Abstract
Stomatal responses to atmospheric change have been well documented through a range of laboratory- and field-based experiments. Increases in atmospheric concentration of CO2 ([CO2]) have been shown to decrease stomatal conductance (gs) for a wide range of species under numerous conditions. Less well understood, however, is the extent to which leaf-level responses translate to changes in ecosystem evapotranspiration (ET). Since many changes at the soil, plant, and canopy microclimate levels may feed back on ET, it is not certain that a decrease in gs will decrease ET in rain-fed crops. To examine the scaling of the effect of elevated [CO2] on gs at the leaf to ecosystem ET, soybean (Glycine max) was grown in field conditions under control (approximately 375 μmol CO2 mol−1 air) and elevated [CO2] (approximately 550 μmol mol−1) using free air CO2 enrichment. ET was determined from the time of canopy closure to crop senescence using a residual energy balance approach over four growing seasons. Elevated [CO2] caused ET to decrease between 9% and 16% depending on year and despite large increases in photosynthesis and seed yield. Ecosystem ET was linked with gs of the upper canopy leaves when averaged across the growing seasons, such that a 10% decrease in gs results in a 8.6% decrease in ET; this relationship was not altered by growth at elevated [CO2]. The findings are consistent with model and historical analyses that suggest that, despite system feedbacks, decreased gs of upper canopy leaves at elevated [CO2] results in decreased transfer of water vapor to the atmosphere.
Soybean (Glycine max) is one of two major species that, with maize (Zea mays), comprises the largest ecosystem type in temperate North America and covers more than 61.8 million ha in the United States (U.S. Department of Agriculture, 2004). Most of this ecosystem is rain fed, and, since it is located in a continental interior, any direct effect of rising concentration of CO2 [CO2] on its transpiration could have a large impact on the regional climate (Sellers et al., 1997). With very few exceptions, decreased stomatal conductance (gs) is one of the most consistent and conserved responses of leaves to growth at elevated [CO2] (Curtis, 1996; Lee et al., 2001; Medlyn et al., 2001; Zheng and Peng, 2001; Ainsworth et al., 2002; Long et al., 2004; Ainsworth and Long, 2005). Although transpiration will be proportional to gs under constant environmental conditions where leaves are tightly coupled with the atmosphere, in the open air the relationship may be more complex. The major resistance pathways between water uptake at the root and transpiration through the stomata influence leaf transpiration, whereas additional resistance pathways exist between the stomata and the bulk atmosphere that can feed back on ecosystem evapotranspiration (ET; Bazzaz and Sombroek, 1996). Resistances occur at the leaf boundary layer, transfer within the plant canopy, transfer to the canopy boundary layer, and entrance into the mixed layer between the canopy and the overlying air. For example, in the short term a lower gs at elevated CO2 will increase leaf temperature and, in turn, water vapor pressure deficit (D), which will tend to increase transpiration partially offsetting the response of gs to CO2. In the longer term, conservation of soil moisture due to decreased gs may result in increased leaf growth and, in turn, more transpiration. Conservation of soil moisture will also allow the plant to maintain gs and ET for longer during a drought. Such feedbacks may decrease or eliminate a long-term effect of decreased gs on ecosystem ET. These uncertainties led the Intergovernmental Panel on Climate Change to conclude that “it is apparent that reductions in gs do not necessarily translate into reductions in catchment-scale evapotranspiration” (Arnell and Liu, 2001).
Some studies in field and laboratory chambers have shown a decrease in ET with elevated CO2 (Chaudhuri et al., 1990; Li et al., 2004; Kim et al., 2006), and others have not (Hileman et al., 1994). However, coupling to the atmosphere, humidity, radiation, and temperature are all altered by chambers, such that they may modify ecosystem response to CO2 (McLeod and Long, 1999). A free air CO2 enrichment (FACE) experiment in Maricopa, AZ, demonstrated decreases in ET for wheat (Triticum aestivum) grown at elevated [CO2] (550 μmol mol−2) of approximately 7% (Kimball et al., 1999) and for sorghum (Sorghum bicolor) about 12% (Triggs et al., 2004), but observed no change in ET for cotton (Gossypium hirsutum; Kimball et al., 1994). FACE experiments in central Italy and in Japan observed a 12% reduction in ET for potato (Solanum tuberosum; Magliulo et al., 2003) and a 8% reduction in ET for rice (Oryza sativa; Yoshimoto et al., 2005) grown at elevated [CO2] (550 μmol mol−2). However, all of these experiments concerned irrigated or flooded systems in which the potential feedback from vegetation to the climate would be expected to be low compared to rain-fed areas in continental interiors (Sellers et al., 1997), such as the Midwestern United States.
The observed decreases in ET for the FACE experiments range from no change to approximately 12% lower with growth in elevated [CO2], whereas meta-analyses across the FACE experiments show a decrease in gs of 20%, with 95% confidence limits ranging from 22.6% to 17.3% (Ainsworth and Long, 2005). This suggests that the decrease in ET is substantially less than the decrease in gs. Does decreased gs result in decreased ET in rain-fed systems, which represent most terrestrial vegetation, and, specifically, the Midwest Corn-Belt ecosystem?
The Soybean Free Air Concentration Enrichment (SoyFACE) experiment located in central Illinois within the most productive portion of the Corn Belt provided an opportunity to test the question of whether rising CO2 will depress ecosystem ET for a rain-fed agricultural ecosystem. Here, across three complete growing seasons, elevation of CO2 from current ambient (approximately 375 ppm) to 550 ppm decreased midday gs of soybean by 16% (Bernacchi et al., 2006). However, leaf area index (LAI) of the same crop in elevated CO2 was increased, on average, by 10% (Dermody et al., 2006), which could potentially offset the decrease in individual leaf gs at the canopy level.
In this study, we present findings from four consecutive years (2002–2005) of season-long ET measurements made at the SoyFACE facility, 3 years of which overlap the leaf-level measurements of gs and photosynthesis (Bernacchi et al., 2006). If feedbacks between the canopy and the atmosphere are sufficiently large to minimize the leaf-level decreases in gs from being expressed at the canopy scale, then plants grown in elevated [CO2] will have rates of ET similar to plants grown under control conditions.
