Abstract
The relative influence of plant age and environmental stress signals in triggering a shift from C3 photosynthesis to Crassulacean acid metabolism (CAM) in the annual halophytic C3-CAM species Mesembryanthemum crystallinum was explored by continuously monitoring net CO2 exchange of whole shoots from the seedling stage until seed set. Plants exposed to high salinity (400 mm NaCl) in hydroponic culture solution or grown in saline-droughted soil acquired between 11% and 24% of their carbon via net dark CO2 uptake involving CAM. In contrast, plants grown under nonsaline, well-watered conditions were capable of completing their life cycle by operating in the C3 mode without ever exhibiting net CO2 uptake at night. These observations are not consistent with the widely expressed view that the induction of CAM by high salinity in M. crystallinum represents an acceleration of preprogrammed developmental processes. Rather, our study demonstrates that the induction of the CAM pathway for carbon acquisition in M. crystallinum is under environmental control.
One of the most intriguing plant adaptations to environmental stress is Crassulacean acid metabolism (CAM), a water-conserving mode of photosynthesis (Winter et al., 2005) expressed by an estimated 6% of vascular plant species (Smith and Winter, 1996; Crayn et al., 2004; Silvera et al., 2005). CAM is characterized by nocturnal uptake of CO2 via phosphoenolpyruvate carboxylase (PEPC) into malic acid (Winter and Smith, 1996; Holtum et al., 2005). Decarboxylation of malic acid during the light internally liberates CO2, which is incorporated into the PCR cycle via Rubisco. The refixation of CO2 in the light does not require stomata to be open, minimizing transpirational water loss during those parts of the day when the driving forces for water loss are high. Considerable interspecific variability exists between CAM species in the degree to which they engage in the CAM cycle relative to C3 photosynthesis in the light (Skillman and Winter, 1997; Holtum and Winter, 1999; Winter and Holtum, 2002; Pierce et al., 2002a, 2002b; Gehrig et al., 2003; Lüttge, 2006), in part a reflection of the range of CAM plant life forms and habitats in which CAM plants are found.
The adaptive significance of CAM is probably best illustrated by species that exhibit a high intraspecific plasticity in the capacity to express CAM (Winter et al., 1992; Zotz and Winter, 1993; Holtum et al., 2004). Of these so-called C3-CAM species, the most widely studied is Mesembryanthemum crystallinum, a halophytic annual in the Aizoaceae (Winter and Holtum, 2005).
The ability of M. crystallinum to switch from photosynthetic metabolism typical of a C3 plant to that of a CAM plant in response to high soil salinity was first described more than 3 decades ago (Winter and von Willert, 1972). Subsequently, it was demonstrated that CAM is also induced by nonsaline treatments that result in leaf-water deficits, indicating that salt stress elicits CAM by adversely affecting plant-water relations and not through ion-specific effects (Winter, 1973, 1974a). Of the environmental stressors demonstrated to induce CAM in M. crystallinum, high salinity leads to the highest and most predictable degree of CAM expression in mature leaves. As a result, comparative studies between salt-treated and non-salt-treated plants operating in either the CAM or C3 mode of photosynthesis, respectively, have become a widely used model system to identify and elucidate key physiological, biochemical, and molecular processes that underlie the functioning of the CAM pathway (Winter et al., 1982, 1986; Demmig and Winter, 1983; Lüttge, 1993; Edwards et al., 1996; Schmitt et al., 1996; Bohnert and Cushman, 2000; Cushman and Borland, 2002).
Soon after the first demonstration of experimentally triggered CAM in M. crystallinum, some CAM activity was detected in old plants that had not been exposed to any particularly designed stress treatment, an observation that led to the conclusion that M. crystallinum automatically exhibits CAM at a certain advanced stage of development and that salt stress merely accelerates normal plant age-dependent processes (von Willert and Kramer, 1972). The notion that the induction of CAM in M. crystallinum is a preprogrammed developmental process gained momentum after its inclusion in Osmond's landmark review on CAM in 1978. Since then, there have been frequent reports of some CAM activity in well-watered, non-salt-treated mature to old plants (e.g. Cushman et al., 1990) and M. crystallinum is now commonly described in the literature as “a halophyte with a developmentally programmed switch from C3 photosynthesis to CAM which is accelerated by salinity and drought” (p. 171; Adams et al., 1998; see also Herppich et al., 1992; Cushman, 2001; Yen et al., 2001; Cushman and Bohnert, 2002; Cushman and Borland, 2002; Grams and Thiel, 2002; Black and Osmond, 2003; Dodd et al., 2003; Popp et al., 2003; Trofimova et al., 2003; Berg et al., 2004; Bozhko et al., 2004; Hurst et al., 2004; Libik et al., 2004; Lüttge, 2004; Niewiadomska et al., 2004).
If environmental stress only acts as an accelerator of CAM in M. crystallinum, then nonstressed plants should eventually attain levels of CAM expression that are similar to those that can be induced experimentally by salt stress. This corollary has never been tested rigorously by comparative lifetime measurements of CO2 assimilation patterns, neither with M. crystallinum nor with any other photosynthetically plastic CAM plant (Dodd et al., 2002). Quantification of the contributions of environmental and developmental factors to the CAM induction process addresses a fundamental question about the biology of CAM, whether distinctions between constitutive and facultative CAM species have merits or are without foundation (Osmond, 1978).
The study documented here measures the contributions of C3 photosynthetic CO2 uptake and CAM to total lifetime carbon gain in M. crystallinum grown under saline or nonsaline conditions. Lifetime carbon gain was determined by continuously monitoring 24-h light-dark patterns of net CO2 exchange of whole shoots, starting with juvenile plants and continuing measurements through to fully mature plants until they flowered and produced seeds. Our observations do not support the hypothesis that salt stress affects carbon assimilation patterns in M. crystallinum primarily by accelerating a developmentally programmed induction of CAM, thereby resolving a debate that has accompanied research into this remarkable C3-CAM species for over 30 years.
RESULTS
Salinity and Drought Treatments
In a first series of experiments, we ascertained that, under the temperature and light conditions used, plants were capable of exhibiting CAM when stressed. Figure 1 depicts the 24-h net CO2 exchange pattern of a shoot showing CAM and refers to the terminology used to describe the gains and losses of CO2 during various phases of the day-night cycle. CAM was readily induced by exposing 31-d-old plants growing in soil or hydroponics to salinity and/or drought stress (Figs. 2, A–C, and 3). When plants were initially exposed to added NaCl, they consisted of four pairs of leaves that were attached to the main stem and exhibited C3 photosynthesis (i.e. net CO2 uptake was restricted to the light).
