Abstract
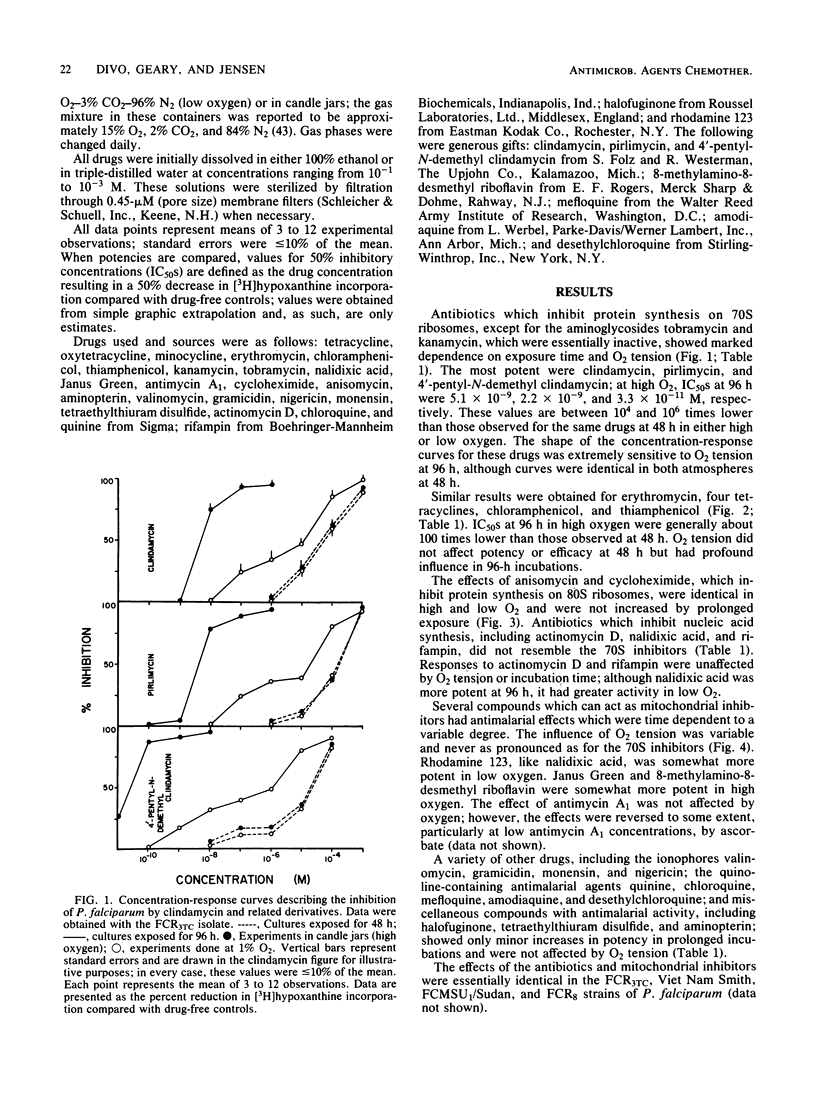
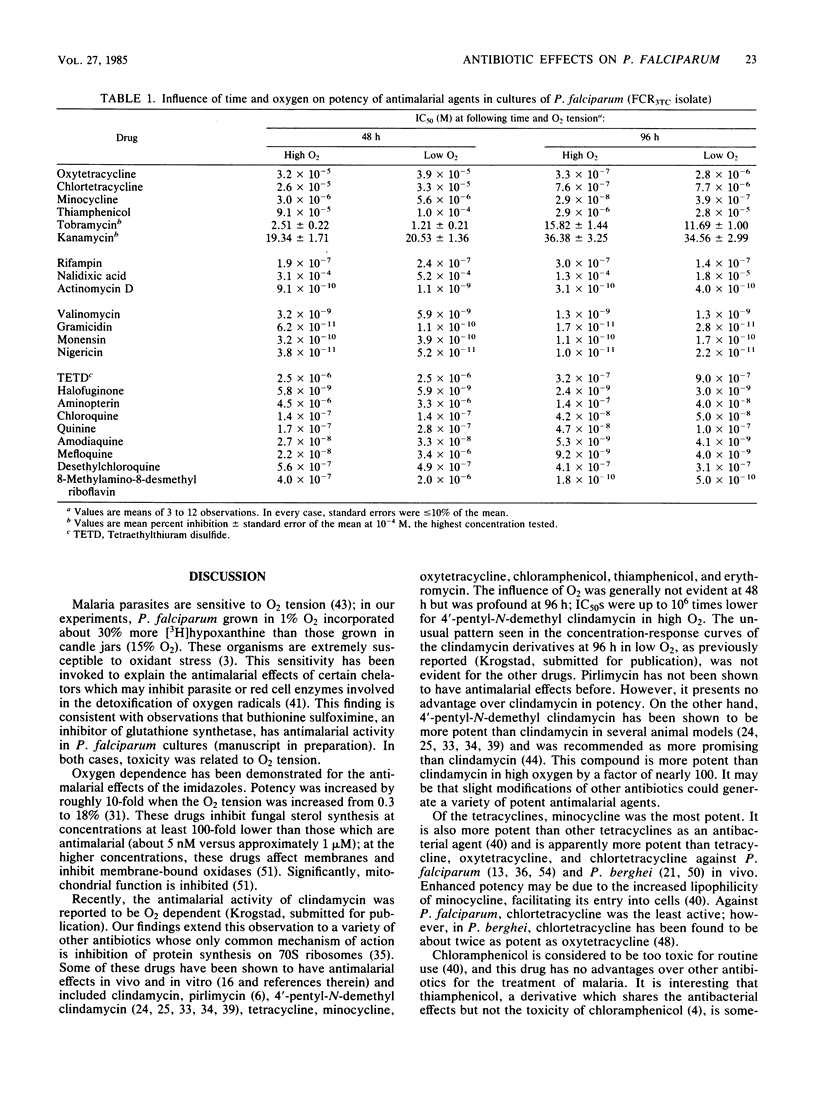
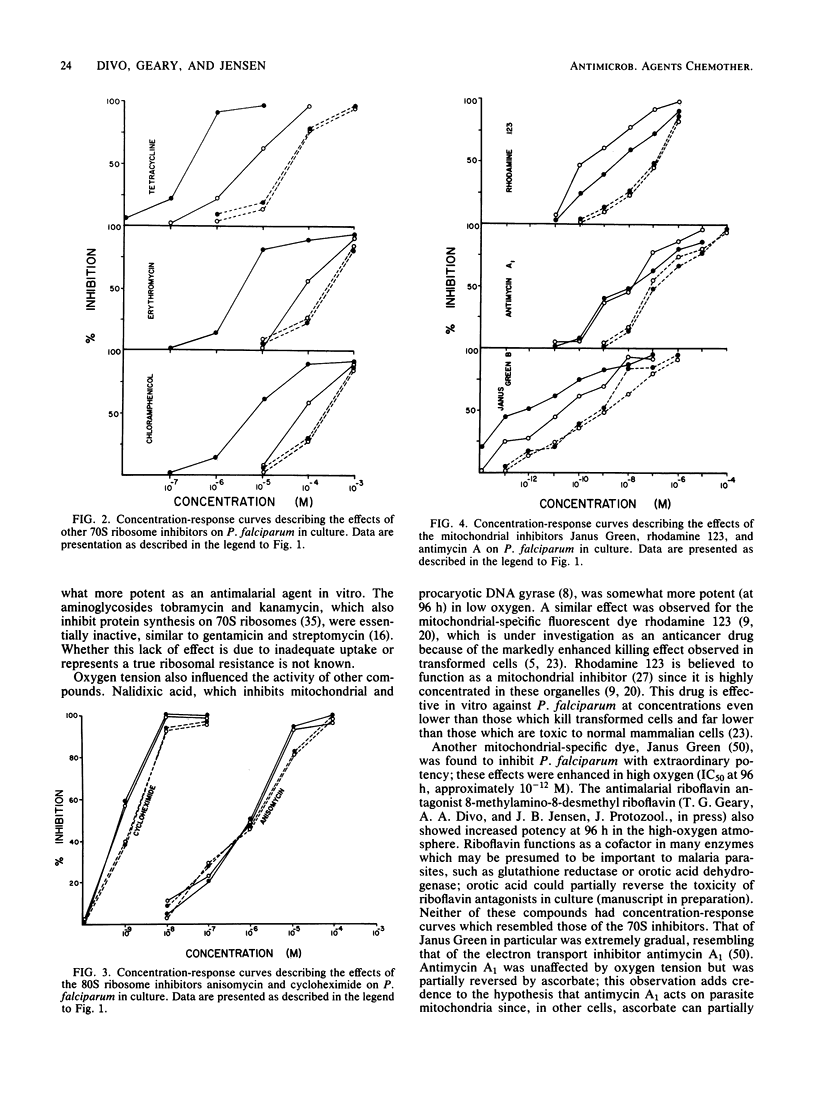
Several antibiotics which inhibit protein synthesis on 70S ribosomes, including clindamycin, pirlimycin, 4'-pentyl-N-demethyl clindamycin, four tetracyclines, chloramphenicol, thiamphenicol, and erythromycin, had antimalarial effects against Plasmodium falciparum in culture which were greatly influenced by the duration of drug exposure and by oxygen tension. In 96-h incubations, potency was increased by a factor of up to 10(6) over the first 48-h period and by a factor of up to 10(4) in 15% O2 versus 1% O2. Two aminoglycosides, kanamycin and tobramycin, had no antimalarial activity. Rifampin and nalidixic acid, which inhibit nucleic acid synthesis, were not similar to the 70S inhibitors. The mitochondrial inhibitors Janus Green, rhodamine 123, antimycin A1, and 8-methylamino-8-desmethyl riboflavin had activities which were influenced by the duration of exposure and oxygen tension. Quinoline-containing antimalarial agents, ionophores, and other antimalarial drugs were influenced to a minor extent by the duration of exposure but were not affected by oxygen tension. These data can best be explained by the hypothesis that antimalarial 70S ribosome-specific protein synthesis inhibitors are toxic to the parasites by acting on the mitochondrion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderounmu A. F., Fleckenstein L. Pharmacokinetics of chloroquine diphosphate in the dog. J Pharmacol Exp Ther. 1983 Sep;226(3):633–639. [PubMed] [Google Scholar]
- Alger N. E., Spira D. T., Silverman P. H. Inhibition of rodent malaria in mice by rifampicin. Nature. 1970 Jul 25;227(5256):381–382. doi: 10.1038/227381b0. [DOI] [PubMed] [Google Scholar]
- Allison A. C., Eugui E. M. A radical interpretation of immunity to malaria parasites. Lancet. 1982 Dec 25;2(8313):1431–1433. doi: 10.1016/s0140-6736(82)91330-7. [DOI] [PubMed] [Google Scholar]