RESULTS
Meteorological and Climatic Conditions Varied over the Duration of This Experiment
The four growing seasons spanning the duration of the experiment (2002–2005) represented a wide range of conditions as is typical with field-based studies on rain-fed sites. Both 2002 and 2005 had slightly above- and 2003 and 2004 slightly below-average temperatures compared with the 30-year mean (Fig. 1A). The Palmer Crop Moisture Index (PCMI) provides a simple indicator of soil moisture status (Palmer, 1968). The mean growing season PCMI for each year from 2002 to 2004 had average or above-average soil moisture status as indicated by the positive PCMI, whereas the 2005 growing season experienced a drought (Fig. 1B). Daily average solar radiation varied slightly over the duration of this study, with 2004 being the only year where the daily mean radiation was less than the 30-year mean (Fig. 1C). The 2003 growing season was unique compared with the other years because a severe hail storm resulted in substantial damage on day of year (DOY) 198. This storm caused an instantaneous reduction in the LAI of at least 55% (Morgan et al., 2005).
Figure 1.
A, Mean daily minimum (black bars) and maximum (gray bars) temperatures (±1 se) recorded at a weather station at the SoyFACE research facility in Champaign, IL, over the duration of this experiment. Seasonal 30-year mean maximum and minimum temperatures are represented by dotted lines from a weather station located within 3 km of SoyFACE. B, Seasonal (May–August) mean PCMI (Palmer, 1968; bars) and the mean (May–August) 30-year average PCMI value (dotted line) for Illinois Climate Division 5, which includes SoyFACE. C, Average daily totals (±1 se; bars) from May to August for each growing season and the 30-year running mean daily total solar radiation (dotted line).
Responses of ET to Elevated [CO2] Varied Based on Meteorological Conditions for a Given Day
The analysis presented here includes data from canopy closure until senescence for four growing seasons (totaling more than 300 d). Data from days prior to canopy closure are disproportionately influenced by the dark soils typically found throughout much of the Midwestern United States and results in substantially high soil heat flux (G0) and sensible heat flux (H) for this period. Latent heat flux (λET) and ET are related, such that λET is the amount of energy (W m−2) consumed when evaporating a quantity of water over time, in this case ET. Strictly, then the units of ET are kg m−2 s−1, but this can be converted to mm s−1 and integrated over the number of seconds in a growing season to obtain total millimeters of water per season. The units specific to the results presented will be referred to throughout the “Results,” but the general term, ET, will be used in the “Discussion.”
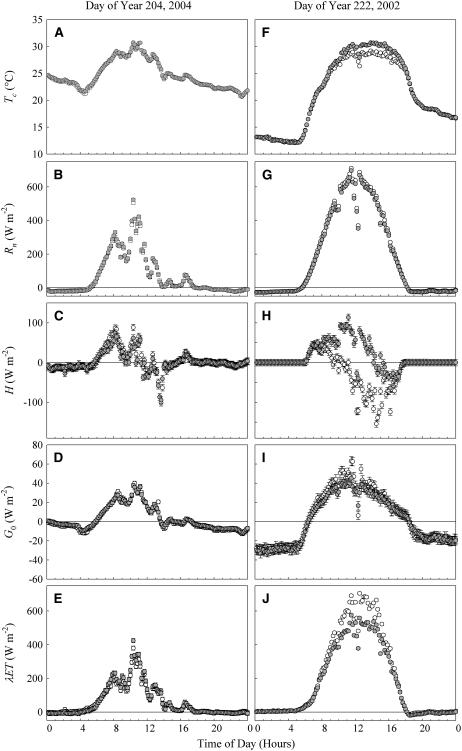
Two days from the four growing seasons are given as examples of energy flux, including λET for an overcast and for a clear day (Fig. 2). The overcast day showed no statistically significant [CO2] effect on any of the measured fluxes (Fig. 2, A–E). The sunny day resulted in a statistically lower λET for elevated [CO2] (F1, 574 = 1,385.9, P < 0.001; Fig. 2J), which was driven primarily by an increase in H during the afternoon hours (F1, 574 = 943.26, P < 0.001; Fig. 2H).
Figure 2.
Tc, Rn, H, G0, and λET for control (white circles) and elevated [CO2] (gray circles) over an example overcast and an example sunny day. The maximum and minimum temperatures on DOY 204 (2004) were 29.5°C and 21.8°C, and were 29.4°C and 13°C for DOY 222 (2002). Each symbol represents a mean of four replicate blocks for DOY 204 (2004) and three replicate blocks for DOY 222 (2002). Error bars represent 1 se around the mean.
Growth in Elevated [CO2] Resulted in Warmer Plant Canopies
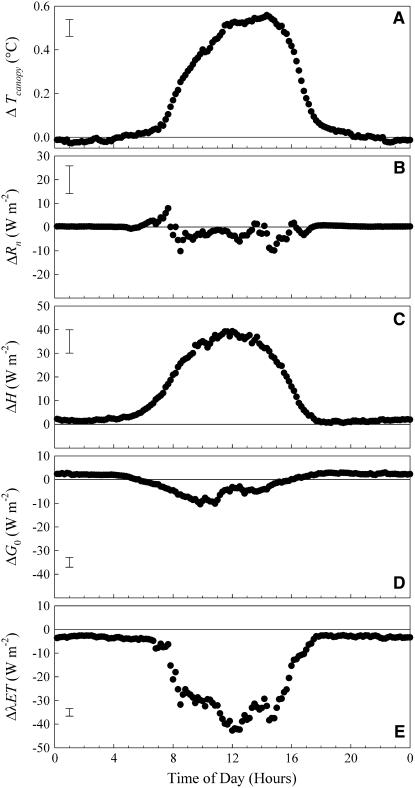
The micrometeorological method used to assess ET responses to elevated [CO2] relies heavily on accurate measurements of canopy temperature (Tc) in determining H. The effect of elevated [CO2] on the diel surface temperatures were analyzed separately for each year, and for each year the differences between control and elevated [CO2] were statistically different. Data were averaged in 10-min intervals for all replicate plots within a year, and these values were averaged over all 4 years, not including measurements prior to canopy closure when the infrared thermometer measurements would be affected by viewing soil as well as canopy surface temperatures (canopy closure occurred at approximately DOY 190 for each of the four growing seasons). This also affected measurements during the 2-week period immediately after the hail storm (DOYs 199–213, 2003), which were also excluded. Across the growing season, the elevated [CO2] canopy was about 0.2°C warmer. This resulted entirely from warmer Tc during the day with no effect at night. On average, Tc was over 0.5°C higher in elevated [CO2] around midday and early afternoon (Fig. 3A); however, this difference exceeded 2°C on a number of individual sunny days (Fig. 2).
Figure 3.
