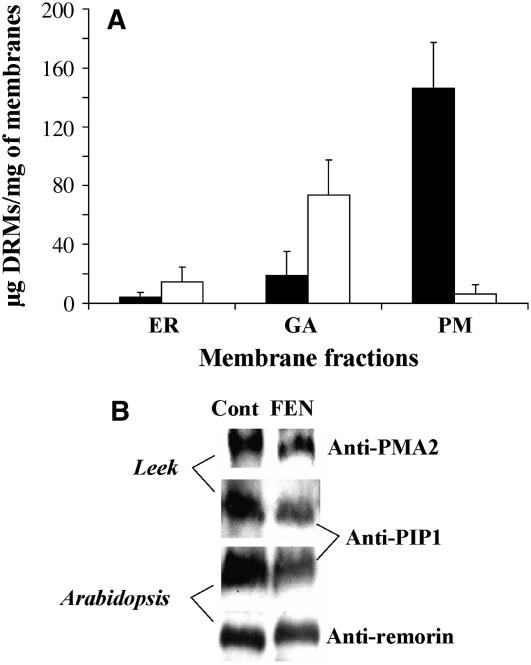
To investigate the protein content of PM DRMs, we used antibodies raised against three putative protein markers of DRMs (Mongrand et al., 2004; Borner et al., 2005): the H+-ATPase PMA2 (Maudoux et al., 2000; Lefebvre et al., 2004), the aquaporin PIP1 (Santoni et al., 2003; Boursiac et al., 2005), and the remorin (Bariola et al., 2004). Figure 1A shows the enrichment of these markers in PM DRMs compared to the PM. An increase in the amount of the three proteins was observed in DRMs isolated from the PM of Arabidopsis seedlings. In DRMs prepared from the PM of leek seedlings, the amounts of PMA2 and PIP1 were also increased, but no band corresponding to remorin could be detected. In both cases, all three proteins were not detected from the corresponding solubilized fractions.
Figure 1.
Characterization of DRMs prepared from the PM of leek and Arabidopsis seedlings. A, The DRM marker proteins PMA2, PIP1, and remorin (Mongrand et al., 2004; Borner et al., 2005) were enriched in the DRM fractions as compared to the PM fractions from which they were isolated. The antibodies raised against the ATPase PMA2 (Maudoux et al., 2000) may recognize PMA4 as well (Lefebvre et al., 2004). The anti-PIP1 antibody may recognize both the PIP1 and PIP2 families (Santoni et al., 2003). Equal amounts of proteins were loaded in each lane. B, Lipid enrichment of DRMs isolated from either the PM of leek seedlings or the PM of Arabidopsis seedlings. The results are expressed as the ratios of the amount (micrograms per milligram protein) of lipids in DRMs to that in the PM and represent the means of three to six analyses ± sd. PL, Total phospholipids; GluCer, glucosylceramides; SG, sterylglucosides; St, free sterols; NL, other neutral lipids (mainly diacylglycerols and free fatty acids).
These DRM preparations were also analyzed for their lipid content. As shown in Figure 1B, DRMs isolated from the PM of leek and Arabidopsis seedlings were clearly enriched in sterols, sterylglucosides (SG), and GluCer and depleted of phospholipids and neutral lipids such as diacylglycerol and free fatty acids. Inositolphosphorylceramides species (Markham et al., 2006) were not determined, as previous reports have already demonstrated that DRMs from Arabidopsis membranes are enriched in these lipids (Borner et al., 2005).
In PM DRMs recovered from leek seedlings, sterols constituted 71% of neutral lipids, compared to 47% in the PM, thus representing 35% of total lipids. The sterol composition of leek membranes and of corresponding DRMs is given in Table II. In microsomes (including the PM), sitosterol was by far the major sterol (63%). The other sterols were represented by 24-methylcholesterol (11%), cholesterol (19%), and stigmasterol (6%). Consistent with previous reports (Mongrand et al., 2004; Borner et al., 2005), the same sterols were found in the corresponding DRMs in similar relative abundances, indicating that all sterol molecules might contribute to generate plant microdomains with the same efficiency.
Table II.
Free sterol composition of microsomal membranes and PM DRMs of leek seedlings grown in the absence or presence of 2 mg/L FEN
Data (percentages of total sterols) are means of two independent experiments.
| Sterols | Control
|
FEN
|
||
|---|---|---|---|---|
| Microsomal Fraction | DRM | Microsomal Fraction | DRM | |
| Cholesterol | 19.2 | 13.6 | 4.6 | 20.4 |
| 24-Methyl cholesterol | 11.4 | 11.3 | 1.9 | 1.9 |
| Stigmasterol | 6.1 | 6.2 | 3.0 | 2.5 |
| Sitosterol | 63.3 | 68.9 | 18.0 | 22.3 |
| Pollinastanol | 0 | 0 | 50.7 | 36.6 |
| 24-Methylene pollinastanol | 0 | 0 | 7.7 | 3.9 |
| 24-Methyl pollinastanol | 0 | 0 | 14.1 | 12.4 |
A new observation was the occurrence of SG in DRM preparations from both the PM of leek and Arabidopsis seedlings. SG amounted to about 7% of total lipids in DRMs isolated from leek seedlings compared to less than 2% in the PM. In DRMs from Arabidopsis seedlings, SG constituted up to 15% to 20% of the total lipids and was even more abundant than GluCer.
Characterization of DRMs from the GA of Leek Seedlings and DRM Distribution through the Secretory Pathway
To determine where the formation of microdomains could take place along the secretory pathway of plant cells, we took advantage of the model of leek seedlings, which allow the isolation of ER- and GA-enriched fractions (Sturbois-Balcerzak et al., 1999; Vincent et al., 1999). ER and GA membranes were treated with a Triton X-100 (1% final concentration)-to-protein ratio of 8. DRMs were isolated from the GA fraction (about 20 μg protein/mg GA protein), although the yield was not as high as from the PM fraction (about 100–150 μg protein/mg PM protein). Only very few or no DRMs could be recovered from ER membranes (4 μg protein/mg ER protein).
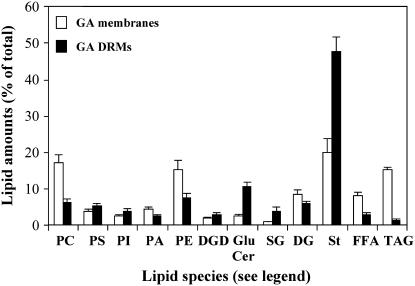
GA and GA DRMs were analyzed for their respective lipid composition (Fig. 2). GA DRMs were found to contain the same lipids as PM DRMs. GA DRMs were enriched in free sterols, which represented 48% of total lipids compared to 20% in GA membranes. They were also enriched in GluCer (11% of total lipids compared to only 2% in GA membranes) and in SG (Fig. 2). In contrast, the levels of phospholipids, especially phosphatidylcholine (PC) and phosphatidylethanolamine (PE), and other lipids (free fatty acids, diacylglycerols, and triacylglycerols) were reduced compared to those of GA membranes.
Figure 2.
Lipid composition of DRMs isolated from the GA (GA-DRMs) of leek seedlings. The results are expressed as percent of total lipids and represent the means of four analyses ± sd. Lipid abbreviations are as in the legend of Figure 1 and as follows: PC, phosphatidylcholine; PS, phosphatidyl-Ser; PI, phosphatidylinositol; PA, phosphatidic acid; PE, phosphatidylethanolamine; DGD, digalactosyl-diacylglycerol; GluCer, glucosylceramides; SG, sterylglucosides; DG, diacylglycerol; St, free sterols; FFA, free fatty acids; TAG, triacylglycerols.
