Abstract
For photoheterotrophic growth, a Chlamydomonas reinhardtii cell requires at least 1.7 × 107 manganese ions in the medium. At lower manganese ion concentrations (typically <0.5 μm), cells divide more slowly, accumulate less chlorophyll, and the culture reaches stationary phase at lower cell density. Below 0.1 μm supplemental manganese ion in the medium, the cells are photosynthetically defective. This is accompanied by decreased abundance of D1, which binds the Mn4Ca cluster, and release of the OEE proteins from the membrane. Assay of Mn superoxide dismutase (MnSOD) indicates loss of activity of two isozymes in proportion to the Mn deficiency. The expression of MSD3 through MSD5, encoding various isoforms of the MnSODs, is up-regulated severalfold in Mn-deficient cells, but neither expression nor activity of the plastid Fe-containing superoxide dismutase is changed, which contrasts with the dramatically increased MSD3 expression and plastid MnSOD activity in Fe-deficient cells. Mn-deficient cells are selectively sensitive to peroxide but not methyl viologen or Rose Bengal, and GPXs, APX, and MSRA2 genes (encoding glutathione peroxidase, ascorbate peroxidase, and methionine sulfoxide reductase 2) are slightly up-regulated. Elemental analysis indicates that the Mn, Fe, and P contents of cells in the Mn-deficient cultures were reduced in proportion to the deficiency. A natural resistance-associated macrophage protein homolog and one of five metal tolerance proteins were induced in Mn-deficient cells but not in Fe-deficient cells, suggesting that the corresponding gene products may be components of a Mn2+-selective assimilation pathway.
Manganese is nutritionally essential for growth and survival of all living organisms because of its function as a redox cofactor in some enzymes or as an activator at a metal binding site of other enzymes (Frieden, 1985; Marschner, 1995; Christianson, 1997; Yocum and Pecoraro, 1999; Keen et al., 2000; Jakubovics and Jenkinson, 2001; Kehres and Maguire, 2003). For example, redox active manganese is present in manganese superoxide dismutase (MnSOD), which is the principal antioxidant enzyme of mitochondria, whereas in arginase, the catalytic Mn2+ activates bound water to generate the nucleophile for hydrolysis of the guanidinium group of Arg (van Loon et al., 1986; Lebovitz et al., 1996). In photosynthetic organisms, manganese is also present as a polynuclear cluster in PSII where it catalyzes the water-splitting reaction (for review, see Merchant and Sawaya, 2005).
The MnSODs and PSII are expected to be the prime targets of Mn deficiency in plants (Yu and Rengel, 1999), and indeed the importance of manganese in the photochemical reactions of photosynthesis was recognized half a century ago because of the impact of deficiency on oxygen evolution and phototrophic growth (Pirson, 1955; Teichler-Zallen, 1969).
Three types of Mn2+ transporting systems are known in bacteria: the MntABC-type proteins that were originally discovered by Pakrasi and coworkers (Bartsevich and Pakrasi, 1995) as being necessary for PSII function in cyanobacteria, the MntH-type proteins that are related to the eukaryotic divalent metal transporters called natural resistance-associated macrophage proteins (Nramps; for review, see Kehres and Maguire, 2003), and a P-type ATPase identified in Lactobacillus plantarum (Hao et al., 1999). The expression of the transporters is regulated by manganese nutrition status and involves specific sensor-regulator signal transduction pathways (e.g. Ogawa et al., 2002; Yamaguchi et al., 2002; Chandler et al., 2003; Guedon et al., 2003). The expression of the MntH-type proteins is also determined by iron status, because these transporters, although more selective for Mn2+, do include Fe2+ among the substrates they handle (Kehres et al., 2002).
Mn assimilation in eukaryotes is attributed to members of the widely distributed Nramp family related to MntH mentioned above. The founding member, Nramp1, was discovered in mouse as a host resistance factor, and its function as a H+-divalent cation symporter, especially for Mn2+, became apparent when a related protein in yeast (Saccharomyces cerevisiae) was shown to be involved in Mn2+ uptake and when transport activity was eventually established for Nramp2 (also called DCT1 or DMT1) by functional assay in the Xenopus oocyte system (Supek et al., 1996; Gunshin et al., 1997; Jabado et al., 2000). Nramp2 is induced by Fe deficiency in animals and probably functions largely in iron assimilation in vivo, indicating that individual Nramp family members may play distinct roles in micronutrient homeostasis.
In plants as well, the Nramps form a family of related proteins but with functionally distinct roles based on subcellular location, organ specific pattern of expression, metal specificity, and pH sensitivity (Belouchi et al., 1997; Curie et al., 2000; Thomine et al., 2003). The Arabidopsis (Arabidopsis thaliana) proteins are capable of transporting both Mn2+ and Fe2+ based on their ability to rescue smf1 or fet3fet4 yeast strains, which are defective in high affinity Mn2+ and Fe2+ uptake, respectively, and at least two members are induced in vivo by Fe deficiency (Curie et al., 2000; Thomine et al., 2000, 2003). Likewise, in tomato (Lycopersicon esculentum), individual members show a different pattern of expression in response to Fe deficiency (Bereczky et al., 2003). The regulation of plant NRAMP gene expression by manganese has received less attention.
In yeast, there are three Nramp-type transporters: Smf1p, Smf2p, and Smf3p (for review, see Culotta et al., 2005). Smf1p and Smf2p are especially involved in manganese homeostasis and the biosynthesis of Mn-containing enzymes, although they do show broad substrate specificity, while Smf3p function is related to iron mobilization from the vacuole in an Fe-deficient situation. The PHO84-type phosphate transporters have also been shown to participate in low affinity Mn2+ uptake in yeast, perhaps because of a substrate preference of the phosphate transporter for a neutral M(II)HPO4 substrate (Fristedt et al., 1999; Jensen et al., 2003). Because the phosphate transporters are not regulated by manganese nutrition status, this activity can lead to accumulation of toxic amounts of manganese in yeast.
In plants, proteins of the cation diffusion facilitator family (named MTP for metal tolerance protein) have been shown to confer Mn tolerance, implicating them in Mn2+ efflux or sequestration into the vacuole (for review, see Hall and Williams, 2003; Kochian et al., 2004; Hanikenne et al., 2005).
Mn deficiency in plants, especially problematic in alkaline soils, is noted by leaf discoloration and impacts freezing tolerance, reproductive fitness, and carbohydrate metabolism (Marschner, 1995). In some regions in Australia, manganese is recognized as one among several micronutrient deficiencies that limits productivity in agricultural zones, and at a reforested site in Europe, Mn deficiency was implicated in needle chlorosis and hence perhaps is a contributing factor to forest decline (Donald and Prescott, 1975; Hiltbrunner and Flückiger, 1996). Yet the molecular details of manganese metabolism and biochemical consequences of deficiency are underinvestigated relative to the metabolism of iron.
Studies of molecular responses to nutrition are facilitated with a microorganism model system because of the ease with which nutrient supply can be manipulated. Chlamydomonas reinhardtii is a widely used model for understanding mechanisms underlying adaptive responses to both macronutrients, like nitrogen, sulfur, and phosphorus, and micronutrients, like the trace transition elements, as they relate to plant metabolism (Grossman, 2000; Merchant et al., 2006). Here, we present the molecular phenotype of Mn deficiency in wild-type Chlamydomonas.
RESULTS
Growth in Mn-Deficient Medium
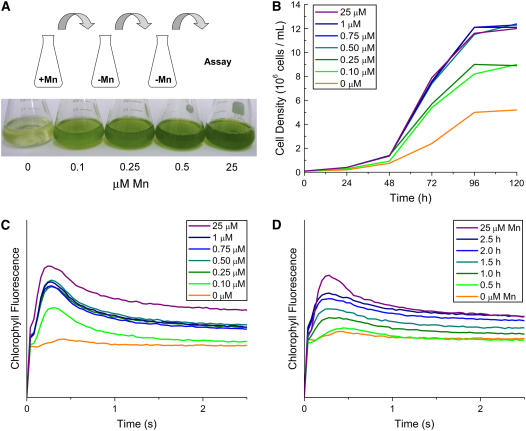
The standard Chlamydomonas Tris-acetate-phosphate (TAP) medium with Hutner's trace elements contains 25 μm Mn ions. To test the manganese requirement for growth, cells were transferred twice sequentially into Mn2+-free medium (see “Materials and Methods”) and then tested after a third transfer into medium containing the indicated amounts of Mn ions. Chlamydomonas will take up more manganese than required when provided with 25 μm in the medium; sequential transfer is therefore required to deplete the excess stores of manganese (Supplemental Fig. S1). As noted previously for Chlorella, growth in the dark under heterotrophic conditions showed a very slight effect of manganese removal (Eyster et al., 1958). Under photoheterotrophic conditions, a growth phenotype (longer doubling time and stationary phase at lower cell density) was evident when the manganese in the medium was reduced to 0.25 μm and became more severe as the manganese supplementation was further reduced (Fig. 1, A and B).
Figure 1.