The average diurnal course of the absolute differences between elevated [CO2] and control plots for Tc (A), Rn (B), H (C), G0 (D) and λET (E). Each point represents a 10-min interval of the day averaged across more than 300 measurement days and three to four replicate blocks, depending on the year. Values are presented as elevated [CO2] minus control, such that negative represents higher values in the control plots and positive higher in the elevated [CO2]. Bars on the upper (A, B, C) and lower (D and E) left side of the figures represent the 1 se around the mean.
Elevated [CO2] Altered Ecosystem Energy Fluxes
Elevated [CO2] resulted in daytime decreases of net radiation (Rn) for all years (Fig. 3B), though not statistically significant in 2002. This decrease was relatively small, with absolute differences between the treatment and control of at most 10 W m−2, compared with maximum midday values of over 500 W m−2 (Fig. 2). Sensible heat flux, H, was substantially higher in elevated [CO2] during the day, and this difference was statistically significant every year. Peak differences between control and elevated [CO2] averaged over the 4 years were approximately 40 W m−2 during the afternoon portion of the day (Fig. 3C). The soybean grown in elevated [CO2] showed less heat flux into the soil during daylight hours and less heat flux from the soil at night compared to control (Fig. 3D). Under elevated [CO2] less heat is transferred through the canopy to the soil during the day and less heat is transferred back from the soil to the canopy at night. This diel difference under elevated [CO2] is statistically significant for all years.
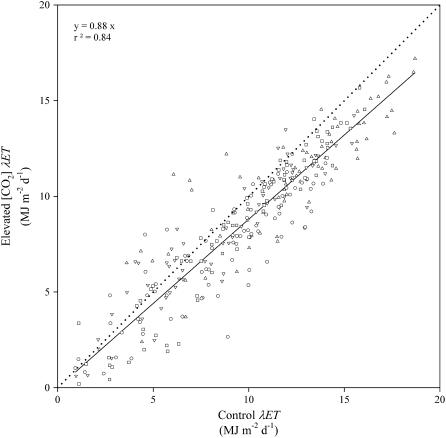
The three measured energy flux components were used to calculate λET as the residual of the energy balance equation and demonstrated a lower λET throughout daylight hours (Fig. 3E). The relationship for daily integrated λET between control and elevated [CO2]-grown soybean for all 4 years results in a linear relationship with a slope of 0.88 (±0.02 se; Fig. 4). The final result from more than 300,000 individual measurements of energy flux across four growing seasons is that there was an average decrease in ET of 12% due to an increase in [CO2] to 550 μmol mol−1.
Figure 4.
The relationship between ET for elevated [CO2]-grown soybean and control-grown soybean over four complete growing seasons. Each symbol represents the mean of three to four replicate blocks, depending on the year. Different symbols represent separate measurement years (▵ for 2002, ∇ for 2003, ○ for 2004, and □ for 2005). The linear regression was fit to all data with an intercept of zero because prior regression analysis showed the intercept not statistically different from zero. The slope is statistically significant from unity with the 99% confidence limits of the slope ranging from 0.86 to 0.90.
Elevated [CO2] Lowers Seasonal Water Use
The daily λET values from canopy closure until senescence were integrated over each growing season and converted to the total amount of water emitted by the ecosystem (mm season−1; Table I). There was variation in ET across the duration of the experiment, with 2002 being the highest and 2004 the lowest water use years; however, data excluded from the analysis as a result of storm damage or equipment maintenance are confounded in the between-years analysis of absolute water use. Both 2002 and 2003 showed remarkable similarity in the difference imposed by elevated [CO2] on water use, with a season-integrated 9% reduction each year (Table I). The largest difference between control and elevated [CO2] was observed in 2004 with a 16% decrease, followed by 2003 with a 13% decrease (Table I). The 2005 growing season was the only year declared a moderate drought; however, exceptionally good soil moisture conditions and precipitation at critical times during the growing season (Kunkel et al., 2006) resulted in adequate moisture availability throughout much of the season. These results show that while the size of decrease in ET depends on season, growth of soybean at elevated [CO2] consistently resulted in lower ET.
Table I.
Total ecosystem ET (mm season−1) and percentage of change for control- and elevated [CO2]-grown soybean at the soybean FACE experiment
Values represent three or four replicate blocks, depending on year. Measurement days include the number of days from canopy closure until senescence included in the analysis.
| Year | Measurement Days | Control | Elevated [CO2] | % Change |
|---|---|---|---|---|
| 2002 | 74 | 407.8 ± 9.3 | 372.6 ± 9.3 | −8.6 |
| 2003 | 70 | 334.5 ± 7.9 | 303.0 ± 7.9 | −9.4 |
| 2004 | 82 | 320.6 ± 7.9 | 268.2 ± 7.9 | −16.3 |
| 2005 | 75 | 363.7 ± 9.3 | 316.8 ± 7.9 | −12.9 |
Decreased ET Was Presaged by Decreased gs
Data for each plot were averaged throughout each growing season and compared with seasonal ET. The relationship of ET to gs was not affected by [CO2] treatment, even though both ET and gs were lower in elevated [CO2] (Fig. 5). This relationship suggests that for a 10% decrease in gs, there was an 8.6% decrease in ET within the range of values illustrated (Fig. 5).
Figure 5.
The relationship of ET to gs based on several days of simultaneous measurements across the 2002, 2003, and 2004 growing seasons. Stomatal conductance of the upper canopy leaves was averaged across the daylight hours and days of measurement (Bernacchi et al., 2006). Total season ET for each plot is plotted against its corresponding gs; control (white symbols) and elevated [CO2] (gray symbols). Since no differences between control and elevated [CO2] were observed based on analysis of covariance with treatment as the main effect and gs as a covariance term (F(1,19) = 1.07, P = 0.31), a linear regression was fit to data for both the control and elevated [CO2] plots over all 4 years.