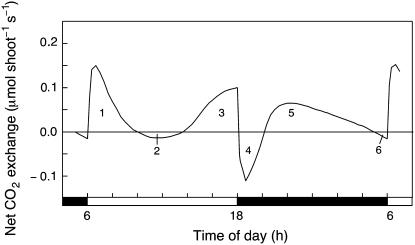
Figure 1.
Diel (24 h) net CO2 exchange pattern of the shoot of a 97-d-old, flowering plant of M. crystallinum grown in hydroponic solution containing 400 mm NaCl. White bar indicates the light period; black bar indicates the dark period. The sum of the areas between the net CO2 exchange trace and the zero line, designated 1 and 3, represents net CO2 uptake during the light period. Area 1 plus area 3 minus area 2 represents net CO2 balance during the light period. Area 5 represents net CO2 uptake during the dark period. Area 5 minus area 4 minus area 6 represents net CO2 balance during the dark period.
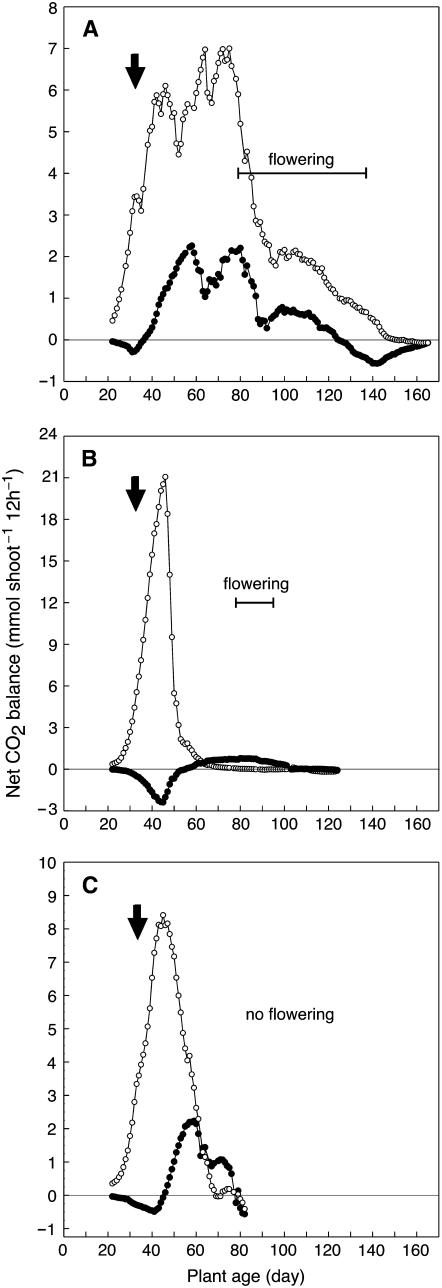
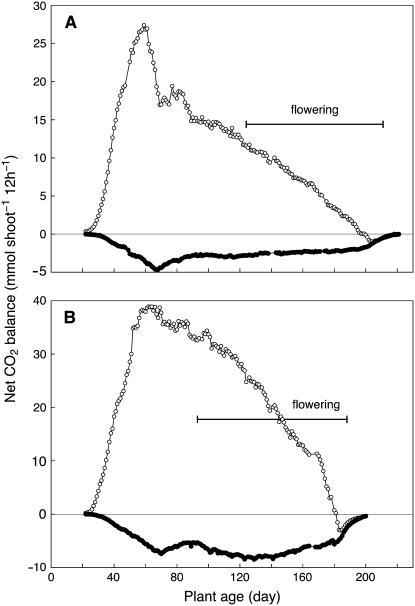
Figure 2.
Net CO2 balance during 12-h light periods (white symbols) and 12-h dark periods (black symbols) during the life cycle of M. crystallinum exposed to salinity and drought stress. Arrow indicates the onset of stress treatments. A, Plant grown in hydroponic solution to which 400 mm NaCl had been added. B, Plant grown in soil and exposed to mild NaCl treatment plus drought stress. C, Plant grown in soil and exposed to severe NaCl treatment plus drought stress. Measurements are of whole, intact shoots (stem, leaves, and branches).
Expression of CAM and the kinetics of its onset varied in response to how stress was applied to the replicates. For a hydroponically cultured plant, the addition of 400 mm NaCl in 100 mm steps over 4 d beginning on day 31 changed CO2 exchange within 24 h (Figs. 2A and 3). A brief period of net CO2 uptake in the dark was observed after 1 d of exposure to 400 mm NaCl (day 35) and the net CO2 balance in the dark became positive after 3 d of exposure (day 37). The daily net CO2 exchange patterns were characterized by increasingly pronounced midday depressions. Between days 45 and 74, net carbon balance in the light and in the dark fluctuated, reflecting the varying contributions of the developing branches, with their young leaves, and the main leaf pairs on the central axis that were shaded progressively and senesced.
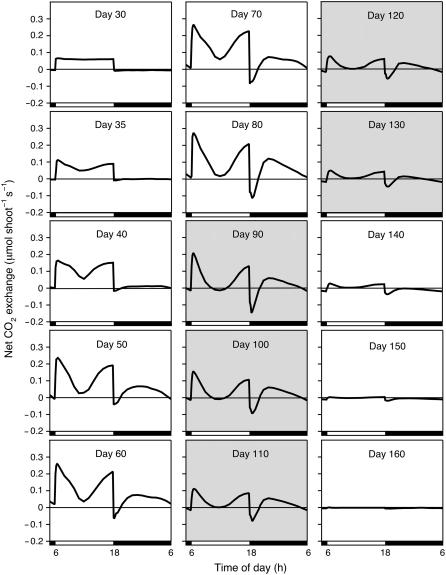
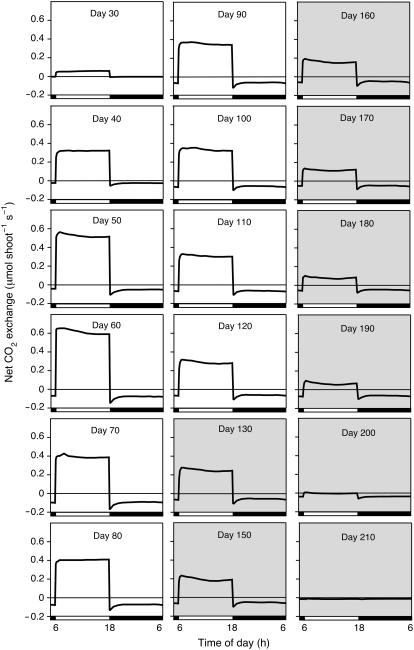
Figure 3.