Manganese is required for photosynthetic electron transfer and growth of Chlamydomonas. Cells adapted to Mn deficiency (see “Materials and Methods”) were transferred to TAP medium containing the indicated amounts of supplemental manganese and grown photoheterotrophically. A, The visual phenotype of Mn deficiency is shown after 3 d growth. B, Growth was measured by counting cells every 24 h with a hemocytometer. C, Room temperature chlorophyll fluorescence induction kinetics of cells grown (96 h) at the indicated manganese concentration. D, Restoration of fluorescence induction was monitored as a function of time after addition of 25 μm manganese to a Mn-deficient culture (0 μm). The pattern from a Mn-replete culture is shown for comparison (25 μm). All experiments were performed in experimental duplicate in strain CC425 and verified in strain CC1021.
PSII Function Is Compromised
It is likely that loss of photosynthetic electron transfer function contributes to the growth phenotype, because there is a strict requirement of manganese for photosynthesis (Fig. 1C, orange trace). The rise phase of the kinetic trace is indicative of PSII function (reduction of primary electron-accepting plastoquinone of PSII [QA]), while the decay phase reports on downstream events through the Cyt b6f complex and PSI (reoxidation of QA). The position of the peak reflects the balance between PSII and PSI function. The shape of the curve in Mn deficiency is therefore consistent with a specific loss of PSII function upstream of QA. Indeed, under phototrophic conditions, Chlamydomonas cells do not grow when the manganese supplementation in the medium falls below 0.1 μm (Merchant et al., 2006).
When manganese was added back to the photoheterotrophic cultures containing no supplemental manganese, photosynthetic electron transfer function was quickly (by 1 h), albeit not immediately (by 0.5 h), restored and increased with time (Fig. 1D). The peak of the chlorophyll fluorescence kinetic curve shifted progressively leftward, indicative of a restoration of PSII function. Measurement of the Mn content of cells indicated an increase within 1 h of supplementation but reached the level maintained in fully replete cells only after 4 h, suggesting that manganese metabolism, including assimilation, compartmentation, and cluster formation, may be the time-dependent step rather than the synthesis of PSII components (see below). The addition of protein synthesis inhibitors cycloheximide and chloramphenicol did not block recovery of PSII, indicating that the polypeptide components are preexisting (data not shown).
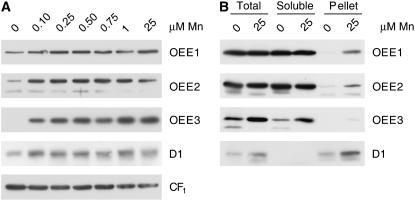
We noted a complete loss of phototrophic growth in cells grown in medium containing <0.1 μm manganese (Merchant et al., 2006). When we monitored the abundance of D1, we noticed an incomplete loss (50%–75% decrease; Fig. 2A). When we monitored the OEE proteins, a fraction of the OEE1 and OEE2 polypeptides and almost all of the OEE3 protein were released from the membrane and recovered instead in the soluble fraction (Fig. 2B). We conclude that manganese is not absolutely required for the accumulation of PSII polypeptides, although it does stabilize the complex on the membrane.
Figure 2.
Loss of the OEE complex in Mn-deficient cells. A, Thirty micrograms of total membrane protein was loaded onto a denaturing polyacrylamide gel and PSII proteins D1, OEE1, OEE2, and OEE3 (PsbO, P, and Q, respectively) were analyzed for abundance in extracts of cells grown in medium with the indicated manganese concentrations. The α- and β-subunits of CF1 (chloroplast ATP synthase) are used as a loading control. The experiment shown is representative of three independent experiments. B, Total cell extract from Mn deficient (0 μm) or Mn replete (25 μm) was separated into soluble and pellet fractions (see “Materials and Methods”). The sum of the soluble and pellet fraction equals total cell extract. Samples were analyzed as for part A. The experiment shown is representative of two independent fractionations.
Reduced MnSOD Activity in Deficient Cells
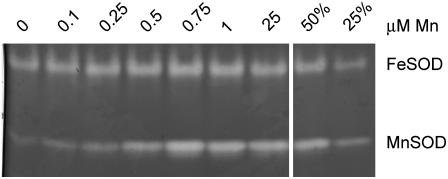
SODs are found with different metal cofactors used for catalysis: CuZnSOD, FeSOD, MnSOD, and NiSOD (Wolfe-Simon et al., 2005). Biochemical analysis identified at least one FeSOD and at least two other MnSODs in Chlamydomonas organelles (Sakurai et al., 1993; Kitayama et al., 1999). This corresponds well with the occurrence of a single FSD1 gene encoding an FeSOD and five genes, MSD1 through MSD5, encoding MnSODs in the version 3.0 draft of the Chlamydomonas genome (http://genome.jgi-psf.org/Chlre3/Chlre3.home.html). We identified two major activities in soluble protein extracts of Chlamydomonas as an FeSOD and a MnSOD (Fig. 3). Mn deficiency had a marked impact on the activity of the major MnSOD; reduced activity was noted when the medium Mn2+ supplement was reduced to 0.5 μm and the loss was proportionally greater as manganese nutrition was reduced (Fig. 3). Other peroxide-resistant minor MnSOD isoforms were occasionally visible in Mn-replete cultures as well, and one of these, migrating just below the major MnSOD isoform, also showed reduced activity in Mn deficiency (data not shown). We assume that the loss of activity is a consequence of the absence of manganese, a necessary cofactor for activity, but we cannot rule out the operation of posttranslational regulatory mechanisms. Surprisingly, we noted also a decrease in the FeSOD activity but only when the manganese supplement in the medium was reduced to 0.1 μm or less. Cell fractionation showed that the major MnSOD is localized to both plastids and mitochondria (Fig. 6B; Kitayama et al., 1999).
Figure 3.
Loss of MnSOD activity in Mn-deficient cells. Twenty micrograms of total soluble protein was loaded onto a nondenaturing polyacrylamide gel and analyzed for SOD activity in extracts of cells grown in medium with the indicated manganese concentrations. Two- and 4-fold dilutions of the 25-μm sample are included for relative activity comparison. All experiments were performed in experimental triplicate.
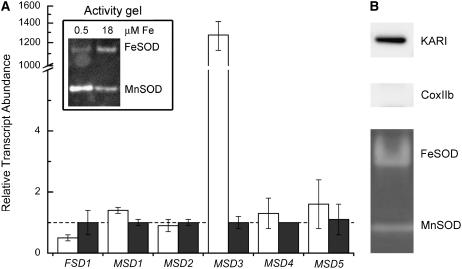
Figure 6.
MnSOD is up-regulated by Fe deficiency. A, Protein and RNA were isolated from cells grown in 0.5 μm (white bars) or 18 μm (gray bars) iron. Gene expression was assessed by real-time PCR as described in the legend to Figure 5, except that the error bars represent the variation in technical triplicates of the PCR reactions. An independent experiment is shown in Supplemental Figure S3. Inset, Twenty micrograms total protein was loaded onto a nondenaturing polyacrylamide gel and SOD activity was analyzed. All experiments were performed in experimental duplicate in strain CC425 and verified in strain CC1021. B, Chloroplasts were isolated from strain CC400 as described in “Materials and Methods.” Samples (20 μg) were loaded onto a denaturing gel, fractions were tested for purity by immunoblotting with compartment-specific markers (keto-acid reductase isomerase [KARI] for the chloroplast, CoxIIb for the mitochondrion). SOD activity was assessed as in Figure 3, except that 40 μg of soluble protein was loaded on the gel.
Mn-Deficient Cells Are Sensitive to Peroxide Stress
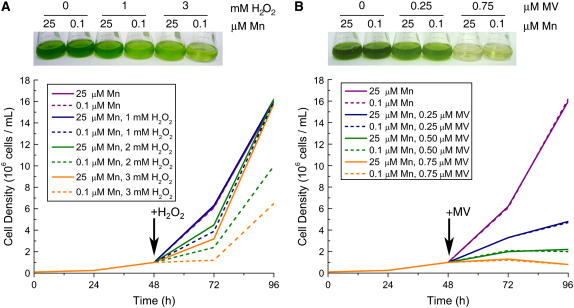
Because MnSOD has antioxidant functions, we tested the impact of manganese nutrition on the ability of Chlamydomonas cells to resist oxidative stress (Fig. 4). We found that there was no impact of manganese nutrition on sensitivity to methyl viologen (Fig. 4B) or metronidazole, which promote the generation of superoxide through PSI activity, nor to Rose Bengal and Neutral Red, which promote singlet oxygen (Supplemental Fig. S2; Ledford and Niyogi, 2005). On the other hand, Mn-deficient cultures (grown in 0.1 μm Mn2+) were more susceptible to hydrogen peroxide (H2O2) relative to Mn-replete cultures (Fig. 4A) as well as to cumene hydroperoxide or t-butyl hydroperoxide (Supplemental Fig. S2). It is possible that plastid FeSOD, whose activity is not greatly reduced at 0.1 μm manganese, is an adequate defense against plastid-generated oxidative stress.
Figure 4.