- Baumelou E., Najean Y. Why still prescribe chloramphenicol in 1983? Comparison of the clinical and biological hematologic effects of chloramphenicol and thiamphenicol. Blut. 1983 Dec;47(6):317–320. doi: 10.1007/BF00320345. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Lampidis T. J., McIsaac R. M., Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983 Oct 14;222(4620):169–172. doi: 10.1126/science.6623064. [DOI] [PubMed] [Google Scholar]
- Birkenmeyer R. D., Kroll S. J., Lewis C., Stern K. F., Zurenko G. E. Synthesis and antimicrobial activity of clindamycin analogues: pirlimycin, 1,2 a potent antibacterial agent. J Med Chem. 1984 Feb;27(2):216–223. doi: 10.1021/jm00368a020. [DOI] [PubMed] [Google Scholar]
- COATNEY G. R., GREENBERG J. The use of antibiotics in the treatment of malaria. Ann N Y Acad Sci. 1952 Dec 30;55(6):1075–1081. doi: 10.1111/j.1749-6632.1952.tb22668.x. [DOI] [PubMed] [Google Scholar]
- Cabrera B. D., Rivera D. G., Lara N. T. Study on clindamycin in the treatment of falciparum malaria. Rev Inst Med Trop Sao Paulo. 1982 Nov-Dec;24(6 Suppl):62–69. [PubMed] [Google Scholar]
- Castora F. J., Vissering F. F., Simpson M. V. The effect of bacterial DNA gyrase inhibitors on DNA synthesis in mammalian mitochondria. Biochim Biophys Acta. 1983 Sep 9;740(4):417–427. doi: 10.1016/0167-4781(83)90090-8. [DOI] [PubMed] [Google Scholar]
- Chen L. B., Summerhayes I. C., Johnson L. V., Walsh M. L., Bernal S. D., Lampidis T. J. Probing mitochondria in living cells with rhodamine 123. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):141–155. doi: 10.1101/sqb.1982.046.01.018. [DOI] [PubMed] [Google Scholar]
- Clyde D. F., Gilman R. H., McCarthy V. C. Antimalarial effects of clindamycin in man. Am J Trop Med Hyg. 1975 Mar;24(2):369–370. doi: 10.4269/ajtmh.1975.24.369. [DOI] [PubMed] [Google Scholar]
- Clyde D. F., Miller R. M., DuPont H. L., Hornick R. B. Antimalarial effects of tetracyclines in man. J Trop Med Hyg. 1971 Nov;74(11):238–242. [PubMed] [Google Scholar]