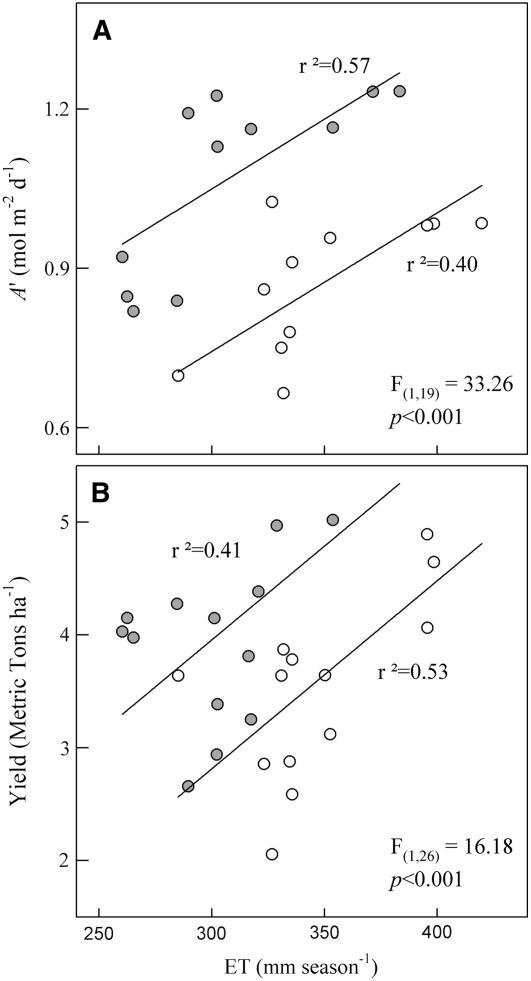
A Decrease in ET with Elevated [CO2] Resulted in Improved Water-Use Efficiency
For any given ET integrated over the season, the average total canopy assimilation (A′) was substantially and significantly greater in elevated [CO2] (Fig. 6A). On average, yield per unit ET was significantly higher in elevated [CO2] (Fig. 6B). Yield and A′ were linearly related to ET, although the slopes of these regressions were significantly different. The intercepts were significantly higher in elevated [CO2] (Fig. 6). Over the range of dates illustrated, this implies that percentage reduction in A′ or yield for a given decrease in ET will be less in elevated [CO2].
Figure 6.
A and B, Daily integrated leaf-level carbon assimilation (A′; Bernacchi et al., 2006; A) and yield (2002–2003 from Morgan et al., 2005; 2004–2005 from R. Nelson, personal communication; B) were plotted against ET. Statistical significance reported in each section represents the results from an analysis of covariance, with ET as the covariance term and treatment as the main effect.
The Effects of Elevated [CO2] on ET Was Not Consistent Over All Meteorological Conditions
Despite the consistent decrease in ET when averaged over the growing seasons, there were times in which the effect of elevated [CO2] was either not apparent or opposite of the general results. For example, no more than 1 mm of precipitation fell in any given day from DOY 226 to 240 during the 2003 growing season. This period was also characterized by high daily maximum temperatures and mostly clear skies (Fig. 7). At about DOY 233, the difference between λET for the control and elevated [CO2] began to reverse with the elevated [CO2] plots showing higher λET than the control (Fig. 7). By the end of this dry period, λET from elevated [CO2] was greater and the canopy cooler than control, in contrast to the pattern seen over most of this 4-year period.
Figure 7.
A subset of data from a period in 2003 showing maximum daily temperature, total daily integrated solar radiation, and precipitation (A); ET from 11 am until 1 pm (Central solar time) for soybean grown at SoyFACE under control (white symbols) and elevated [CO2] (gray symbols; B); and the difference of λET for elevated [CO2] versus control treatments (C). After 9 d with only minimal precipitation, the elevated [CO2] plots begin to show higher rates of λET compared with the control, the opposite to the usual response seen when water is not limiting. Each symbol represents a mean of four replicates and error bars represent 1 se around the mean.
DISCUSSION
Short- and long-term decrease in gs is one of the best-documented effects of elevated [CO2] on terrestrial plants. Whether this results in decreased ET under open-air field conditions has not previously been assessed for rain-fed crops. This study showed that elevation of [CO2] to expected 2050 levels (Intergovernmental Panel on Climate Change, 2001) results in a highly significant 12% decrease in ecosystem ET based on over 300,000 individual measurements across four full growing seasons. This decrease was shown to be closely correlated with decrease in upper canopy gs.
There was considerable variation in response of ET within and among years (Fig. 4). The effect of elevated [CO2] on ET, when integrated over each growing season, ranged from approximately 9% to 16% (Table I). Soybean grown in 2004 showed the highest percentage of decrease in ET with elevated [CO2] (Table I). What could cause the wettest year to have the greatest difference? A greater difference during wet years might be explained by the fact that elevated [CO2] plots conserve more soil moisture, and as drought develops these canopies can continue transpiring for longer. The hypothesis that soil moisture would be higher in elevated [CO2] has been confirmed through measurements at the SoyFACE facility (A.D.B. Leakey, personal communication). Thus, during dry periods the differences in ET between control and elevated [CO2] are minimized or even reversed (Fig. 7). These conditions are rare or nonexistent during wet years, maximizing differences in ET between control and elevated [CO2].
Overall, decreases in ET were compensated by increases in H in the elevated [CO2] plots. The measurements used in calculating H are numerous and presented as supplemental online material; however, the measure with the largest difference between the control and elevated [CO2] plots affecting H was Tc. The warmer Tc in elevated [CO2] is consistent with previous FACE experiments (e.g. Magliulo et al., 2003; Triggs et al., 2004; Yoshimoto et al., 2005; Kimball and Bernacchi, 2006). These studies, however, were of irrigated fields, and none was representative of large continental ecosystems.
Soybean represents a large amount of the land cover in the Midwest Corn Belt, and, therefore, any decrease in ET compensated by increased Tc and H is likely to have a more profound influence on atmospheric conditions (e.g. Sellers et al., 1997). While soybean only represents half of the maize-soybean ecosystem, leaf-level gs for maize has been shown to decrease in elevated [CO2] at SoyFACE by as much if not more than soybean (Leakey et al., 2004, 2006b). Although 20-m-diameter open-air FACE plots may better represent feedbacks at the soil and canopy levels than small chambers, while maintaining natural atmospheric coupling, they cannot represent regional atmospheric feedbacks on ET (McLeod and Long, 1999). For example, if ET is suppressed for the whole region, then humidity of the bulk atmosphere may drop and air temperature increase. Both factors would act to increase water vapor pressure deficit, which would tend to increase ET and offset the effect of elevated [CO2]. Similarly, lower ET at the regional level might decrease precipitation, increasing the incidence of dry periods, in which the soil moisture conservation effect of elevated [CO2] may result in more frequent occurrences of the reversal in the ET response illustrated in Figure 7. This too could offset the decrease in ET due to elevated [CO2] across the season. Could these regional feedbacks nullify the decreased ET and increased H observed here?