Diel (24 h) net CO2 exchange patterns at various stages during the life cycle of a M. crystallinum plant grown in hydroponic solution to which 400 mm NaCl was added in steps of 100 mm/d beginning on day 31. White horizontal bars indicate 12-h light period; black horizontal bars indicate 12-h dark period; shading indicates the presence of open flowers. Measurements are of the whole, intact shoot (stem, leaves, and branches). Same experiment as in Figure 2A.
From day 75 onward, net CO2 balance in the light and in the dark declined for the rest of the life cycle. During this phase, the plant flowered, with flowers open between days 79 and 137, and began to senesce from the base upward until the only photosynthetic tissues were the fruit capsules and a few regions of stem. At day 125, the net CO2 balance in the dark became negative, but the net CO2 balance in the light remained positive. After day 137, all net CO2 uptake occurred in the light, although an underlying CAM rhythm was present (Fig. 3, day 140). At this stage, net CO2 exchange was restricted to the green fruit capsules and stems. From day 151 onward, the carbon balance was negative in the light and in the dark. A general feature of CO2 exchange by the plant in hydroponics was that, although it exhibited net CO2 uptake in the dark between days 35 and 137, at no time during its life cycle was the carbon gain in the dark greater than that in the light (Fig. 2A).
Salinity treatments were also applied to plants in 3-L pots containing well-watered, fertilized soil. Both mild and severe salinity treatments were applied. In the mild salinity treatment, 100 mL of 100, 200, 300, and 400 mm NaCl were added to a plant at daily intervals beginning on day 31 (Fig. 2B). Watering ceased following the addition of NaCl. The treatment had no immediate effect on the time course of photosynthesis and respiration, presumably due to the strong dilution of the NaCl solutions by the water in the pot. CO2 gain, which occurred exclusively in the light, increased markedly, as did plant size. The plant wilted on day 47, a response to the diminishing soil-water content and correspondingly increasing concentration of NaCl. Net CO2 balance in the light declined rapidly, midday reductions in CO2 uptake became evident, and, on day 56, the net CO2 balance at night became positive (i.e. CAM was present). By day 63, CO2 gain in the night exceeded that in the light, a situation that did not develop in the hydroponically grown plant that was exposed to NaCl (Fig. 2A). Net CO2 balance in the light became negative on day 77. Flowers opened between days 78 and 95. From day 87 until day 110, positive net CO2 balance was restricted to the dark. Net CO2 balance in the dark became negative on day 111 and remained so until the end of the experiment. The plant produced 15 fruit capsules containing viable seed.
The severe salinity treatment involved adding to the soil, at daily intervals beginning on day 31, 500 mL of 100, 200, 300, and 400 mm NaCl (Fig. 2C). Again, watering ceased following the addition of NaCl. As in the experiment outlined in Figure 2B, the treatment had no immediate effect on the time course of photosynthesis and respiration. The net CO2 balance in the light continued to increase, but growth was constrained in contrast to the effects of the mild salinity treatment (Fig. 2B). On day 46, net CO2 balance during the light began to decline and net CO2 balance in the dark became positive. The latter continued to rise until day 59, when CO2 balance in the light and in the dark was similarly positive. From day 60 onward, net CO2 gain in the dark declined, although it continued to contribute more carbon than did CO2 gain in the light from day 64 until day 79. Thereafter, CO2 balance in the light and dark was negative.
The side branches of the plant exposed to severe salinity (and subsequent drought) were stunted (up to 5 cm long) in contrast to those of the plant exposed to mild salinity and subsequent drought treatment, which developed leafy side branches, up to 12 cm long, that shaded the leaves on the central axis. By day 71, the side branches of the severely stressed plant were chlorotic, but leaves 3 and 4 on the main axis were still green. By day 79, leaves 3 and 4 began to senesce and the rest of the shoot had died. The stress treatment killed the plant during bud initiation and no flowers were produced.
Nonsaline Treatments—Plants in Hydroponics
M. crystallinum was grown under nonsaline conditions to test whether plants could complete a life cycle without exhibiting CAM. Indeed, three hydroponically cultured plants grew to flowering and seed set without ever exhibiting positive net CO2 balance in the dark (Fig. 4, A–C). However, in each of the plants, low rates of net CO2 uptake were measured for short time periods in the dark, generally on days when wilting of leaves on the main axis was noticeable during the light.
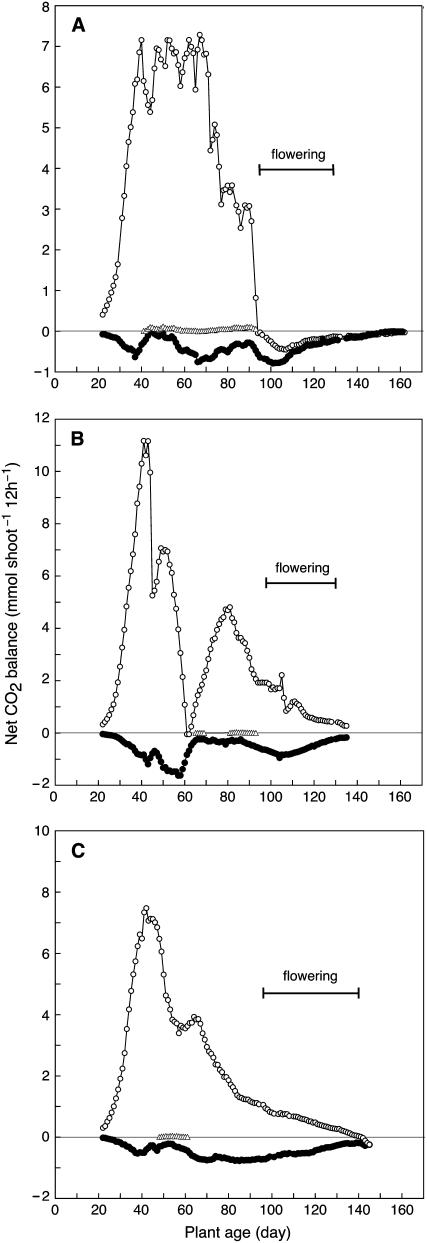
Figure 4.
