Abstract
Using a new Arabidopsis (Arabidopsis thaliana) mutant (Atnrt2.1-nrt2.2) we confirm that concomitant disruption of NRT2.1 and NRT2.2 reduces inducible high-affinity transport system (IHATS) by up to 80%, whereas the constitutive high-affinity transport system (CHATS) was reduced by 30%. Nitrate influx via the low-affinity transport system (LATS) was unaffected. Shoot-to-root ratios were significantly reduced compared to wild-type plants, the major effect being upon shoot growth. In another mutant uniquely disrupted in NRT2.1 (Atnrt2.1), IHATS was reduced by up to 72%, whereas neither the CHATS nor the LATS fluxes were significantly reduced. Disruption of NRT2.1 in Atnrt2.1 caused a consistent and significant reduction of shoot-to-root ratios. IHATS influx and shoot-to-root ratios were restored to wild-type values when Atnrt2.1-nrt2.2 was transformed with a NRT2.1 cDNA isolated from Arabidopsis. Disruption of NRT2.2 in Atnrt2.2 reduced IHATS by 19% and this reduction was statistically significant only at 6 h after resupply of nitrate to nitrogen-deprived plants. Atnrt2.2 showed no significant reduction of CHATS, LATS, or shoot-to-root ratios. These results define NRT2.1 as the major contributor to IHATS. Nevertheless, when maintained on agar containing 0.25 mm KNO3 as the sole nitrogen source, Atnrt2.1-nrt2.2 consistently exhibited greater stress and growth reduction than Atnrt2.1. Evidence from real-time PCR revealed that NRT2.2 transcript abundance was increased almost 3-fold in Atnrt2.1. These findings suggest that NRT2.2 normally makes only a small contribution to IHATS, but when NRT2.1 is lost, this contribution increases, resulting in a partial compensation.
Physiological evidence based upon nitrate influx versus nitrate concentration plots has demonstrated that nitrate uptake by roots of higher plants occurs via both high-affinity transport systems (HATS) and low-affinity transport systems (LATS; for review, see Glass and Siddiqi, 1995; Crawford and Glass, 1998; Forde, 2000). In barley (Hordeum vulgare), for example, this physiological evidence suggested the existence of a constitutive high-affinity transport system (CHATS) and a nitrate-inducible high-affinity transport system (IHATS), whose Vmax was 30 times that of the CHATS (Siddiqi et al., 1990). In addition, a constitutive LATS showed no saturation even at 50 mm external nitrate. This appears to be the case for nitrate uptake by other species, including white spruce (Picea glauca), aspen (Populus tremuloides), and lodgepole pine (Pinus contorta; Min et al., 2000). In Arabidopsis (Arabidopsis thaliana), there are also CHATS and IHATS, but LATS is mediated by both constitutive and nitrate-inducible transport systems (Crawford and Glass, 1998).
Physiological evidence based upon biphasic Lineweaver-Burk and Hofstee plots (Aslam et al., 1992; Kronzucker et al., 1995) indicates that, in nitrate-induced plants, both CHATS and IHATS operate independently and additively, suggesting the existence of discrete transporters for CHATS and IHATS. Likewise, total influx at elevated nitrate concentrations is the sum of CHATS, IHATS, and LATS. At first, it was anticipated that a single gene might encode each physiologically defined nitrate transport system. However, isolation of the first high-affinity eukaryotic nitrate transporter, initially designated CRNA, now renamed NRTA from Aspergillus nidulans (Unkles et al., 1991), was soon followed by the isolation of a second member of the family, namely, NRTB (Unkles et al., 2001). Nitrate influx studies with appropriate mutants of A. nidulans revealed that, although both are HATS, nrtA and nrtB exhibit large differences in Km and Vmax values. The adaptive value of this duplication (with kinetic differentiation) remains unclear. Likewise, when genes encoding HATS belonging to the same family were cloned from Chlamydomonas reinhardtii (Quesada et al., 1994), two genes (initially named CnNAR3 and CnNAR4 and renamed CnNRT2.1 and CnNRT2.2) were found to have quite different Km and Vmax values. Both high- and low-affinity nitrate transporters have subsequently been characterized from this species (Galván and Fernández, 2001).
Higher plant representatives of this same family of genes were subsequently isolated from several plant groups (Trueman et al., 1996; Quesada et al., 1997; Filleur and Daniel-Vedele, 1999; Zhuo et al., 1999; Vidmar et al., 2000a). In the case of Arabidopsis, two members, AtNRT2.1 and AtNRT2.2, were cloned (Zhuo et al., 1999). The completion of the Arabidopsis genome project revealed that, in total, there were seven members of the NRT2 family present in this species. These genes were presumed to encode high-affinity nitrate transporters based upon the high degree of sequence identity with the Aspergillus and Chlamydomonas genes. Nevertheless, direct evidence for the roles of these several family members was not available.