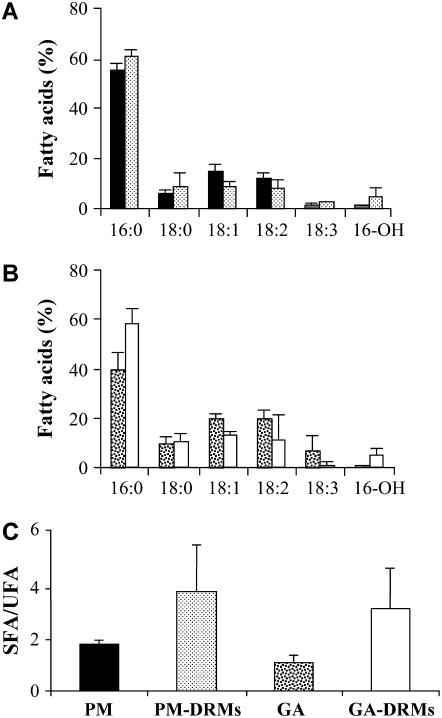
We also analyzed the fatty acid composition of lipids in GA DRMs and PM DRMs. The glycerolipids of DRMs contained preferentially saturated palmitic acid compared to the glycerolipids of the PM and GA membranes (Fig. 3, A–C). Gas chromatography (GC) analyses ran on the individual lipid species clearly indicated that 16-OH fatty acids exclusively arose from GluCer.
Figure 3.
Fatty acid composition of DRMs isolated from the PM (PM DRMs) and GA (GA DRMs) of leek seedlings. A, Values for PM (black) and PM DRMs (small dots) represent the means ± sd of four and seven replicates, respectively. B, Values for GA (black dots) and GA DRMs (white) represent the means ± sd of three replicates. C, Saturation degree of fatty acyl chains present in PM, PM DRMs, GA, and GA DRMs. According to the Student's law, P values were, respectively, <0.05 for PM and PM DRMs and <0.10 for GA and GA DRMs. SFA, Saturated fatty acids; UFA, unsaturated fatty acids.
Thus, our results demonstrate that DRMs can be isolated from GA membranes with a lipid composition similar to that of PM DRMs, suggesting that microdomain formation starts at the level of the GA membranes in the plant secretory pathway.
Involvement of Sterols in the Intracellular Distribution and Lipid Composition of DRMs
To further investigate the role of sterols in DRM formation along the secretory pathway, we deregulated the sterol biosynthesis in leek and Arabidopsis seedlings. Seedlings from both leeks and Arabidopsis were grown in the presence of 2 mg/L of FEN, a fungicide that inhibits the sterol pathway at the level of the cycloeucalenol-obtusifoliol-isomerase. In leek seedlings, such a treatment was found to trigger an almost complete replacement of the usual sterols (Δ5-sterols) by 9β,19-cyclopropylsterols (Table II; Hartmann et al., 2002). In addition, significant changes in the morphology of the GA in treated plants, consisting in highly fenestrated cisternae, were observed (Hartmann et al., 2002). After treatment of Arabidopsis seedlings with FEN, a change in the sterol profile was also observed, as evidenced by the large increase (from 0.17 ± 0.01 to 1.3 ± 0.3) in the ratio of sterol precursors to Δ5-sterols, as determined on high-performance thin-layer chromatography (HPTLC) plates.
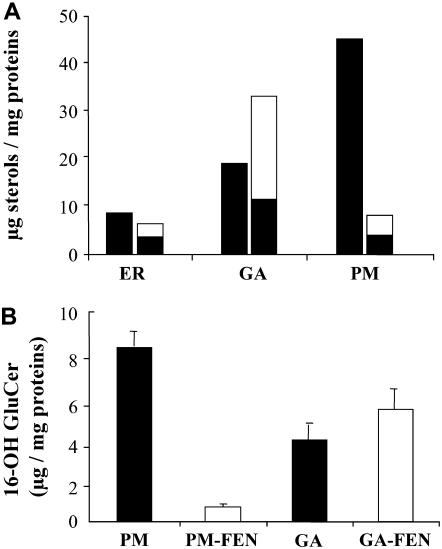
In leek treated with FEN, the modification of the sterol profile consisted of a significant decrease in the amount of Δ5-sterols in all the membranes of the secretory pathway, particularly the PM. At the same time, the remaining Δ5-sterols and new 9β,19-cyclopropylsterols were found to greatly accumulate in the GA membranes (Fig. 4A). To investigate whether the normal distribution of specific GluCer species through the secretory pathway was also disrupted, we analyzed by GC the fatty acid content of GluCer in the membrane fractions isolated from FEN-treated and untreated plants. Although the various fatty acid methyl esters were identified by comparison with standards, we further characterized GluCer species by mass spectrometry. Lithium iodide was added to GluCer at a 5 mm final concentration prior to infusion in the electrospray source to observe only (M + Li)+ ions. We identified three compounds, detected at mass-to-charge ratio (m/z) 703.7, m/z 720.5, and m/z 736.5, as GluCer made from ceramide with 4,8-sphingadiene (d18:2) and either palmitic acid, α-hydroxypalmitic acid (16-OH GluCer), or dihydroxypalmitic acid (Sullards et al., 2000). The GluCer species at m/z 736.5 could not be identified on GC analyses without an available standard. For structural identification of ceramides, mass measurements were associated to MS2 and MS3 fragment ions (see Supplemental Table S1). As in tobacco (Nicotiana tabacum; Mongrand et al., 2004) and soybean (Glycine max; Sullards et al., 2000), the major GluCer species contained 16-OH GluCer and represented at least 70% of total GluCer. In addition to its effect on sterol distribution, GC analyses of GluCer from PM and GA membranes revealed that FEN treatment also led to a high and specific decrease of 16-OH GluCer in the PM of leek seedlings, whereas these lipid species were still present and increased in the GA membranes (Fig. 4B).
Figure 4.
Effect of FEN (2 mg/L) on sterol and 16-OH GluCer distribution in the membranes of the secretory pathway of leek seedlings. A, Δ5-Sterols in control and FEN-treated seedlings are shown in black. Cyclopropysterols in FEN-treated seedlings are shown in white. For each membrane compartment, the first column corresponds to the control seedlings and the second one to the FEN-treated seedlings. Values in the figure represent one of two representative experiments. B, Amounts of 16-OH GluCer isolated from PM and GA from FEN-treated or untreated plants. Values represent means ± sd of five replicates for PM and PM-FEN and three for GA and GA-FEN. According to the Student's law, the P value for GA and GA-FEN was <0.10.
Therefore, FEN treatment induced both an accumulation of sterols and 16-OH GluCer in the GA membranes. Because sterols and GluCer (and especially 16-OH containing GluCer) can be considered as critical lipid species required for the formation of lipid microdomains (Mongrand et al., 2004), we determined the effect of FEN treatment on the distribution of DRMs in the secretory pathway (Fig. 5A). In agreement with the sterol and GluCer analyses, we observed a shift of the DRMs from the PM to the GA membranes in FEN-treated leek seedlings.
Figure 5.