Net CO2 balance during 12-h light (white circles) and 12-h dark periods (black circles) and net CO2 uptake during 12-h dark periods (white triangles) during the life cycle of M. crystallinum grown under nonsaline conditions in hydroponic solution. Results from experiments with three plants (A, B, and C) are shown. In B and C, nutrient solutions were not replaced after days 69 and 36, respectively. Measurements are of whole, intact shoots (stem, leaves, and branches).
In the first experiment, a plant completed its life cycle in 163 d under conditions in which the dew point of the air entering the gas-exchange cuvette was maintained at 10°C and the dew point inside the chamber never exceeded 18.5°C (66% relative humidity [RH] in the light). Leaves 3 and 4 on the main axis began to wilt on day 41 and a low level of CAM was observed until the onset of flowering when net CO2 balance during the day became negative (Fig. 4A).
A second plant was monitored until day 135 (Fig. 4B). The dew point of the incoming air was 15°C and the nutrient solution was not changed after day 69. The dew point inside the chamber reached a maximum of 22.5°C (85% RH) when the plant showed the highest daytime CO2 gain around day 40. Leaves 3 and 4 wilted on day 44 and, for unknown reasons, the plant became chlorotic at around day 47. Photosynthesis and respiration declined as tissue died, but the remaining tissue did not exhibit net CO2 uptake in the dark during this period. The plant recovered and produced new leaves on the side branches. As the plant recovered, it exhibited low rates of net CO2 uptake in the dark beginning at day 65 and at day 81, following accidental water stressing of its roots.
In a third experiment in which the dew point of the incoming air was 15°C and the nutrient solution was not changed after day 36, a plant was monitored until day 145 (Fig. 4C), when it was close to completing its life cycle. During this time, the dew point inside the chamber never exceeded 21°C in the light (77% RH). Short periods of net CO2 uptake in the dark were observed between days 48 and 61 after leaves 3 and 4 exhibited wilting in the afternoon.
Although low levels of CAM were observed in the three hydroponic plants cultured under nonsaline conditions, the contributions of net CO2 uptake in the dark were small, never exceeding eight molecules of CO2 per 1,000 molecules of CO2 fixed in the light (Table I). In contrast, the plant grown under saline hydroponic conditions fixed 321 molecules of CO2 in the dark per 1,000 molecules fixed in the light.
Table I.
Cumulative net CO2 uptake in the light and the dark determined from day 22 until the day when net CO2 uptake in the light and the dark ceased and the life cycle of plants was close to completion
| Treatment | Net CO2 Uptake
|
Relevant Figures | ||
|---|---|---|---|---|
| Light | Dark | Dark:Light | ||
| mmol | mmol | mmol mol−1 | ||
| Nonsaline culture | ||||
| Hydroponics | 338.8 | 2.7 | 8.0 | Figure 4A |
| Hydroponicsa | 340.7 | 0.2 | 0.6 | Figure 4B |
| Hydroponics | 263.5 | 0.3 | 1.1 | Figure 4C |
| Soil | 2,043.4 | 0 | 0 | Figure 5A, Figure 6 |
| Soil | 3,718.1 | 0 | 0 | Figure 5B |
| Salinity (and drought) | ||||
| Hydroponics | 403.2 | 129.5 | 321 | Figure 2A, Figure 3 |
| Soil | 288.8 | 35.6 | 123 | Figure 2B |
| Soil | 187.5 | 46.2 | 246 | Figure 2C |
Measurements stopped on day 135 (flowering had terminated 5 d beforehand) when a small net CO2 uptake during the 12-h light period (0.3 mmol) still occurred.
Nonsaline Treatments—Plants in Soil
Two plants, grown for 220 and 200 d in 3- and 5-L pots, respectively, containing fertilized, well-watered soil, were C3 plants throughout their life cycles (Fig. 5, A and B). They never exhibited net CO2 uptake in the dark. Their gas exchange was characterized by relatively constant rates of CO2 uptake during light periods and relatively constant rates of respiratory net CO2 loss during dark periods, even when flowering and setting seed (Fig. 6). In both plants, carbon gain during the light period peaked at around day 60 when the plants had seven leaf pairs on the central axis and three pairs of leafy side branches. Subsequently, daytime carbon gain declined as the leaves on the main axis were shaded and senesced and throughout flowering and seed set. For most of the life cycle of the plants grown in soil, the humidity levels were high inside the gas-exchange cuvette, with an air dew point of about 24°C at a chamber temperature of 25.3°C in the light (93% RH).
Figure 5.
Net CO2 balance during 12-h light periods (white symbols) and 12-h dark periods (black symbols) during the life cycle of M. crystallinum grown in nonsaline, well-watered soil. Pot size = 3 L (A) and 5 L (B). Measurements are of whole, intact shoots (stem, leaves, and branches).
Figure 6.
Diel (24 h) net CO2 exchange patterns at various stages during the life cycle of a M. crystallinum plant grown in well-watered, nonsaline soil. White horizontal bars indicate 12-h light period; black horizontal bars indicate 12-h dark period; shading indicates the presence of open flowers. Measurements are of the whole, intact shoot (stem, leaves, and branches). Same experiment as in Figure 5A.
The form and development of the plants that completed their life cycles without ever exhibiting net CO2 uptake in the dark were typical of healthy M. crystallinum and 85% germination was obtained for 20 seeds randomly chosen from the plant featured in Figures 5A and 6.
DISCUSSION
After 4 years of study and the continuous monitoring of CO2 exchange over 1,067 complete 24-h light/dark cycles, we cannot but conclude that the expression of CAM in M. crystallinum is under environmental control. The plants exploited a range of photosynthetic options while completing their life cycles, from salt- and drought-stressed plants in which CAM contributed significantly to lifetime carbon gain (Figs. 2 and 3), to plants that showed a modicum of CAM (Fig. 4), to those in which CAM did not contribute to carbon gain but which, nevertheless, produced viable seeds (Figs. 5 and 6). Thus, physiologically, M. crystallinum is a truly facultative CAM plant.
Ecologically, the facultative capacity of M. crystallinum is not manifested as rapidly induced, rapidly reversible C3-CAM shifts that respond to rapidly fluctuating environmental conditions. Rather, the expression of photosynthetic pathway reflects the habitats to which the species is typically adapted, coastal areas with Mediterranean climates where cool, wet winters alternate with hot, dry summers. During the wet season, plants are C3 and during the dry season plants exhibit CAM. In its natural habitat, M. crystallinum is always CAM during the hot, dry summers, not because it is developmentally programmed to do so, but, rather because the dry season brings with it the environmental stressors that trigger the induction of CAM (Winter et al., 1978). This is well illustrated by a field study in which the development of CAM in two Californian populations that germinated at different times was closely correlated with decreasing soil moisture, not plant age (Bloom and Troughton, 1979).