Evidence for a role of AtNRT2.1 in high-affinity nitrate transport was provided by the observation that, among the seven NRT2 genes, only AtNRT2.1 exhibited statistically significant correlations between transcript abundance and high-affinity nitrate influx following provision of nitrate to nitrate-deprived plants (Okamoto et al., 2003). This correlation was also evident in plants maintained at different levels of nitrate and following exposure to various inhibitors of nitrate assimilation (Zhuo et al., 1999; Vidmar et al., 2000b). Transcript abundance of AtNRT2.2 was shown to be much lower than that of AtNRT2.1 (Zhuo et al., 1999; Orsel et al., 2002; Okamoto et al., 2003), although, like AtNRT2.1, AtNRT2.2 is strongly up-regulated following resupply of nitrate to plants that have been nitrate-deprived for several days (Okamoto et al., 2003). However, the up-regulation of AtNRT2.2 is transient and transcript abundance declines quite rapidly after 3 h of reexposure to nitrate (Okamoto et al., 2003). Evidence that AtNRT2.1 and/or AtNRT2.2 encoded the IHATS was further supported by the demonstration that high-affinity nitrate uptake was reduced to 27% of wild-type values in a T-DNA mutant, originally designated atnrt2a (Filleur et al., 2001) and subsequently renamed Atnrt2.1-1 (Little et al., 2005), and disrupted in both NRT2.1 and NRT2.2. The question remains whether both NRT2.1 and NRT2.2, or only one of these genes, encodes IHATS activity. Recently, it has been demonstrated that, in addition to functioning in nitrate transport, AtNRT2.1 may serve to integrate lateral root initiation (LRI) and lateral root growth (Little et al., 2005; Remans et al., 2006). Here also, the role of NRT2.2, if any, has not been clarified. In this study, we have isolated T-DNA mutants disrupted in both NRT2.1 and NRT2.2 and in either NRT2.1 or NRT2.2 and examined their growth in hydroponic systems and on agar, as well as their 13NO3− influxes at low and high external nitrate concentrations. To simplify reference to these mutant lines, we have used the designation Atnrt2.1-nrt2.2 for the T-DNA mutants disrupted in NRT2.1 and NRT2.2, Atnrt2.1 for the mutant disrupted only in AtNRT2.1, and Atnrt2.2 for the mutant disrupted only in AtNRT2.2. By use of these mutants, we have been able to dissect the separate functions of these genes by genetic means. Our data suggest that only disruption of NRT2.1 caused substantial impairment of IHATS nitrate influx. By contrast, disruption of NRT2.2 caused only a modest reduction of influx. cDNA encoding NRT2.1 was used to transform Atnrt2.1-nrt2.2. This transformation restored IHATS and other characteristics that were altered in mutant plants. Nevertheless, in Atnrt2.1, in which AtNRT2.1 is expressed at approximately 10% of the wild-type level, AtNRT2.2 expression was increased almost 3-fold, suggesting that loss of AtNrt2.1 function may be partially compensated for by overexpression of AtNRT2.2 and hence reduce the extent of growth reduction. Thus AtNRT2.2 appears to serve a small, but important, contributory and compensatory role in IHATS nitrate influx.
RESULTS
Location of T-DNA Insertions
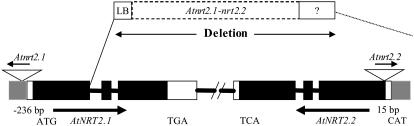
Figure 1 provides a schematic representation of the AtNRT2.1 and AtNRT2.2 genes with T-DNA insertions. Gray boxes indicate the location of the promoter, whereas black and white boxes represent exons and untranslated regions, respectively. We have used different nomenclature for mutants than that employed by Little et al. (2005), as detailed in the introduction. Atnrt2.1-nrt2.2 (Salk_035429) is actually disrupted in AtNRT2.1 and AtNRT2.2 with a T-DNA insertion 33 bp after the first exon. This insertion completely deleted AtNRT2.2 and partially deleted AtNRT2.1. This line was named nrt2.1-2 by Little et al. (2005). A second Atnrt2.1 mutant (Atnrt2.1; Salk_141712) had a T-DNA insertion at 236 bp before the putative start codon, whereas a third mutant, Atnrt2.2 (Salk_043543), was disrupted in AtNRT2.2 by a T-DNA insertion 15 bp upstream of the putative start codon.
Figure 1.
Schematic representation of AtNRT2.1 and AtNRT2.2 genes with T-DNA insertions. Gray boxes indicate locations of promoters; black and white boxes represent exons and untranslated regions, respectively. In Atnrt2.1-nrt2.2 mutants (Salk_035429), the T-DNA insertion has deleted all of AtNRT2.2 and part of the AtNRT2.1 gene; in Atnrt2.1 mutants (Salk_141712), the T-DNA insertion is at 236 bp before the putative start codon; in Atnrt2.2 mutants (Salk_043543), the T-DNA insertion is 15 bp upstream of the putative start codon. The diagram is not drawn to scale.
mRNA Expression Patterns
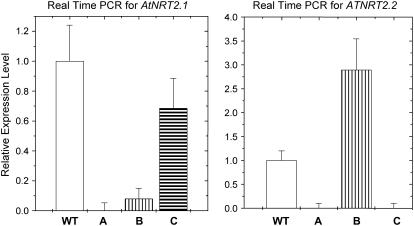
Real-time PCR (Fig. 2) established that, after exposure to inducing conditions (6 h in 1 mm KNO3 after 4-week growth on 1 mm NH4NO3 followed by 1 week without exogenous nitrogen), Atnrt2.1-nrt2.2 and Atnrt2.1 expressed AtNRT2.1 at 1% and 9% of wild-type values, respectively. In the Atnrt2.2 mutant, AtNRT2.1 was expressed at wild-type levels. Under the same conditions, AtNRT2.2 was undetectable in Atnrt2.1-nrt2.2 and Atnrt2.2, but expression levels were increased 3-fold compared to wild-type plants in the Atnrt2.1 mutant. These results were confirmed by semiquantitative PCR (data not shown).
Figure 2.
Real-time PCR expression patterns for AtNRT2.1 and AtNRT2.2 in wild-type (WT), Atnrt2.1-nrt2.2 (A), Atnrt2.1 (B), and Atnrt2.2 (C) plants, respectively. Expression levels of mutants are given relative to wild type (set at 1). Results are the means of two separate experiments using three replicate analyses for each experiment.
Analysis of Growth
Atnrt2.1-nrt2.2 (Salk_035429)
When grown together with wild-type plants in a common hydroponic tank containing 1 mm NH4NO3 for 4 weeks, followed by 1 week of nitrogen deprivation, growth of the double-mutant seedlings was visibly affected. Whereas mutant shoots were typically smaller than those of wild-type plants, root growth was usually equivalent to that of the wild type or even surpassed wild-type root growth, suggesting increased allocation of resources to root growth. In preliminary experiments, dry weight analyses among various lines gave the same relative results as fresh weights. Thereafter, only fresh weights were recorded. Shoot-to-root ratios and shoot and root weights (g plant−1 fresh weight) are shown in Table I for the genotypes employed in this study. Compared to wild-type plants, mutants disrupted in AtNRT2.1 (Atnrt2.1-nrt2.2 and Atnrt2.1) exhibited significantly reduced shoot-to-root ratios, significantly reduced shoot weights, and significantly elevated root weights (P < 0.05). The reductions of shoot-to-root ratios (64% and 55%, respectively) and absolute shoot weights (47% and 30%, respectively) were consistently greater for Atnrt2.1-nrt2.2 than for Atnrt2.1, but the elevation of root weight was similar among these mutants. In contrast to the effects of AtNRT2.1 disruption in Atnrt2.1-nrt2.2 and Atnrt2.1, disruption of AtNRT2.2 in the Atnrt2.2 mutant caused no reduction of shoot-to-root ratios in five of six experiments. After transformation of the Atnrt2.1-nrt2.2 line with cDNA encoding the NRT2.1 sequence, shoot-to-root ratios and absolute shoot and root weights were no longer significantly different from corresponding values for wild-type plants (P > 0.05).
