Abstract
We investigated the response of Helianthus species nighttime conductance (gnight) and transpiration (Enight) to soil nutrient and water limitations in nine greenhouse studies. The studies primarily used wild Helianthus annuus, but also included a commercial and early domesticate of H. annuus and three additional wild species (Helianthus petiolaris Nutt., Helianthus deserticola Heiser, and Helianthus anomalus Blake). Well-watered plants of all species showed substantial gnight (0.023–0.225 mol m−2 s−1) and Enight (0.29–2.46 mmol m−2 s−1) measured as instantaneous gas exchange. Based on the potential for transpiration to increase mass flow of mobile nutrients to roots, we hypothesized that gnight and Enight would increase under limiting soil nutrients but found no evidence of responses in all six studies testing this. Based on known daytime responses to water limitation, we hypothesized that gnight and Enight would decrease when soil water availability was limited, and results from all four studies testing this supported our hypothesis. We also established that stomatal conductance at night was on average 5 times greater than cuticular conductance. Additionally, gnight and Enight varied nocturnally and across plant reproductive stages while remaining relatively constant as leaves aged. Our results further the ability to predict conditions under which nighttime water loss will be biologically significant and demonstrate that for Helianthus, gnight can be regulated.
It is widely accepted that plants regulate stomatal aperture both to minimize water loss for a given amount of carbon assimilated and to minimize xylem cavitation (Cowan, 1977; Sperry, 2000). C3 and C4 plants fix carbon during the day and lose water from leaves as an unavoidable cost of getting CO2 to the site of carboxylation. Although these plants are generally expected to close their stomata at night to conserve water when carbon gain is not occurring, significant nighttime leaf conductance (gnight) and transpiration (Enight) have been observed in many C3 species across a wide range of habitats (for review, see Musselman and Minnick, 2000; Caird et al., 2007). Reported rates for gnight typically range from 0.01 to 0.25 mol m−2 s−1 and can represent greater than 50% of daytime conductance (gday). Enight depends on both gnight and leaf-to-air vapor pressure deficit (VPDl) but is usually 5% to 15% of daytime transpiration (Eday). To date, most studies document the magnitude of gnight and Enight and several have correlated these traits with environmental or physiological variables (Benyon, 1999; Oren et al., 2001; Kavanagh et al., 2007). However, there have been few manipulative experiments that individually test the effect of environmental factors on the regulation of stomata at night.
Several researchers have speculated that nighttime water loss could enhance nutrient uptake by increasing mass flow of soluble nutrients to plant roots (Snyder et al., 2003; Daley and Phillips, 2006; Caird et al., 2007). The Barber-Cushman model predicts that increasing water flux to the rhizoplane minimizes or eliminates the formation of a nitrate depletion zone around plant roots when conditions are appropriate for Enight (Barber and Cushman, 1981; Barber, 1995). Empirically, McDonald et al. (2002) demonstrated a benefit of increased transpiration on nitrate delivery and uptake by Populus plants. Although the Tanner and Beevers (2001) study is sometimes cited as contrary evidence, it dealt only with effects of transpiration on long-distance nitrogen transport within the xylem, not with mass flow delivery to roots. Thus, increased nutrient acquisition may represent a benefit that counters the cost of water loss at night.
If nighttime water loss increases nutrient acquisition, then plants may benefit from the ability to regulate gnight in response to nutrient conditions. The effects of nitrate availability on gday and Eday have been investigated and are variable (Chapin, 1990; Fredeen et al., 1991; Ciompi et al., 1996; Cechin and Fumis, 2004). Potential regulatory pathways are still being debated (Dodd et al., 2003; Sakakibara et al., 2006). Two recent field studies with nutrient addition treatments found that gnight declined in response to nutrient additions (Ludwig et al., 2006; Scholz et al., 2007). However, the experimental designs of these studies did not permit direct effects due to reduced plant demand for nutrient acquisition regulating gnight to be separated from indirect effects of plant size or water status. More studies are needed that experimentally manipulate soil nutrient availability and test its effect on gnight and Enight, independent of confounding variation in soil and plant water potential.
During the day, stomatal conductance is regulated with respect to changing soil water potential and atmospheric demand to minimize use of available water during CO2 uptake and maintain soil-to-leaf hydraulic continuity (Sperry et al., 2002). To further optimize use of limited soil water, regulation may also occur at night, reducing gnight and consequently Enight. This expectation held true for droughted wheat plants, where gnight decreased as compared to well-watered controls (Rawson and Clarke, 1988). However, variable results have been obtained from studies that manipulated soil water potential with salt addition (Donovan et al., 1999) or through irrigation in the field (Donovan et al., 2003). At this time, generalization about the effect of soil water availability on gnight and Enight is not possible, and further examination in controlled experiments is needed.
The magnitude of gnight and Enight may also vary temporally as leaves age or across plant reproductive stages (e.g. prereproductive, reproductive). Field studies have shown that small juvenile plants have higher gday and Eday and lower water use efficiency than larger adults (Donovan and Ehleringer, 1991, 1992). Leaf age has been shown to cause a decline in gday in sunflowers (Helianthus annuus; Cechin and Fumis, 2004). Similar to these daytime responses, Grulke et al. (2004) found higher gnight in large saplings than in mature trees, and Blom-Zandstra et al. (1995) found gnight of rose leaves declined as leaves aged from 3 to 6 weeks. However, in both of these cases, direct effects of reproductive stage and leaf age cannot be differentiated from additional variables such as plant size and age. Controlled studies are needed to accurately assess the role of plant reproductive stage and leaf age on gnight.
Most measures of plant water loss include loss across both the cuticular and stomatal pathways operating in parallel. Because cuticular conductance (gcuticular) is very small compared to daytime conductance through open stomata (gstomata), its contribution to gday has traditionally been ignored. However, when considering much lower magnitude gnight and Enight, cuticular losses may represent a substantial portion of the total measurement. Estimates of gcuticular, ranging from 0.004 to 0.016 mol m−2 s−1, have been derived from gas exchange measurements of intact leaves where stomatal closure has been induced by either leaf wilting (water stress) or exogenous abscisic acid (ABA) application (Rawson and Clarke, 1988; Kerstiens, 1995; Boyer et al., 1997; Burghardt and Riederer, 2003; Nobel, 2005). These estimates include water loss through the cuticle and maximally closed stomata and thus represent a functional definition of gcuticular. New techniques are available for estimating conductance and permeability of the cuticle separate from the stomatal pores, and they highlight the potential for variability in cuticular permeability (Schreiber et al., 2001; Santrucek et al., 2004; Kerstiens, 2006). However, it is still useful to measure water loss occurring though the cuticle plus stomata at maximal closure, because this represents a baseline that is not subject to short-term stomatal regulation.
We examined gnight and Enight in controlled greenhouse studies using wild H. annuus, H. annuus domesticates (commercial cultivar and Hopi domesticate), and a group of closely related wild species (Helianthus anomalus Blake, Helianthus deserticola Heiser, and Helianthus petiolaris Nutt.). Substantial gnight (0.08–0.10 mol m−2 s−1) has been reported for H. annuus and H. anomalus in their native habitats (Snyder et al., 2003; Ludwig et al., 2006). The inclusion of several species allowed us to assess whether results for regulation of gnight and Enight can be generalized across closely related species. As large annuals, the Helianthus species were easily grown in the greenhouse, allowing experimental manipulation of soil treatments under controlled environmental conditions. This allowed for robust tests of environmentally stimulated regulation and nighttime water loss at different phases of maturity.