The low-level CAM reported repeatedly for M. crystallinum grown in glasshouses or in controlled environment chambers under well-watered, nonsaline conditions without intentional imposition of stress probably occurs because it is technically difficult to grow M. crystallinum completely stress free. For example, in the latter half of the light period, in the absence of any particular stress treatment, mature highly elastic M. crystallinum leaves (low bulk elastic modulus of the cell wall) can routinely experience transpirationally induced water deficits that are characterized by decreases in the relative water content of around 15% and decreases of turgor pressure by 0.02 MPa (Winter and Gademann, 1991). Although both water content and turgor pressure usually recover during the following dark period, the water deficits often lead to visible wilting in the light of large leaves on the main axis, particularly leaves 3 and 4, and are accompanied by the induction of CAM (Fig. 4). The imbalance between water uptake and water loss may be exacerbated in plants grown in the absence of added NaCl because M. crystallinum is a halophyte that requires moderate concentrations of NaCl for optimal growth and maintenance of turgor (Winter and Lüttge, 1976).
Wilting is not necessarily the trigger, or the only trigger, that induces CAM, but it definitely is an indicator of nonoptimal growth conditions (i.e. stress). Split-root experiments suggest that root signals can be involved in CAM induction in the absence of noticeable changes in leaf-water relations (Eastmond and Ross, 1997). On the other hand, PEPC transcripts increase in detached leaves exposed to water deficits and decrease when leaves rehydrate (Schmitt, 1990), suggesting that the presence of roots is not obligatory for CAM induction. We are only beginning to understand the interplay between roots and shoots, and the cellular physicochemical signal transduction mechanisms involved in the expression of CAM in M. crystallinum (Taybi and Cushman, 2002; Taybi et al., 2002).
We coaxed M. crystallinum growing in a relatively small-volume gas-exchange cuvette to complete its life cycle as a C3 plant by culturing plants for long periods at extremely high humidity and a moderate light regime (approximately 15 mol photons m−2 day−1, equivalent to about one-third of integrated photon flux density [PFD] on a bright day in the field; Figs. 5 and 6). Our approach originated with observations that the occurrence and extent of expression of CAM in leaves of plants under nonsaline conditions are reduced substantially when plants are grown under conditions of low evaporative demand (e.g. high RH and low light; Winter, 1973). The fine environmental control required to provide high humidity and small differences between leaf temperatures and air dew point temperatures over prolonged periods is much easier to achieve and to track in a small-volume gas-exchange cuvette in comparison to a glasshouse or an environmental growth chamber. Furthermore, M. crystallinum plants that completed their life cycles without ever expressing CAM features of CO2 exchange were grown in soil in 3- and 5-L pots to minimize possible detrimental effects of small pot size on plant water availability (Schmitt and Piepenbrock, 1992; Piepenbrock et al., 1994). Soil-grown plants were more vigorous than hydroponically grown plants and attained greater biomass as shown by their higher lifetime carbon gains (Table I).
Maintaining plants at higher irradiance levels than used by us increases leaf temperatures and enlarges the leaf-to-air vapor pressure difference, the driving force for transpirational water loss. Thus, the induction of CAM in well-watered plants at high irradiance (Broetto et al., 2002; Epimashko et al., 2006) is presumably not the consequence of high PFD per se; rather, it results from adverse effects of vapor pressure difference on leaf-water relations.
The gas-exchange measurements reported here are the net signals for whole shoots that include stem, branches, and leaves and, when present, reproductive tissues. One cannot exclude the possibility that the integrated signal from the shoots may mask CAM signals from a small proportion of tissue. In the day/night cycles shown in Figure 6 for a plant that never exhibited net CO2 uptake in the dark, there is no indication at any time during its life cycle of even the most minimal type of CAM, CAM cycling (Ting, 1985), which is characterized by transient reductions in net CO2 loss at night due to refixation of respiratory CO2. If at all present, any contribution of CAM-type CO2 uptake in the dark to the overall CO2 balance would have been extremely small, considerably less than the 0.06% of the total CO2 fixed by a plant under nonsaline conditions in hydroponics (Fig. 4B; Table I).
The induction of CAM in M. crystallinum is primarily a response to the environment, but the speed and intensity of the response is influenced by developmental factors, particularly leaf age. Consistent with constitutive CAM plants in which the CAM cycle is increasingly expressed as leaves mature (Deleens and Queiroz, 1984; Gehrig et al., 2005), the propensity to respond to stress by inducing CAM increases in M. crystallinum with leaf age (Winter and Lüttge, 1979; Cushman et al., 1990; Piepenbrock and Schmitt, 1991). Although there are conflicting reports about the inducibility of CAM in very young plants with young leaves (Adams et al., 1998), both acid fluctuations and increased PEPC activity have been reported in leaves of M. crystallinum 2 to 3 weeks after germination, obviously when the leaf tissue investigated was of sufficient maturity (Piepenbrock and Schmitt, 1991; Winter and Smith, 1996).
Full reversibility of the C3-to-CAM shift following the relaxation of stress would provide a strong argument in support of the strictly environmental control of the induction of CAM in M. crystallinum. However, absence of reversibility is not necessarily an argument against a predominantly environmental control of CAM induction. The reversibility of CAM in M. crystallinum is understudied, but it has been reported (Winter, 1974b; Vernon et al., 1988). Reversibility appears more difficult to achieve in older than younger tissues and its demonstration may be problematic because leaves of M. crystallinum live only for a few weeks and, because it can take about 9 d for PEPC activity to revert from rates found in stressed plants to rates typical of unstressed plants (Vernon et al., 1988), the reversion may be obscured by processes associated with tissue senescence. Another potential complication of reversibility experiments is that osmotic damage to roots may occur if plants are abruptly switched from highly saline to nonsaline growth media.