Effect of FEN (2 mg/L) on the distribution of DRMs in the membranes of the secretory pathway of leek seedlings. A, Control and FEN-treated leek seedlings are shown in black and white, respectively. Values in the figure represent means ± sd of three replicates. According to the Student's law, the P value for GA (control) and GA (FEN) was <0.05. B, Effect of FEN on the amount of DRM markers present in the PM. PM was isolated from either FEN-treated or untreated plants, and the content of different markers was estimated in each preparation by western blots with equal amounts of proteins loaded in each lane. Cont, Control.
In untreated plants, 86.4% of total purified DRMs were isolated from the PM (145 μg protein/mg PM protein). In contrast, in FEN-treated plants, 87.2% of the total amount of purified DRMs was recovered from the GA membranes (73.4 ± 23.9 μg protein/mg GA protein). It must be pointed out that these results cannot be attributed to differences of recoveries between the various membrane fractions. The corresponding recoveries were as follows: for ER, control: 26.3 ± 11.7 μg protein/g seedlings and FEN: 28.1 ± 1.8 μg protein/g seedlings; for GA, control: 77.4 ± 2.2 μg protein/g seedlings and FEN: 54.2 ± 6.4 μg protein/g seedlings; for PM, control: 47.3 ± 7.9 μg protein/g seedlings and FEN: 39.7 ± 7.2 μg protein/g seedlings. Thus, on a protein basis, the PM fraction represented 31.3% and 32.5% of the sum of the three membrane fractions in control and treated plants, respectively. In addition, no increase in the recovery of GA membranes was observed in FEN-treated plants (44.5% versus 51.2% in control plants). Therefore, our results clearly demonstrate that inhibition of sterol biosynthesis by FEN induces a preferential formation of DRMs in the GA and that these DRMs are not delivered to the PM with the same efficiency.
Such a finding is supported by the results presented in Figure 5B, which shows respective recoveries of DRM protein markers in the PM fractions from control and FEN-treated leek and Arabidopsis seedlings, as detected by their respective antibodies. Although the amount of remorin in the PM from Arabidopsis seedlings was less affected by FEN treatment than expected, the levels of the other marker proteins were clearly decreased after FEN treatment.
Impact of the Fatty Acid Unsaturation Degree on Lipid Microdomain Formation in Arabidopsis fad2 and Fad3+ Mutants
For this study, we took advantage of two Arabidopsis cultured cell lines, fad2 and Fad3+, which are affected in fatty acid desaturases. Because of their deficit in 18:1 desaturase (Okuley et al., 1994), fad2 cells accumulate fatty acyl chains enriched in 18:1 (Caiveau et al., 2001), whereas the Fad3+ mutant accumulates 18:3 fatty acids (Shah et al., 1997).
DRMs were isolated directly from suspension cultures of Arabidopsis cells. In this case, we applied a methodology already used with success with animal cell cultures (Legembre et al., 2002, 2005; see “Materials and Methods” for more details). Using this technique, DRMs can be isolated either directly from broken cells or from microsomal membranes. We first prepared microsomes from Arabidopsis cell cultures before treatment with the detergent, and DRMs were recovered by centrifugation on Suc gradients as described in “Materials and Methods” section. DRMs recovered in this manner represented 13.5 μg protein ± 2.6/mg microsomal membrane proteins.
The lipid profile of DRMs isolated from microsomes of Arabidopsis cells was found to be very similar to that of DRMs isolated from the PM of Arabidopsis seedlings. The results in the wt column of Figure 6A correspond, therefore, to the mean values determined from both analyses run separately.
Figure 6.
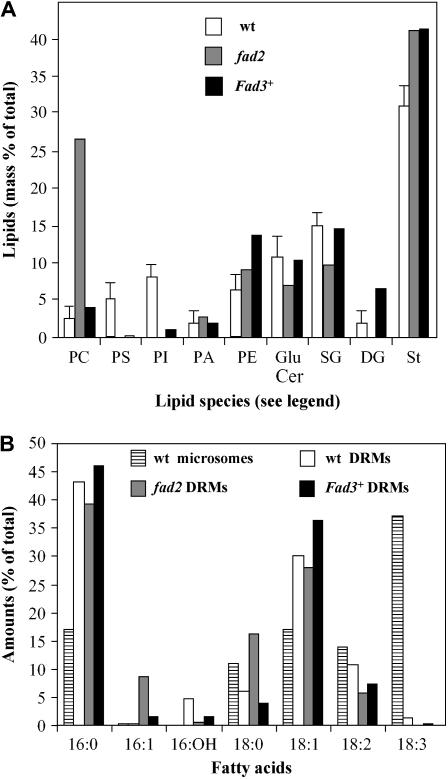
Lipid and fatty acid composition of DRMs from wild-type Arabidopsis seedlings, wild-type Arabidopsis cells, and fad2 and Fad3+ mutant cells. A, Wild-type Arabidopsis seedlings and cells were analyzed independently. Their lipid profiles being similar, the data were combined in the wild-type column. The membranes of fad2 and Fad3+Arabidopsis mutant cells are shown as compared to the combined controls. Because very low amounts of DRMs were recovered from the mutant cell lines, we grouped three to four DRM fractions from different experiments to perform lipid analyses. The results are expressed as percent of total lipids (means of two analyses corresponding to 6–8 experiments). Lipid abbreviations are as in the legends of Figures 1 and 2. B, Total fatty acid composition of DRMs isolated from microsomes of Arabidopsis wild-type and fad2 and Fad3+ mutants. The results are expressed as percent of total fatty acids (as in A, means correspond to two analyses from 6–8 grouped experiments).
Using the same methodology as for the wild-type cells, we isolated DRMs from Arabidopsis fad2 and Fad3+ cells, two mutants characterized, respectively, by the accumulation of 18:1 and 18:3 fatty acids. In both cases, the amount of DRMs recovered dramatically decreased and represented only 3.3 μg protein ± 0.6 and 2.1 μg protein ± 0.3/mg microsomal membrane proteins for fad2 and Fad3+ cells, respectively (i.e. about 20% of the amount of DRMs recovered from Arabidopsis wild-type cells).
The lipid composition of DRMs isolated from both mutant cell lines was compared to that of DRMs isolated from wild-type cells (Fig. 6A). DRMs from fad2 and Fad3+ cells were highly enriched in GluCer, SG, and free sterols, indicating that these DRMs likely represented lipid microdomains. However, it should be noted that phosphatidyl-Ser and phosphatidylinositol contents in DRMs from both mutants were strongly reduced, and an unexpected high amount of PC was observed in DRMs isolated from fad2 cells (Fig. 6A).
These results prompted us to investigate in more details the fatty acid composition of the different lipid molecular species of DRMs. Figure 6B shows the total fatty acid composition of microsomal membranes from wild-type cells and of DRMs from wild-type and mutant cell lines. As expected, the major fatty acid found in wild-type microsomal membranes was 18:3, but this fatty acid was barely detectable in the DRMs (Fig. 6B). The 18:3 fatty acids also disappeared from the DRMs isolated from mutant cells. Compared to the wild-type microsomal fraction, we observed a high increase in 16:0 in all DRMs (Fig. 6B) and higher amounts of 16:1 and 18:0 fatty acids in DRMs isolated from fad2 cell membranes (Fig. 6B). Thus, the saturated-to-unsaturated fatty acid ratios of DRM lipids from Arabidopsis wild-type and mutant cells were found to be 1.24 ± 0.07, compared to only 0.43 ± 0.06 for wild-type microsomal membranes.