Our objective was to investigate issues of regulation and variation in gnight and Enight. Specifically, we addressed three questions: Are gnight and Enight regulated in response to soil nutrient and water availability? Under optimal soil conditions, do gnight and Enight vary nocturnally (within a night) and across leaf lifespan and plant reproductive stage? Finally, is gnight substantially larger than gcuticular when the latter is defined functionally as conductance though the cuticle and maximally closed stomata?
RESULTS
In all nine greenhouse studies (summarized in Table I; Supplemental Table S1), the four species of wild Helianthus plus domesticated H. annuus and H. annuus Hopi all showed substantial loss of water at night. For sufficiently watered plants, gnight averaged 0.098 mol m−2 s−1 (range, 0.023–0.225) and Enight averaged 1.19 mmol m−2 s−1 (range, 0.29–2.46). Where available, gday averaged 0.893 mol m−2 s−1 and Eday averaged 15.60 mmol m−2 s−1. VPDl for the gas exchange measurements averaged 1.30 kPa at night and 2.14 kPa during the day.
Table I.
Overview of nine studies including Helianthus species, water and nutrient treatments (trts), and experimental design
The H. annuus was wild, except where designated; H. annuus dom. is a commercial domesticate and H. annuus Hopi is an early domesticate. See “Materials and Methods” for composition of sufficient and limiting nitrate modified Hoagland solution. For experimental design, RCBD is randomized complete block and CR is completely randomized.
| Study | Species | Nutrient Treatment | Water Stress Treatment | Additional Tests | Experimental Design |
|---|---|---|---|---|---|
| Fall 2003-1 | H. annuusH. anomalusH. deserticolaH. petiolaris | Yes: 40 g or 4 g Osmocote | No | N/A | RCBD: four species × two NPK trts × three blocks × three replicates = 72 plants (gas exchange measures taken on each species separately) |
| Fall 2003-2 | H. annuus dom. | Yes: Hydrosol, then 10 or 1 g Osmocote | Yes: sustained | N/A | RCBD: two NPK trts × two water trts × three blocks × four replicates = 48 plants |
| Fall 2004-1 | H. annuusH. anomalusH. deserticolaH. petiolaris | Yes: 140 or 7 μg mL−1 N (as nitrate) Hoagland | No | Species, cuticular wilting, nocturnal time course | RCBD: four species × two nitrogen trts × three blocks × three replicates = 72 plants |
| Fall 2004-2 | H. annuusH. annuus Hopi | No: 20:10:20 NPK soluble fertilizer | Yes: before measurements | Accession (wild versus Hopi) | RCBD: two accessions × two water trts × three blocks × three replicates = 36 plants |
| Spring 2005 | H. annuus | Yes: 140 or 7 μg mL−1 N (as nitrate) Hoagland | No | Leaf age, cuticular wilting | RCBD: two nitrogen trts × three blocks × three replicates = 18 plants |
| Summer 2005 | H. annuus | Yes: 140 or 7 μg mL−1 N (as nitrate) Hoagland | Yes: before measurements | N/A | RCBD: two nitrogen trts × two water trts × three blocks × three replicates = 36 plants |
| Fall 2005-1 | H. annuus | Yes: 140 or 7 μg mL−1 N (as nitrate) Hoagland | No | Plant age, leaf age | RCBD: three ages × two nitrogen trts × three blocks × three replicates = 54 plants |
| Fall 2005-2 | H. annuus | No: 140 μg mL−1 N (as nitrate) Hoagland | Yes: before measurements | 24-h time course gravimetric E | CR: two water trts × 13 to 14 replicates (10 for gas exchange and three to four for xylem pressure potential) = 27 plants |
| Spring 2006 | H. annuusH. annuus dom. | No: 140 μg mL−1 N (as nitrate) Hoagland | No | Cuticular ABA | CR: two accessions × two trts (ABA or control) × three to seven replicates = 19 plants |
Response of gnight and Enight to Soil Nutrient and Water Manipulation
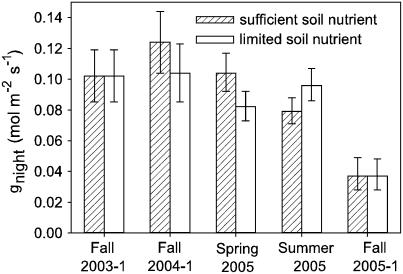
Six studies applied a soil nutrient treatment, four of which only manipulated soil nitrate (Table I). There was no effect of nutrient limitation on gnight and Enight in any of these studies of Helianthus species (Fig. 1; Supplemental Table S1; P > 0.05 for all). The nutrient limitation was substantial enough to significantly reduce vegetative shoot biomass in all six studies (Table II) and reproductive biomass in the studies where plant growth continued into the reproductive stage (Fall 2003-1 micro- and macronutrient manipulation, P < 0.05 for all species except H. deserticola; Fall 2004-1, Spring 2005, Summer 2005 nitrogen manipulation, P < 0.001; data not shown). Leaf total nitrogen content was also measured in four of the six nutrient manipulation studies. The limited nitrate treatment imposed as a modified Hoagland solution resulted in lower leaf nitrogen content (Table II). Leaf nitrogen was measured in only one study involving total macro- and micronutrient manipulation, and here the limited treatment resulted in significantly lower leaf nitrogen concentrations for H. annuus but not for H. anomalus or H. petiolaris.
Figure 1.
Effect of manipulating soil nutrient availability on gnight showing all of the tests for wild H. annuus. In Fall 2003-1, availability of all macro- and micronutrients was manipulated, whereas only nitrogen, available as nitrate, was manipulated in the additional four studies. Bars are lsmeans (least square means) ± 1 se. See Supplemental Table S1 for nutrient treatment comparisons for H. annuus domesticate, H. annuus Hopi, and other Helianthus species.
Table II.