Regional feedbacks can, however, be included in models. By linking a physiological model of stomatal response to elevated [CO2] at the canopy level to an Atmospheric Global Circulation model, Sellers et al. (1996) and Bounoua et al. (1999) simulated the extent to which decreased gs in elevated [CO2] would translate into decreased ET when these regional and larger-scale feedbacks were included. They showed that for a decrease in gs similar to that observed here that annual continental ET would be decreased by 2.3% to 3.5% and air temperature increased by 0.3°C to 0.7°C. This suggests that while larger-scale feedbacks would decrease the effect observed at the FACE plot level, a very significant effect in terms of regional climate would result. Decreases in ET in continental regions have been identified as a potential amplifier for abrupt climate change (Alley et al., 2003). The findings are also consistent with the recent mechanistic modeling and statistical fingerprinting analysis of global trends in increasing river discharge across the 20th century. This suggests that suppression of plant transpiration due to the effect of rising [CO2] on stomatal closure is the most consistent factor in explaining observed changes (Gedney et al., 2006). Since ET and H are coupled inversely, warming due to atmospheric change will be greater than that previously predicted, based on the enhanced greenhouse gas effect alone (e.g. Intergovernmental Panel on Climate Change, 2001).
The ET calculated in this study, as in previous studies, is by difference, i.e. from the measured components of the energy balance equation. Its accuracy therefore depends on internal consistency between the measured components and absence of unexplained variation. The responses of the component fluxes for the energy balance were highly consistent. For example, both H and Rn were measured independently, yet the slight decrease in Rn during the day for elevated [CO2] is in agreement with the warmer canopies. From Figure 3A, midday Tc increased about 0.5°C in elevated [CO2]. From Figure 1A, this corresponds to higher Tc, from about 29.0°C to 29.5°C. This would cause increases in up-welling, long-wave radiation of about 3 W m−2, in close agreement with the observed decreases in down-welling midday Rn (Fig. 3B). Similarly, the G0 data agree well with the increase in aboveground biomass and leaf area observed at SoyFACE (Morgan et al., 2005; Dermody et al., 2006). With more aboveground biomass and a larger canopy, it is expected that less energy would penetrate through the canopy. Thus, there was less heat flowing into the soil during the day in elevated [CO2]. It follows that at night more heat would be released from the soil in the control plots from the increased daytime warming, as observed. While G0 represents a small portion of the overall energy balance for a closed canopy and the measurements are prone to more variability from sunflecks, soil heterogeneity, soil moisture, etc., these results were consistent over all 4 years.
Previously, we showed greater decreases in leaf-level gs in 2004 compared with 2002 and 2003 for soybean grown in elevated [CO2] (Bernacchi et al., 2006). Seasonal decreases in gs for soybean grown in elevated [CO2] averaged 14%, 11%, and 20% in 2002, 2003, and 2004, respectively (Bernacchi et al., 2006), mimicking the pattern of observed decrease in ET for the first 3 years of this experiment. When ET data from individual replicates were plotted against gs, a single relationship, independent of [CO2] treatment, is apparent (Fig. 5). While the elevated [CO2] treatment points cluster at the lower end of the relationship and vice versa, there is no statistical evidence that the [CO2] treatment alters the dependence of ET on gs. This implies that soil, canopy, and microclimate feedbacks have a negligible effect on ET. This is despite significant measured increases in LAI and Tc, both of which must remove a part of the effect of decreased gs on ET at elevated [CO2]. Acclimation of the stomatal response to growth at elevated [CO2] could also alter the response of ET to gs. However, it has been shown independently for these plants that gs at the leaf level did not acclimate to elevated [CO2] (Leakey et al., 2006a).
The relationship also shows a coupling between upper canopy gs and ET, such that a 10% decrease in gs at the level of individual upper canopy leaves across the season would translate into an 8.6% decrease in ET. This suggests that the upper canopy leaves either reflect the behavior of the lower canopy leaves or/and account for most of the ET. The latter would explain why a significant increase in LAI at elevated [CO2] did not significantly alter the relationship of ET on gs (Fig. 5). Unfortunately, there are no other data with other crops with which to compare this finding, since no other FACE studies appear to have measured gs across the day at multiple dates and simultaneously and independently measured ET. If this may be extrapolated more widely to productive row crops, then it suggests that decreases in ET at elevated [CO2] might be predicted effectively from decreases in gs.
Although there is no apparent change in the relationship of ET to gs with [CO2] treatment, both are substantially and significantly decreased at elevated [CO2], yet A′ and seed yield are substantially higher, resulting in large increases in the efficiency of water use (Fig. 6). Overall, this first study of the effect of elevated [CO2] on water use on a rain-fed crop under fully open-air conditions confirms that at the scale of FACE plots, gs appears closely coupled to ecosystem ET. This suggests that feedbacks at the plant, canopy, and soil levels have little impact, in contrast to previous speculation (Arnell and Liu, 2001). The effects are consistent with both model and regional analyses that suggest that decreasing gs with rising [CO2] will lower water transfer to the atmosphere, so altering regional hydrology and climate (Sellers et al., 1996; Gedney et al., 2006).
MATERIALS AND METHODS
Site Description
The SoyFACE facility is situated in a 32-ha (80-acre) field at the University of Illinois at Urbana-Champaign (40°03′21.3″N, 88°12′3.4″, 230-m elevation; www.soyface.uiuc.edu). Soybean (Glycine max L. Merr. cv Pioneer 93B15) and maize (Zea mays) each occupied one-half of the field and followed an annual rotation. The agricultural practices at SoyFACE followed those typical for Illinois rain-fed agriculture and have been described previously (Ainsworth et al., 2004; Rogers et al., 2004; Bernacchi et al., 2006).
The treatment plots of 20-m diameter were arranged in a randomized complete block design (n of 4 for the control and elevated [CO2] treatment) to allow for topographic and soil variation across the field. All were equipped for micrometeorological measurement of ET, except in 2002 when three of the four blocks were equipped. A complete description of the SoyFACE experiment is given elsewhere (Rogers et al., 2004; Bernacchi et al., 2005; Morgan et al., 2005). The target elevated [CO2] was 550 μmol mol−1 based on projected future mean global tropospheric concentrations for 2050 (Intergovernmental Panel on Climate Change, 2001). Actual seasonal daytime average [CO2] was 552 μmol mol−1 in 2002 and 2003 and 550 μmol mol−1 in 2004 and 2005, compared to an ambient daytime [CO2] of approximately 375 μmol mol−1. No enrichment of [CO2] was undertaken at night.