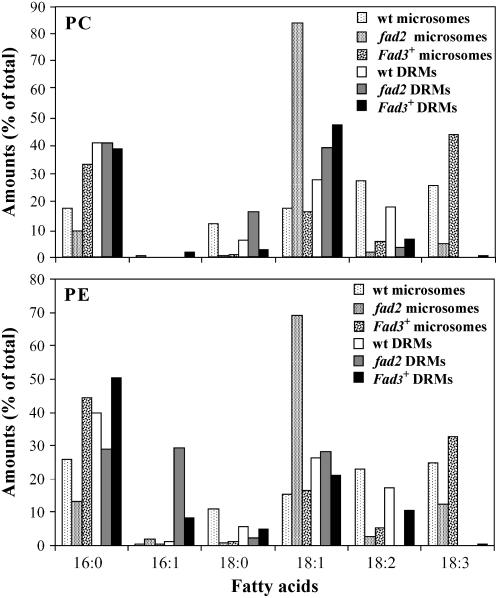
We then compared the fatty acid composition of PC and PE in the microsomes and DRMs recovered from wild-type and mutant cells. An interesting observation was that 16:0 and 18:1 were the two major fatty acids of PC and PE in all DRM preparations, regardless of the initial abundance of these fatty acids in the microsomal fractions from where each DRM fraction was recovered (Fig. 7). This result strongly suggests that the fatty acid composition of these phospholipids governs their recovery into DRMs. In addition, we observed that DRMs from fad2 cells contained a higher amount of PC species with 18:0 and 18:1 and lower amounts of PC with 18:2 compared to wild-type DRMs. The significant increase in 16:1 fatty acids found in the DRMs from fad2 cell membranes (Fig. 6B) was due to the segregation of 16:1-PE into DRMs (Fig. 7). In DRMs from Fad3+ cells, we observed an increase of 18:1-PC, a decrease of 18:0-PC, and an increase of 16:0- and 16:1-PE compared to wild-type DRMs (Fig. 7).
Figure 7.
Fatty acid composition of PC and PE in microsomes and DRMs from fad2 and Fad3+Arabidopsis mutants as compared to microsomes and DRMs from control cells. The results are expressed as percent of total fatty acids as in Figure 6A.
Thus, the important changes in the fatty acid composition of fad2 and Fad3+ membranes (Shah et al., 1997; Caiveau et al., 2001), and therefore their different fluidity and dynamic properties (Vaultier et al., 2006), have an impact not only on the amount of DRMs recovered from the membranes but also on the nature of the lipid species present in these DRMs.
DISCUSSION
Evidence for the existence of lipid microdomains within the PM of higher plant cells has been given only recently (Peskan et al., 2000; Mongrand et al., 2004; Borner et al., 2005). To gain more insight into the role of microdomains in the regulation of the lipid (and protein) composition of the PM in plant cells, we addressed two major questions in this article: (1) What are the lipid species playing a critical role in lipid microdomain organization? and (2) Where are lipid microdomains formed along the secretory pathway?
To investigate where lipid microdomain formation may take place, we isolated DRMs from ER-, GA-, and PM-enriched fractions from leek seedlings. This article clearly demonstrates that DRMs can be isolated from the GA of plant cells, a result in line with the presence of lipid microdomains in the GA of animal cells (Hansen et al., 2000; Gkantiragas et al., 2001) and yeast (Bagnat et al., 2000). However, although the presence of lipid microdomains in the ER of animal cells (Sevlever et al., 1999) and yeast (Bagnat et al., 2000; Lee et al., 2002) has been reported, our attempts to isolate significant amounts of DRMs from the ER of leek cells were unsuccessful. In this context, it should be pointed out that cold detergent insolubility is only one of the criteria to judge the existence of microdomains. In addition, limitations of this approach have been highlighted (Shogomori and Brown, 2003), and different detergents can lead to the isolation of DRMs with different lipid and protein compositions (Schuck et al., 2003). In other words, it is possible that detergent-insoluble membrane domains already exist in the ER but cannot be recovered after cold Triton treatment.
To further examine the involvement of lipid microdomains in membrane trafficking in higher plant cells, our approach has been to manipulate the PM sterol content by using FEN, a sterol biosynthetic inhibitor. This compound is known to mainly inhibit the cycloeucalenol-obtusifoliol isomerase (Rahier et al., 1986). In a previous report (Hartmann et al., 2002), we have shown that treatment of leek seedlings with FEN induced an accumulation of 9β,19-cyclopropylsterols in place of the usual Δ5-sterols. Here, we show that cyclopropylsterols as well as the remaining Δ5-sterols mainly accumulate in the GA of leek seedlings. In FEN-treated leek seedlings, the highest amount of DRMs was isolated from the GA instead of the PM. Therefore, such a modification of the sterol profile was found to trigger a drastic shift in detergent resistance of GA membranes versus PM membranes, which is consistent with a redistribution of microdomains in the secretory pathway. Another interesting observation was the increase of GluCer with hydroxylated fatty acyl chains in GA DRMs after treatment with FEN and their strong decrease or disappearance in PM DRMs.
These results raise several comments. In all DRMs, sterols were present as a mixture of the same sterol molecules as that of the PM or microsomal fractions and in similar proportions. Such a result confirms that no specific enrichment in a given sterol occurs in plant DRMs, indicating either that all sterol molecules may contribute to microdomain formation with the same efficiency, as recently pointed out for sitosterol and stigmasterol (Xu et al., 2001), or that some of them may diffuse passively to these domains. The replacement of Δ5-sterols by 9β,19-cyclopropylsterols in membranes from treated plants did not inhibit lipid microdomain formation in the GA, indicating that these compounds remained able to promote microdomains. In this context, biosynthetic precursors of cholesterol, such as 7-dehydrocholesterol or Δ7-cholesterol, have been shown to promote lipid microdomain formation with the same efficiency as cholesterol (Xu and London, 2000; Xu et al., 2001; Keller et al., 2004). In contrast, lanosterol was found to be less efficient. According to Xu and London (2000), those sterols that promote tight packing of lipids also promote domain formation. Our results are therefore consistent with previous studies that gave evidence for 24-methylpollinastanol to be able to order soybean PC bilayers with the same efficiency as sitosterol as assessed by DPH fluorescence polarization or 2H RMN (Hartmann, 1998).
The fact that the highest amount of DRMs was found in the GA instead of the PM after treatment of leek seedlings by FEN indicates that lipid microdomain delivery to or formation at the PM was perturbed. A concomitant decrease of sterols and hydroxy-palmitic acid-containing GluCer in the PM may provide new evidence that close relationships exist between sterol and sphingolipid biosynthetic pathways (Hartmann et al., 2002). We have previously shown that extensive fenestration of the Golgi cisternae takes place in FEN-treated leek seedlings (Hartmann et al., 2002). These changes in the morphology of the Golgi may affect the steady state of membrane flux between GA and PM, emphasizing the key role played by this organelle in the transport of newly synthesized sterols to the cell surface of plant cells, as in animal cells (Heino et al., 2000). In higher eukaryotes, sterols and sphingolipids are gradually enriched along the secretory pathway (van Meer and Sprong, 2004). In Zea mays coleoptiles (Hartmann and Benveniste, 1987) as well as in leek seedlings (Moreau et al., 1998b), sterols also mainly accumulate in the PM, with a progressive increase through the secretory pathway (Moreau et al., 1998b). A similar situation can be expected for sphingolipids; both sterols and GluCer were found to be highly enriched in the DRMs isolated from the PM and from the GA membranes of leek seedlings.