Table I.
Shoot-to-root ratios, shoot fresh weights, and root fresh weights (g FW plant−1) of wild type (WT) and various mutant lines
Atnrt2.1-nrt2.2, disrupted in AtNRT2.1 and AtNRT2.2 (Salk_035429); Atnrt2.1, disrupted in AtNRT2.1 (Salk_141712); and Atnrt2.2, disrupted in AtNRT2.2 (Salk_042543). Plants were grown in 1 mm NH4NO3 for 4 weeks and then deprived of nitrogen for 1 week. Roots were then exposed to 1 mm KNO3 for the times shown prior to measuring 13NO3− influx from 100 μm K13NO3. Values shown are means (n = 24) ± ses; values that differ significantly from those of wild type (WT; P < 0.05) are shown in bold.
| Genotype | Shoot-to-Root Ratio | Shoot Weight | Root Weight |
|---|---|---|---|
| WT | 6.43 ± 0.3 | 0.66 ± 0.06 | 0.11 ± 0.01 |
| Atnrt2.1-nrt2.2 | 2.3 ± 0.01 | 0.35 ± 0.03 | 0.15 ± 0.01 |
| Atnrt2.1 | 2.9 ± 0.2 | 0.46 ± 0.05 | 0.16 ± 0.02 |
| Atnrt2.2 | 6.34 ± 0.4 | 0.69 ± 0.08 | 0.11 ± 0.01 |
| Complemented Atnrt2.1nrt2.2 | 6.44 ± 0.6 | 0.76 ± 0.1 | 0.12 ± 0.04 |
Growth on Agar
While growing the different mutant lines alongside wild type on agar containing various concentrations of different nitrogen sources, a number of characteristics became apparent that were not revealed in hydroponic growth. This may be because in agar, compared to 8 L of nutrient solution, the absolute quantity of nitrogen may be more severely limited. Furthermore, effects of lower nitrogen concentrations were explored than in the hydroponic experiments. When wild-type plants and the three mutant lines, Atnrt2.1-nrt2.2, Atnrt2.1, and Atnrt2.2, were grown on 2.5 mm KNO3, there was virtually no impact upon root growth (Fig. 3). Similar results were observed in 0.5 mm NH4NO3 and 0.5 mm (NH4)2SO4 (data not shown). Nevertheless, shoot growth was slightly reduced in Atnrt2.1-nrt2.2 and there was some pink coloration of leaves and petioles (Fig. 3). Growth of the other mutants was less affected and no reddening of the leaves was evident. By contrast, growth on 0.25 mm KNO3 reduced lateral root growth in all mutant lines, especially Atnrt2.1-nrt2.2 (Fig. 4). Compared to wild type and other mutants, shoot growth was strongly reduced in Atnrt2.1-nrt2.2 and leaves were pink in color. This coloration effect was not evident in the other mutants, although shoot growth was slightly reduced. Figure 4 also shows that transformation of Atnrt2.1-nrt2.2 with an AtNRT2.1 cDNA restored growth to normal.
Figure 3.
Growth of wild type (WT) and Atnrt2.1-nrt2.2, Atnrt2.1, and Atnrt2.2 mutants on agar containing 2.5 mm KNO3.
Figure 4.
Growth of wild type (WT) and Atnrt2.1-nrt2.2, Atnrt2.1, and Atnrt2.2 mutants on agar containing 0.25 mm KNO3.
13NO3− Fluxes
Wild-type and mutant plants were compared with respect to three types of treatments. Plants that had been grown hydroponically for 4 weeks in 1 mm NH4NO3 were deprived of nitrogen for 7 d to deinduce the HATS and to reduce internal nitrogen reserves. When such plants are first exposed to 13NO3− after such treatment, the flux measured is the CHATS. Alternatively, plants that had been deprived of nitrogen for 7 d were treated with 1 mm KNO3 for periods up to 24 h and then exposed to 13NO3−. This flux represents the combined activities of the CHATS plus the IHATS. By subtracting the CHATS flux from the latter, it is possible to estimate the IHATS. Typically, peak influx of the IHATS was observed at 6 h; hence, in the last kind of treatment, to measure the concentration responses and determine Km and Vmax values for IHATS, fluxes were estimated after 6 h of induction with 1 mm KNO3. Thus, plants that had been deprived of nitrogen for 7 d were induced with 1 mm KNO3 for 6 h and then exposed to various concentrations of K13NO3 in the concentration range representative of the HATS (25–150 μm). LATS influx was also measured after 6 h of induction using 1 mm KNO3 in the concentration range that is representative of the LATS (1–10 mm).
CHATS Influx
Table II summarizes the results of several experiments in which the CHATS was measured in wild-type and mutant plants. There were no significant differences between wild-type and mutant CHATS influx for the Atnrt2.1 mutant or for the Atnrt2.2 mutant in seven of eight experiments. By contrast, CHATS influx in the Atnrt2.1-nrt2.2 was significantly reduced by 29% (P < 0.05).
Table II.