Vegetative shoot biomass at harvest and total leaf nitrogen (N) content of gas exchange leaves for studies that included a nutrient limitation treatment
If no treatment is designated (–), then all plants in that study received sufficient levels of that resource. Values are lsmeans ± 1 se. F values and associated degrees of freedom (Fdf num, df denom) are presented for each model effect (PROC MIXED ANOVA, block as random). F values in bold indicate statistical significance (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
| Study and Species | Nutrient Treatment | Water Treatment | Model Effects | Shoot | N for Gas Exchange Leaf |
|---|---|---|---|---|---|
| g | mg g−1 | ||||
| Fall 2003-1 | |||||
| H. annuus | Sufficient | – | 7.3 ± 3.3 | 5.54 ± 0.39 | |
| Limited | – | 1.8 ± 3.3 | 4.17 ± 0.41 | ||
| Nutrient effect | 29.571,14*** | 26.571,6** | |||
| H. anomalus | Sufficient | – | 14.5 ± 3.0 | 4.30 ± 0.22 | |
| Limited | – | 5.1 ± 3.0 | 4.13 ± 0.22 | ||
| Nutrient effect | 10.461,14** | 1.241,6 | |||
| H. deserticola | Sufficient | – | 15.4 ± 3.3 | Not assessed | |
| Limited | – | 3.3 ± 3.9 | Not assessed | ||
| Nutrient effect | 5.61,8* | Not assessed | |||
| H. petiolaris | Sufficient | – | 10.4 ± 2.9 | 5.85 ± 0.36 | |
| Limited | – | 4.7 ± 2.9 | 4.89 ± 0.32 | ||
| Nutrient effect | 7.011,12* | 4.101,5 | |||
| Fall 2003-2 | |||||
| H. annuus dom. | Sufficient | Sufficient | 8.0 ± 0.4 | Not assessed | |
| Limited | Sufficient | 4.2 ± 0.4 | Not assessed | ||
| Sufficient | Limited | 2.8 ± 0.4 | Not assessed | ||
| Limited | Limited | 1.7 ± 0.4 | Not assessed | ||
| Water effect | 78.331,42*** | ||||
| Nutrient effect | 30.691,42*** | ||||
| Water×nutrient | 9.831,42** | ||||
| Fall 2004-1 | |||||
| H. annuus | Sufficient | – | 4.5 − 1.0, +1.2 | 2.86 ± 0.23 | |
| Limited | – | 1.6 − 0.3, +0.4 | 2.82 ± 0.223 | ||
| H. anomalus | Sufficient | – | 7.3 − 1.6, +2.1 | 3.70 ± 0.24 | |
| Limited | – | 1.0 − 0.3, +0.3 | 3.44 ± 0.26 | ||
| H. deserticola | Sufficient | – | 7.8 − 1.7, +2.2 | 3.53 ± 0.24 | |
| Limited | – | 2.9 − 0.6, +0.8 | 3.19 ± 0.23 | ||
| H. petiolaris | Sufficient | – | 7.9 − 1.7, +2.1 | 4.17 ± 0.23 | |
| Limited | – | 1.2 − 0.3, +0.4 | 3.44 ± 0.26 | ||
| Nitrate effect | 70.561,56*** | 6.781,56* | |||
| Species effect | 2.453,56 | 10.163,56*** | |||
| Nitrate×species | 2.383,56 | 1.213,56 | |||
| Spring 2005 | |||||
| H. annuus | Sufficient | – | 81.7 − 5.5, +5.9 | Not assessed | |
| Limited | – | 7.8 − 0.5, +0.6 | Not assessed | ||
| Nitrate effect | 561.231,33*** | Not assessed | |||
| Summer 2005 | |||||
| H. annuus | Sufficient | Sufficient | 114.0 − 10.5, +11.6 | 3.83 ± 0.10 | |
| Sufficient | Limited | 71.6 − 6.3, +6.8 | Not assessed | ||
| Limited | Sufficient | 6.2 − 0.6, +0.6 | 2.17 ± 0.10 | ||
| Limited | Limited | 4.6 − 0.4, +0.4 | Not assessed | ||
| Water effect | 38.41,29*** | – | |||
| Nitrate effect | 2,065.791,29*** | 130.651,32*** | |||
| Water×nitrate | 1.621,29 | – | |||
| Fall 2005-1 | |||||
| H. annuus | Sufficient; 15.5 week age | – | 87.2 − 16.6, +20.5 | 2.75 ± 0.18 | |
| Sufficient; 10 week age | – | 10.2 − 2.0, +2.5 | 3.87 ± 0.18 | ||
| Sufficient; 5.5 week age | – | 0.6 ± 0.1 | 5.17 ± 0.18 | ||
| Limited; 15.5 week age | – | 7.6 − 1.4, +1.8 | 1.82 ± 0.18 | ||
| Limited; 10 week age | – | 1.3 − 0.2, +0.3 | 2.81 ± 0.18 | ||
| Limited; 5.5 week age | – | 0.3 ± 0.1 | 4.27 ± 0.18 | ||
| Nitrate effect | 180.161,46*** | 42.811,46*** | |||
| Plant age effect | 351.872,46*** | 91.462,46*** | |||
| Nitrate×plant age | 18.492,46*** | 0.112,46 |
In one of the nutrient limitation studies, Fall 2004-1, differences between wild Helianthus species were tested. A significant species effect was found (gnight, F-statistic3,51 = 3.08, P < 0.05; Enight, F3,51 = 3.03, P < 0.05), but a means separation test with Tukey's honestly significant difference showed differences to be minimal and only significant between H. deserticola, with the highest mean gnight and Enight, and H. petiolaris with the lowest (P < 0.05).
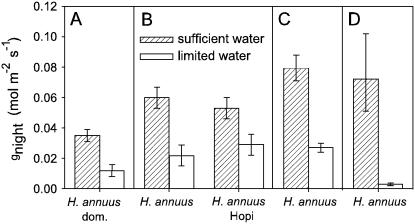
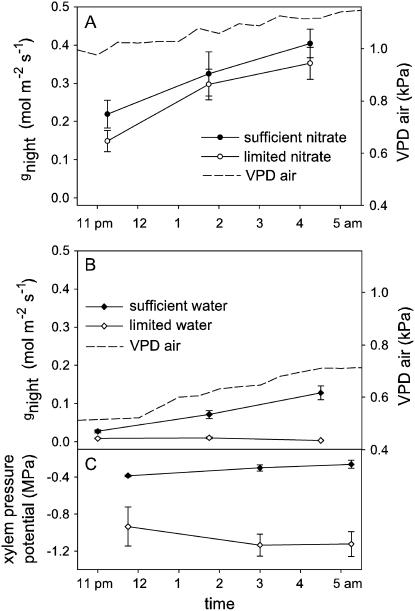
Four studies applied soil water treatments (Table I): sufficient (maintained near field capacity) and limited. Plants with limited water showed substantially reduced gnight, Enight (P < 0.001), gday, Eday, and photosynthesis (P < 0.05–0.001; Fig. 2; Supplemental Table S1). In the Fall 2004-2 study, gnight and Enight were assessed in both wild H. annuus and H. annuus Hopi, but there was no interaction between accession and response to soil water limitation for these traits (P > 0.05). During Fall 2005-2, xylem pressure potentials were measured at three points though the night and were consistently and substantially lower in the water-limited H. annuus (F1,14 = 30.82, P < 0.001; Fig. 3).
Figure 2.
Effect of manipulating soil water availability on gnight during Fall 2003-2 (A), Fall 2004-2 (B), Summer 2005 (C), and Fall 2005-2 (D). In studies where both a water and nutrient treatment were applied (A and C), bars represent data from the high nutrient treatment only. Bars are lsmeans ± 1 se. gcuticular for H. annuus and H. annuus domesticate, measured in Fall 2004-1, Spring 2005, and Spring 2006, ranged from 0.013 to 0.023 mol m−2 s−1.
Figure 3.
Variation in H. annuus gnight and Enight across a single night during Fall 2004-1 (A) and Fall 2005-2 (B) studies. Included are independent measurements of VPDa. Fall 2005-2 included measurements of xylem pressure potential (C) made on separate, randomly chosen plants from each treatment level. Points represent means ± 1 se, n = 5 to 6 for gnight and Enight and n = 3 to 4 for xylem pressure potential. gcuticular for H. annuus, measured in Fall 2004-1, Spring 2005, and Spring 2006, ranged from 0.013 to 0.023 mol m−2 s−1.
Variation in gnight and Enight Nocturnally and across Leaf Lifespan and Plant Reproductive Stages
A 24-h time course was measured for H. annuus in Fall 2005-2. gday, Eday, and photosynthesis showed typical patterns, increasing rapidly in the morning and declining during the afternoon. gnight and Enight, though low compared to daytime rates, increased through the night in the sufficiently watered plants despite a small increase in atmospheric VPD (VPDa) though the night (Fig. 3; time effect for gnight and Enight, respectively, F2,11 = 31.2, P < 0.001; F2,11 = 32.37, P < 0.001). In addition to instantaneous gas exchange measures, gravimetric measures were used to estimate total Enight and total Eday during the same time period. Enight of sufficiently watered plants was 0.86 (se = 0.10) for instantaneous gas exchange and 0.22 (se = 0.01) mmol m−2 s−1 for gravimetric measures. These rates were 5.7% and 6.5%, respectively, of the daytime rates measured by the same methods. Measures of Enight and Eday made with instantaneous and gravimetric methods were correlated (Enight r2 = 0.78, P < 0.001, and Eday r2 = 0.87, P < 0.001; Spearman rank correlations). During this same night and day period, average VPDa in the greenhouse was 0.6 kPa (se = 0.02) and 1.5 kPa (se = 0.12), respectively.