In mammalian cells, cholesterol levels can influence both the domain association and biological activity of associated proteins (Friedrichson and Kurzchalia, 1998; Varma and Mayor, 1998; Hancock, 2006). In yeast cells, a similar role is played by ergosterol (Bagnat et al., 2000). Within lipid microdomains, sterols and sphingolipids interact with each other but may also interact with proteins. The specificity of lipid-protein interactions might be higher than that of lipid-lipid interactions. The importance of lipid structure is likely to involve more than its ability to promote lipid microdomains. In mammalian cells, replacement of cholesterol by 7-dehydrocholesterol triggers a drastic change in the protein pattern of DRMs (Keller et al., 2004). In the erg6 yeast mutant (defective in ergosterol synthesis), the tryptophan permease Tat2p is not properly targeted to the PM at low tryptophan concentration but is rerouted to the vacuole for degradation (Umebayashi and Nakano, 2003), indicating that specific structural determinants of the sterol molecule may be required for the association of this protein with lipid microdomains. In the same context, an elo3-erg6 double mutant (respectively blocked in C26-sphingolipid and ergosterol synthesis) was found to be lethal due to a defect in the association of the ATPase Pma1p with lipid microdomains (Eisenkolb et al., 2002). The lethality could be rescued by addition of ergosterol but not cholesterol, suggesting a specific requirement of the ergosterol methyl group. However, other data indicate that C26-sphingolipids may be much more critical than ergosterol for Pma1p sorting to the yeast PM (Gaigg et al., 2005). In addition, the leek orc mutant, defective in sterol methyltransferase 1, shows defects in cell polarity and PM distribution of PIN proteins (implicated in auxin efflux), but their vesicular transport to the cell surface is not blocked, suggesting that either their polarized targeting or assembly in the PM was affected (Willemsen et al., 2003). The apparent discrepancies between these studies may reflect that lipids in microdomains can have different roles and specificities in interactions, which may govern protein sorting, targeting, and microdomain association in different ways. Moreover, we must be aware that protein-protein interactions may also drive protein segregation, as suggested, for example, in the case of T-cell-receptor signaling (Nichols, 2005). In our case, we can highlight the presence of GluCer species with hydroxy and dihydroxy fatty acids in DRMs isolated from leek seedlings, which would be more efficient to form hydrogen bonds with other components (sterols and/or proteins). In agreement with a possible requirement of sterols and/or sphingolipids for protein targeting, FEN treatment of both leek and Arabidopsis seedlings led to a decrease of two DRM protein markers in the PM of FEN-treated plants.
In addition, cholesterol was proposed to regulate protein sorting by a bilayer-mediated mechanism, in which proteins are targeted to bilayers whose hydrophobic thickness matches the length of their transmembrane domain (Bretscher and Munro, 1993; Lundbaeck et al., 2003). Thus, replacement of usual Δ5-sterols (sitosterol, stigmasterol, and 24-methylcholesterol) by cyclopropylsterols might affect the width of the bilayer, which would no longer be able to match with specific proteins to be targeted to the PM.
Another critical point that has been investigated in this article was whether the degree of unsaturation of phospholipid fatty acyl chains may have an impact on the formation of lipid microdomains in plant cells. We took advantage of two mutant Arabidopsis cell lines, fad2 and Fad3+, which are affected in their fatty acid desaturases. The 18:1 desaturase is shut down in the fad2 mutant (Okuley et al., 1994), resulting in an accumulation of 18:1 fatty acids (Caiveau et al., 2001), whereas the Fad3+ mutant accumulates 18:3 fatty acids (Shah et al., 1997). Given the important role played by lipids with saturated fatty acyl chains in regulating the physicochemical properties of biological membranes, it was particularly interesting to check DRM formation in these two cell lines, which exhibit significant differences in the mean fluidity (defined as temperature-dependent lipid movements in the membrane) of their PM, compared to the PM of wild-type cells (Vaultier et al., 2006). In both fad2 and Fad3+cell lines, a dramatic decrease in the amount of DRMs was found, indicating a much-reduced efficiency of their respective membrane components to segregate into lipid microdomains. Another critical observation was that, independent of the initial levels in the respective microsomal membranes, 16:0 and 18:1 were systematically the two major fatty acids found in the PC and PE of DRMs isolated from fad2, Fad3+, and wild-type cell lines. Therefore, the recovery of these phospholipids into DRMs is influenced by the nature of their fatty acyl chains, regardless of the initial general degree of unsaturation of these phospholipids in the whole membrane. In addition, DRMs isolated from fad2 cells showed an increase of saturated PC species and of 16:1-PE, compared to DRMs isolated from wild-type cells. An increase of 18:1-PC, 16:0-, and 16:1-PE was also observed in DRMs from Fad3+ cells compared to wild-type DRMs. In all cases, this corresponded to saturated and monounsaturated phospholipid species that were systematically segregated to these domains.
Taken together, these results suggest that the unsaturation degree of lipid acyl chains of plant cell membranes plays a crucial role in the ability of lipids to partition into lipid microdomains, and two aspects may be highlighted: (1) it is more the fatty acyl moieties than the polar heads that drive lipid segregation in plant cell membranes; and (2) the overall degree of unsaturation of the membrane and therefore its fluidity has an impact on the amount of lipids that can be segregated into microdomains.
In conclusion, we have given evidence that sterols, GluCer, and the unsaturation degree of lipid acyl chains play critical roles in the formation of lipid microdomains in the PM and GA of plant cells. Recent evidence suggests that the wide functional diversity in which lipid microdomains may be involved is provided by the coexistence of distinct lipid microdomain populations with different protein and lipid compositions (Pike, 2004). The next challenges will be to determine whether individual lipids or microdomains are trafficking between GA and PM membranes and whether different lipid microdomains may govern the sorting and targeting of different proteins through the plant secretory pathway.
MATERIALS AND METHODS
Plant and Cell Growth Conditions
Leek (Allium porrum) seeds were purchased from Vilmorin and stored overnight at 4°C before being hydrated with distilled water for 2 h. The seeds were allowed to germinate and grow in the dark for 7 d at 22°C to 24°C on a culture medium containing 0.8 mm NaH2PO4, 1 mm MgSO4, 7 mm NaNO3, 0.8 mm CaCl2, 10 mm KCl, and 5% (w/v) agar. Arabidopsis (Arabidopsis thaliana) ecotype Columbia seedlings were grown according to Krysan et al. (1996). Arabidopsis (ecotype Columbia) cell suspensions were cultured as described by Ruelland et al. (2002) and Vergnolle et al. (2005).
FEN, which was kindly supplied by BASF, was added to the culture medium at a final concentration of 2 mg/L (6.6 μm).