Micrometeorological Measurements
A residual energy balance approach was used to determine ET from individual plots (Huband and Monteith, 1986; Jackson et al., 1987; Kimball et al., 1994, 1995, 1999; Triggs et al., 2004) according to the relationship:
 |
(1) |
where λ is latent heat of vaporization (J kg−1), ET is evapotranspiration (kg m−2 s−1; positive upward), Rn is net radiation (W m−2; positive downward), G0 is soil surface heat flux (W m−2; positive downward), and H is sensible heat flux (W m−2; positive upward). Each of the energy flux components will be briefly discussed below, and a list of symbols, units, and equations is presented in supplemental online material.
The residual energy balance approach, while not directly measuring ET, has proven effective in obtaining quantitative estimates of ET (e.g. Kimball et al., 1999). Other meteorological methods that provide more direct measures of ET, such as eddy covariance or flux gradient analysis (Baldocchi et al., 1988), require a much larger fetch than can be provided using FACE (Kimball et al., 1999; Triggs et al., 2004). The residual energy balance approach used here depends on four energy fluxes into and out of soybean canopies. This assumes that energy fluxes due to photosynthesis, respiration, and heat storage within the canopy over a 24-h period, which will be less than 1% of incoming radiation, can be ignored (Meyers and Hollinger, 2004).
Each plot contained a micrometeorological station equipped to measure each of the three major energy flux terms on the right side of Equation 1 (Rn, heat flux to the soil, H to the atmosphere) connected to a datalogger (CR10, CR10x, CR21, CR23X, or CR7 Micrologger; Campbell Scientific), which transmitted its measurements to a central computer via radio. Measurements were made in 10-s intervals and averaged over 10 min throughout the growing season, and then relayed to and stored on the central computer. Each station was operated with online power, but provided with battery back-up to minimize down time in the event of a power outage. Measurements were made from planting until harvest. Data were checked for errors daily, and instruments were inspected, cleaned, and adjusted in height to maintain a constant distance of 1 m above the canopy surface, weekly. Averaged across all plots and all years, periods in which data were not obtained due to storm damage, equipment failure, and maintenance were <10% of the growing season.
Rn
Rn is a measure of total incoming radiation minus the fraction of up-welling radiation from the canopy. Measurements of Rn were collected using single-channel net radiometers (model Q*6 or Q*7; Radiation and Energy Balance Systems) in each plot, complete with ventilators to remove the need for wind corrections and to minimize condensation on the net radiometer domes. Net radiometers were positioned 1 m above the crop surface and were raised as the crop canopy grew. A cross-calibration was performed prior to, or immediately after, each growing season. All net radiometers were placed over a carefully raked portion of bare soil for at least 1 d and compared with a “standard” factory calibrated net radiometer (Q*7; Radiation and Energy Balance Systems) that was reserved for cross-calibration and not otherwise deployed at any point into the field. During the 2002 and 2003 growing seasons, the net radiometers were rotated weekly to a new plot, the data from that day being excluded. This accounted for the bulk of missing data (see above). The rotation scheme in 2002 ensured that each net radiometer was situated for at least 1 week in all experimental plots. In 2003 the net radiometers were rotated to all plots within a block. It was determined after the 2003 growing season that the four replicates for the control and elevated [CO2] plots were sufficient to overcome any systematic error associated with stationing the net radiometers in the same plot for the duration of the growing season.
G0
The replication of G0 measurements differed throughout the experiment depending on the number of available instruments. G0 measurements were collected in one block in 2002 and three blocks in 2003 to 2005. Soil heat flux plates (model HFT-3; Radiation and Energy Balance Systems) were buried at 10-mm depths between and within planting rows. The total number of heat flux plates in each replicate was four in 2002 and two in 2003 to 2005 and were arranged perpendicular to the direction of planting, such that equal numbers of measurements were made within and between rows. Measurements of G0 also included the heat storage in the 10 mm of soil above each heat flux plate, obtained by placing thermocouples below the soil surface and above each plate (Kimball et al., 1994). Measurements of G0 were not as well replicated as Rn and H, but it is a much smaller portion of the total energy balance. Further, variability in G0 within plots was assumed more critical than variation between plots.
Sensible Heat Flux
Sensible heat flux relied on a number of sensors and independent measurements, and was calculated as:
 |
(2) |
where ρa is air density (kg m−3), cp the heat capacity of air (J kg−1 °C−1), Ts and Ta the surface and air temperatures (°C), and ra the aerodynamic resistance (s m−1). Air temperature was measured using custom-built aspirated psychrometers, from the design of Peresta et al. (1991), which were located in each plot. Surface temperatures, Ts, were measured using infrared thermometers (IRT-P; Apogee Instruments), which were calibrated between each growing season according to the method of Triggs et al. (2004). Aerodynamic resistance was calculated based on a previously described model (Jackson et al., 1987; Kimball et al., 1994, 1999; Triggs et al., 2004), which can be found in the supplemental material. This model relies on wind speed measured using a 3-cup anemometer (model 12102D; R.M. Young Company), Ta, Ts, dew point temperatures (Td), and canopy height. Canopy height was measured in weekly intervals throughout each season. Upon the completion of each growing season, height data were fitted to an equation describing the sigmoidal increase in height throughout the season and the equation was incorporated into the appropriate equations. The infrared thermometers were similarly rotated among the plots in 2002 and 2003 as with the net radiometers above.
Meteorology
Precipitation and solar radiation were recorded at a weather station located in the center of the SoyFACE facility. Temperature and humidity were from the individual plot sensors described above. Thirty-year mean averages (1971–2000) of meteorological variables were from a weather station within 3 km of the SoyFACE (Midwestern Climate Information System; http://mrcc.sws.uiuc.edu/). The PCMI is an estimate of short-term moisture conditions calculated from temperature, precipitation, and modeled soil water content (Palmer, 1968). As PCMI decreases below zero, it indicates progressively greater drought stress conditions. PCMI from 1973 to 2004 for East Central Illinois were provided by the Climate Operation Branch of the National Oceanic and Atmospheric Administration (http://www.usda.gov/oce/waob/jawf/).