Repeated measures of gnight and Enight were also made on sufficiently watered H. annuus in the Fall 2004-1 study and showed similar trends to those documented in 2005 (Fig. 3). gnight and Enight increased through the night (time effect, respectively: F2,29 = 145.84, P < 0.001; F2,29 = 358.69, P < 0.001) despite increasing VPDa, and these trends were not affected by nitrate treatment (P > 0.5).
The effect of leaf aging on gnight and Enight was initially assessed in the Spring 2005 study. Repeated measures of gnight and Enight were made on the same leaves of H. annuus across 4 weeks, starting when leaves were recently fully expanded. Start dates for the 4-week measurement sets were staggered across several weeks and used in the analysis to account for random environmental variation between nights. There was no decline in gnight or Enight due to leaf aging (F1,321 = 0.83, P > 0.3; F1,321 = 0.57, P > 0.4, respectively; Supplemental Table S1). In the Fall 2005-1 study, leaf age effects were further assessed by comparing a young fully mature and older fully mature leaf of the same plant using sufficient nitrate treatment, 10-week-old plants. Here again, gnight and Enight did not differ with leaf age (t14 = 1.21, P = 0.2; t14 = 1.22, P = 0.2, respectively).
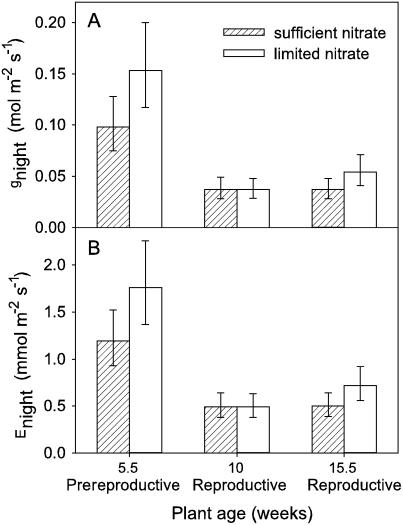
The effect of plant reproductive stage on gnight and Enight was assessed in the Fall 2005-1 study. For H. annuus, plant reproductive stage affected gnight and Enight under both sufficient and limited nitrate availability (F2,46 = 17.45, P < 0.001; F2,46 = 15.96, P < 0.001, respectively; Fig. 4; Supplemental Table S1). Prereproductive plants (5.5 weeks old) had higher gnight and Enight than did reproductive plants (10 or 15.5 weeks old).
Figure 4.
Effect of plant reproductive stage on gnight (A) and Enight (B) in H. annuus during the Fall 2005-1 study. Measurements were made on most recently fully mature leaves produced concurrently. Five and one-half-week-old plants were prereproductive, while 10- and 15.5-week-old plants were both reproductive. Bars are lsmeans ± 1 se, n = 8 to 9.
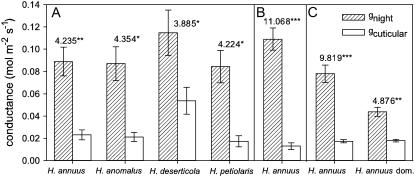
The Contribution of gcuticular to gnight
During the Fall 2004-1 and Spring 2005 studies, gcuticular, functionally defined as water loss though the cuticle with stomata at maximal closure, was measured on excised, wilted leaves. In Fall 2004-1, gnight (stomatal and cuticular conductances combined) was higher than gcuticular for all four wild Helianthus species (Fig. 5). In Spring 2005, gnight was again higher than gcuticular (Fig. 5). In both studies, gcuticular measured on leaves was higher than instrument error (P < 0.001), which averaged −7.5 × 10−6 mol m−2 s−1 during gcuticular measurements. During Spring 2006, gcuticular was measured on intact leaves of plants infused with exogenous ABA into the xylem. gcuticular was lower than gnight measured on intact leaves of control plants for both wild H. annuus and domesticated H. annuus (Fig. 5).
Figure 5.
Instantaneous measures of gnight and gcuticular, functionally defined as conductance though both the cuticle and stomata at maximal closure. Measures of gcuticular were made on excised, wilted leaves during the Fall 2004-1 study (A) and Spring 2005 study (B) and on intact leaves of plants infused with exogenous ABA during the Spring 2006 study (C). Bars are means ± 1 se, n = 5 to 6 bulked across nitrate treatment in Fall 2004-1, n = 9 bulked across nitrate treatment in Spring 2005, and n = 3 to 7 sufficient nitrate treated plants during Spring 2006. Measures were made on different leaves of the same plant in Fall 2004-1and Spring 2005 and made on separate control or ABA treatment plants during one night during Spring 2006. t values and associated degrees of freedom are presented from a paired t test in Fall 2004-1 and Spring 2005 and from an independent t test in Summer 2006 (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Looking across all three studies, gcuticular for wild H. annuus ranged from 0.013 to 0.023 mol m−2 s−1 and there was good agreement between measures made with the two different techniques (Fig. 5). Of the other three wild species, only the estimate of gcuticular for H. deserticola was substantially larger than the range for H. annuus. Not considering H. deserticola, calculated gstomata for wild Helianthus was on average 5 times greater than gcuticular.
DISCUSSION
The Helianthus gnight reported here for greenhouse-grown plants (0.023–0.225 mol m−2 s−1) are within the range reported for two of these species in their native habitats (Snyder et al., 2003; Ludwig et al., 2006) and for C3 and C4 plants in general (Caird et al., 2007). The wild and domesticated Helianthus species in our studies had typical values for gday, Eday, and photosynthesis (Supplemental Table S1), and the gnight values were relatively large and greater than explained by gcuticular.
In the Fall 2005-2 study, gravimetric measures were compared to instantaneous measures of transpiration. The gravimetric measures were approximately 4-fold lower, reflecting their integration over the entire night or day period, whereas instantaneous measures were timed to capture maximal Enight and Eday rates. However, there was a strong correlation between the two measurement techniques. Additionally, the percentage total Enight of total Eday measured gravimetrically over the 24 h gave an estimate of 6%, which agreed well with the 5% estimate from instantaneous gas exchange measures during the same day/night period. This added validity to our estimates based on instantaneous measures.
Response of gnight and Enight to Soil Nutrient and Water Manipulation
We hypothesized that regulation might occur for increased gnight under limited nutrient conditions to increase bulk flow of soil solution to the roots and reduce the development of a nutrient depletion zone in the rhizosphere. Although the soil nutrient limitations were sufficient to limit shoot and reproductive biomass and generally to reduce leaf nitrogen concentration, they did not affect gnight and Enight in any of the wild Helianthus species or in domesticated H. annuus. Thus, for Helianthus, there is no evidence of nighttime stomatal regulation in response to soil nutrient limitations. Contrary to our Helianthus results, we have evidence that other species do respond to soil nutrient limitations imposed while controlling for plant water status, some with higher gnight (Distichlis spicata, Populus balsamifera subsp. trichocarpa) and others with lower gnight (Arabidopsis [Arabidopsis thaliana]; M. Caird and A. Howard, unpublished data). A broader range of species needs to be tested to support any generalizations. The variable response of gnight to nutrient limitation may involve the same mechanisms that are currently being investigated for gday responses, such as ABA, pH, and cytokinin signals (Dodd et al., 2003; Sakakibara et al., 2006).