Selective sensitivity of Mn-deficient cells to H2O2. Cells were grown in 0.1-μm supplemental Mn or 25-μm Mn conditions for 2 d. The growth rate of cells in 0.1 μm supplemental Mn is similar to that of cells grown with 25 μm Mn. Cultures were diluted to 1 × 106 cells/mL before the addition of the indicated concentrations of H2O2 (A) or methyl viologen (MV; B). Growth was monitored by counting cells in a hemocytometer (bottom). Cultures were photographed 24 (H2O2) and 48 (MV) h after exposure to the chemical (top). A duplicate experiment is shown in Supplemental Figure S3.
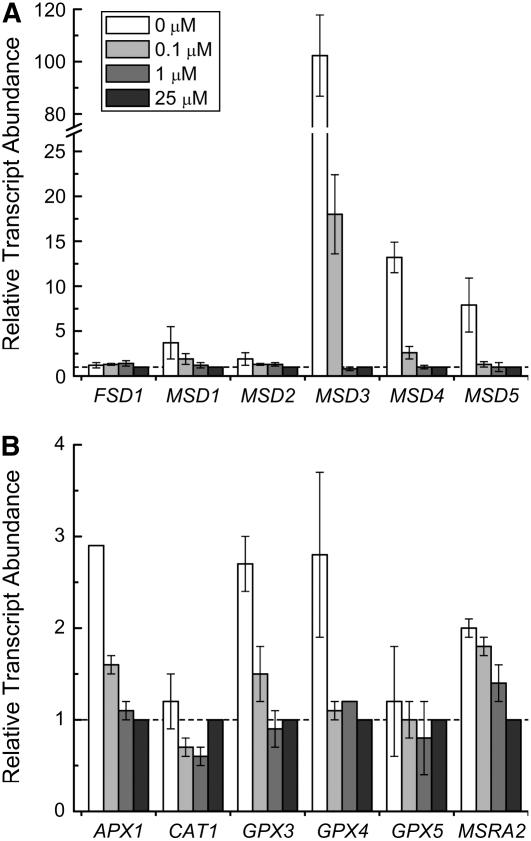
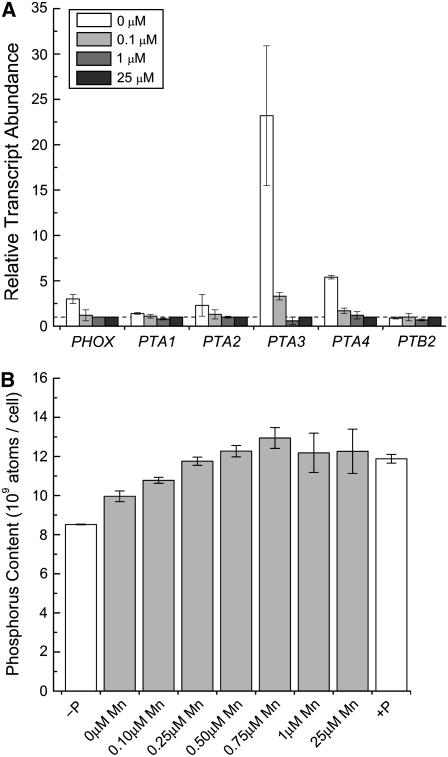
When we tested the expression of antioxidant enzymes (Table I), we noted that of the six SOD-encoding genes, three were induced by Mn deficiency: MSD3, MSD4, and MSD5. Increased expression was evident only upon severe Mn deficiency: for MSD3 at a supplement of 0.1 μm Mn2+ or less, and for the other two only in the zero-supplement growth medium (Fig. 5A). The increase of MSD mRNAs lagged behind the loss of MnSOD activity (Fig. 3). This suggests that increased abundance of MSD3, MSD4, and MSD5 mRNAs is not directly responsive to manganese nutrition and loss of MnSOD activity but rather to a downstream consequence (see “Discussion”). When Mn2+ was resupplied to Mn-deficient cells, MSD3 mRNAs were reduced in abundance with a time course that paralleled the recovery of PSII activity (compare Fig. 1 with Fig. 8, discussed below). The genes encoding glutathione peroxidases and ascorbate peroxidase were only slightly induced in Mn-deficient versus Mn-replete cells (Fig. 5B), which is comparable to the increase in peroxide- or methyl viologen-treated cells and much less than the 102-fold increase in GPX gene expression noted in cells treated with singlet oxygen generating photosensitizers (Leisinger et al., 2001). The expression of antioxidant selenoenzymes was likewise not increased in Mn-deficient cultures.
Table I.
Primers used for real-time PCR
The primer pairs for each gene model (corresponding to the Version 3.0 draft genome) are shown. The upper row of each set corresponds to the forward direction for the gene model and the lower row to the reverse direction. All primer sequences are written 5′ to 3′. The percent efficiency for each primer pair is calculated based on the theoretical doubling of product at each cycle. For 100% efficient primers, the amount of product doubles at every cycle.
| Name | Protein ID | Percent Efficiency | Primer Pair |
|---|---|---|---|
| APX1 | 186597 | 91 | TCAAGGAGATCAAGGCCAAG |
| GCCGCTCAGTCCAGAGTAAC | |||
| CAT1 | 150104 | 86 | CACGAGGGCTTCATGAACTT |
| CTTGGCGATCACCATCTTCT | |||
| COPT1 | 196102 | 106 | TCTGGTTTGCTGGGCTCTCC |
| GTACACCGCCAGTCCCTCGT | |||
| COT1 | 116945 | 101 | CGCCGACCACCACCTGTATC |
| GAGCACGCTGTAGGCAAGCA | |||
| CTP1 | 121438 | 92 | GGTGTTGACCTGGCTACGCTGTG |
| GCCACCGGTGCCAAGGGAGGA | |||
| CTR1 | 196101 | 81 | CCTTCAACATCGGCTTCTTC |
| CCCTTCTTGTGGTCAGCAGT | |||
| CTR2 | 196096 | 87 | CGCTGCTGAACCTCATCTC |
| GCAGGTGGCCCAGGAATAG | |||
| CβLP | 105734 | 108 | GCCACACCGAGTGGGTGTCGTGCG |
| CCTTGCCGCCCGAGGCGCACAGCG | |||
| FEA1 | 129929 | 93 | TGCTGGGCCTGCAGGGCGTGTC |
| ACGGCGGAGGCCTTGAAGTTGC | |||
| FSD1 | 182933 | 95 | CCGTGGTGGAGGGCAAGACCCCCAT |
| TCGCCTGGCGCCGACATGCTTGTGA | |||
| GPX3 | 137012 | 108 | GACAAGGCGGTTCTGGTCGT |
| AGCCCTGCTTGCCGTACTTG | |||
| GPX4 | 188373 | 96 | GCAAGGACCTCATCTGGAAC |
| TATAGATCGCCCCCTCAACA | |||
| GPX5 | 143122 | 87 | CTAGCAACTGCGGCTTTACG |
| GCTTCTTTTGGCTCTTCAGG | |||
| HMA1 | 195998 | 97 | ACGGATGACGAACAGCAGCA |
| CTCACACAGCCCCTTCCACA | |||
| IRT1 | 196644 | 104 | GGGCTCTTCGCACGTCTTCA |
| AGGGGACCAGGAAGCCAAAA | |||
| IRT2 | 174212 | 97 | GGGGCTACTGATGGCGGTGA |
| GTAGATGGGTGCGGCGATGA | |||
| MSD1 | 53941 | 102 | TGATGACCGCGCTGCCGGACGTGGC |
| TCACAGCGGCACGGTGCCGGCCTGG | |||
| MSD2 | 193511 | 101 | ACAAGCTGGAGGTGGTGACGCTGCC |
| TTACTTCTTGGCGGCGGCCAGGCG | |||
| MSD3 | 196645 | 109 | ACCAGACCCACCCAGGAG |
| CAGCCCCAACCAGGATAAC | |||
| MSD4 | 114882 | 88 | CCATGAGTGGGTTGTCCTG |
| GACACTGGAACCACCAGATG | |||
| MSD5 | 115390 | 90 | CACAGCCAACCAGGACAACC |
| CGCGGACCAGTTTATCAG | |||
| MSRA2 | 196103 | 100 | ACCGCGTGCGGCTGGCCACCA |
| GAGCCACGGCGCATCATGGA | |||
| MTP1 | 119301 | 95 | CGTGTGGCTTGAGCGAAGAG |
| GCTTGCGTGCGATGATACGA | |||
| MTP2 | 150298 | 97 | ATGAGTGTGCGGGAGTCGCA |
| GGCAGTGGCTTCATCACGTC | |||
| MTP3 | 195957 | 96 | ATCGAGGCTCTTGACACTGT |
| GTAAGGCGCTGCTCAGCGTC | |||
| MTP4 | 150298 | 101 | ACATGTGTGTGCGGGAGTCG |
| CTTGTGCCGGTGCAGGGACC | |||
| MTP5 | 149470 | 96 | CCTTCATCCTCAGCCATAGCA |
| CGAGAGGTTGGGAAGTGAG | |||
| NIK1 | 173008 | 102 | CCACCTACGCCACAGGCATC |
| TGCCGATCACGAACATGGAG | |||
| NRAMP1 | 128400 | 109 | GCGCCGCATTATCACCCGTGGCGCCGCCATC |
| CAGCGGTTGGCGAAGTTGCCGAGGTAC | |||
| NRAMP2 | 113049 | 87 | GCGACGCCTACGAGCTGCTGGCCACCTC |
| ACTTGATGGCCAGAAAGCCCTCC | |||
| ZIP1 | 122719 | 100 | GCTGGCTTCTACAGCCTCACC |
| ACGAACCTGCCCATGTCCTC | |||
| ZIP3 | 100805 | 92 | CCGTCATCTCGCTGTTCGTG |
| CCCTTTGGGTCCTGTTCGTG | |||
| ZIP6 | 183171 | 86 | GGCAAGAACCGGCTGCTAAA |
| CTCCTGCGATGGGTGTTCCT | |||
| ZRT1 | 146152 | 90 | TCGTCTTCGGGTTCCTCCTG |
| CGGGGGTGTTATCCTCGTTG | |||
| ZRT2 | 107206 | 97 | GGCTGCGTGTTCCACTCCTT |
| CTGATGCCCTCCGCAAACTG | |||
| ZRT3 | 168584 | 96 | CCTTTCCCACAAACACGCAAG |
| TGCCGATGATGATGCTGTGA | |||
| ZRT4 | 196642 | 92 | GGGCTTCCTGTGGTCTGTGG |
| GCCGTACATGAGGGGGTCTG | |||
| ZRT5 | 196756 | 100 | TGCGTGTTCCACAGCTTCATC |
| ACCACAGACGCCAGCGAGAC |
Figure 5.