CHATS influx (μmol g−1 FW h−1) in wild type (WT) and in Atnrt2.1-nrt2.2 (Salk_035429), disrupted in AtNRT2.1 and AtNRT2.2; Atnrt2.1 (Salk_141712), disrupted in AtNRT2.1; and Atnrt2.2 (Salk_042543), disrupted in AtNRT2.2
Plants were grown in 1 mm NH4NO3 for 4 weeks and then deprived of nitrogen for 1 week. 13NO3− influx was then measured from solutions containing 100 μm K13NO3 without pretreatment with 1 mm KNO3 to reinduce IHATS. Values shown are means (n = 6) ± ses; values that are significantly different from wild-type (WT) values (P < 0.05) are shown in bold.
| Genotype | CHATS Influx |
|---|---|
| WT | 2.41 ± 0.16 |
| Atnrt2.1-nrt2.2 | 1.56 ± 0.17 |
| Atnrt2.1 | 1.94 ± 0.19 |
| Atnrt2.2 | 2.69 ± 0.23 |
| Complemented Atnrt2.1-nrt2.2 | 2.57 ± 0.22 |
Time Course of IHATS Induction by 1 mm KNO3
Figure 5 shows a pattern of IHATS induction in wild-type plants that is well documented (Okamoto et al., 2003). This figure also shows data for the three mutant lines. Influx was measured in 100 μm external 13KNO3 and, to determine the IHATS, the measured HATS influx at time 0 was subtracted from the HATS flux at the times shown. The maximal value of IHATS in wild-type plants, 4.3 μmol g−1 fresh weight h−1 was observed after 6 h of induction. At 6 h, all mutant lines showed a statistically significant reduction of influx (P < 0.05) when compared to wild-type plants. This was equal to 62% (Atnrt2.1-nrt2.2), 56% (Atnrt2.1), and 35% (Atnrt2.2). The reduction of HATS in Atnrt2.1-nrt2.2 is similar to that observed by Filleur et al. (2001) using a different mutant (Atnrt2.1-1) also disrupted in AtNRT2.1 and AtNRT2.2. Although IHATS influx was reduced at all times evaluated in Atnrt2.2, only at 6 h was IHATS influx significantly reduced (P < 0.05) in two separate experiments by an average of 25%.
Figure 5.
Time course of IHATS influx in wild-type and mutant lines following resupply of nitrate to plants nitrogen deprived for 7 d. Plants were grown in 1 mm NH4NO3 for 4 weeks and then deprived of nitrogen for 1 week. Plant roots were then exposed to 1 mm KNO3 for the times shown prior to measuring 13NO3− influx from 100 μm KNO3. Values shown are means (n = 6) together with ses. •, Wild type; ○, Atnrt2.2; ▴, Atnrt2.1; ▪, Atnrt2.1-nrt2.2.
Concentration Dependence of HATS Influx
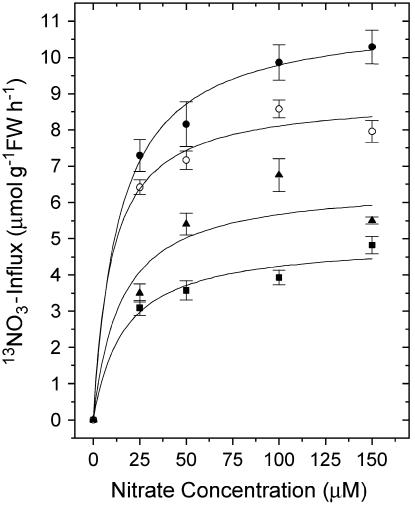
Figure 6 presents HATS influx for the same four lines shown in Figure 5 evaluated for concentration dependence after 6 h of the standard induction. Influx was again reduced in the order Atnrt2.1-nrt2.2 > Atnrt2.1 > Atnrt2.2. At all concentrations evaluated, each mutant line showed similar values for the reduction of influx. Vmax and Km values were generated from these data by means of Hofstee plots. The resulting data are shown in Table III. The reductions of Vmax in the three mutant lines were 56% (Atnrt2.1-nrt2.2), 45% (Atnrt2.1), and 20% (Atnrt2.2), respectively.
Figure 6.
Concentration dependence of the HATS influx in wild-type and mutant lines after 6-h resupply of nitrate to plants nitrogen deprived for 7 d. Plants were grown in 1 mm NH4NO3 for 4 weeks and then deprived of nitrogen for 1 week. Plant roots were then exposed to 1 mm KNO3 for 6 h prior to measuring 13NO3− influx at various concentrations of KNO3. Values shown are means (n = 6) together with ses. •, Wild type; ○, Atnrt2.2; ▴, Atnrt2.1; ▪, Atnrt2.1-nrt2.2.
Table III.
Vmax (μmol g−1 FW h−1) and Km (μm) values for 13NO3− influx in wild type (WT); Atnrt2.1-nrt2.2 mutants, disrupted in AtNRT2.1 and AtNRT2.2; Atnrt2.1 mutants, disrupted in AtNRT2.1; and Atnrt2.2 mutants, disrupted in AtNRT2.2
Plants were grown in 1 mm NH4NO3 for 4 weeks and then deprived of nitrogen for 1 week. Roots were then exposed to 1 mm KNO3 for 6 h prior to measuring 13NO3− influx in solutions containing from 10 to 150 μm K13NO3. Values shown are means (n = 24) ± ses. WT, Wild type.
| WT | Atnrt2.1-nrt2.2 | Atnrt2.1 | Atnrt2.2 | |
|---|---|---|---|---|
| Vmax | 11.16 ± 0.58 | 4.9 ± 0.28 | 6.1 ± 0.19 | 8.9 ± 0.3 |
| Km | 14.1 ± 3.7 | 16.5 ± 4.4 | 14.8 ± 3.2 | 9.9 ± 1.9 |
Complementation Line
HATS influx in the Atnrt2.1-nrt2.2 mutant transformed with cDNA encoding the AtNRT2.1 under the 35S promoter was restored to wild-type values (P > 0.05), whereas influx in the original mutant line remained strongly and significantly reduced (P < 0.05). Plants were deprived of nitrogen for 7 d and then reexposed to 1 mm KNO3 for 6 h before influx was determined using 100 μm KNO3. Wild-type influx was 6.69 ± 0.49 μmol g−1 fresh weight h−1, whereas that for the Atnrt2.1-nrt2.2 mutant was 2.66 ± 0.22 μmol g−1 fresh weight h−1 (a 60% reduction) and influx in the transformed line was 6.64 ± 0.58 μmol g−1 fresh weight h−1.