Whether or not a species regulates gnight in response to soil nutrients, a plant that is transpiring at night may have increased uptake of nutrients such as nitrate. McDonald et al. (2002) demonstrated that Populus plants transpiring continuously (day and night), instead of only during the day, took up more nitrogen. Given that there is genetic variation for gnight and Enight (Arabidopsis; M. Caird, unpublished data), selection may favor high gnight and Enight in nutrient poor habitats if Enight provides a nutrient uptake benefit. The four wild Helianthus species studied here are native to habitats differing in nutrient availability; H. anomalus and H. deserticola are endemic to nutrient poor desert dune habitats (Rosenthal et al., 2002; Brouillette et al., 2007). We found that H. deserticola did have higher gnight and Enight than H. petiolaris, consistent with the direction predicted by selection for higher gnight in lower nutrient habitats, but the magnitude of difference was relatively small and appeared largely driven by greater gcuticular in H. deserticola.
gnight and Enight did decline in response to water limitations that were generally sufficient to decrease leaf predawn xylem pressure potential, gday, Eday, and photosynthesis. Declines were such that gnight in the limited water treatments was generally within the range we recorded for functionally defined gcuticular. For three of the four studies, the water limitation was short term and consisted of withholding water just prior to measurements on fully mature leaves, so that the effect on gnight could not be due to a long-term change in leaf structure, stomatal density or size, or cuticle. The decline in gnight and Enight due to water limitation demonstrates that guard cell regulation of nighttime water loss is possible, analogous to daytime regulation of water loss in response to soil drying. Our results agree with previous results showing lower gnight associated with decreased plant water status in Hibiscus cannabinus (Muchow et al., 1980), Pseudostuga menziesii (Running, 1976; Blake and Ferrell, 1977), and H. anomalus (Ludwig et al., 2006), and a water stress treatment resulting in decreased water loss at night in wheat plants (Rawson and Clarke, 1988). The nighttime stomatal response to drought likely involves many of the same mechanisms that are currently being investigated for daytime responses, such as ABA and pH signals (Dodd, 2003; Davies et al., 2005; Li et al., 2006), although this remains to be determined.
Variation in gnight and Enight Nocturnally and across Leaf Lifespan and Plant Reproductive Stages
Assessing temporal variation is necessary for interpreting the significance of instantaneous leaf-level measures of gnight and Enight. We complemented single instantaneous measures on most recently fully expanded leaves of mature plants with studies that assessed variation nocturnally, across leaf lifespan, and across plant reproductive stages. Beginning with nocturnal variation (across a single night), repeated measures during the night in two studies both showed a significant increase in gnight and Enight. A similar gradual increase in gnight has been observed in several other species, including Arabidopsis, desert shrubs, and trees (Lasceve et al., 1997; Leymarie et al., 1998, 1999; Donovan et al., 2003; Bucci et al., 2004; Dodd et al., 2004, 2005), and potential regulatory mechanisms are being investigated (Lasceve et al., 1997; Gardner et al., 2006).
When gnight was measured across three nocturnal time points for sufficiently watered H. annuus in Fall 2005-2, the increase in gnight was associated with an increase in VPDa, although of small magnitude (from approximately 0.5–0.7 kPa; Fig. 3). Thus, over this range of VPDa, the correlation between gnight and VPD was not the negative relationship expected from daytime VPDa responses (Franks and Farquhar, 1999). However, a larger range of VPDa is needed to test nighttime VPD responses. Correlative data from other studies suggest that gnight does decline in response to increased VPDa, similar to gday, and responses may be species specific (Oren et al., 2001; Bucci et al., 2004; but see Barbour et al., 2005 for contrast). Bakker (1991) found a decline in gnight in response to experimentally manipulated VPD. However, more studies are needed that experimentally manipulate VPDa and VPDl and account for potentially confounding factors such as gcuticular, circadian rhythms, and xylem pressure potential recovery.
We assessed variation in gnight and Enight across entire leaf lifetime. In contrast to Cechin and Fumis (2004), who found that gday declined as Helianthus leaves aged, and Blom-Zandstra et al. (1995) who found that gnight declined as rose leaves aged, we found no decline in H. annuus gnight and Enight as recently matured (i.e. fully expanded) leaves aged over the following 4 weeks. Measures on the same plants indicated that leaf lifespan (number of days from 1-cm leaf blade length to 50% of leaf senesced) averaged 40 d (5.7 weeks). Thus, our gas exchange measurements captured the majority of leaf lifespan. The lack of decline in nighttime gas exchange rates over leaf lifespan suggests that for Helianthus, instantaneous gnight on a recently matured leaf may be used to scale up to instantaneous gnight for whole-plant leaf area, provided that there is an open canopy structure.
To generalize across plant life stages, we investigated variation in gnight and Enight across plant reproductive stages, controlling for leaf age. Prereproductive H. annuus showed significantly higher gnight and Enight than individuals that were flowering or setting seeds. Our results are consistent with those of Grulke et al. (2004) who found gnight to be higher in large saplings compared to mature ponderosa pine.
Young plants, during rapid vegetative growth, expend a large portion of respiratory energy on nutrient uptake, and this proportion generally declines as plants age (Marschner, 1995). Thus, although Helianthus species appear unable to regulate nighttime water loss in response to soil nutrient conditions, an inherently higher Enight for younger plants may be beneficial if it reduces formation of a nutrient depletion zone around roots at night, as suggested by results with the Barber-Cushman model (Barber and Cushman, 1981). Nutrient depletion zones may be more pronounced around roots of prereproductive plants due to a significantly lower root mass ratio (F2,46 = 6.89, P < 0.01). Whether or not increased Enight represents a nutrient uptake benefit for prereproductive phase plants, it is possible that estimates of total water flux in mixed-aged stands or integrated over the life of a crop are underestimated when based on a combination of Eday and Enight measured only on reproductive-aged individuals.
The Contribution of gcuticular to gnight
Measures of gnight and Enight include cuticular and stomatal pathways in parallel, yet only water loss through stomata, at an aperture greater than maximal possible closure, may be subject to guard cell regulation. For all wild Helianthus species except H. deserticola, gstomata was 5 times greater than gcuticular, suggesting that most nighttime water loss can be regulated. With the exception of the extremely high gcuticular for H. deserticola, which deserves further investigation, the remaining gcuticular for Helianthus were in the upper range of those reported in the literature using comparable techniques (Rawson and Clarke, 1988; Kerstiens, 1995; Boyer et al., 1997; Burghardt and Riederer, 2003; Nobel, 2005). More characterizations are needed of inter- and intraspecific variation in gcuticular, including the extent to which growth conditions and atmospheric humidity can change gcuticular components (Schreiber et al., 2001; Kerstiens, 2006; Kock et al., 2006).