Relative expression of oxidative stress response genes. RNA was isolated from cells grown in TAP medium containing the indicated manganese supplement plus excess Fe (50 μm). Relative expression was normalized to CβLP expression, and average CT value was calculated from technical triplicates. The error bars represent variation from biological duplicates. Expression was calculated by the 2−ΔΔCT method. All experiments were performed in experimental duplicate and also at two different concentrations of iron (18 and 50 μm).
Figure 8.
(Legend appears on following page.)
Plastid MnSOD Activity and MSD3 Expression Increase in Fe Deficiency
Because there is no increase in the accumulation of FSD1 mRNA nor any increase in FeSOD activity, it is evident that FeSOD cannot cover the loss of MnSOD function in Mn-deficient cells. On the other hand, in Fe-deficient cells, where FeSOD activity is reduced, the activity of the major MnSOD isoform was increased to compensate for the deficiency (Fig. 6, inset). This isoform is likely the product of the MSD3 gene, because its expression is dramatically (103-fold) increased in Fe deficiency (Fig. 6; Supplemental Fig. S3). We suggest that the MSD3 gene is directly responsive to iron nutrition rather than secondarily to reactive oxygen species, because we cannot mimic this pattern of expression by imposition of various oxidative stress conditions (high light, peroxide, methyl viologen, or Rose Bengal treatment; J. Long and S. Merchant, unpublished data). Because the major MnSOD activity was found in the chloroplast fraction, the increased expression of the MSD3 gene may compensate for loss of chloroplast FeSOD in Fe deficiency by MnSOD (Fig. 6B).
Candidate Manganese Transporters
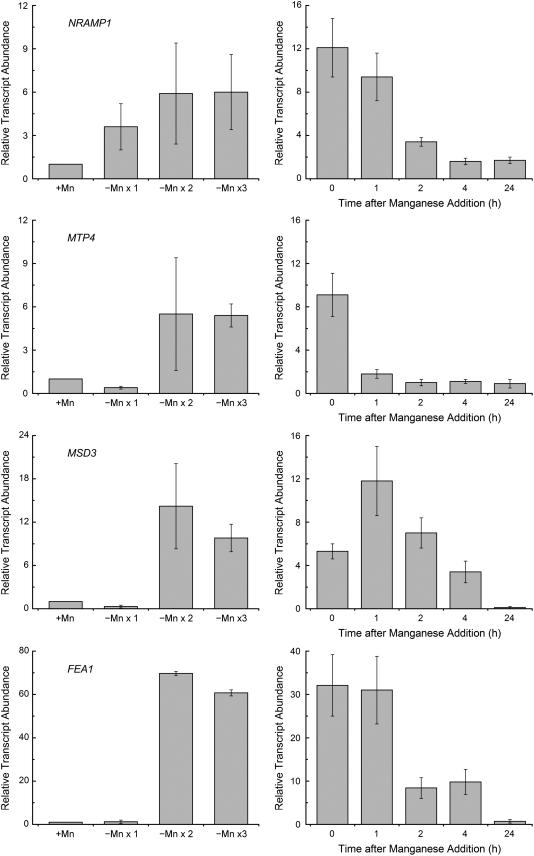
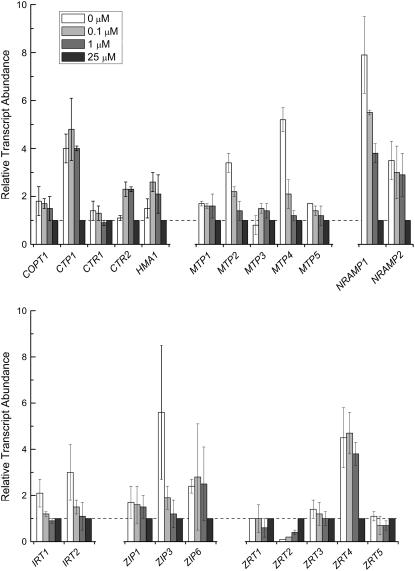
When cells are starved for a nutrient, the first line of defense is the activation of assimilatory transporters. The identity of the manganese uptake transporter(s) is not known, but an NRAMP homolog is an excellent candidate (see introduction). RNAs corresponding to both NRAMP genes of Chlamydomonas increased in Mn-deficient relative to Mn-replete cells, with NRAMP1 showing a pattern of expression typical for an assimilatory transporter (Fig. 7). Specifically, the RNA was increased in abundance already at 1 μm manganese supplementation, prior to the appearance of symptoms (Figs. 1–4), but at a concentration that is just barely adequate to support the accumulation of manganese to the level maintained in a replete culture at stationary phase (1.3–1.5 × 107 Mn atoms/cell) and continued to increase (up to 8-fold relative to Mn-replete cells) as the Mn2+ content of the medium was further reduced. NRAMP2 expression also increased in cells grown in 1 μm Mn2+ but did not increase further with reduced Mn2+ supplementation, and the magnitude of the change was less than that for NRAMP1 (Fig. 7). For MTP4, the increase in expression was not evident until the cells were severely Mn deficient, which is not typical of an assimilatory transporter.
Figure 7.
Relative expression of genes encoding candidate manganese transporters. RNA was isolated from cells grown in TAP medium containing the indicated amounts of manganese supplement plus excess Fe (50 μm). Gene expression was assessed by real-time PCR as described in the legend to Figure 5. Genes encoding transporters of the CTR family (COPT1, CTR1, and CTR2), HMA family (CTP2 and HMA1), cation diffusion facilitator family (MTP1-MTP5), Nramp family (NRAMP1 and NRAMP2), and ZIP family (IRT1, IRT2, ZIP1, ZIP3, ZIP6, and ZRT1-ZRT5) were identified in the Chlamydomonas draft genome by sequence homology. All experiments were performed in experimental duplicate in 18 and 50 μm Fe.
When we analyzed the expression of NRAMP1 upon transfer of Mn-replete cells to Mn-deficient medium, a 4-fold increase in expression was evident already upon the first round of transfer, and this correlates nicely with a reduced manganese content of cells after the first transfer (Fig. 8). Maximum expression was achieved after the second sequential transfer to Mn-deficient medium, again correlated with maximally reduced total manganese content of cells. When Mn2+ was added back to the deficient cells, NRAMP1 expression did not decrease immediately and remained high even 2 h later (by which time the manganese content of cells had reached the level maintained in a replete situation) but returned to basal levels within 24 h (Fig. 8). This contrasts with the immediate change in expression of the MTP4 gene (within 1 h) upon replenishment of Mn2+ to the deficient culture and suggests that regulatory mechanisms that affect the NRAMP1 protein directly may be operational. In plants, members of the cation diffusion facilitator family or cation efflux family (called MTPs) have been implicated in manganese homeostasis, particularly in a situation of manganese excess (Mäser et al., 2001). We tested five members of this family and noted that one, MTP4, showed significantly (9-fold) increased accumulation but only when the manganese content of the medium was severely reduced (Fig. 8). Unlike NRAMP1, whose expression was increased upon the first transfer to medium lacking manganese supplementation, MTP expression was actually depressed in this situation and increased only after the second transfer to deficient medium (Fig. 8). Given the role of the MTPs in cation efflux, this pattern of expression would be more consistent with an intracellular distributive transporter that could function to prioritize manganese distribution from one organelle to another in a situation of limiting intracellular manganese. This may account for the loss of MnSOD but not PSII at 0.5 μm manganese in the medium (Fig. 1 versus Fig. 3).