LATS Influx
To evaluate the effect on LATS influx of mutations to AtNRT2.1 and AtNRT2.2, 13NO3− influx was also determined in wild-type plants and in the three mutant lines, namely, Atnrt2.1-nrt2.2, Atnrt2.1, and Atnrt2.2. After 6 h induction in 1 mm KNO3, influx was measured at KNO3 concentrations of 1, 5, and 10 mm. Plots of influx versus external nitrate were linear and slopes of wild-type plants and mutants were not significantly different (P > 0.05; data not shown).
DISCUSSION
Earlier studies that sought to determine the identity of higher plant genes encoding IHATS by plant roots made use of three lines of evidence to conclude that one of the NRT2 family of genes, probably AtNRT2.1 in Arabidopsis, was the most likely candidate. These were (1) sequence comparisons with genes cloned from A. nidulans and C. reinhardtii earlier established to encode HATS by use of mutants defective in high-affinity nitrate transport (Unkles et al., 1991; Quesada et al., 1994); (2) correlations between high-affinity NO3− influx and NRT2.1 gene expression (Zhuo et al., 1999; Vidmar et al., 2000b; Okamoto et al., 2003); and (3) reduced high-affinity NO3− influx in the T-DNA mutant Atnrt2.1-1 (Filleur et al., 2001).
Unfortunately, the Atnrt2.1-1 mutant referred to above was disrupted in both AtNRT2.1 and AtNRT2.2. Earlier work by Zhuo et al. (1999) showed that, in plants grown under sterile conditions with Suc and a continuous supply of KNO3, AtNRT2.2 was expressed at low abundance compared to AtNRT2.1. However, Okamoto et al. (2003) showed that transcript abundance of AtNRT2.2 increased in parallel with AtNRT2.1 during the first hours after induction, using nonsterile plants grown in the absence of exogenous Suc. Thus, notwithstanding the findings based upon the Atnrt2.1-1 mutant (Filleur et al., 2001), the role of AtNRT2.2 in IHATS influx remains in doubt. In this work, we have been able to dissect the contributions of the AtNRT2.1 and AtNRT2.2 complex by use of mutants individually disrupted in AtNRT2.1 and AtNRT2.2. Our first selection, Salk_035429 (Atnrt2.1-nrt2.2) was thought to be a line disrupted only in AtNRT2.1 (Little et al., 2005), but semiquantitative PCR and real-time PCR using plants induced for 6 h in 1 mm KNO3 revealed that transcript abundance of both AtNRT2.1 and AtNRT2.2 was reduced by >99%. Thus, this mutant is similar to, but distinct from, the line used by Filleur et al. (2001) and Remans et al. (2006) that was also disrupted in AtNRT2.1 and AtNRT2.2. Results of real-time PCR (Fig. 2) revealed that, after 6 h of KNO3 induction, the mutant line Atnrt2.1 expressed transcript abundance of AtNRT2.2 at 3 times those of wild-type levels, whereas AtNRT2.1 was reduced to below 10% of wild-type levels, making this a legitimate knockdown mutant of AtNRT2.1. Similarly, Atnrt2.2 was revealed to be a valid AtNRT2.2 knockout line by virtue of undetectable levels of AtNRT2.2 together with normal levels of AtNRT2.1. These results were confirmed by semiquantitative PCR (data not shown).
Growth of Atnrt2.1-nrt2.2 was visibly impaired in both hydroponic growth and on agar with 0.25 mm KNO3. In particular, shoot growth was consistently reduced, whereas root growth was usually increased, resulting in significantly reduced shoot-to-root ratios. Growth reduction in Atnrt2.1 was less obvious (Figs. 3 and 4; Table I), but in hydroponic growth, shoot-to-root ratios decreased significantly and absolute root growth was also increased (Table I). This was not true of Atnrt2.2. Thus, insofar as growth is concerned, only disruption of AtNRT2.1 caused altered partitioning of growth between roots and shoots. By contrast, growth was only slightly affected in Atnrt2.1 and Atnrt2.2 when grown on 5 mm KNO3. This is consistent with the observed absence of any effect of disruption of both AtNRT2.1 and AtNRT2.2 on LATS influx (Filleur et al., 2001; this study). Growth was also unaffected when nitrogen was supplied as NH4+ (data not shown). Morphological anomalies associated with simultaneous disruption of both AtNRT2.1 and AtNRT2.2 have been reported earlier by Orsel et al. (2004), Little et al. (2005), and Remans et al. (2006) in the mutant named Atnrt2.1-1. This mutant line (Atnrt2.1-nrt2.2) is distinct from Atnrt2.1-1, but our line is also disrupted in both genes (Fig. 2 and reverse transcription [RT]-PCR analyses).
Decreased shoot-to-root ratios, as well as reddening of leaves observed in Atnrt2.1-nrt2.2 (Fig. 4), is typical of nutrient deprivation. The study by Orsel et al. (2004) noted a reduced shoot-to-root ratio in their Atnrt2.1-1 mutant when maintained on low nitrogen. The study by Little et al. (2005) reported that the normal repression of LRI was circumvented in Atnrt2.1-1 when grown on low nitrate and high Suc. Moreover, the authors suggest that this role for AtNRT2.1 is independent of nitrate uptake and that AtNRT2.1 also serves as a repressor of LRI to coordinate root development with nutritional cues. A more recent study of Arabidopsis root system architecture using media devoid of Suc support this hypothesis (Remans et al., 2006) while establishing that effects of nitrogen limitation on LRI are transient and dependent upon the extent of nitrogen limitation. In this study, shoot-to-root ratios were restored to wild-type values in the Atnrt2.1-nrt2.2 line transformed with AtNRT2.1 cDNA consistent with the hypothesis that AtNRT2.1 is largely responsible for altered patterns of growth.