As has been the case with past investigations, researchers of M. crystallinum can continue to expect to repeatedly observe the occurrence of some CAM activity in well-watered, apparently nonstressed plants when these reach an advanced stage of development. Comparative studies between such plants and those in which CAM is a direct response to experimentally imposed salt stress will continue to help identify salt-specific metabolic stress responses unrelated to CAM. But there is no automatic, preprogrammed induction of CAM during the life cycle of M. crystallinum. Under carefully managed conditions, well-watered plants can complete their life cycle without ever showing net CO2 gas-exchange features indicative of CAM. It is therefore clear that what has repeatedly been interpreted as developmentally triggered CAM has an environmental origin, most likely involving similar stress signals and physicochemical signal transduction mechanisms that lead to the induction of CAM in M. crystallinum when plants are exposed to high salinity and drought. Genotypically, M. crystallinum is a CAM species because of its capacity to perform CAM. Phenotypically and ecologically, it is a C3-CAM species because of its directional, seasonal shift from C3 to CAM in its natural habitat. Physiologically, M. crystallinum is a facultative CAM plant because, as demonstrated here, it can, but need not, use CAM for growth and reproduction.
MATERIALS AND METHODS
Plant Culture
Seeds of Mesembryanthemum crystallinum (Aizoaceae) were germinated in cactus and succulent potting mix (Schultz Co.) inside a controlled-environment cabinet (GEC) under a 12-h light (23°C)/12-h dark (17°C) cycle. PFD during initial growth was 200 μmol m−2 s−1. RH ranged from 50% during the light periods to 80% during the dark periods.
After about 12 d, when leaf pair 1 on the main axis was just visible between the two cotyledons, seedlings were transferred to an aerated hydroponic solution composed of 3 mm KNO3, 2 mm Ca(NO3)2, 0.5 mm NH4H2PO4, 0.5 mm (NH4)2HPO4, 0.5 mm MgSO4, 12.5 μm H3BO3, 1 μm MnSO4, 1 μm ZnSO4, 0.25 μm MoO3, and 10 μm EDTA Fe(III)-sodium salt.
CO2 Gas Exchange
Eighteen-day-old plant shoots were sealed inside a Plexiglas cuvette (interior dimensions: 30 cm × 30 cm × 15 cm) in the controlled-environment chamber. Roots were inserted from above through a 0.8-cm-diameter hole in the center of the cuvette base. The hole was sealed with a nonporous synthetic rubber sealant, Terostat VII (Henkel-Teroson), such that the entire shoot system was inside the cuvette and the attached entire root system outside the cuvette. The roots were placed in either 0.8 L of nutrient solution that was continuously aerated and replaced weekly unless stated otherwise or in soil. Three-liter pots contained potting soil plus (Schultz) and a 5-L pot contained moisture control potting mix (Miracle-Gro; Scotts) to which 10 g of Osmocote Plus (Scotts) had been added. Plants were watered daily.
PFD inside the cuvette ranged from 320 μmol m−2 s−1 at the bottom of the cuvette to 370 μmol m−2 s−1 at the top.
Net CO2 exchange by the intact shoots was measured using a flow-through gas-exchange system (most components from Walz; Holtum and Winter, 2003) operating between 2.33 and 7.21 L air min−1. The CO2 concentration of the air, sourced 16 m above ground level and passed through a 1 m3 container, varied between 360 and 430 μL L−1. The container served to buffer the air source against short-term fluctuations in CO2 concentration. The temperature inside the cuvette was 25.3°C during the light and 17°C during the dark. Net CO2 exchange was monitored with a LI-6252 CO2 analyzer (LI-COR) operating in the absolute mode.
The dew point of the air entering the gas-exchange cuvette was 10°C for one plant grown in nonsaline hydroponics. For all other plants, a dew point of 15°C was used, at least initially. As plants increased in size, transpiration increased and the concentration of water vapor in the cuvette approached saturation. In some cases (e.g. with two plants grown in soil under nonsaline conditions), the dew point of the incoming air was adjusted to minimize condensation but to maintain high humidity. A small amount of condensate in the cuvette was tolerated to guarantee the highest possible humidity. Condensate did not interfere with measurements of net CO2 exchange because air leaving the cuvette and air bypassing the cuvette was dehumidified in a KF-18/2 electronically controlled cold trap (Walz) at 2°C before passage through the CO2 analyzer at 0.5 L min−1 in an alternating fashion (2 min/gas stream).
Gas-exchange measurements were conducted without interruption from the day the shoots were transferred into the gas-exchange cuvette until they ceased to exhibit net CO2 uptake in the light and in the dark, unless stated otherwise.
During the study, the absence or presence of open flowers was determined daily. Seed viability was quantified by studying germination of seeds placed on moist filter paper in petri dishes.
This work was supported by the A.W. Mellon Foundation (to K.W.), by the Smithsonian Tropical Research Institute (to K.W.), and by the James Cook University Special Study Program (to J.A.M.H.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Klaus Winter (winterk@si.edu).
References
- Adams P, Nelson DE, Yamada S, Chmara W, Jensen RG, Bohnert HJ, Griffiths H (1998) Growth and development of Mesembryanthemum crystallinum (Aizoaceae). New Phytol 138 171–190 [DOI] [PubMed] [Google Scholar]
- Berg A, Orthen B, de Mattos EA, Duarte HM, Lüttge U (2004) Expression of Crassulacean acid metabolism in Clusia hilariana Schlechtendal in different stages of development in the field. Trees (Berl) 18 553–558 [Google Scholar]
- Black CC, Osmond CB (2003) Crassulacean acid metabolism photosynthesis: “working the night shift.” Photosynth Res 76 329–341 [DOI] [PubMed] [Google Scholar]
- Bloom AJ, Troughton JH (1979) High productivity and photosynthetic flexibility in a CAM plant. Oecologia 38 35–43 [DOI] [PubMed] [Google Scholar]
- Bohnert HJ, Cushman JC (2000) The ice plant cometh: lessons in abiotic stress tolerance. J Plant Growth Regul 19 334–346 [Google Scholar]
- Bozhko KN, Zhestkova IM, Trofimova MS, Kholodova VP, Kuznetsov VV (2004) Aquaporin content in cell membranes of Mesembryanthemum crystallinum as affected by plant transition from C3 to CAM type of photosynthesis. Russ J Plant Physiol 51 798–805 [Google Scholar]