We tested also the expression of genes encoding candidate copper transporters (assimilatory molecules of the CTR family, distributive molecules of the HMA family), zinc and iron transporters of the ZIP family and components of the high affinity iron uptake pathway, ferroxidase, ferric transporter, and ferrireductase (Merchant et al., 2006). None of the genes showed a pattern of expression consistent with a function in manganese assimilation (Fig. 7; Supplemental Fig. S4). The mRNA for one Zn-deficiency-responsive transporter, ZRT2, was consistently reduced in expression in Mn deficiency, and the pattern was evident already at 1 μm manganese in the medium (where no symptoms are evident), suggesting a direct response to manganese nutrition rather than an indirect response to stress.
Secondary Fe Deficiency Results from Mn Deficiency
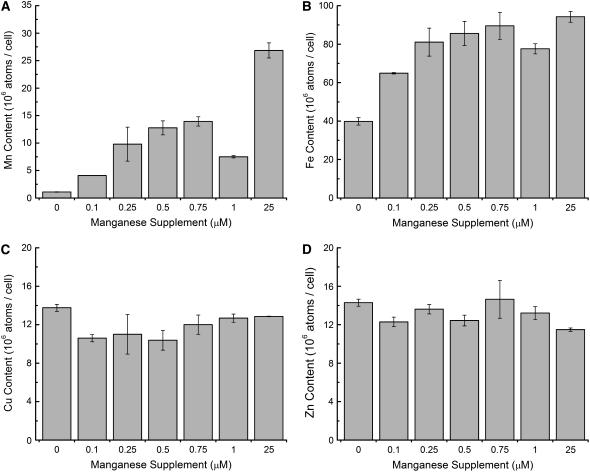
When we tested the expression of components of the iron assimilation pathway (La Fontaine et al., 2002), we noted that the FOX1, FTR1, FEA1, and FRE1 genes were up-regulated (Table II). The increase in gene expression was only about 10% of that noted in Fe-deficient (1 μm iron) cells. The loss of FeSOD activity and the appearance of mild chlorosis in the Mn-deficient culture together suggest the cells are Fe deficient. Analysis of the metal content of Chlamydomonas cells indicated a reduction in the Fe content correlated with a progressive decrease from 0.25 μm manganese nutrition in the medium (Fig. 9). Without manganese supplementation, the cells contained about one-half the iron of a Mn-replete culture. The effect is specific for iron, because the Cu and Zn content of cells are not affected by manganese nutrition (Fig. 9). The amount of iron in the cells is comparable to the iron content of cells grown in Fe-deficient conditions (1–3 μm iron, 25 μm Mn2+), but, as noted above, the increase in gene expression was only a fraction of that observed in Fe deficiency. Because the iron assimilation pathway relies on proteins that are processed through the secretory pathway and presumably glycosylated, we wondered whether the phenotype might be explained by loss of function of Mn-dependent protein-modifying glycosyl transferases. Nevertheless, when we monitored the synthesis of plasma membrane ferroxidase, periplasmic arylsulfatase, and carbonic anhydrase, we saw no evidence for reduced secretion of any of these proteins (data not shown). In fact, the ferroxidase was increased in abundance in Mn deficiency, as expected from the increased abundance of the mRNA (Table II). Some phenotypes of Mn deficiency could be partially restored by provision of extra iron (50 μm) in the medium, and reduction of iron from the standard 18 to 1 μm exacerbated those phenotypes, suggesting that a component of the Mn-deficiency phenotype is related to iron nutrition; yet, the iron content of the Mn-deficient cells could not be restored by excess iron supplementation to the level maintained by nutrient-replete Chlamydomonas, and the expression of genes involved in iron assimilation remained slightly elevated.
Table II.
Fe-responsive gene expression during Mn deficiency
Gene expression was assessed by real-time PCR. Cells were made Fe or Mn deficient and harvested as described in “Materials and Methods.” Relative expression was normalized to CβLP expression, and average CT value was calculated from technical triplicates and compared to expression in replete conditions. Expression was calculated by the 2−ΔΔCT method (Livak and Schmittgen, 2001).
| Gene | Relative Gene Expression
|
|
|---|---|---|
| 1 μm Iron | 0 μm Supplemental Mn2+ | |
| FEA1 | 2.0 × 102 | 2.4 × 101 |
| FOX1 | 3.5 × 101 | 4.1 × 100 |
| FRE1 | 3.3 × 102 | 1.9 × 102 |
| FTR1 | 5.3 × 101 | 4.7 × 100 |
Figure 9.
Intracellular metal content. Cells were grown in medium supplemented with Mn2+ to the indicated concentration, and total Mn (A), Fe (B), Cu (C), and Zn (D) content was measured by inductively coupled plasma-mass spectrometry (see “Materials and Methods”). All experiments were performed in biological duplicate.
Phosphorus Content Is Reduced
The total P content of Mn-deficient cells was also reduced relative to the Mn-replete condition in proportion to the deficiency and approached but did not reach the P content of cells starved for P for 24 h (Fig. 10). One of the four genes, PTA3, encoding a PHO84-type phosphate transporter, was 25-fold up-regulated, and a second gene, PTA4, was 5-fold up-regulated by Mn deficiency. The PHO84 transporters use a divalent cation as the counterion (van Veen et al., 1994; Persson et al., 1999): the up-regulation of a subset suggests that Mn2+ may be the preferred counterion for these transporters. In yeast, up-regulation of PHO84 can suppress Δsmf2, indicative of Mn2+ cotransport (Jensen et al., 2003).
Figure 10.
Mn-deficient cells are phosphorus deficient. A, RNA was isolated from cells grown in TAP medium containing the indicated amounts of Mn2+ supplement. Gene expression was assessed by real-time PCR as described in the legend to Figure 5. The results shown are representative of experimental duplicates. The iron content of the medium (18 and 50 μm) had no impact on the result. B, Phosphorus accumulation was measured by inductively coupled plasma-atomic emission spectroscopy (see “Materials and Methods”) during Mn deficiency (gray bars). Phosphorus-starved (for 24 h) and -replete cultures are included as controls (white bars). Experiments were performed in biological duplicate in strain CC425 and verified in strain CC1021.
The pattern of PTA gene expression in Mn-deficient Chlamydomonas is different from that in P deficiency, where PTA3 is unaffected while PTA4 is 30- to 50-fold up-regulated. In addition, PTA1 is unresponsive in Mn deficiency, while it is 103-fold repressed by P deficiency (Moseley et al., 2006). These results indicate independent regulation of PTA genes by manganese and phosphorus nutrient status. Note that PTB2, which encodes a different type of phosphate transporter, and PHOX, which encodes a phosphatase, were not up-regulated in Mn-deficient cells, suggesting that the P-deficiency response was not turned on.
DISCUSSION
Assimilation and Transport Mechanisms
During acclimation to a metal cofactor deficiency, an organism activates assimilation mechanisms to acquire that metal or mechanisms that conserve utilization of the nutrient. These mechanisms are induced early in the transition from sufficiency to deficiency. When cofactor supply is exceeded by metabolic demand, symptoms of deficiency ensue (Merchant et al., 2006). We therefore expect that changes in the expression of assimilatory transporters would precede the establishment of deficiency phenotypes. Accordingly, we surveyed the Chlamydomonas genome for the pattern of expression of genes encoding candidate transition metal transporters and noted that only one, NRAMP1, showed a pattern consistent with a function as a manganese assimilation component. Its expression was increased already in the first transfer from cells grown in Mn excess (25 μm) to a medium without manganese supplementation (Fig. 8) and in cells grown with supplementation in the medium to 1 μm (Fig. 7), which is just barely enough to support the accumulation of PSII and MnSODs.
This analysis relies on the assumption that the transporters are regulated, at least in part, by supply-and-demand-dependent changes in the abundance of the mRNAs. The assumption is validated by the known transcriptional responses in Chlamydomonas to Cu, Fe, and Zn deficiency (for review, see Merchant et al., 2006), but we certainly cannot rule out mechanisms that act directly at the level of the protein. In fact, because of the toxicity of most of the essential transition metals, the transporters do seem to be subject to nutrient-excess-dependent posttranslational modifications/degradation (e.g. Gitan et al., 1998; Guo et al., 2004; Wang et al., 2004). Therefore, we conclude that NRAMP1 is likely to be one but perhaps not the only manganese assimilation transporter. Chlamydomonas NRAMP1 is a member of the MntH group C family, which includes many proteins from prokaryotes (Cellier et al., 2001). The possibility that NRAMP1 functions in organellar manganese uptake should therefore be considered.
The expression of MTP4 was also increased but only in symptomatic Mn deficiency (when expression of MSD3 and FEA1 was also increased), suggesting that it is either a high affinity assimilatory transporter or that it might play a role in preferential intracellular compartmentation of manganese, for example by catalyzing efflux from the mitochondrion (see below). The latter function would be consistent with the function of the MTPs in plants where they sequester metal ions in the vacuole and hence confer metal tolerance. Subcellular localization of the gene product in Mn-replete versus -deficient medium would be informative in terms of distinguishing these models.