CHATS influx was reduced by 30%, 12%, and 3.5% in Atnrt2.1-nrt2.2, Atnrt2.1, and Atnrt2.2, respectively, but this reduction was statistically significant (P < 0.05) only in the case of Atnrt2.1-nrt2.2. Even in the case of Atnrt2.1-nrt2.2, the reduction of influx was small compared to the effect on IHATS. Furthermore, it is well recognized that nutrient fluxes are dependent upon growth rates, particularly shoot growth rate. Thus, we consider that this effect on CHATS in Atnrt2.1-nrt2.2, which was not statistically significant in the other mutants, may result from reduced growth rather than via direct effects of AtNRT2.1 disruption on CHATS.
In the time-course experiments, as in the CHATS analysis (Fig. 4), disruption of both AtNRT2.1 and AtNRT2.2 or AtNRT2.1 or AtNRT2.2 in the three mutant lines reduced IHATS at 6 h and at almost all other times analyzed in the order Atnrt2.1-nrt2.2 > Atnrt2.1 > Atnrt2.2. Likewise, in the study of concentration dependence, over the whole range of low concentration (Fig. 6) from 25 to 150 μm K13NO3, influx reduction was again in the order Atnrt2.1-nrt2.2 > Atnrt2.1 > Atnrt2.2, as was the order of Vmax values (Table III). Clearly, even after subtracting the CHATS influx values (Table II) from those of the Atnrt2.1-nrt2.2 mutant (Fig. 6), influx was not reduced to zero. This suggests that other transporters may be contributing to the measured influx at low external nitrate concentration. This may be due, in part, to a role for CHL1 (AtNRT1.1), as suggested by Wang et al. (1998). Alternatively, there is evidence that the CHATS influx measured in nitrate-deprived plants is significantly increased following exposure to nitrate (Aslam et al., 1993; Kronzucker et al., 1995). This would mean that the CHATS influx estimated in nitrate-deprived plants is an underestimate of CHATS influx in nitrate-induced plants.
In the high concentration range typical of LATS influx (from 1–10 mm), the reduction of influx was only that due to disruption of IHATS. Thus, it can be concluded that disruption of AtNRT2.1 and AtNRT2.2 impacts only indirectly upon LATS, the measured LATS influx being the sum of HATS and LATS. Using the Atnrt2.1-1 mutant, disrupted in AtNRT2.1 and ATNRT2.2, Filleur et al. (2001) also recorded a lack of effect on LATS influx.
It is clear from analyses of transcript abundances by real-time PCR and semiquantitative PCR that Atnrt2.1-nrt2.2 and Atnrt2.2 are valid knockout mutants. The situation with Atnrt2.1 is less clear. As reported above (and Fig. 2), real-time PCR demonstrated that AtNRT2.1 expression in the Atnrt2.1 mutant was reduced by 90%, suggesting a knockdown mutant. As this pertains to this study, two hypotheses may be advanced: (1) AtNRT2.1 is solely responsible for the IHATS. The consistently greater effects on growth and influx in Atnrt2.1-nrt2.2 compared to that in Atnrt2.1 is due to the remaining low level of AtNRT2.1 expression. This hypothesis would preclude a contribution from AtNRT2.2; (2) AtNRT2.1 is largely responsible for IHATS, but AtNRT2.2 makes a small, but consistent, contribution to IHATS, particularly during recovery from nitrogen deprivation (note the 6-h maximum effect). This contribution may be enhanced when AtNRT2.1 is disrupted.
We favor the second hypothesis for the following reasons. (1) Disruption of AtNRT2.2 consistently reduced IHATS influx. At 6 h, this reduction reached statistical significance (P < 0.05). It is interesting that, in an earlier article from this group, Okamoto et al. (2003) recorded that AtNRT2.2 transcript increased rapidly following the first 3 h after restoration of NO3− supply to nitrogen-deprived plants, but then rapidly declined. (2) Transcript abundance of AtNRT2.2 increased at least 3-fold when AtNRT2.1 was disrupted in the Atnrt2.1 mutant. (3) The reduction of influx in Atnrt2.1-nrt2.2 was consistently greater than in Atnrt2.1 at almost all times during induction (Fig. 5) and at all low concentrations investigated (Fig. 6). Typically, IHATS influx was reduced by 60% to 70% in Atnrt2.1-nrt2.2, but in one experiment the value was as high as 80%. (4) Growth effects and the reduction of CHATS influx in Atnrt2.1-nrt2.2 were consistently greater than in Atnrt2.1.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia-0 was grown hydroponically under nonsterile conditions as described in earlier articles (Zhuo et al., 1999; Okamoto et al., 2003). Briefly, 0.64-cm-thick Styrofoam platforms were cut to fit and float on the surface of 8 L of the hydroponic nutrient medium contained within plastic washing bowls. Each platform contained 25 holes (diameter 1.6 cm), into which were fitted 1.5-cm plastic cylinders cut from the tops of disposable 5-mL pipette tips. The bottoms of these cylinders were covered with plastic mesh and the cylinders filled with clean sand. The advantages of this method are that seedling roots quickly grow into the nutrient solutions and intact plant units (seedlings, together with sand and plastic cylinders) can be removed from the platform for pretreatment, tracer study, or other manipulations. Seeds were imbibed in a cold room at 4°C for 3 d and sown directly on the moistened sand in the platform. The nutrient solution that was used to support plant growth contained 1 mm KH2PO4, 0.5 mm MgSO4, 0.25 mm CaSO4, 20 μm Fe-EDTA, 25 μm H3BO3, 2 μm ZnSO4, 2 μm MnSO4, 0.5 μm CuSO4, 0.5 μm (NH4)6Mo7O24, and 1 mm NH4NO3. The pH of the nutrient solution was maintained at around 6.2 by addition of a small amount of powdered CaCO3 to the 8 L of nutrient solution. This solution was completely replaced after 2 weeks and weekly thereafter. For induction of the IHATS, 5-week-old plants were transferred to nitrogen solution for 1 week (other nutrients remained as before), then reinduced with 1 mm KNO3 contained in the same nutrient solution described above, except that KNO3 replaced NH4NO3 because in short-term exposures NH4+ is inhibitory to NO3− influx. In previous trials, we have observed that withholding NO3− for 1 week is sufficient to deplete roots and shoots of NO3− and return plants to a preinduced condition with respect to IHATS NO3− uptake (Okamoto et al., 2003). Plants were reinduced for from 0 to 24 h. The walk-in environment chamber was maintained under the following conditions: light/dark = 8/16 h, 25°C/20°C; relative humidity = 70%. Fluorescent tubes (Vita-Lite; Duro-Test Lighting) were used to provide light of 400 μE m−2 s−1 at the plant level. All flux determinations and plant harvesting for RNA extraction were undertaken at 4 h after the light period began, and all pretreatments were appropriately staggered to meet this requirement. All experiments were repeated at least twice.