- Broetto F, Lüttge U, Ratajczak R (2002) Influence of light intensity and salt-treatment on mode of photosynthesis and enzymes of the antioxidative response system of Mesembryanthemum crystallinum. Funct Plant Biol 29 13–23 [DOI] [PubMed] [Google Scholar]
- Crayn DM, Smith JAC, Winter K (2004) Multiple origins of Crassulacean acid metabolism and the epiphytic habit in the neotropical family Bromeliaceae. Proc Natl Acad Sci USA 101 3703–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman JC (2001) Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol 127 1439–1448 [PMC free article] [PubMed] [Google Scholar]
- Cushman JC, Bohnert HJ (2002) Induction of Crassulacean acid metabolism by salinity—molecular aspects. In A Läuchli, U Lüttge, eds, Salinity: Environment—Plants—Molecules. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 361–393
- Cushman JC, Borland AM (2002) Induction of Crassulacean acid metabolism by water limitation. Plant Cell Environ 25 295–310 [DOI] [PubMed] [Google Scholar]
- Cushman JC, Michalowski CB, Bohnert HJ (1990) Developmental control of CAM inducibility by salt stress in the common ice plant. Plant Physiol 94 1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleens E, Queiroz O (1984) Effects of photoperiod and aging on the carbon isotope composition of Bryophyllum daigremontianum Berger. Plant Cell Environ 7 279–283 [Google Scholar]
- Demmig B, Winter K (1983) Photosynthetic characteristics of chloroplasts from Mesembryanthemum crystallinum L., a halophilic plant capable of Crassulacean acid metabolism. Planta 159 66–76 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53 569–580 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Griffiths H, Taybi T, Cushman JC, Borland AM (2003) Integrating diel starch metabolism with the circadian and environmental regulation of Crassulacean acid metabolism in Mesembryanthemum crystallinum. Planta 216 789–797 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ, Ross JD (1997) Evidence that the induction of Crassulacean acid metabolism by water stress in Mesembryanthemum crystallinum (L.) involves root signalling. Plant Cell Environ 20 1559–1565 [Google Scholar]
- Edwards GE, Dai Z, Cheng SH, Ku MSB (1996) Factors affecting the induction of Crassulacean acid metabolism in Mesembryanthemum crystallinum. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism. Springer, Berlin, pp 119–134
- Epimashko S, Fischer-Schliebs E, Christia A-L, Thiel G, Lüttge U (2006) Na+/H+- transporter, H+-pumps and an aquaporin in light and heavy tonoplast membranes from organic acid and NaCl accumulating vacuoles of the annual facultative CAM plant and halophyte Mesembryanthemum crystallinum L. Planta 224 944–951 [DOI] [PubMed] [Google Scholar]
- Gehrig HH, Aranda J, Cushman MA, Virgo A, Cushman JC, Hammel BE, Winter K (2003) Cladogram of Panamanian Clusia based on nuclear DNA: implications for the origins of Crassulacean acid metabolism. Plant Biol 5 59–70 [Google Scholar]
- Gehrig HH, Wood JA, Cushman MA, Virgo A, Cushman JC, Winter K (2005) Large gene family of phosphoenolpyruvate carboxylase in the Crassulacean acid metabolism plant Kalanchoe pinnata (Crassulaceae) characterized by partial cDNA sequence analysis. Funct Plant Biol 32 467–472 [DOI] [PubMed] [Google Scholar]
- Grams TEE, Thiel S (2002) High light-induced switch from C3-photosynthesis to Crassulacean acid metabolism is mediated by UV- A/blue light. J Exp Bot 53 1475–1483 [PubMed] [Google Scholar]
- Herppich WB, Herppich M, von Willert DJ (1992) The irreversible C3 to CAM shift in well-watered and salt-stressed plants of Mesembryanthemum crystallinum is under strict ontogenetic control. Bot Acta 105 34–40 [Google Scholar]
- Holtum JAM, Aranda J, Virgo A, Gehrig HH, Winter K (2004) δ13C values and Crassulacean acid metabolism in Clusia species from Panama. Trees (Berl) 18 658–668 [Google Scholar]
- Holtum JAM, Smith JAC, Neuhaus HE (2005) Intracellular transport and pathways of carbon flow in plants with Crassulacean acid metabolism. Funct Plant Biol 32 429–449 [DOI] [PubMed] [Google Scholar]
- Holtum JAM, Winter K (1999) Degrees of Crassulacean acid metabolism in tropical epiphytic and lithophytic ferns. Aust J Plant Physiol 26 749–757 [Google Scholar]
- Holtum JAM, Winter K (2003) Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218 152–158 [DOI] [PubMed] [Google Scholar]
- Hurst AC, Grams TEE, Ratajczak R (2004) Effects of salinity, high irradiance, ozone, and ethylene on mode of photosynthesis, oxidative stress and oxidative damage in the C3/CAM intermediate plant Mesembryanthemum crystallinum L. Plant Cell Environ 27 187–197 [Google Scholar]
- Libik M, Pater B, Elliot S, Slesak I, Miszalski Z (2004) Malate accumulation in different organs of Mesembryanthemum crystallinum L. following age-dependent or salinity-triggered CAM metabolism. Zeitschrift Naturforschung 59c 223–228 [DOI] [PubMed] [Google Scholar]
- Lüttge U (1993) The role of Crassulacean acid metabolism (CAM) in the adaptation of plants to salinity. New Phytol 125 59–71 [DOI] [PubMed] [Google Scholar]
- Lüttge U (2004) Ecophysiology of Crassulacean acid metabolism (CAM). Ann Bot (Lond) 93 629–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U (2006) Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytol 171 7–25 [DOI] [PubMed] [Google Scholar]
- Niewiadomska E, Karpinska B, Romanowska E, Slesak I, Karpinski S (2004) A salinity-induced C3-CAM transition increases energy conservation in the halophyte Mesembryanthemum crystallinum L. Plant Cell Physiol 45 789–794 [DOI] [PubMed] [Google Scholar]
- Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annu Rev Plant Physiol 29 379–414 [Google Scholar]
- Piepenbrock M, Schmitt JM (1991) Environmental control of phosphoenolpyruvate carboxylase induction in mature Mesembryanthemum crystallinum L. Plant Physiol 97 998–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenbrock M, von Albert C, Schmitt JM (1994) Decreasing leaf water-content induces Crassulacean acid metabolism in well-irrigated Mesembryanthemum crystallinum. Photosynthetica 30 623–628 [Google Scholar]
- Pierce S, Winter K, Griffiths H (2002. a) The role of CAM in high rainfall cloud forests: an in situ comparison of photosynthetic pathways in Bromeliaceae. Plant Cell Environ 25 1183–1192 [Google Scholar]