In yeast, manganese is also assimilated at low affinity by a PHO84 type of transporter, which moves phosphate with a divalent counterion (Fristedt et al., 1999; Jensen et al., 2003). This pathway is not regulated by manganese overload in yeast and therefore can lead to overaccumulation of intracellular manganese when cells are grown in Mn-excess conditions. The connection between manganese and phosphorus assimilation may be obligatory for Chlamydomonas, because Mn-deficient cells showed reduced phosphorus content and two of the four PHO84-type transporters of Chlamydomonas were induced in Mn deficiency.
To estimate how much manganese is required in the growth medium for Chlamydomonas and to establish conditions for the study of Mn deficiency, we measured the metal content of cells grown with various levels of manganese supplementation (Fig. 9). With 25 μm manganese in the medium, the cells overaccumulate manganese beyond what is required to maintain manganese enzymes. The excess manganese may constitute a storage pool as described in cyanobacteria (Keren et al., 2002). As the supplementation is reduced to the submicromolar range (from 0.5 to 0), the manganese content of the cells is reduced in proportion to the supply. Based on assay of MnSOD and PSII function, we conclude that 1 μm manganese is just barely sufficient, and hence we recommend an optimum supply of about 3 to 5 μm for photoheterotrophically grown Chlamydomonas.
We noted also that Mn-deficient cells had reduced Fe content, and this correlated well with the appearance of chlorosis (data not shown). In fact, chlorosis could be relieved by provision of extra iron in the medium, suggesting a connection between manganese and iron homeostasis. A simple explanation might be that manganese nutrition reduced the expression of the high affinity iron transport pathway consisting of FOX1, FTR1, and FEA1 (Merchant et al., 2006). But this was clearly not the case. In fact, the expression of these components was actually increased, although by only 10% of the increase expected based on the iron content of cells (50% of a replete cell). We suspect that the iron nutrition phenotype is a complex balance between the need to reduce the intracellular ferrous availability in response to peroxide stress resulting from Mn deficiency and the compensatory need to assimilate more iron because of the reduced intracellular iron availability.
Hierarchy of Mn Distribution
Two of the more abundant manganese enzymes in a photosynthetic cell are PSII and the MnSODs (Raven, 1990), and the impact of Mn deficiency on the activity of these two enzymes (Figs. 1 and 3) is well known and therefore predictable. What is interesting, however, is that the loss of activity of the major MnSOD precedes loss of PSII activity, indicative of regulated manganese supply to intracellular or intraorganellar compartments.
Oxidative Stress
The loss of MnSOD activity, which is not compensated by an increase in FeSOD, should result in increased sensitivity to superoxide. Nevertheless, this was not the case. This might be explained by the finding that methyl viologen-induced superoxide stress acts predominantly in the chloroplast in light grown cells (Bowler et al., 1991), where FeSOD activity may be an adequate protectant.
On the other hand, the cells did appear to be more sensitive to H2O2 (Fig. 4) or organic peroxides (Supplemental Fig. S2) relative to Mn-replete cultures, and they also showed an increase in the expression of genes encoding antioxidant enzymes (Fig. 5B). We suspect that these increases, including that of the MSD3 through MSD5 genes, occur in response to oxidative stress rather than directly to manganese nutrition, because they are noted only coincident with the appearance of symptoms and when the manganese content is severely depleted, and they parallel the pattern of expression of the MSD genes in response to H2O2 treatment (J. Long and S. Merchant, unpublished data). Iron toxicity is exacerbated in the presence of H2O2, because ferrous ion promotes the production of hydroxyl radical from H2O2, and the decreased cellular content of iron (Fig. 9) may be a protective mechanism (Storz and Imlay, 1999). A connection between manganese nutrition and peroxide and iron metabolism has been noted already in various bacteria (Horsburgh et al., 2002). An increase in lipid peroxidation in Mn-deficient rats was correlated with damage to mitochondrial membranes, suggesting that Mn deficiency in Chlamydomonas may result in localized oxidative stress (Zidenberg-Cherr et al., 1983).
Replacement of FeSOD by MnSOD
Although FSD1 expression was not increased to compensate for the loss of MnSOD in Mn deficiency, MnSOD activity was increased in Fe-deficient cells. One of the five MSD genes was dramatically up-regulated in Fe-deficient cells (Fig. 6A). This response is quite distinct in terms of magnitude as well as specificity from the response of the MSD genes to oxidative stress (Fig. 5), suggesting that the MSD3 gene, but not the MSD4 and MSD5 genes, also responds separately to iron nutrition. Interestingly, cell fractionation experiments suggest that the major MnSOD isoform shows dual localization to both the mitochondria and chloroplast, indicating that this isoform may cover the loss of the chloroplast FeSOD. The replacement of an FeSOD by a MnSOD has been observed in bacteria and suggested as well in diatoms that show an increased requirement for manganese in an Fe-deficient growth environment (Privalle and Fridovich, 1993; Campbell and Laudenbach, 1995; Peers and Price, 2004).
CONCLUSION
The individual MSD genes show unique patterns of expression in response to Fe deficiency or oxidative stress. It is likely that the major plastid-localized MnSOD noted on activity gels is the product of the MSD3 gene, which is independently regulated by peroxide stress and Fe deficiency. The up-regulation of MSD3 in Fe deficiency is viewed as a mechanism to compensate for loss of FeSOD in the chloroplast. Manganese assimilation and homeostasis may involve three different types of transporters: NRAMP1, MTP4, and PTA3/4. The lowered P content of Mn-deficient cells may reflect a preference for Mn2+ as the counterion for phosphate transport by one or more of the PHO84-type transporters. The Fe-deficiency phenotype of Mn-depleted cells may result from an active mechanism to reduce Fe to combat oxidative stress.
MATERIALS AND METHODS
Growth Conditions
Chlamydomonas reinhardtii wild-type strain CC425 (Chlamydomonas culture collection, Duke University, NC) was maintained in the light (60–80 μmol m−2 s−1) in TAP medium supplemented with 100 μg/mL Arg (Harris, 1989). For Mn-deficiency experiments, cells in TAP medium (25 μm MnCl2) were transferred through two rounds of TAP medium without manganese supplement (1:500 dilution). We estimated a residual manganese content of between 0.02 to 0.1 μm from: (1) the calculated manganese content of the medium based on the measured manganese abundance in the high purity chemicals used for preparing stocks; (2) direct measurement of Mn content of the medium by inductively coupled plasma-mass spectrometry; and (3) the extrapolated Mn content of the medium based on measurement of Mn in the cell paste collected from cells grown without Mn supplementation. Fresh cultures were inoculated with these Mn-deficient cells (1:500 dilution) into medium containing the indicated concentration of manganese provided as MnCl2 chelated with EDTA. For Fe-deficiency experiments, replete cells (18 μm Fe) were transferred to Fe-free TAP, and iron was supplemented to the indicated concentration as Fe-EDTA chelate (Moseley et al., 2002). For phosphorus deficiency experiments (Quisel et al., 1996), TAP-grown cells were resuspended in medium lacking phosphate (but supplemented instead with 1.5 mm KCl to retain the K content of the medium) and collected for analysis after 24 to 48 h of continued growth.
Fluorescence Rise and Decay Kinetics
Room temperature fluorescence rise and decay kinetics were analyzed using a FluorCam 700MF (Photon Systems Instruments). Twenty-five microliters of mid-log phase liquid culture (2–8 × 106 cells/mL) was spotted onto a solid plastic surface and dark adapted for 10 min prior to measurement of the Kautsky effect in continuous red light at 150 μmol m−2 s−1 (Moseley et al., 2002).
Chloroplast Isolation
Cells of strain CC400, cw15 mt+ (2 L) from mid-log phase cultures were collected by centrifugation (1,000g, 10 min) and resuspended in 50 mL 0.3 m sorbitol, 50 mm HEPES-KOH, pH 7.8, 2 mm EDTA, 5 mm MgCl2, 0.1% bovine serum albumin, 0.5% polyvinylpyrrolidone-40. The cells were lysed by passage of the suspension through a Yeda press (4.5 bar, 30 s). The lysed cells (25 mL) were used to isolate chloroplasts (Rolland et al., 1997). The chloroplast fraction was resuspended in 10 mm sodium phosphate, pH 7.0, and assayed for purity by immunoblot.
Protein Isolation
Chlamydomonas cultures were collected by centrifugation (1,000g, 5 min) and washed twice with 10 mm sodium phosphate, pH 7.0. The total protein fraction was further subfractionated into soluble and membrane components as described in Howe and Merchant (1992). Protein concentration was determined as described in Lowry et al. (1951) against a bovine serum albumin standard.
Immunoblot Analysis
For immunoblot analysis, proteins were separated on an SDS-containing polyacrylamide gel (10% monomer for CF1, 11% for D1, or 15% monomer for OEE1, OEE2, and OEE3) and transferred onto polyvinylidene difluoride (0.45 μm, Millipore) for 1 h at 4°C under constant voltage (100 V) in 25 mm Tris, 192 mm Gly, 0.01% SDS, and 20% methanol. Membranes were blocked with 5% dry milk in Tris-buffered saline (10 mm Tris-Cl, 150 mm NaCl, pH 7.5) + Tween 20 (0.05% [w/v]). Primary antibodies were used at 1:1,000 (D1), 1:2,000 (OEE1), or 1:20,000 (OEE2, OEE3, and CF1), and a 1:5,000 dilution of goat anti-rabbit horseradish peroxidase (Pierce Biotechnology) was used as the secondary antibody. Signals were detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology).