T-DNA Mutant Analysis
The Salk T-DNA insertion mutant lines were obtained from the Arabidopsis Biological Resource Center. These were Atnrt2.1-nrt2.2 (Salk_035429), disrupted in AtNRT2.1 and AtNRT2.2; Atnrt2.1 (Salk_141712), disrupted in AtNRT2.1; and Atnrt2.2 (Salk_042543), disrupted in AtNRT2.2. Homozygous T2 lines for the mutants were selected by genomic PCR using the specific primers for Atnrt2.1-nrt2.2 (LP, 5′-TGGCGACGTTTGTGTCTTGTC-3′; RP, 5′-CACAATTGCAAAGGTGGCTCC-3′) and the specific primer for the Atnrt2.2 (LP, 5′-ATCCGCAGCGCCTAATGATTT-3′; RP, 5′-TTGTCATCTGAGAGGTGAATATCGG-3′); the T-DNA primer LB1a (5′-TGGTTCACGTAGTGGGCCATCG-3′) was used for selection of all three mutants. Each of the mutant lines exhibited a 3:1 ratio to the selection marker (kanomycin) in the T2 generations. Salk_035429 was backcrossed once and Salk_141712 was backcrossed twice.
Complementation Experiment
Complete AtNRT2.1 cDNA was PCR amplified from plasmid pCRNRT21 (isolated by Zhuo et al., 1999) using Expand High Fidelity Tag DNA polymerase with primers (forward, 5′-CCGACAAGACGGCCAAGTTCGACCT-3′ [XbaI is underlined] and reverse, 5′-TCCGTAGAGAAGAACGAAGATCCAAG-3′ [SalI is underlined]), and cloned into the cauliflower mosaic virus 35S promoter binary vector pBin-Hygro (gift of Hugo). Homozygous Atnrt2.1-nrt2.2 mutants were recipients in the transformation. Transgenic plants were generated by Agrobacterium-mediated transformation using the floral-dip method and selected on 25 mg L−1 hygromycin (Sigma). T1 seedlings that showed hygromycin resistance were transferred to soil and grown for 4 to 6 weeks. Mature T2 seeds were harvested and used for subsequent experiments.
RT-PCR Analysis
Total RNA was extracted from roots using TRIzol reagent (Life Technology), according to the manufacturer's method, followed by additional chloroform isolation and sodium acetate precipitation steps. RT-PCR was performed following the protocol of the One-Step RT-PCR kit (Qiagen). As a positive control, QuantumRNA 18S Internal Standards (Ambion) was amplified at the same time under the following conditions: 50°C for 30 min; 95°C for 15 min; 21 cycles of 94°C for 30 s, 58°C for 30 s, 72°C for 1 min; 72°C for 10 min. The primers used were: AtNRT2.1 (forward, 5′-CCGACAAGACGGCCAAGTTCGACCT-3′; reverse, 5′-TCCGTAGAGAAGAACGAAGATCCAAG-3′) and AtNRT2.2 (forward, 5′-CAGGTGGAAACAGAGCTGCCATGG-3′; reverse, 5′-GGACCATAGATACAACGGCAGTGACGAG-3′).
Complementation Experiment
Complete AtNRT2.1 cDNA was PCR amplified from plasmid pCRNRT21 (isolated by Zhuo et al., 1999) using Expand High-Fidelity Tag DNA polymerase with primers (forward, 5′-CCGACAAGACGGCCAAGTTCGACCT-3′ [XbaI is underlined] and reverse, 5′-TCCGTAGAGAAGAACGAAGATCCAAG-3′ [SalI is underlined]), and cloned into the cauliflower mosaic virus 35S promoter binary vector pBin-Hygro (gift of Hugo). Homozygous Atnrt2.1-nrt2.2 mutants were recipients in the transformation. Transgenic plants were generated by Agrobacterium-mediated transformation using the floral-dip method and selected on 25 mg L−1 hygromycin (Sigma). T1 seedlings that showed hygromycin resistance were transferred to soil and grown for 4 to 6 weeks. Mature T2 seeds were harvested and used for subsequent experiments.
Quantitative Real-Time PCR
For gene expression analysis, total RNA was isolated from approximately 100 mg fresh weight with the RNeasy plant mini kit (Qiagen). RNase-free DNAase treatment was also carried out during the isolation. Gene expression levels were analyzed by two-step real-time RT-PCR. cDNAs were synthesized from 250 ng of total RNA using SuperScript II transcriptase (Invitrogen) and the reaction mixture was diluted 20 times for subsequent PCR. Quantitative real-time PCR was performed by using MiniOpticon with the SYBR Green I detection system under the following conditions: 95°C for 15 min; 40 cycles of 94°C for 15 s, 60°C for 30 s, 72°C 30 s followed by melting curve analysis. PCR mixtures with final volumes of 20 μL contained 5 μL cDNA, 0.3 μm of each primer, 2.5 mm Mg2+, and 10 μL of 2× QuantiTect SYBR Green Master mix (Qiagen). As a control, a reaction in which reverse transcriptase was omitted in the RT step was included. The following primer sets were used: AtNRT2.1 (forward, 5′-CCACAGATCCAGTGAAAGG; reverse, 5′-CATTGTTGGGTGTGTTCTCA); AtNRT2.2 (forward, 5′-CGGAGCACTATTATGTTGGC; reverse, 5′-GGTTGCGTTCCCTTTGT); and Clathrin-At4g24550 (internal control gene forward, 5′-ATACGCGCTGAGTTCCC; reverse, 5′-CTGACTGGCCCTGCTT).