Isolation of Membrane Fractions from Leek and Arabidopsis Seedlings
ER-, GA-, and PM-rich fractions from leek seedlings were isolated and characterized as described earlier (Vincent et al., 1999). Briefly, leek seedlings were homogenized in the presence of 10 mm KH2PO4, pH 8.2, with 0.5 m sorbitol, 5% (w/v) polyvinylpyrrolidone 40, 0.5% (w/v) bovine serum albumin (BSA), 2 mm salicylhydroxamic acid, and 1 mm phenylmethylsulfonyl fluoride. After filtration, the homogenate was subjected to successive centrifugations at 1,000g for 10 min, 10,000g for 10 min, and 150,000g for 60 min. The resulting microsomal pellet was suspended in 10 mm KH2PO4, pH 8.2, containing 0.5 m sorbitol (washing buffer). The microsomal suspension was then loaded onto a discontinuous Suc density gradient consisting of 10 mL of 37% (w/v), 15 mL of 25%, and 6 mL of 18% Suc. After centrifugation at 150,000g for 150 min, membranes at the 18%/25% (ER fraction) and at the 25%/37% (Golgi fraction) interfaces were collected, diluted with the washing buffer, centrifuged for 60 min at 150,000g, and finally resuspended in the washing buffer. PM was isolated by phase partitioning of microsomes between 6.0% (w/w) PEG 4000 (Sigma) and 6.0% (w/w) dextran T500 (Sigma) in 10 mm KH2PO4, pH 7.8, with 0.5 m sorbitol and 40 mm NaCl. After centrifugation at 1,000g for 15 min, the upper PEG-enriched phase, which contained most of PM vesicles, was recovered and diluted with the washing buffer. PM vesicles were pelleted by centrifugation at 150,000g for 60 min, resuspended in the washing buffer, and immediately used for the isolation of DRMs.
PM-rich fractions from Arabidopsis seedlings were isolated by the same procedure and under similar conditions as described above.
Protein concentrations were determined according to either Lowry et al. (1951) or Bradford (1976) using BSA as a standard.
Isolation of DRMs from the Membrane Fractions Prepared from Leek and Arabidopsis Seedlings and from Arabidopsis Cell Suspension Cultures
The various membrane fractions isolated from leek and Arabidopsis seedlings were treated at 4°C for 30 min with 1% Triton X-100 (1% final concentration) using detergent-to-protein ratios varying from 2 to 16. Membranes were then brought to a final concentration of 48% (w/w) Suc, overlaid with 2 mL of 40%, 35%, 30%, and 5% (w/w) Suc in the washing buffer, and spun for 16 h at 150,000g at 4°C. DRMs were recovered from below the 30% to 35% interface, washed with water to remove residual Suc, and recentrifuged at 200,000g for 15 min.
Membrane microdomains from Arabidopsis cell cultures were isolated according to the method of Ko et al. (1999). Briefly, 500 to 700 g of Arabidopsis cells (wild type), 130 to 200 g of fad2 mutant cell lines, and 400 to 500 g of Fad3+ mutant cell lines were homogenized in 100 mm MOPS-KOH buffer containing 600 mm Suc, 4 mm EDTA, 0.5 g/L Cys, 2 g/L BSA, 6 g/L polyvinylpyrrolidone 40, 5 mm ascorbic acid, 5 mm dithiothreitol, and 100 μm phenylmethylsulfonyl fluoride. The homogenates were filtered on Miracloth (Calbiochem) layers, then centrifuged at 15,000g for 35 min, and corresponding supernatants were spun down at 100,000g for 90 min. The resulting microsomal pellets were suspended in 25 mm HEPES buffer with 3 mm EDTA and 150 mm NaCl, pH 7.4. The membranes were then incubated with 1% Triton X-100 for 30 min at 4°C and mixed with an equal volume of 85% Suc (w/v) in HEPES buffer, transferred to a centrifuge tube, and overlaid with 30% Suc and 5% Suc solutions. The Suc gradient was centrifuged 21 h at 150,000gmax. DRMs were recovered in the 30% Suc phase, washed with water to remove residual Suc, and centrifuged at 200,000g for 15 min.
SDS-PAGE and Immunoblots
SDS-PAGE was carried out on 12% polyacrylamide gels. Membrane protein samples were mixed with an equal volume of Laemmli sample buffer (Sigma) and heated at 90°C for 3 min before being loaded on the gels. After electrophoresis, the bands were blotted on polyvinylidene difluoride membranes (Perkin Elmer) by transverse electrophoresis. Three different antibodies were used: the anti-PMA2 antibody was generously provided by M. Boutry (University of Louvain, Belgium), the anti-PIP1 antibody was kindly provided by V. Santoni and C. Maurel (Centre National de la Recherche Scientifique/Institut National de la Recherche Agronomique/Université Montpellier 2, France), and the anti-remorin antibody, which was generously provided by E.E. Farmer (University of Lausanne, Switzerland). The anti-PMA2 antibody may recognize PMA4 as well (Lefebvre et al., 2004). The anti-PIP1 antibody was raised against a 42-amino acid N-terminal peptide of PIP1-1, and due to sequence homologies, may recognize the PIP1 and PIP2 families (Santoni et al., 2003). The immunolabeling with the different antibodies was carried out at room temperature; the polyvinylidene difluoride membranes were sequentially incubated, at room temperature, with the saturation solution (phosphate-buffered saline, 1% BSA) for 1 h, with IgGs (1/8,000 or 1/4,000) for 2 h, and with anti-IgG-peroxidase conjugate (1/10,000) for 30 min. Labeling was revealed using a chemiluminescent reagent kit (Perkin Elmer). The membranes were then exposed to Kodak Biomax Light films.
Lipid Analyses
Lipids were extracted by chloroform:methanol (2:1, v/v) for 30 min at room temperature and then washed three times with 9% NaCl. The solvent was evaporated and lipids were dissolved in an appropriate volume of chloroform/methanol (1:1, v/v).
Polar lipids were analyzed by loading total lipids onto HPTLC plates (60F254, Merck), which were developed in methyl acetate/n-propanol/chloroform/methanol/0.25% aqueous KCl (25:25:25:10:9, v/v) according to Heape et al. (1985). Under these conditions, GluCer and SG were not separated. To separate these two lipid species, the corresponding spot was further applied on a TLC plate developed with chloroform/methanol (85:15, v/v; Hillig et al., 2003). To isolate neutral lipids, total lipids were loaded onto HPTLC plates developed in hexane/ethylether/acetic acid (90:15:2, v/v) and separated in diacylglycerols (RF 0.08), sterols (RF 0.17), fatty alcohols (RF 0.22), and free fatty acids (RF 0.29). Lipids were identified by comigration with known standards and quantified by densitometry analysis (Macala et al., 1983) using a TLC scanner 3 (CAMAG). The different families of lipids had to be analyzed on different HPTLC plates. To take into account any variation in lipid charring and therefore quantification between the different HPTLC plates, several known amounts of the same standards were deposited on each plate before charring.
Fatty acids of individual lipids were determined and quantified by GC (using a Hewlett-Packard 5890 series II chromatograph) after conversion to the corresponding methyl esters by hot methanolic H2SO4 according to Browse et al. (1986). Fatty acid methyl esters were identified by comparison to known standards for 16:0, 18:0, 18:1, 18:2, 18:3, 20:0, 20:1, 22:0, 22:1, and 24:0. Soybean (Glycine max) GluCer (Avanti Lipids), which contains >98% of hydroxypalmitic acid, was used as a standard to determine the RF of the corresponding methyl ester.
Free sterols from leek membrane fractions and DRMs were analyzed as previously reported (Hartmann et al., 2002). Sterols were extracted with hexane and isolated by TLC. They were quantified as acetate derivatives by GC and identified by GC-MS.
Mass spectrometry of GluCer was performed as follows: ceramide extracts from DRMs were evaporated to dryness and dissolved in a methanol/dichloromethane 3:1 mixture prior to addition of aqueous lithium iodide (5 mm final concentration). Exact mass measurements of lipids were made in the electrospray mode using a Waters LCT Premier mass spectrometer fitted with a dual sprayer (Lockspray) source. Lockmass correction used the doubly charged ion of a peptide reference compound (Glufibrinopeptide from Sigma). MS2 and MS3 mass measurements were made with an ion trap mass spectrometer (Thermo Instruments LCQ Advantage) operated in the electrospray ionization mode. A 4-D isolation width was applied for precursor selection.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Characterization of GluCer species by mass spectrometry.