Variation among Studies in Magnitude of gnight
Although our tests of gnight responses to nutrients and water occurred within each study, and cross study comparisons were not preplanned, the study differences in maximum gnight deserve some comments. For wild H. annuus in the nutrient and water manipulation studies, gnight of sufficiently watered plants ranged from 0.04 to 0.12 mol m−2 s−1 (Figs. 1 and 2; Supplemental Table S1). Because studies were conducted in different seasons and years, some of the variation may have been due to differences in the growth environment and to VPDl differences during the nights and days of gas exchange measurements. However, the study with the lowest gnight (Fall 2005-1) did not stand out as having the highest VPDl on the night or accompanying day of gas exchange measurements or an unusual VPDa across the growth interval of the study. It is possible that using study means obscures a specific time interval where VPDa affected leaf development and maximum gnight, but there are many other potential contributing factors. We recommend more exploration of growth environment (temperature, humidity, CO2 levels, light quantity and quality, plant nutritional status, growth medium, etc.) on leaf structure, stomatal density and size, cuticular properties, and maximum gnight (Hetherington and Woodward, 2003; Bergmann et al., 2006; Kock et al., 2006). Additionally, the effects of VPDa and VPDl prior to and during the gas exchange measurements deserve more attention (Franks and Farquhar, 1999; Schreiber et al., 2001).
Across multiple studies, we demonstrate substantial gnight and Enight in Helianthus wild species and domesticates. For Helianthus, nighttime water loss occurs largely through stomata and is regulated in response to plant water stress but not soil nutrient availability. Additionally, Helianthus gnight varies nocturnally and across plant reproductive stages but does not vary for individual leaves as they age. More research is needed to test the commonality of these findings in plants of various life histories and native to diverse habitats. Building generalities for variation and regulation of gnight and Enight is necessary for predicting the conditions under which nighttime water loss will be biologically significant.
MATERIALS AND METHODS
The objectives were addressed in nine greenhouse studies carried out at the Biological Sciences Plant Growth Facility at the University of Georgia, Athens (Table I). The studies included four wild annual Helianthus species (Helianthus annuus, Helianthus anomalus Blake, Helianthus deserticola Heiser, and Helianthus petiolaris Nutt.), commercial H. annuus cv Gray Stripe (referred to as H. annuus domesticate), and the Hopi domesticate of H. annuus (referred to as H. annuus Hopi). Achenes of the four wild Helianthus species were collected in Juab County, Utah, except for the H. annuus from Keith County, Nebraska used in the Fall 2003-2 and Fall 2004-2 studies, and the H. petiolaris collected in Washington County, Utah. The achenes of H. annuus domesticate used in Fall 2003-2 and Spring 2006 studies were obtained from Carolina Biological. The achenes of H. annuus Hopi (PI 432504 NPGS accession) used in the Fall 2004-2 study were originally collected from Shungopovi Village, Hopi Indian Reservation, Navajo County, Arizona.
The wild Helianthus species and the H. annuus Hopi achenes were germinated in petri dishes and transferred to pots after the seedlings developed root hairs. The H. annuus domesticate achenes were sown directly into the study pots. The study pots (20–25 cm diameter) contained a mix of sand and Turface (fritted clay, Profile Products), except for the Fall 2003-1 and Fall 2003-2 studies that used all sand. All plants were grown in a greenhouse with natural daylight supplemented to 12 to 14 h with metal-halide lamps. Temperatures were generally set to be at or above 26°C (day) and 16°C (night). For the six studies that had greenhouse weather available for the growth interval (Fall 2004-1, Fall 2004-b, Spring 2005, Summer 2005, Fall 2005-1, and Spring 2006), the average night VPDa and day VPDa across studies (n = 6) was 0.88 (se = 0.11) and 1.57 (se = 0.10) kPa, respectively.
Nutrient and Water Treatments
Nutrient treatments manipulated either total macro- and micronutrients (slow-release fertilizer, Osmocote Plus, Scotts-Sierra Horticultural Products) or manipulated just nitrogen (available only as nitrate). The latter was achieved with thrice weekly applications of a modified Hoagland solution containing 140 or 7 μg mL−1 nitrogen as nitrate. The sufficient and limited nitrate Hoagland solutions contained equal amounts of potassium (176 μg mL−1 K) and phosphorus (31 μg mL−1 P). Additional macronutrients were calcium (50 μg mL−1 in high; 10 μg mL−1 in low), sulfur (8 μg mL−1 in high; 120 μg mL−1 in low), and magnesium (55 μg mL−1 in high; 6 μg mL−1 in low). Micronutrients included: Cl (0.443 μg mL−1), B (0.068 μg mL−1), Mn (0.027 μg mL−1), Zn (0.033 μg mL−1), Cu (0.008 μg mL−1), Mo (0.012 μg mL−1), and Fe (0.698 μg mL−1 as FeEDTA). In the three studies without a nutrient treatment, the plants received either the high nitrate Hoagland solution or weekly application of 20:10:20 NPK soluble fertilizer (Peter's Peat-Lite Special, Scotts-Sierra Horticultural Products).
The soil water treatments consisted of supplying plants with ample water to maintain soils near field capacity (sufficient) and limiting the soil water availability (limited) either just prior to gas exchange measures or as a sustained treatment throughout the study. The limitation of soil water availability prior to gas exchange measures consisted of withholding water until visual wilting and depression of daytime gas exchange rates were achieved. The sustained water limitation in the Fall 2003-2 study consisted of watering every 4 to 5 d, beginning 2 weeks after germination. For the Fall 2005-2 study, leaf predawn xylem pressure potentials were sampled to accompany gas exchange measurements using a pressure chamber (Soil Moisture Equipment).
Gas Exchange Procedures
Leaf level measurements of daytime and nighttime gas exchange were made with a portable photosynthesis system (LI-6400, LI-COR). Measurements were made on a young fully expanded leaf of each plant, except when testing leaf age effects in the Spring 2005 study. The chamber light level was set to be 0 or 2,000 μmol m−2 s−1 during the night and day, respectively. To view equipment and plants at night, we used green safety headlamps with intensity not detectable by an LI-190 sensor (0 μmol m−2 s−1 photosynthetic photon flux density (LiCor) to avoid promoting stomatal opening. During the Fall 2004-1 study and part of the Spring 2005 study, leaves of some species were too small for the standard chamber, and an Arabidopsis (6400-15, LiCor) chamber was used. This chamber lacks an internal light source, and daytime measurements were therefore only taken on sunny days when photosynthetically active radiation exceeded 1,500 μmol m−2 s−1.
For both chambers, air temperature was set to ambient, and CO2 was supplied at 400 μmol mol−1. Flow was set to 125 to 200 μmol s−1 at night and 700 μmol s−1 during the day. Chamber fan speed was set to high. To partially compensate for removal of the boundary layer due to the chamber mixing fan, chamber relative humidity was manually manipulated to a target 5% to 10% above ambient (assessed with open chamber). The standard chamber directly measures leaf temperature, and before every set of measurements, the leaf thermocouple was checked to ensure it was reading accurately to within 0.1°C. Sample and reference infrared gas analyzers were matched prior to every plant for nighttime measurements. Measurements were also made with an empty chamber or with dry paper in the chamber every four to six leaf measures to assess instrument error. Averaged by study, estimates of instrument error obtained with the standard or Arabidopsis chamber at night yielded values for g from 0.001 to 0.016 mol m−2 s−1, which was always substantially lower than plant measures. Plant measures were logged when readings were stable and typically within 1 to 2 min of clamping onto the leaf.
Whenever possible, leaves were chosen that would fill the leaf chamber (6 cm2 for standard chamber; 0.8 cm2 for Arabidopsis chamber). When leaves that did not fill the chamber were used, all leaves in the measurement set (including those that filled the chamber) were marked before removal from the chamber to indicate placement of the chamber gaskets. The following day, gas exchange leaves were cut to remove all area that was not inside the chamber and scanned (Winfolia, Regent Instruments) to determine area. Leaves that did not fill the chamber were used in the Arabidopsis chamber in Fall 2004-1 (minimum area 0.45 cm2) and in the standard chamber in Fall 2005-1 (minimum area 4.5 cm2).