13NO3− Influx Experiments
Nitrate influx using 13NO3− was measured as described before (Zhuo et al., 1999; Okamoto et al., 2003). The basic components of the solution for pretreatment, influx, and desorption were the same as those of the growth media, except that KNO3 replaced NH4NO3. Prior to measuring 13NO3− influx, plants were pretreated for 5 min with solution containing KNO3− at the same NO3− concentration as used for influx and then transferred for 10 min to the influx solution, which was labeled with 13NO3−. After the influx period, plant roots were desorbed with cold solution (identical to pretreatment solution) for 3 min to remove 13NO3− from the cell wall. Radioactivity was determined using a gamma-counter (MINAXI Auto-Gamma 5000 series, Packard Instruments).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AF019748 and AF019749.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (to A.D.M.G.) and by the National Institutes of Health (grant no. GM40672 to N.M.C.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Anthony D.M. Glass (aglass@interchange.ubc.ca).
Open Access articles can be viewed online without a subscription.
References
- Aslam M, Travis R, Huffaker RC (1993) Comparative induction of nitrate and nitrite uptake and reduction systems by ambient nitrate and nitrite in intact roots of barley (Hordeum vulgare L.) seedlings. Plant Physiol 102 811–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Travis RL, Huffaker RC (1992) Comparative kinetics and reciprocal inhibition of nitrate and nitrite uptake in roots of uninduced and induced barley (Hordeum vulgare L.) seedlings. Plant Physiol 99 1124–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford NM, Glass ADM (1998) Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci 3 389–395 [Google Scholar]
- Filleur S, Daniel-Vedele F (1999) Expression analysis of a high-affinity nitrate transporter isolated from Arabidopsis thaliana by differential display. Planta 207 461–469 [DOI] [PubMed] [Google Scholar]
- Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, Daniel-Vedele F (2001) An Arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett 489 220–224 [DOI] [PubMed] [Google Scholar]
- Forde BG (2000) Nitrate transporters in plants: structure, function and regulation. Biochim Biophys Acta 1465 219–236 [DOI] [PubMed] [Google Scholar]
- Galván A, Fernández E (2001) Eukaryotic nitrate and nitrite transporters. Cell Mol Life Sci 58 225–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass ADM, Siddiqi MY (1995) Nitrogen absorption by plant roots. In HS Srivastava, RP Singh, eds, Nitrogen Nutrition in Higher Plants. Associated Publishing Co., New Delhi, India, pp 21–56
- Kronzucker HJ, Siddiqi MY, Glass ADM (1995) Kinetics of NO3− influx in spruce. Plant Physiol 109 319–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little YD, Rao H, Oliva S, Daniel-Vedel F, Krapp A, Malamy JE (2005) The putative high-affinity nitrate transporterNRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci USA 102 13693–13698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min X, Siddiqi MY, Guy RD, Glass ADM, Kronzucker HJ (2000) A comparative kinetic analysis of nitrate and ammonium influx in two early-successional tree species of temperate and boreal forest ecosystems. Plant Cell Environ 23 321–328 [Google Scholar]
- Okamoto M, Vidmar JJ, Glass ADM (2003) Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol 44 304–317 [DOI] [PubMed] [Google Scholar]
- Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F (2004) Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta 219 714–721 [DOI] [PubMed] [Google Scholar]
- Orsel M, Krapp A, Daniel-Vedele F (2002) Analysis of the NRT2 nitrate transporter family in Arabidopsis: structure and gene expression. Plant Physiol 129 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada A, Galvan A, Fernandez E (1994) Identification of nitrate transporter genes in Chlamydomonas reinhardtii. Plant J 5 407–419 [DOI] [PubMed] [Google Scholar]
- Quesada A, Krapp A, Trueman LJ, Daniel-Vedele F, Fernandez E, Forde BG, Caboche M (1997) PCR-identification of a Nicotiana plumbaginifolia cDNA homologous to the high-affinity nitrate transporters of the crnA family. Plant Mol Biol 34 265–274 [DOI] [PubMed] [Google Scholar]
- Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, Gojon A (2006) A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol 140 909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi MY, Glass ADM, Ruth TJ, Rufty TW (1990) Studies of the uptake of nitrate in barley. I. Kinetics of 13NO3−influx. Plant Physiology 93 1426–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueman LJ, Richardson A, Forde BG (1996) Molecular cloning of higher plant homologues of the high-affinity nitrate transporters of Chlamydomonas reinhardtii and Aspergillus nidulans. Gene 175 223–231 [DOI] [PubMed] [Google Scholar]
- Vidmar JJ, Zhuo D, Siddiqi MY, Glass ADM (2000. a) Isolation and characterization of HvNRT2.3 and HvNRT2.4, cDNAs encoding high-affinity nitrate transporters from roots of Hordeum vulgare. Plant Physiol 122 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidmar JJ, Zhuo D, Siddiqi MY, Schjoerring JK, Touraine B, Glass ADM (2000. b) Regulation of HvNRT2 expression and high-affinity nitrate influx in roots of Hordeum vulgare by ammonium and amino acids. Plant Physiol 123 307–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkles SE, Hawker KL, Grieve C, Campbell EI, Montague P, Kinghorn JR (1991) crnA encodes a nitrate transporter in Aspergillus nidulans. Proc Natl Acad Sci USA 88 204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unkles SE, Zhuo D, Siddiqi MY, Kinghorn JR, Glass ADM (2001) Apparent genetic redundancy facilitates ecological plasticity for nitrate transport. EMBO J 22 6246–6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu D, Crawford NM (1998) The Arabidopsis CHL1 protein plays a major role in high-affinity nitrate uptake. Proc Natl Acad Sci USA 95 15134–15139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo D, Okamoto M, Vidmar JJ, Glass ADM (1999) Regulation of a putative high-affinity nitrate transporter (Nrt2;1At) in roots of Arabidopsis thaliana. Plant J 17 563–568 [DOI] [PubMed] [Google Scholar]