Data Analysis
Data for each plot were collected in 10-min intervals throughout the day and separated into individual files by day. Comparisons of ET, Rn, H, and G0 between control and elevated [CO2] for each 10-min time point throughout the day was performed using a complete block repeated-measures ANOVA with DOY as the repeated measure, treatment as a main effect, and DOY and block as random factors (The Mixed Procedure, SAS 9.1; The SAS Institute). This analysis was conducted separately for each year because of the annual rotation of the experiment to different sides of the field. The seasonal 10-min means from each season were averaged to provide a 4-year mean and se for each of the flux components. The daily totals of ET were accumulated over the each growing season and converted to total crop water use (mm season−1). Differences in seasonal water use between control and elevated [CO2] plots were tested using a complete block ANOVA with treatment and year as main effects and block as a random factor. The relationship of ET between control and elevated [CO2] treatments were fit to a linear regression and the 95% confidence intervals for the slope of the line calculated (The Regression Procedure, SAS 9.1). Seasonal mean gs and A′ for each plot over the 2002 to 2004 growing seasons were calculated from Bernacchi et al. (2006), and grain harvested from each plot from 2002 to 2005 were provided by R. Nelson (personal communication). Comparisons of these versus total seasonal crop water use were performed using analysis of covariance with treatment as the main effect and ET as a covariance term (The GLM Procedure, SAS 9.1). Since the number of measurement days varied for each season, the total crop water use for these comparisons was normalized to the mean number of measurement days across all four growing seasons, which worked out to be 76 d.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Data S1. All equations used in the calculation of ET using the residual energy balance approach (Huband and Monteith, 1986; Jackson et al., 1987; Kimball et al., 1994, 1995, 1999; Triggs et al., 2004) are presented.
Supplementary Material
Acknowledgments
We thank Justin McGrath, Chrissy Whitacre, Mark Harrison, Mathew Conley, and Carrie O'Brien for technical assistance for this project.
The views expressed in this work are those of the authors and do not necessarily reflect those of the U.S. Department of Energy or the Illinois State Water Survey.
This work was supported in part by the Office of Science (Biological and Environmental Research program), U.S. Department of Energy (grant no. DE–FG02–03ER63685 to C.J.B.). SoyFACE was funded by the Illinois Council for Food and Agricultural Research, Archer Daniels Midland Company, Pioneer Hi-Bred International, and the U.S. Department of Agriculture Agricultural Research Service.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Carl J. Bernacchi (bernacch@uiuc.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Ainsworth EA, Davey PA, Bernacchi CJ, Dermody OC, Heaton EA, Moore DJ, Morgan PB, Naidu SL, Ra HSY, Zhu XG, et al (2002) A meta-analysis of elevated [CO2] effects on soybean (Glycine max) physiology, growth and yield. Glob Change Biol 8 695–709 [Google Scholar]
- Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy. New Phytol 165 351–371 [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the “source-sink” hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric For Meteorol 122 85–94 [Google Scholar]
- Alley RB, Marotzke J, Nordhaus WD, Overpeck JT, Peteet DM, Pielke RA Jr, Pierrehumbert RT, Rhines PB, Stocker TF, Talley LD, et al (2003) Abrupt climate change. Science 299 2005–2010 [DOI] [PubMed] [Google Scholar]
- Arnell N, Liu C (2001) Hydrology and water resources. Climate change 2001: impacts, adaptation, and vulnerability. In JJ McCarthy, OF Canziani, NA Leary, DJ Dokken, KS White, eds, Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK, pp 192–236
- Baldocchi DD, Hicks BB, Meyers TP (1988) Measuring biosphere-atmosphere exchanges of biologically related gases with micrometeorological methods. Ecology 69 1331–1340 [Google Scholar]
- Bazzaz FA, Sombroek WG (1996) Global Climate Change and Agricultural Production: Direct and Indirect Effects of Changing Hydrological, Pedological, and Plant Physiological Processes. Food and Agriculture Organization of the United Nations/Wiley, Chichester, UK/New York
- Bernacchi CJ, Leakey ADB, Heady LE, Morgan PB, Dohleman FG, McGrath JM, Gillespie KM, Wittig VE, Rogers A, Long SP, et al (2006) Hourly and seasonal variation in photosynthesis and stomatal conductance of soybean grown at future CO2 and ozone concentrations for 3 years under fully open-air field conditions. Plant Cell Environ 29 2077–2090 [DOI] [PubMed] [Google Scholar]
- Bernacchi CJ, Morgan PB, Ort DR, Long SP (2005) The growth of soybean under free air [CO2] enrichment (FACE) stimulates photosynthesis while decreasing in vivo Rubisco capacity. Planta 220 434–446 [DOI] [PubMed] [Google Scholar]
- Bounoua L, Collatz GJ, Sellers PJ, Randall DA, Dazlich DA, Los SO, Berry JA, Fung I, Tucker CJ, Field CB, et al (1999) Interactions between vegetation and climate: radiative and physiological effects of doubled atmospheric CO2. J Clim 12 309–324 [Google Scholar]
- Chaudhuri UN, Kirkham MB, Kanemasu ET (1990) Carbon-dioxide and water level effects on yield and water-use of winter-wheat. Agron J 82 637–641 [Google Scholar]
- Curtis PS (1996) A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ 19 127–137 [Google Scholar]
- Dermody O, Long SP, DeLucia EH (2006) How does elevated CO2 or ozone affect the leaf-area index of soybean when applied independently? New Phytol 169 145–155 [DOI] [PubMed] [Google Scholar]
- Gedney N, Cox PM, Betts RA, Boucher O, Huntingford C, Stott PA (2006) Detection of a direct carbon dioxide effect in continental river runoff records. Nature 439 835–838 [DOI] [PubMed] [Google Scholar]
- Hileman DR, Huluka G, Kenjige PK, Sinha N, Bhattacharya NC, Biswas PK, Lewin KF, Nagy J, Hendrey GR (1994) Canopy photosynthesis and transpiration of field-grown cotton exposed to free-air CO2 enrichment (FACE) and differential irrigation. Agric For Meteorol 70 189–207 [Google Scholar]