Daytime measurements were typically made between 9 am and 2 pm, and nighttime measurements were typically made between 1 am and the beginning of astronomical twilight (sun 12° below the horizon). Measures made at three times spaced though the night confirmed that this period captured maximum gnight but was well before a predawn stomatal opening would occur.
In the Fall 2005-2 study, nighttime water loss was measured both instantaneously using the LI-6400 as well as gravimetrically. Gravimetric measures of transpiration made over a 24-h time span were achieved by sealing the pot and root system in a bag, bagging all flower heads, and weighing at the beginning and end of the day and night periods. To obtain water loss per area, all leaves were harvested the following day, and total leaf area was measured using a LI-3100 leaf area meter (LiCor).
Assessment of Cuticular Water Loss
gcuticular was defined functionally as conductance through the cuticle and stomata at maximum closure induced by either leaf wilting (water stress) or exogenous ABA application. As such, it includes both water loss through the cuticle and water loss through stomata at minimum aperture. The conductance provided by the LI-6400 (gnight or gday in this study) is a total of both gcuticular and gstomata in parallel. Stomatal conductance at night was calculated as gnight minus gcuticular (Nobel, 2005).
Cuticular water loss for excised, wilted leaves was estimated both by weighing (Rawson and Clarke, 1988) and by gas exchange measurements with the LI-6400. For weighing, excised leaves (cut end of petiole sealed with wax) were allowed to dry and wilt in the dark at ambient room temperature and VPD. Weights were taken approximately every 15 min, and, after initial rapid loss of water during which time stomata presumably closed, the linear relationship of water loss and time was used to estimate Ecuticular. During this period of linear water loss, gcuticular and Ecuticular were also measured with the LI-6400 set to match ambient temperature and VPD. In the Fall 2004-1 study, Ecuticular from these methods, including all four species of wild Helianthus, were highly correlated (r2 = 0.939, P < 0.0001, n = 24). Thus, only the instantaneous LI-6400 measurements of gcuticular and Ecuticular are reported.
gcuticular and Ecuticular were also measured on leaves for which stomatal closure had been induced by exogenous ABA application. ABA was fed into the xylem sap of sufficiently watered plants (Borel et al., 2001). Funnels were sealed around the stems of treatment plants and filled at predawn with degassed ABA solution [1.6 mol m−3 synthetic (±) ABA, 0.4 mol m−3 Ca(NO3)2, and 2.0 mol m−3 KH2PO4]. Stems were drilled radially below the surface of the solution. Funnels were covered with silver foil and the solution was topped off as needed during the following day and night to ensure the drill hole was always below the surface of the solution. Plants were watered amply throughout the period of experimentation, and gas exchange measures were made on the first leaf above the infusion point.
Leaf Tissue Analysis
In most of the nutrient treatment studies, leaves used for gas exchange were collected after measurement, dried, ground, and analyzed for nitrogen content (Carbo Era NA 1500 CN analyzer). When a factorial design of water and nutrient treatments was present, only the plants in the high water treatment were analyzed for leaf nitrogen. In the Fall 2004-1 study, gas exchange measurements were made on two dates per plant, and these two leaves were combined for analysis of nitrogen content.
Biomass Measures
Plants were generally harvested after reaching reproductive maturity and when plants began to show shoot senescence. Plants in the Fall 2003-2, Fall 2005-2, and younger age classes in Fall 2005-1 studies were harvested before or shortly after the appearance of first flower. Plant shoots were divided into vegetative and reproductive components, dried at 60°C, and weighed.
Experimental Design and Statistical Analysis
Experiments were either complete randomized block designs or completely randomized (Table I). When gas exchange measurements were made across several days and nights, plants were grouped by block so that random effects due to night of measurement (e.g. VPDa) were accounted for by the block effect. Measurements of different species or treatments made in one night and block were randomized to avoid confounding treatment results with effects of circadian rhythm or changing VPD though the night.
Most data were analyzed using a mixed-model ANOVA, with block treated as a random effect (PROC MIXED; SAS Institute, 2004, version 9.1) or with a general linear model ANOVA when blocking was not present (Fall 2005-2 study; PROC GLM; SAS Institute, 2004, version 9.1). In some cases, plant death, outliers, or difficulties with treatment application (e.g. ABA application, Spring 2006) resulted in an unbalanced design. When additional tests only involved two levels of a single variable, paired or independent t tests were used as appropriate. The Fall 2004-1 and Fall 2005-2 studies included repeated gas exchange measures during a 24-h period, and these data were analyzed in a repeated-measurement mixed model in PROC MIXED with an unstructured covariance matrix. In all analyses, variables were log transformed when necessary to approach model assumptions of normality of residuals and homogeneity of variance.
Supplemental Data
The following materials are available in the online version of this article.
Table S1. A summary of means and statistical results for results summarized in text for gnight, Enight, gday, Eday, and photosynthesis.
Supplementary Material
Acknowledgments
The authors thank A. Tull, M. Boyd, M. Gebremedhin, F. Ludwig, M. Spharago, S. Howard, and B. Brouillette for assistance in the greenhouse, and J.H. Richards for comments on earlier drafts.
This work was supported by the National Science Foundation (grant nos. 0416627 to L.A.D. and 0416581 to J.H.R.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ava R. Howard (ahoward@plantbio.uga.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bakker JC (1991) Leaf conductance of four glasshouse vegetable crops as affect by air humidity. Agricul Forest Meterol 55 23–36 [Google Scholar]
- Barber SA (1995) Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley & Sons, New York
- Barber SA, Cushman JH (1981) Nitrogen uptake model for agronomic crops. In IK Iskander, ed, Modeling Waste Water Renovation-Land Treatment. Wiley-Interscience, New York
- Barbour MM, Cernusak LA, Whitehead D, Griffin KL, Turnbull MH, Tissue DT, Farquhar GD (2005) Nocturnal stomatal conductance and implications for modeling δ18O of leaf-respired CO2 in temperate tree species. Funct Plant Biol 32 1107–1121 [DOI] [PubMed] [Google Scholar]
- Benyon R (1999) Nighttime water use in an irrigated Eucalyptus grandis plantation. Tree Physiol 19 853–859 [DOI] [PubMed] [Google Scholar]
- Bergmann D (2006) Stomatal development: from neighborly to global communication. Curr Opin Plant Biol 9 478–483 [DOI] [PubMed] [Google Scholar]
- Blake J, Ferrell WK (1977) Association between soil and xylem water potential, leaf resistance, and abscisic acid content in droughted seedlings of Douglas fir (Pseudotsuga menziesii). Physiol Plant 39 106–109 [Google Scholar]
- Blom-Zandstra M, Pot CS, Mass FM, Schapendonk HCM (1995) Effects of different light treatment on the nocturnal transpiration and dynamics of stomatal closure of two Rose cultivars. Sci Hortic 61 251–262 [Google Scholar]
- Borel C, Frey A, Marion-Poll A, Tardieu F, Simonneau T (2001) Does engineering abscisic acid biosynthesis in Nicotiana plumbaginifolia modify stomatal response to drought? Plant Cell Environ 24 477–489 [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD (1997) CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol 114 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouillette LC, Gebremedhin M, Rosenthal DM, Donovan LA (2007) Testing hypothesized evolutionary shifts toward stress tolerance in hybrid Helianthus species. West N Am Nat (in press)