SOD Activity Gels
Total soluble proteins were separated on a polyacrylamide gel (10% monomer) and analyzed in gel for SOD activity as described by Beauchamp and Fridovich (1971). FeSOD activity was identified by sensitivity to 20 μm H2O2 treatment prior to activity staining.
Nucleic Acids Analysis
Total Chlamydomonas RNA was prepared as described by Quinn and Merchant (1998).
Quantitative Real-Time PCR
Genomic DNA was removed from the total RNA preparation by treatment with RQ1 DNAse (Promega) according to the manufacturer's instructions. Complementary DNA, primed with oligo(dT), was generated with reverse transcriptase (Invitrogen), also according to the manufacturer's instructions, and used in the amplification reaction directly after dilution. The amplification reaction was carried out with reagents from the iQ SYBR Green Supermix qPCR kit (Bio-Rad Laboratories). Each reaction contained the vendor's master mix, 0.3 μm of each primer, and cDNA corresponding to 20 ng input RNA in the reverse transcriptase reaction. The reaction conditions for the Opticon 2 from MJ Research were: 95°C for 5 min, followed by cycles of 95°C for 10 s, 65°C for 30 s, 72°C for 30 s, up to a total of 40 cycles. The fluorescence was measured at each cycle at 72°C and 83°C. The 2−ΔΔCT method was used to analyze the database on the fluorescence at 83°C (Livak and Schmittgen, 2001). Melting curves were performed after the PCR reaction to assess the presence of a unique final product, and the product was analyzed by gel electrophoresis and sequenced from one reaction to verify that it represented the gene of interest. The data are presented as the fold change in mRNA abundance, normalized to an endogenous reference gene (CβLP), relative to the RNA sample from cells grown in 25 μm Mn (considered Mn replete). The results presented are the averages of technical triplicates but are representative of at least two independent experiments.
Measurement of Metal and Phosphorus Content
Cells were grown in the indicated metal concentration to stationary phase (>1 × 107 cells/mL) so that we could establish the minimal manganese requirement for growth of a Chlamydomonas culture. Cells (5 × 108) were collected for each measurement by centrifugation at 1,700g for 5 min. The cell paste was washed twice with 1 mm EDTA and once with deionized water (MilliQ, Millipore). The washed cell paste was then overlaid with nitric acid corresponding to a final concentration of 30% in 1 mL and digested at 65°C for at least 48 h. To obtain a corresponding blank, the volume of the cell paste was replaced by deionized water and treated the same way as the cell paste. Digested cell paste and blank were diluted with 9 mL deionized water prior to measurement. Total metal content was measured at the Interdisciplinary Center for Plasma Mass Spectrometry (University of California, Davis) by the standard addition method. Total phosphorus content was measured by inductively coupled plasma-atomic emission spectroscopy (detection limit 100 ppb) at the University of California, Los Angeles Molecular Instrumentation Center with reference to a standard solution of esterified phosphate.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Intracellular metal content in response to manganese depletion.
Supplemental Figure S2. Selective sensitivity of Mn-deficient cells to peroxides.
Supplemental Figure S3. MnSOD is up-regulated by Fe deficiency.
Supplemental Figure S4. Time course of changes in gene expression in response to Mn deficiency and resupply.
Supplementary Material
Acknowledgments
We are grateful to the Joint Genome Institute for the draft sequence of the Chlamydomonas genome. We thank Dr. Susanne Preiss for D1 antibody, Dr. Maryse Block for KARI antibody, Dr. Cheryl Kerfeld for OEE1 antibody, Dr. Jean-David Rochaix and Dr. Olivier Vallon for OEE2 and OEE3 antibody, and Dr. Jeffrey Moseley for primers for analyzing the expression of PHOX, PTA1 through PTA4, and PTB2.
This work was supported by the Department of Energy (grant no. DE–FG02–04ER15529), by the National Institutes of Health (grant no. GM42143), by the Institutional and Individual Kirschstein Fellowships (GM07185 and GM077066 to M.D.A.), by the Spanish Ministry for Education (a postdoctoral fellowship to J.A.D.C.), and by the University of California Toxic Substances Research and Teaching Program (S.T.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Sabeeha S. Merchant (merchant@chem.ucla.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bartsevich VV, Pakrasi HB (1995) Molecular identification of an ABC transporter complex for manganese: analysis of a cyanobacterial mutant strain impaired in the photosynthetic oxygen evolution process. EMBO J 14 1845–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44 276–287 [DOI] [PubMed] [Google Scholar]
- Belouchi A, Kwan T, Gros P (1997) Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol Biol 33 1085–1092 [DOI] [PubMed] [Google Scholar]
- Bereczky Z, Wang HY, Schubert V, Ganal M, Bauer P (2003) Differential regulation of nramp and irt metal transporter genes in wild type and iron uptake mutants of tomato. J Biol Chem 278 24697–24704 [DOI] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, Van Montagu M, Inzé D (1991) Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. EMBO J 10 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell WS, Laudenbach DE (1995) Characterization of four superoxide dismutase genes from a filamentous cyanobacterium. J Bacteriol 177 964–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellier MF, Bergevin I, Boyer E, Richer E (2001) Polyphyletic origins of bacterial Nramp transporters. Trends Genet 17 365–370 [DOI] [PubMed] [Google Scholar]
- Chandler LE, Bartsevich VV, Pakrasi HB (2003) Regulation of manganese uptake in Synechocystis 6803 by RfrA, a member of a novel family of proteins containing a repeated five-residues domain. Biochemistry 42 5508–5514 [DOI] [PubMed] [Google Scholar]
- Christianson DW (1997) Structural chemistry and biology of manganese metalloenzymes. Prog Biophys Mol Biol 67 217–252 [DOI] [PubMed] [Google Scholar]
- Culotta VC, Yang M, Hall MD (2005) Manganese transport and trafficking: lessons learned from Saccharomyces cerevisiae. Eukaryot Cell 4 1159–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000) Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347 749–755 [PMC free article] [PubMed] [Google Scholar]
- Donald CM, Prescott JA (1975) Trace elements in Australian crop and pasture production, 1924–1974. In DJD Nicholas, AR Egan, eds, Trace Elements in Soil-Plant-Animal Systems. Academic Press, New York, pp 7–37
- Eyster C, Brown TE, Tanner HA, Hood SL (1958) Manganese requirement with respect to growth, Hill reaction and photosynthesis. Plant Physiol 33 235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieden E (1985) New perspectives on the essential trace elements. J Chem Ed 62 917–923 [Google Scholar]
- Fristedt U, van Der Rest M, Poolman B, Konings WN, Persson BL (1999) Studies of cytochrome c oxidase-driven H+-coupled phosphate transport catalyzed by the Saccharomyces cerevisiae Pho84 permease in coreconstituted vesicles. Biochemistry 38 16010–16015 [DOI] [PubMed] [Google Scholar]
- Gitan RS, Luo H, Rodgers J, Broderius M, Eide D (1998) Zinc-induced inactivation of the yeast ZRT1 zinc transporter occurs through endocytosis and vacuolar degradation. J Biol Chem 273 28617–28624 [DOI] [PubMed] [Google Scholar]
- Grossman A (2000) Acclimation of Chlamydomonas reinhardtii to its nutrient environment. Protist 151 201–224 [DOI] [PubMed] [Google Scholar]
- Guedon E, Moore CM, Que Q, Wang T, Ye RW, Helmann JD (2003) The global transcriptional response of Bacillus subtilis to manganese involves the MntR, Fur, TnrA and σB regulons. Mol Microbiol 49 1477–1491 [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero MF, Boron WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388 482–488 [DOI] [PubMed] [Google Scholar]
- Guo Y, Smith K, Lee J, Thiele DJ, Petris MJ (2004) Identification of methionine-rich clusters that regulate copper-stimulated endocytosis of the human Ctr1 copper transporter. J Biol Chem 279 17428–17433 [DOI] [PubMed] [Google Scholar]
- Hall JL, Williams LE (2003) Transition metal transporters in plants. J Exp Bot 54 2601–2613 [DOI] [PubMed] [Google Scholar]
- Hanikenne M, Krämer U, Demoulin V, Baurain D (2005) A comparative inventory of metal transporters in the green alga Chlamydomonas reinhardtii and the red alga Cyanidioschizon merolae. Plant Physiol 137 428–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Chen S, Wilson DB (1999) Cloning, expression, and characterization of cadmium and manganese uptake genes from Lactobacillus plantarum. Appl Environ Microbiol 65 4746–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris EH (1989) The Chlamydomonas Sourcebook: A Comprehensive Guide to Biology and Laboratory Use. Academic Press, San Diego [DOI] [PubMed]