- Pierce S, Winter K, Griffiths H (2002. b) Carbon isotope ratio and the extent of daily CAM use by Bromeliaceae. New Phytol 156 75–84 [Google Scholar]
- Popp M, Janett HP, Lüttge U, Medina E (2003) Metabolite gradients and carbohydrate translocation in rosette leaves of CAM and C3 bromeliads. New Phytol 157 649–656 [DOI] [PubMed] [Google Scholar]
- Schmitt JM (1990) Rapid concentration changes of phosphoenolpyruvate carboxylase mRNA in detached leaves of Mesembryanthemum crystallinum L. in response to wilting and rehydration. Plant Cell Environ 13 845–850 [Google Scholar]
- Schmitt JM, Fißlthaler B, Sheriff A, Lenz B, Bäßler M, Meyer G (1996) Environmental control of CAM induction in Mesembryanthemum crystallinum—a role for cytokinin, abscisic acid and jasmonate? In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism. Springer, Berlin, pp 159–175
- Schmitt JM, Piepenbrock M (1992) Induction of mRNA for phosphoenolpyruvate carboxylase is correlated with a decrease in shoot water content in well-irrigated Mesembryanthemum crystallinum. Plant Physiol 99 759–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvera K, Santiago LS, Winter K (2005) Distribution of Crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Funct Plant Biol 32 397–407 [DOI] [PubMed] [Google Scholar]
- Skillman JB, Winter K (1997) High photosynthetic capacity in a shade-tolerant Crassulacean acid metabolism plant. Plant Physiol 113 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JAC, Winter K (1996) Taxonomic distribution of Crassulacean acid metabolism. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism. Springer, Berlin, pp 427–436
- Taybi T, Cushman JC (2002) Abscisic acid signaling and protein synthesis requirements for phosphoenolpyruvate carboxylase transcript induction in the common ice plant. J Plant Physiol 159 1235–1243 [Google Scholar]
- Taybi T, Cushman JC, Borland AM (2002) Environmental, hormonal and circadian regulation of Crassulacean acid metabolism expression. Funct Plant Biol 29 669–678 [DOI] [PubMed] [Google Scholar]
- Ting IP (1985) Crassulacean acid metabolism. Annu Rev Plant Physiol 36 595–622 [Google Scholar]
- Trofimova MS, Zhestkova IM, Kholodova VP, Andreev IM, Sorokin EM, Kruglova AG, Kuznetsov VV (2003) Osmotic water permeability of cell membranes from Mesembryanthemum crystallinum leaves: effects of age and salinity. Physiol Plant 118 232–239 [Google Scholar]
- Vernon DM, Ostrem JA, Schmitt JM, Bohnert HJ (1988) PEPCase transcript levels in Mesembryanthemum crystallinum decline rapidly upon relief from salt stress. Plant Physiol 86 1002–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Willert DJ, Kramer D (1972) Feinstruktur und Crassulaceensäurestoffwechsel in Blättern von Mesembryanthemum crystallinum während natürlicher und NaCl-induzierter Alterung. Planta 107 227–237 [DOI] [PubMed] [Google Scholar]
- Winter K (1973) CO2-Fixierungsreaktionen bei der Salzpflanze Mesembryanthemum crystallinum unter variierten Außenbedingungen. Planta 114 75–85 [DOI] [PubMed] [Google Scholar]
- Winter K (1974. a) Evidence for the significance of Crassulacean acid metabolism as an adaptive mechanism to water stress. Plant Sci Lett 3 279–281 [Google Scholar]
- Winter K (1974. b) NaCl-induzierter Crassulaceen-Säurestoffwechsel bei der Salzpflanze Mesembryanthemum crystallinum. Oecologia 15 383–392 [DOI] [PubMed] [Google Scholar]
- Winter K, Aranda J, Holtum JAM (2005) Carbon isotope composition and water-use efficiency in plants with Crassulacean acid metabolism. Funct Plant Biol 32 381–388 [DOI] [PubMed] [Google Scholar]
- Winter K, Arron GP, Edwards GE (1986) Malate decarboxylation by mitochondria of the inducible Crassulacean acid metabolism plant Mesembryanthemum crystallinum. Plant Cell Physiol 27 1533–1539 [Google Scholar]
- Winter K, Foster JG, Edwards GE, Holtum JAM (1982) Intracellular localization of enzymes of carbon metabolism in Mesembryanthemum crystallinum exhibiting C3 photosynthetic characteristics or performing Crassulacean acid metabolism. Plant Physiol 69 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Gademann R (1991) Daily changes in CO2 and water vapor exchange, chlorophyll fluorescence, and leaf water relations in the halophyte Mesembryanthemum crystallinum during the induction of Crassulacean acid metabolism in response to high NaCl salinity. Plant Physiol 95 768–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM (2002) How closely do the δ13C values of Crassulacean acid metabolism plants reflect the proportion of CO2 fixed during day and night? Plant Physiol 129 1843–1851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter K, Holtum JAM (2005) The effects of salinity, Crassulacean acid metabolism and plant age on the carbon isotope composition of Mesembryanthemum crystallinum L., a halophytic C3-CAM species. Planta 222 201–209 [DOI] [PubMed] [Google Scholar]
- Winter K, Lüttge U (1976) Balance between C3 and CAM pathway of photosynthesis. In OL Lange, L Kappen, ED Schulze, eds, Water and Plant Life. Springer, Berlin, pp 323–334
- Winter K, Lüttge U (1979) C3-Photosynthese und Crassulaceen-Säurestoffwechsel bei Mesembryanthemum crystallinum L. Ber Dtsch Bot Ges 92 117–132 [Google Scholar]
- Winter K, Lüttge U, Winter E, Troughton JH (1978) Seasonal shift from C3 photosynthesis to Crassulacean acid metabolism in Mesembryanthemum crystallinum growing in its natural environment. Oecologia 34 225–237 [DOI] [PubMed] [Google Scholar]
- Winter K, Smith JAC (1996) Crassulacean acid metabolism. Current status and perspectives. In K Winter, JAC Smith, eds, Crassulacean Acid Metabolism. Springer, Berlin, pp 389–426
- Winter K, von Willert DJ (1972) NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Z Pflanzenphysiol 67 166–170 [Google Scholar]
- Winter K, Zotz G, Baur B, Dietz KJ (1992) Light and dark CO2 fixation in Clusia uvitana as affected by plant water status and CO2 availability. Oecologia 91 47–51 [DOI] [PubMed] [Google Scholar]
- Yen S-K, Chung M-C, Chen P-C, Yen H-E (2001) Environmental and developmental regulation of the wound-induced cell wall protein WI12 in the halophyte ice plant. Plant Physiol 127 517–528 [PMC free article] [PubMed] [Google Scholar]
- Zotz G, Winter K (1993) Short-term regulation of Crassulacean acid metabolism activity in a tropical hemiepiphyte, Clusia uvitana. Plant Physiol 102 835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]