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Hinojosa JA, Hoffman WA, Franco AC (2004) Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiol 24 1119–1127 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Riederer M (2003) Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. J Exp Bot 54 1941–1949 [DOI] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA (2007) Nighttime stomatal conductance and transpiration in C3 and C4 plants. Plant Physiol 143 4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechin I, Fumis TD (2004) Effect of nitrogen supply on growth and photosynthesis of sunflower plants grown in the greenhouse. Plant Sci 166 1379–1385 [Google Scholar]
- Chapin FS III (1990) Effects of nutrient deficiency on plant growth: evidence for a centralized stress-response system. In WJ Davies, B Jeffcoat, eds, Importance of Root to Shoot Communication in the Responses to Environmental Stress. British Society for Plant Regulation, Bristol, United Kingdom, pp 135–148
- Ciompi S, Gentili E, Guidi L, Soldanti GF (1996) The effect of nitrogen deficiency on leaf gas exchange and chlorophyll fluorescence parameters in sunflower. Plant Sci 118 177–184 [Google Scholar]
- Cowan IR (1977) Stomatal behavior and environment. Adv Bot Res 4 117–228 [Google Scholar]
- Daley MJ, Phillips NG (2006) Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol 26 411–419 [DOI] [PubMed] [Google Scholar]
- Davies WJ, Kudoyarova G, Hartung W (2005) Long-distance ABA signaling and its relation to other signaling pathways in the detection of soil drying and the mediation of the plant's response to drought. J Plant Growth Regul 24 285–295 [Google Scholar]
- Dodd AN, Parkinson K, Webb AAR (2004) Independent circadian regulation of assimilation and stomatal conductance in the ztl-1 mutant of Arabidopsis. New Phytol 162 63–70 [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309 630–633 [DOI] [PubMed] [Google Scholar]
- Dodd IC (2003) Hormonal interactions and stomatal responses. J Plant Growth Regul 22 32–46 [Google Scholar]
- Dodd IC, Tan LP, He J (2003) Do increases in xylem sap pH and/or ABA concentration mediate stomatal closure following nitrate deprivation? J Exp Bot 54 1281–1288 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Ehleringer JR (1991) Ecophysiological differences among juvenile and reproductive plants of several woody species. Oecologia 86 594–597 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Ehleringer JR (1992) Contrasting water-use patterns among size and life-history classes of a semiarid shrub. Funct Ecol 6 482–488 [Google Scholar]
- Donovan LA, Grise DJ, West JB, Pappert RA, Alder NN, Richards JH (1999) Predawn disequilibrium between plant and soil water potentials in two cold-desert shrubs. Oecologia 120 209–217 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84 463–470 [Google Scholar]
- Franks PJ, Farquhar GD (1999) A relationship between humidity response, growth form, and photosynthetic operating point in C3 plants. Plant Cell Environ 22 1337–1349 [Google Scholar]
- Fredeen AL, Gamon JA, Field CB (1991) Responses of photosynthesis and carbohydrate-partitioning to limitation in nitrogen and water availability in field-grown sunflower. Plant Cell Environ 14 963–970 [Google Scholar]
- Gardner MJ, Hubbard KE, Hotta CT, Dodd AN, Webb AAR (2006) How plants tell the time. Biochem J 397 15–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grulke NE, Alonso R, Nguyen T, Cascio C, Dobrowolski W (2004) Stomata open at night in pole-sized and mature ponderosa pine: implications for O3 exposure metrics. Tree Physiol 24 1001–1010 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424 901–908 [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Pangle R, Schotzko A (2007) Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol (in press) [DOI] [PubMed]
- Kerstiens G (1995) Cuticular water permeance of European trees and shrubs grown in polluted and unpolluted atmospheres, and its relation to stomatal response to humidity in beech (Fagus sylvatica L.). New Phytol 129 495–503 [Google Scholar]
- Kerstiens G (2006) Water transport in plant cuticles: an update. J Exp Bot 57 2493–2499 [DOI] [PubMed] [Google Scholar]
- Kock K, Hartmann KD, Schreiber L, Barthlott W, Neinhuis C (2006) Influences of air humidity during the cultivation of plant wax chemical composition, morphology and leaf surface wettability. Environ Exp Bot 56 1–9 [Google Scholar]
- Lasceve G, Leymarie J, Vavasseur A (1997) Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant Cell Environ 20 350–358 [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A (1998) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25 785–791 [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A (1999) Elevated CO2 enhances stomatal responses to osmotic stress and abscisic acid in Arabidopsis thaliana. Plant Cell Environ 22 301–308 [Google Scholar]
- Li S, Assman SM, Albert R (2006) Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol 4 1732–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig F, Jewett RA, Donovan LA (2006) Nutrient and water addition effects on day and night-time conductance and transpiration in a C3 desert annual. Oecologia 148 219–225 [DOI] [PubMed] [Google Scholar]
- Marschner HM (1995) Mineral Nutrition of Higher Plants, Ed 2. Academic Press, San Diego
- McDonald EP, Erickson JE, Kruger EL (2002) Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct Plant Biol 29 1115–1120 [DOI] [PubMed] [Google Scholar]
- Muchow RC, Ludlow MM, Fisher MJ, Myers RJK (1980) Stomatal behavior of kenaf and sorghum in a semiarid tropical environment. I. During the night. Aust J Plant Physiol 7 609–619 [Google Scholar]
- Musselman RC, Minnick TJ (2000) Nocturnal stomatal conductance and ambient air quality standards for ozone. Atmos Environ 34 719–733 [Google Scholar]
- Nobel PS (2005) Physiochemical and Environmental Plant Physiology, Ed 3. Academic Press, San Diego
- Oren R, Sperry JS, Ewers BE, Pataki DE, Phillips N, Megonigal JP (2001) Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia 126 21–29 [DOI] [PubMed] [Google Scholar]
- Rawson HM, Clarke JM (1988) Nocturnal transpiration in wheat. Aust J Plant Physiol 15 397–406 [Google Scholar]
- Rosenthal DM, Schwarzbach AE, Donovan LA, Raymond O, Rieseberg LH (2002) Phenotypic differentiation between three ancient hybrid taxa and their parental species. Int J Plant Sci 163 387–398 [Google Scholar]
- Running SW (1976) Environmental control of leaf water conductance in conifers. Can J For Res 6 104–112 [Google Scholar]
- Sakakibara H, Takei K, Hirose N (2006) Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends Plant Sci 11 440–448 [DOI] [PubMed] [Google Scholar]
- Santrucek J, Simanova E, Karbulkova J, Simkova M, Schreiber L (2004) A new technique for measurement of water permeability of stomatous cuticular membranes isolated from Hedera helix leaves. J Exp Bot 55 1411–1422 [DOI] [PubMed] [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol (in press) [DOI] [PubMed]
- Schreiber L, Skrabs M, Hartman KD, Diamantopoulos P, Simanova E, Santrucek J (2001) Effect of humidity on cuticular water permeability of isolated cuticular membranes and leaf disks. Planta 214 274–282 [DOI] [PubMed] [Google Scholar]
- Snyder KA, Richards JH, Donovan LA (2003) Night-time conductance in C3 and C4 species: do plants lose water at night? J Exp Bot 54 861–865 [DOI] [PubMed] [Google Scholar]
- Sperry JS (2000) Hydraulic constraints on plant gas exchange. Agricul Forest Meterol 1041 13–23 [Google Scholar]
- Sperry JS, Hacke UG, Oren R, Comstock JP (2002) Water deficits and hydraulic limits to leaf water supply. Plant Cell Environ 25 251–263 [DOI] [PubMed] [Google Scholar]
- Tanner W, Beevers H (2001) Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci USA 98 9443–9447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.