- Hiltbrunner E, Flückiger W (1996) Manganese deficiency of silver fir trees (Abies alba) at a reforested site in the Jura mountains, Switzerland: aspects of cause and effect. Tree Physiol 16 963–975 [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Wharton SJ, Cox AG, Ingham E, Peacock S, Foster SJ (2002) MntR modulates expression of the PerR regulon and superoxide resistance in Staphylococcus aureus through control of manganese uptake. Mol Microbiol 44 1269–1286 [DOI] [PubMed] [Google Scholar]
- Howe G, Merchant S (1992) The biosynthesis of membrane and soluble plastidic c-type cytochromes of Chlamydomonas reinhardtii is dependent on multiple common gene products. EMBO J 11 2789–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabado N, Jankowski A, Dougaparsad S, Picard V, Grinstein S, Gros P (2000) Natural resistance to intracellular infections: natural resistance-associated macrophage protein 1 (Nramp1) functions as a pH-dependent manganese transporter at the phagosomal membrane. J Exp Med 192 1237–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Jenkinson HF (2001) Out of the iron age: new insights into the critical role of manganese homeostasis in bacteria. Microbiology 147 1709–1718 [DOI] [PubMed] [Google Scholar]
- Jensen LT, Ajua-Alemanji M, Culotta VC (2003) The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J Biol Chem 278 42036–42040 [DOI] [PubMed] [Google Scholar]
- Keen CL, Ensunsa JL, Clegg MS (2000) Manganese metabolism in animals and humans including the toxicity of manganese. In A Sigel, H Sigel, eds, Manganese and Its Role in Biological Processes, Vol 37. Marcel Dekker, New York, pp 89–121 [PubMed]
- Kehres DG, Janakiraman A, Slauch JM, Maguire ME (2002) Regulation of Salmonella enterica serovar Typhimurium mntH transcription by H2O2, Fe2+, and Mn2+. J Bacteriol 184 3151–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehres DG, Maguire ME (2003) Emerging themes in manganese transport, biochemistry and pathogenesis in bacteria. FEMS Microbiol Rev 27 263–290 [DOI] [PubMed] [Google Scholar]
- Keren N, Kidd MJ, Penner-Hahn JE, Pakrasi HB (2002) A light-dependent mechanism for massive accumulation of manganese in the photosynthetic bacterium Synechocystis sp. PCC 6803. Biochemistry 41 15085–15092 [DOI] [PubMed] [Google Scholar]
- Kitayama K, Kitayama M, Osafune T, Togasaki RK (1999) Subcellular localization of iron and manganese superoxide dismutase in Chlamydomonas reinhardtii (Chlorophyceae). J Phycol 35 136–142 [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55 459–493 [DOI] [PubMed] [Google Scholar]
- La Fontaine S, Quinn JM, Nakamoto SS, Page MD, Göhre V, Moseley JL, Kropat J, Merchant S (2002) Copper-dependent iron assimilation pathway in the model photosynthetic eukaryote Chlamydomonas reinhardtii. Eukaryot Cell 1 736–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J Jr, Dionne L, Lu N, Huang S, Matzuk MM (1996) Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA 93 9782–9787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledford HK, Niyogi KK (2005) Singlet oxygen and photo-oxidative stress management in plants and algae. Plant Cell Environ 28 1037–1045 [Google Scholar]
- Leisinger U, Rüfenacht K, Fischer B, Pesaro M, Spengler A, Zehnder AJ, Eggen RI (2001) The glutathione peroxidase homologous gene from Chlamydomonas reinhardtii is transcriptionally up-regulated by singlet oxygen. Plant Mol Biol 46 395–408 [DOI] [PubMed] [Google Scholar]
-
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the
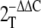 method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar] - Lowry OH, Rosebrough NJ, Farr LA, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193 265–275 [PubMed] [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, London
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant S, Sawaya MR (2005) The light reactions: a guide to recent acquisitions for the picture gallery. Plant Cell 17 648–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant SS, Allen MD, Kropat J, Moseley JL, Long JC, Tottey S, Terauchi AM (2006) Between a rock and a hard place: trace element nutrition in Chlamydomonas. Biochim Biophys Acta 1763 578–594 [DOI] [PubMed] [Google Scholar]
- Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, Hippler M (2002) Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J 21 6709–6720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley JL, Chang CW, Grossman AR (2006) Genome-based approaches to understanding phosphorus deprivation responses and PSR1 control in Chlamydomonas reinhardtii. Eukaryot Cell 5 26–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Bao DH, Katoh H, Shibata M, Pakrasi HB, Bhattacharyya-Pakrasi M (2002) A two-component signal transduction pathway regulates manganese homeostasis in Synechocystis 6803, a photosynthetic organism. J Biol Chem 277 28981–28986 [DOI] [PubMed] [Google Scholar]
- Peers G, Price NM (2004) A role for manganese in superoxide dismutases and growth of iron-deficient diatoms. Limnol Oceanogr 49 1774–1783 [Google Scholar]
- Persson BL, Petersson J, Fristedt U, Weinander R, Berhe A, Pattison J (1999) Phosphate permeases of Saccharomyces cerevisiae: structure, function and regulation. Biochim Biophys Acta 1422 255–272 [DOI] [PubMed] [Google Scholar]
- Pirson A (1955) Functional aspects in mineral nutrition of green plants. Annu Rev Plant Physiol 6 71–114 [Google Scholar]
- Privalle CT, Fridovich I (1993) Iron specificity of the Fur-dependent regulation of the biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem 268 5178–5181 [PubMed] [Google Scholar]
- Quinn JM, Merchant S (1998) Copper-responsive gene expression during adaptation to copper deficiency. Methods Enzymol 297 263–279 [DOI] [PubMed] [Google Scholar]
- Quisel JD, Wykoff DD, Grossman AR (1996) Biochemical characterization of the extracellular phosphatases produced by phosphorus-deprived Chlamydomonas reinhardtii. Plant Physiol 111 839–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven JA (1990) Predictions of Mn and Fe use efficiencies of phototrophic growth as a function of light availability for growth and of C assimilation pathway. New Phytol 116 1–18 [Google Scholar]
- Rolland N, Dorne A-J, Amoroso G, Sültemeyer DF, Joyard J, Rochaix J-D (1997) Disruption of the plastid ycf10 open reading frame affects uptake of inorganic carbon in the chloroplast of Chlamydomonas. EMBO J 16 6713–6726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai H, Kusumoto N, Kitayama K, Togasaki RK (1993) Isozymes of superoxide dismutase in Chlamydomonas and purification of one of the major isozymes containing Fe. Plant Cell Physiol 34 1133–1137 [Google Scholar]
- Storz G, Imlay JA (1999) Oxidative stress. Curr Opin Microbiol 2 188–194 [DOI] [PubMed] [Google Scholar]
- Supek F, Supekova L, Nelson H, Nelson N (1996) A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc Natl Acad Sci USA 93 5105–5110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichler-Zallen D (1969) The effect of manganese on chloroplast structure and photosynthetic ability of Chlamydomonas reinhardi. Plant Physiol 44 701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomine S, Lelievre F, Debarbieux E, Schroeder JI, Barbier-Brygoo H (2003) AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J 34 685–695 [DOI] [PubMed] [Google Scholar]
- Thomine S, Wang R, Ward JM, Crawford NM, Schroeder JI (2000) Cadmium and iron transport by members of a plant metal transporter family in Arabidopsis with homology to Nramp genes. Proc Natl Acad Sci USA 97 4991–4996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loon AP, Pesold-Hurt B, Schatz G (1986) A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci USA 83 3820–3824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veen HW, Abee T, Kortstee GJ, Konings WN, Zehnder AJ (1994) Substrate specificity of the two phosphate transport systems of Acinetobacter johnsonii 210A in relation to phosphate speciation in its aquatic environment. J Biol Chem 269 16212–16216 [PubMed] [Google Scholar]
- Wang F, Dufner-Beattie J, Kim BE, Petris MJ, Andrews G, Eide DJ (2004) Zinc-stimulated endocytosis controls activity of the mouse ZIP1 and ZIP3 zinc uptake transporters. J Biol Chem 279 24631–24639 [DOI] [PubMed] [Google Scholar]
- Wolfe-Simon F, Grzelryk D, Schofield O, Falkowski PG (2005) The role and evolution of superoxide dismutases in algae. J Phycol 41 453–465 [Google Scholar]
- Yamaguchi K, Suzuki I, Yamamoto H, Lyukevich A, Bodrova I, Los DA, Piven I, Zinchenko V, Kanehisa M, Murata N (2002) A two-component Mn2+-sensing system negatively regulates expression of the mntCAB operon in Synechocystis. Plant Cell 14 2901–2913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum CF, Pecoraro VL (1999) Recent advances in the understanding of the biological chemistry of manganese. Curr Opin Chem Biol 3 182–187 [DOI] [PubMed] [Google Scholar]
- Yu Q, Rengel Z (1999) Micronutrient deficiency influences plant growth and activities of superoxide dismutases in narrow-leafed lupins. Ann Bot (Lond) 83 175–182 [Google Scholar]
- Zidenberg-Cherr S, Keen CL, Lonnerdal B, Hurley LS (1983) Superoxide dismutase activity and lipid peroxidation in the rat: developmental correlations affected by manganese deficiency. J Nutr 113 2498–2504 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.