- Huband NDS, Monteith JL (1986) Radiative surface-temperature and energy-balance of a wheat canopy. 2. Estimating fluxes of sensible and latent-heat. Bound-Lay Meteorol 36 107–116 [Google Scholar]
- Intergovernmental Panel on Climate Change (2001) Climate Change 2001: The Scientific Basis. Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, UK
- Jackson RD, Moran MS, Gay LW, Raymond LH (1987) Evaluating evaporation from field crops using airborne radiometry and ground-based meteorological data. Irrig Sci 8 81–90 [Google Scholar]
- Kim SH, Sicher RC, Bae H, Gitz DC, Baker JT, Timlin DJ, Reddy VR (2006) Canopy photosynthesis, evapotranspiration, leaf nitrogen, and transcription profiles of maize in response to CO2 enrichment. Glob Change Biol 12 588–600 [Google Scholar]
- Kimball B, Bernacchi CJ (2006) Evapotranspiration, canopy temperature, and plant water relations. In J Nösberger, H Blum, eds, Managed Ecosystems and Rising CO2: Case Studies, Processes, and Perspectives. Springer Verlag, Berlin, pp 311–324
- Kimball BA, LaMorte RL, Pinter PJ Jr, Wall GW, Hunsaker DJ, Adamsen FJ, Leavitt SW, Thompson TL, Matthias AD, Brooks TJ (1999) Free-air CO2 enrichment and soil nitrogen effects on energy balance and evapotranspiration of wheat. Water Resour Res 35 1179–1190 [Google Scholar]
- Kimball BA, LaMorte RL, Seay RS, Pinter JPJ, Rokey RR, Hunsaker DJ, Dugas WA, Heuer ML, Mauney JR (1994) Effects of free-air CO2 enrichment on energy balance and evapotranspiration of cotton. Agric For Meteorol 70 259–278 [Google Scholar]
- Kimball BA, Pinter PJ, Garcia RL, LaMorte RL, Wall GW, Hunsaker DJ, Wechsung G, Wechsung F, Kartschall T (1995) Productivity and water use of wheat under free-air CO2 enrichment. Glob Change Biol 1 429–442 [Google Scholar]
- Kunkel KE, Angel JR, Changnon SA, Claybrooke R, Hilberg SD, Knapp HV, Larson RS, Palecki M, Scott RW, Winstanley D, editors (2006) The 2005 Illinois Drought. Illinois State Water Survey, Champaign, IL
- Leakey ADB, Bernacchi CJ, Dohleman FG, Ort DR, Long SP (2004) Will photosynthesis of maize (Zea mays) in the US Corn Belt increase in future [CO2] rich atmospheres? An analysis of diurnal courses of CO2 uptake under free-air concentration enrichment (FACE). Glob Change Biol 10 951–962 [Google Scholar]
- Leakey ADB, Bernacchi CJ, Ort DR, Long SP (2006. a) Long-term growth of soybean at elevated [CO2] does not cause acclimation of stomatal conductance under fully open-air conditions. Plant Cell Environ 29 1794–1800 [DOI] [PubMed] [Google Scholar]
- Leakey ADB, Uribelarrea M, Ainsworth EA, Naidu SL, Rogers A, Ort DR, Long SP (2006. b) Photosynthesis, productivity, and yield of maize are not affected by open-air elevation of CO2 concentration in the absence of drought. Plant Physiol 140 779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TD, Tjoelker MG, Ellsworth DS, Reich PB (2001) Leaf gas exchange responses of 13 prairie grassland species to elevated CO2 and increased nitrogen supply. New Phytol 150 405–418 [Google Scholar]
- Li FS, Kang SZ, Zhang JH (2004) Interactive effects of elevated CO2, nitrogen and drought on leaf area, stomatal conductance, and evapotranspiration of wheat. Agric Water Manag 67 221–233 [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: Plants FACE the future. Annu Rev Plant Biol 55 591–628 [DOI] [PubMed] [Google Scholar]
- Magliulo V, Bindi M, Rana G (2003) Water use of irrigated potato (Solanum tuberosum L.) grown under free air carbon dioxide enrichment in central Italy. Agric Ecosyst Environ 97 65–80 [Google Scholar]
- McLeod A, Long SP (1999) Free air carbon dioxide enrichment (FACE) in global change research: a review. Adv Ecol Res 28 1–55 [Google Scholar]
- Medlyn BE, Barton CVM, Broadmeadow MSJ, Ceulemans R, De Angelis P, Forstreuter M, Freeman M, Jackson SB, Kellomaki S, Laitat E, et al (2001) Stomatal conductance of forest species after long-term exposure to elevated CO2 concentration: a synthesis. New Phytol 149 247–264 [DOI] [PubMed] [Google Scholar]
- Meyers TP, Hollinger SE (2004) An assessment of storage terms in the surface energy balance of maize and soybean. Agric For Meteorol 125 105–115 [Google Scholar]
- Morgan PB, Bollero GA, Nelson RL, Dohleman FG, Long SP (2005) Smaller than predicted increase in aboveground net primary production and yield of field-grown soybean under fully open-air [CO2] elevation. Glob Change Biol 11 1856–1865 [Google Scholar]
- Palmer W (1968) Keeping track of crop moisture conditions, nationwide: the new Crop Moisture Index. Weatherwise 21 156–161 [Google Scholar]
- Peresta GJ, Kimball BA, Johnson SM (1991) Procedures for CO2-Enrichment Chamber Construction and Data Acquisition and Analysis. WCL Report 18. United States Water Conservation Laboratory, Phoenix, AZ
- Rogers A, Allen DJ, Davey PA, Morgan PB, Ainsworth EA, Bernacchi CJ, Cornic G, Dermody O, Dohleman FG, Heaton EA, et al (2004) Leaf photosynthesis and carbohydrate dynamics of soybeans grown throughout their life-cycle under Free-Air Carbon dioxide Enrichment. Plant Cell Environ 27 449–458 [Google Scholar]
- Sellers PJ, Bounoua L, Collatz GJ, Randall DA, Dazlich DA, Los SO, Berry JA, Fung I, Tucker CJ, Field CB, et al (1996) Comparison of radiative and physiological effects of doubled atmospheric CO2 on climate. Science 271 1402–1406 [Google Scholar]
- Sellers PJ, Dickinson RE, Randall DA, Betts AK, Hall FG, Berry JA, Collatz GJ, Denning AS, Mooney HA, Nobre CA, et al (1997) Modeling the exchanges of energy, water, and carbon between continents and the atmosphere. Science 275 502–509 [DOI] [PubMed] [Google Scholar]
- Triggs JM, Kimball BA, Pinter PJ, Wall GW, Conley MM, Brooks TJ, LaMorte RL, Adam NR, Ottman MJ, Matthias AD, et al (2004) Free-air CO2 enrichment effects on the energy balance and evapotranspiration of sorghum. Agric For Meteorol 124 63–79 [Google Scholar]
- U.S. Department of Agriculture (2004) Crop Production 2003 Summary (Cr Pr 2-1 (04)). Agricultural Statistics Board, National Agricultural Statistics Service, U.S. Government Printing Office, Washington, DC
- Yoshimoto M, Oue H, Kobayashi K (2005) Energy balance and water use efficiency of rice canopies under free-air CO2 enrichment. Agric For Meteorol 133 226–246 [Google Scholar]
- Zheng FY, Peng SL (2001) Meta-analysis of the response of plant ecophysiological variables to doubled atmospheric CO2 concentrations. Acta Bot Sin 43 1101–1109 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.