Supplementary Material
Acknowledgments
We thank Professor M. Boutry (University of Louvain, Belgium), Drs. V. Santoni and C. Maurel (Centre National de la Recherche Scientifique/Institut National de la Recherche Agronomique/Université Montpellier 2, France), and Professor E.E. Farmer (University of Lausanne, Switzerland) for providing us with anti-PMA2, anti-PIP1, and anti-remorin antibodies, respectively.
This work was supported by the Centre National de la Recherche Scientifique (postdoctoral fellowship to M.L.), by the Victor Segalen University of Bordeaux 2, by the Conseil Régional d'Aquitaine (M.L., A.M.P., L.C., M.V., R.L., P.M.; mass spectrometry equipment to J.M.S.), and by the Pierre and Marie Curie University of Paris (C.C., M.N.V., A.Z.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Patrick Moreau (pmoreau@biomemb.u-bordeaux2.fr).
The online version of this article contains Web-only data.
References
- Bagnat M, Chang A, Simons K (2001) Plasma membrane proton ATPase Pma1p requires raft association for surface delivery in yeast. Mol Biol Cell 12 4129–4138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Keranen S, Shevchenko A, Simons K (2000) Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA 97 3254–3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnat M, Simons K (2002) Cell surface polarization during yeast mating. J Biol Chem 383 1475–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bariola PA, Retelska D, Stasiak A, Kammerer RA, Fleming A, Hijri M, Frank S, Farmer EE (2004) Remorins form a novel family of coiled coil-forming oligomeric and filamentous proteins associated with apical, vascular and embryonic tissues in plants. Plant Mol Biol 55 579–594 [DOI] [PubMed] [Google Scholar]
- Bhat RA, Panstruga R (2005) Lipid rafts in plants. Planta 223 5–19 [DOI] [PubMed] [Google Scholar]
- Borner GH, Sherrier DJ, Weimar T, Michaelson LV, Hawkins ND, Macaskill A, Napier JA, Beale MH, Lilley KS, Dupree P (2005) Analysis of detergent-resistant membranes in Arabidopsis: evidence for plasma membrane lipid rafts. Plant Physiol 137 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boursiac Y, Chen S, Luu DT, Sorieul M, van den Dries N, Maurel C (2005) Early effects of salinity on water transport in Arabidopsis roots: molecular and cellular features of aquaporin expression. Plant Physiol 139 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Munro S (1993) Cholesterol and the Golgi apparatus. Science 261 1280–1281 [DOI] [PubMed] [Google Scholar]
- Brown DA, London E (1998) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164 103–114 [DOI] [PubMed] [Google Scholar]
- Brown DA, London E (2000) Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem 275 17221–17224 [DOI] [PubMed] [Google Scholar]
- Brown RE (1998) Sphingolipid organization in biomembranes: what physical studies of model membranes reveal. J Cell Sci 111 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152 141–145 [DOI] [PubMed] [Google Scholar]
- Caiveau O, Fortune D, Cantrel C, Zachowski A, Moreau F (2001) Consequences of omega-6-oleate desaturase deficiency on lipid dynamics and functional properties of mitochondrial membranes of Arabidopsis thaliana. J Biol Chem 276 5788–5794 [DOI] [PubMed] [Google Scholar]
- Eisenkolb M, Zenzmaier C, Leitner E, Schneiter R (2002) A specific structural requirement for ergosterol in long-chain fatty acid synthesis mutants important for maintaining rafts domains in yeast. Mol Biol Cell 13 4414–4428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichson T, Kurzchalia TV (1998) Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394 802–805 [DOI] [PubMed] [Google Scholar]
- Gaigg B, Timischl B, Corbino L, Schneiter R (2005) Synthesis of sphingolipids with very long chain fatty acids but not ergosterol is required for routing of newly synthesized plasma membrane ATPase to the cell surface of yeast. J Biol Chem 280 22515–22522 [DOI] [PubMed] [Google Scholar]
- Gkantiragas I, Brugger B, Stuven E, Kaloyanova D, Li XY, Lohr K, Lottspeich F, Wieland FT, Helms JB (2001) Sphingomyelin-enriched micro-domains at the Golgi complex. Mol Biol Cell 12 1819–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock JF (2006) Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7 456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen GH, Niels-Christiansen LL, Thorsen E, Immerdal L, Danielsen EM (2000) Cholesterol depletion of enterocytes: effect on the Golgi complex and apical membrane trafficking. J Biol Chem 275 5136–5142 [DOI] [PubMed] [Google Scholar]
- Hartmann MA (1998) Plant sterols and the membrane environment. Trends Plant Sci 3 170–175 [Google Scholar]
- Hartmann MA, Benveniste P (1987) Plant membrane sterols: isolation, identification and biosynthesis. Methods Enzymol 148 632–650 [Google Scholar]
- Hartmann MA, Perret AM, Carde JP, Cassagne C, Moreau P (2002) Inhibition of the sterol pathway in leek seedlings impairs phosphatidylserine and glucosylceramide synthesis but triggers an accumulation of triacylglycerols. Biochim Biophys Acta 1583 285–296 [DOI] [PubMed] [Google Scholar]
- Heape AM, Juguelin H, Boiron F, Cassagne C (1985) Improved one dimensional thin layer chromatographic technique for polar lipids. J Chromatogr 332 391–395 [DOI] [PubMed] [Google Scholar]
- Heino S, Lusa S, Somerharju P, Ehnholm C, Olkkonen VM, Ikonen E (2000) Dissecting the role of the golgi complex and lipid rafts in biosynthetic transport of cholesterol to the cell surface. Proc Natl Acad Sci USA 97 8375–8380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillig I, Leipelt M, Ott C, Zahringer U, Warnecke D, Heinz E (2003) Formation of glucosylceramide and sterol glucoside by a UDP-glucose-dependent glucosylceramide synthase from cotton expressed in Pichia pastoris. FEBS Lett 553 365–369 [DOI] [PubMed] [Google Scholar]
- Keller P, Simons K (1998) Cholesterol is required for surface transport of influenza virus hemagglutinin. J Cell Biol 140 1357–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller RK, Arnold TP, Fliesler SJ (2004) Formation of 7-dehydrocholesterol-containing membrane rafts in vitro and in vivo, with relevance to the Smith-Lemli-Opitz syndrome. J Lipid Res 45 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko YG, Lee JS, Kang YS, Ahn JH, Seo JS (1999) TNF-a-mediated apoptosis is initiated in caveolae-like domains. J Immunol 162 7217–7223 [PubMed] [Google Scholar]
- Krysan PJ, Young JC, Tax F, Sussman MR (1996) Identification of transferred DNA insertions within Arabidopsis genes involved in signal transduction and ion transport. Proc Natl Acad Sci USA 93 8145–8150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MC, Hamamoto S, Schekman R (2002) Ceramide biosynthesis is required for the formation of the oligomeric H+-ATPase Pma1p in the yeast endoplasmic reticulum. J Biol Chem 277 22395–22401 [DOI] [PubMed] [Google Scholar]