- Colwell E. J., Hickman R. L., Intraprasert R., Tirabutana C. Minocycline and tetracycline treatment of acute falciparum malaria in Thailand. Am J Trop Med Hyg. 1972 Mar;21(2):144–149. doi: 10.4269/ajtmh.1972.21.144. [DOI] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary T. G., Divo A. A., Jensen J. B. An in vitro assay system for the identification of potential antimalarial drugs. J Parasitol. 1983 Jun;69(3):577–583. [PubMed] [Google Scholar]
- Geary T. G., Jensen J. B. Effects of antibiotics on Plasmodium falciparum in vitro. Am J Trop Med Hyg. 1983 Mar;32(2):221–225. doi: 10.4269/ajtmh.1983.32.221. [DOI] [PubMed] [Google Scholar]
- Golenser J., Casuto D., Pollack Y. Plasmodium falciparum: in vitro induction of resistance to aminopterin. Exp Parasitol. 1981 Dec;52(3):371–377. doi: 10.1016/0014-4894(81)90095-3. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Capps T. C., Carlin J. M. Clinical drug-resistant falciparum malaria acquired from cultured parasites. Am J Trop Med Hyg. 1981 May;30(3):523–525. doi: 10.4269/ajtmh.1981.30.523. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: use of outdated erthrocytes and description of the candle jar method. J Parasitol. 1977 Oct;63(5):883–886. [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lampidis T. J., Bernal S. D., Summerhayes I. C., Chen L. B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983 Feb;43(2):716–720. [PubMed] [Google Scholar]
- Lewis C. Antiplasmodial activity of 7-halogenated lincomycins. J Parasitol. 1968 Feb;54(1):169–170. [PubMed] [Google Scholar]
- Miller L. H., Glew R. H., Wyler D. J., Howard W. A., Collins W. E., Contacos P. G., Neva F. A. Evaluation of clindamycin in combination with quinine against multidrug-resistant strains of Plasmodium falciparum. Am J Trop Med Hyg. 1974 Jul;23(4):565–569. doi: 10.4269/ajtmh.1974.23.565. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Weiss M. J., Chen L. B., Aprille J. R. Rhodamine 123 inhibits bioenergetic function in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1984 Feb 14;118(3):717–723. doi: 10.1016/0006-291x(84)91453-0. [DOI] [PubMed] [Google Scholar]
- Nguyen-Dinh P., Trager W. Plasmodium falciparum in vitro: determination of chloroquine sensitivity of three new strains by a modified 48-hour test. Am J Trop Med Hyg. 1980 May;29(3):339–342. doi: 10.4269/ajtmh.1980.29.339. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Krogstad D. J. Oxygen enhances the antimalarial activity of the imidazoles. Am J Trop Med Hyg. 1983 Jul;32(4):660–665. doi: 10.4269/ajtmh.1983.32.660. [DOI] [PubMed] [Google Scholar]
- Powers K. G. Activity of chlorinated lincomycin analogues against Plasmodium cynomolgi in rhesus monkeys. Am J Trop Med Hyg. 1969 Jul;18(4):485–490. doi: 10.4269/ajtmh.1969.18.485. [DOI] [PubMed] [Google Scholar]
- Powers K. G., Jacobs R. L. Activity of two chlorinated lincomycin analogues against chloroquine-resistant falciparum malaria in owl monkeys. Antimicrob Agents Chemother. 1972 Jan;1(1):49–53. doi: 10.1128/aac.1.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUIZ-SANCHEZ F., QUEZADA M., PAREDES M., CASILLAS J., RIEBELING R. Chloramphenicol in malaria. Am J Trop Med Hyg. 1952 Nov;1(6):936–940. doi: 10.4269/ajtmh.1952.1.936. [DOI] [PubMed] [Google Scholar]
- Rieckmann K. H., Powell R. D., McNamara J. V., Willerson D., Jr, Lass L., Frischer H., Carson P. E. Effects of tetracycline against chloroquine-resistant and chloroquine-sensitive Plasmodium falciparum. Am J Trop Med Hyg. 1971 Nov;20(6):811–815. doi: 10.4269/ajtmh.1971.20.811. [DOI] [PubMed] [Google Scholar]
- Rivera D. G., Cabrera B. D., Lara N. T. Treatment of falciparum malaria with clindamycin. Rev Inst Med Trop Sao Paulo. 1982 Nov-Dec;24(6 Suppl):70–75. [PubMed] [Google Scholar]
- Rozman R. S., Canfield C. J. New experimental antimalarial drugs. Adv Pharmacol Chemother. 1979;16:1–43. doi: 10.1016/s1054-3589(08)60241-0. [DOI] [PubMed] [Google Scholar]
- Scheibel L. W., Adler A. Antimalarial activity of selected aromatic chelators. II. Substituted quinolines and quinoline-N-oxides. Mol Pharmacol. 1981 Jul;20(1):218–223. [PubMed] [Google Scholar]
- Scheibel L. W., Adler A., Trager W. Tetraethylthiuram disulfide (Antabuse) inhibits the human malaria parasite Plasmodium falciparum. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5303–5307. doi: 10.1073/pnas.76.10.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel L. W., Ashton S. H., Trager W. Plasmodium falciparum: microaerophilic requirements in human red blood cells. Exp Parasitol. 1979 Jun;47(3):410–418. doi: 10.1016/0014-4894(79)90094-8. [DOI] [PubMed] [Google Scholar]