- Lefebvre B, Batoko H, Duby G, Boutry M (2004) Targeting of a Nicotiana plumbaginifolia H+ -ATPase to the plasma membrane is not by default and requires cytosolic structural determinants. Plant Cell 16 1772–1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legembre P, Daburon S, Moreau P, Ichas F, de Giorgi F, Moreau JF, Taupin JL (2005) Amplification of Fas-mediated apoptosis in type II cells via microdomain recruitment. Mol Cell Biol 25 6811–6820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legembre P, Moreau P, Daburon S, Moreau JF, Taupin JL (2002) Potentiation of Fas-mediated apoptosis by an engineered glycosylphosphatidylinositol-linked Fas. Cell Death Differ 9 329–339 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough HJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin reagent. J Biol Chem 193 265–275 [PubMed] [Google Scholar]
- Lundbaek JA, Andersen OS, Werge T, Nielsen C (2003) Cholesterol-induced protein sorting: an analysis of energetic feasibility. Biophys J 84 2080–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macala LJ, Yo RK, Ando S (1983) Analysis of brain lipids by high performance TLC and densitometry. J Lipid Res 24 1243–1250 [PubMed] [Google Scholar]
- Markham JE, Li J, Cahoon EB, Jaworski JG (2006) Plant sphingolipids: separation and identification of major sphingolipid classes from leaves. J Biol Chem 281 22684–22694 [DOI] [PubMed] [Google Scholar]
- Maudoux O, Batoko H, Oecking C, Gevaert K, Vandekerckhove J, Boutry M, Morsomme P (2000) A plant plasma membrane H+-ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J Biol Chem 275 17762–17770 [DOI] [PubMed] [Google Scholar]
- Mongrand S, Morel J, Laroche J, Claverol S, Carde JP, Hartmann MA, Bonneu M, Simon-Plas F, Lessire R, Bessoule JJ (2004) Lipid rafts in higher plant cells: purification and characterization of TX100-insoluble micro-domains from tobacco plasma membrane. J Biol Chem 279 36277–36286 [DOI] [PubMed] [Google Scholar]
- Moreau P, Bessoule JJ, Mongrand S, Testet E, Vincent P, Cassagne C (1998. a) Lipid trafficking in plant cells. Prog Lipid Res 37 371–391 [DOI] [PubMed] [Google Scholar]
- Moreau P, Hartmann MA, Perret AM, Sturbois-Balcerzak B, Cassagne C (1998. b) Transport of sterols to the plasma membrane of leek seedlings. Plant Physiol 117 931–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S (2003) Lipid rafts: elusive or illusive? Cell 115 377–388 [DOI] [PubMed] [Google Scholar]
- Nichols B (2005) Cell biology: without a raft. Nature 436 638–639 [DOI] [PubMed] [Google Scholar]
- Okuley J, Lightner J, Feldmann K, Yadav N, Lark E, Browse J (1994) Arabidopsis FAD2 gene encodes the enzyme that is essential for polyunsaturated lipid synthesis. Plant Cell 6 147–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskan T, Westermann M, Oelmuller R (2000) Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur J Biochem 267 6989–6995 [DOI] [PubMed] [Google Scholar]
- Pike LJ (2004) Lipid rafts: heterogeneity on the high seas. Biochem J 378 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier A, Schmitt P, Huss B, Benveniste P, Pommer EH (1986) Chemical structure activity relationships of the inhibition of sterol biosynthesis by N-substituted morpholines in higher plant cells. Pestic Biochem Physiol 25 112–124 [Google Scholar]
- Röper K, Corbeil D, Huttner WB (2000) Retention of prominin in microvilli reveals distinct cholesterol-based lipid micro-domains in the apical plasma membrane. Nat Cell Biol 2 582–592 [DOI] [PubMed] [Google Scholar]
- Ruelland E, Cantrel C, Gawer M, Kader JC, Zachowski A (2002) Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol 130 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoni V, Vinh J, Pflieger D, Sommerer N, Maurel C (2003) A proteomic study reveals novel insights into the diversity of aquaporin forms expressed in the plasma membrane of plant roots. Biochem J 373 289–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck S, Honsho M, Ekroos K, Shevchenko A, Simons K (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci USA 100 5795–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever D, Pickett S, Mann KJ, Sambamurti K, Medof ME, Rosenberry TL (1999) Glycosylphosphatidylinositol-anchor intermediates associate with triton-insoluble membranes in subcellular compartments that include the endoplasmic reticulum. Biochem J 343 627–635 [PMC free article] [PubMed] [Google Scholar]
- Shah S, Xin Z, Browse J (1997) Overexpression of the FAD3 desaturase gene in a mutant of Arabidopsis. Plant Physiol 114 1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shogomori H, Brown DA (2003) Use of detergents to study membrane rafts: the good, the bad, and the ugly. Biol Chem 384 1259–1263 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E (1997) Functional rafts in cell membranes. Nature 387 569–572 [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1 31–39 [DOI] [PubMed] [Google Scholar]
- Simons K, Vaz WL (2004) Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 33 269–295 [DOI] [PubMed] [Google Scholar]
- Sperling P, Heinz E (2003) Plant sphingolipids: structural diversity, biosynthesis, first genes and functions. Biochim Biophys Acta 1632 1–15 [DOI] [PubMed] [Google Scholar]
- Sturbois-Balcerzak B, Vincent P, Maneta-Peyret L, Duvert M, Satiat-Jeunemaitre B, Cassagne C, Moreau P (1999) ATP-dependent formation of phosphatidylserine-rich vesicles from the ER of leek cells. Plant Physiol 120 245–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullards MC, Lynch DV, Merrill AH Jr, Adams J (2000) Structure determination of soybean and wheat glucosylceramides by tandem mass spectrometry. J Mass Spectrom 35 347–353 [DOI] [PubMed] [Google Scholar]
- Umebayashi K, Nakano A (2003) Ergosterol is required for targeting of tryptophan permease to the yeast plasma membrane. J Cell Biol 161 1117–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G, Sprong H (2004) Membrane lipids and vesicular traffic. Curr Opin Cell Biol 16 373–378 [DOI] [PubMed] [Google Scholar]
- Vaultier MN, Cantrel C, Vergnolle C, Justin AM, Demandre C, Benhassaine-Kesri G, Cicek D, Zachowski A (2006) Desaturase mutants reveal that membrane rigidification acts as a cold perception mechanism upstream of the diacylglycerol kinase pathway in Arabidopsis cells. FEBS Lett 580 4218–4223 [DOI] [PubMed] [Google Scholar]
- Varma R, Mayor S (1998) GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394 798–801 [DOI] [PubMed] [Google Scholar]
- Vergnolle C, Vaultier MN, Taconnat L, Renou JP, Kader JC, Zachowski A, Ruelland E (2005) The cold-induced early activation of phospholipase C and D pathways determines the response of two distinct clusters of genes in Arabidopsis cell suspensions. Plant Physiol 139 1217–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent P, Maneta-Peyret L, Sturbois-Balcerzak B, Duvert M, Cassagne C, Moreau P (1999) One of the origins of plasma membrane phosphatidylserine in plant cells is a local synthesis by a serine exchange activity. FEBS Lett 464 80–84 [DOI] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B (2003) Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE 1 function. Plant Cell 15 612–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E (2001) Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts): comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem 276 33540–33546 [DOI] [PubMed] [Google Scholar]
- Xu X, London E (2000) The effect of sterol structure on membrane lipid domains reveals how cholesterol can induce lipid domain formation. Biochemistry 39 843–849 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.