- Schmidt L. H., Harrison J., Ellison R., Worcester P. The activities of chlorinated lincomycin derivatives against infections with Plasmodium cynomolgi in Macaca mulatta. Am J Trop Med Hyg. 1970 Jan;19(1):1–11. doi: 10.4269/ajtmh.1970.19.1. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THURSTON J. P. The action of dyes, antibiotics, and some miscellaneous compounds against Plasmodium berghei. Br J Pharmacol Chemother. 1953 Jun;8(2):162–165. doi: 10.1111/j.1476-5381.1953.tb00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Mikkelsen R. B., Wallach D. F. Transport of ions in erythrocytes infected by plasmodia. Ciba Found Symp. 1983;94:64–73. doi: 10.1002/9780470715444.ch5. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Marichal P., Lauwers W. Hypothesis on the molecular basis of the antifungal activity of N-substituted imidazoles and triazoles. Biochem Soc Trans. 1983 Dec;11(6):665–667. doi: 10.1042/bst0110665. [DOI] [PubMed] [Google Scholar]
- Warhurst D. C., Robinson B. L., Peters W. The chemotherapy of rodent malaria, XXIV. The blood schizontocidal action of erythromycin upon Plasmodium berghei. Ann Trop Med Parasitol. 1976 Sep;70(3):253–258. doi: 10.1080/00034983.1976.11687121. [DOI] [PubMed] [Google Scholar]
- Willerson D., Jr, Rieckmann K. H., Carson P. E., Frischer H. Effects of minocycline against chloroquine-resistant falciparum malaria. Am J Trop Med Hyg. 1972 Nov;21(6):857–862. doi: 10.4269/ajtmh.1972.21.857. [DOI] [PubMed] [Google Scholar]
- Wyler D. J. Malaria--resurgence, resistance, and research. (First of two parts). N Engl J Med. 1983 Apr 14;308(15):875–878. doi: 10.1056/NEJM198304143081505. [DOI] [PubMed] [Google Scholar]
- Yayon A., Vande Waa J. A., Yayon M., Geary T. G., Jensen J. B. Stage-dependent effects of chloroquine on Plasmodium falciparum in vitro. J Protozool. 1983 Nov;30(4):642–647. doi: 10.1111/j.1550-7408.1983.tb05336.x. [DOI] [PubMed] [Google Scholar]



