Abstract
Given that stomatal movement is ultimately a mechanical process and that stomata are morphologically and mechanically diverse, we explored the influence of stomatal mechanical diversity on leaf gas exchange and considered some of the constraints. Mechanical measurements were conducted on the guard cells of four different species exhibiting different stomatal morphologies, including three variants on the classical “kidney” form and one “dumb-bell” type; this information, together with gas-exchange measurements, was used to model and compare their respective operational characteristics. Based on evidence from scanning electron microscope images of cryo-sectioned leaves that were sampled under full sun and high humidity and from pressure probe measurements of the stomatal aperture versus guard cell turgor relationship at maximum and zero epidermal turgor, it was concluded that maximum stomatal apertures (and maximum leaf diffusive conductance) could not be obtained in at least one of the species (the grass Triticum aestivum) without a substantial reduction in subsidiary cell osmotic (and hence turgor) pressure during stomatal opening to overcome the large mechanical advantage of subsidiary cells. A mechanism for this is proposed, with a corollary being greatly accelerated stomatal opening and closure. Gas-exchange measurements on T. aestivum revealed the capability of very rapid stomatal movements, which may be explained by the unique morphology and mechanics of its dumb-bell-shaped stomata coupled with “see-sawing” of osmotic and turgor pressure between guard and subsidiary cells during stomatal opening or closure. Such properties might underlie the success of grasses.
Although the morphological diversity of stomata is widely documented (Haberlandt, 1884; Meidner and Mansfield, 1968; Allaway and Milthorpe, 1976; Ziegler, 1987; Willmer and Fricker, 1996), little is known of how this translates into functional diversity and what the environmental context of this might be. Throughout the 400 million year (Ma) history of vascular plants on land, long-term decline in atmospheric CO2 concentration and shifts in prevailing moisture patterns have placed selective pressures on stomata to increase epidermal conductance to CO2 diffusion and also to increase transpiration efficiency (CO2 fixed per unit water transpired). This posed two separate problems, upon which the combination of mutation and time might have worked to give rise to the current diversity of stomatal form and function. The first centered on the simple geometric practicalities of fitting enough functional stomatal units per unit leaf surface area to meet the desired CO2 flux as atmospheric CO2 concentration changed, or to service an increase in photosynthetic capacity. The second centered on the performance characteristics of any new stomatal structure or configuration in relation to transpiration efficiency. Here, by examining the mechanical and performance characteristics of stomata in four different species, we explore the nature of these two problems and how they might have been resolved.
The mechanical characteristics of stomata are central to their performance in gas-exchange regulation, but relatively little is known about these properties, particularly how they vary across different stomatal forms. The simple quantitative relationship between guard cell turgor (Pg), epidermal (or subsidiary) cell turgor (Pe), and stomatal aperture (a), which defines the operational potential of all stomata, is known for only a few species, and most of these have structurally similar stomata (Meidner and Bannister, 1979; Franks et al., 1998, 2001). No such data are available for the distinctive “dumb-bell”-shaped stomata of grasses, for example, or for the more mechanically isolated stomata that are common in many pteridophytes. Without this information on the mechanical diversity of stomata, the power to predict the behavior of stomata in diverse types of vegetation or to understand key stages in the evolution of plant gas-exchange characteristics remains limited. Our first objective was to address this shortfall by measuring and analyzing the Pg-Pe-a relationship in four species with distinctively different stomatal morphologies: (1) the lycopod Huperzia prolifera; (2) the fern Nephrolepis exaltata; (3) the herbaceous angiosperm Tradescantia virginiana; and (4) the grass Triticum aestivum.
Stomata of the four species chosen for the study cover a broad morphological and evolutionary spectrum (Fig. 1). H. prolifera is a living representative of one of the most ancient vascular plant taxa (Lycopodiaceae), with fossilized remains of its close relatives (having the same stomatal morphology) dating back to the lower Devonian (Stubblefield and Banks, 1978; Sun et al., 2005). Its stomata are anomocytic (lacking subsidiary cells), with large and comparatively fat guard cells, which undergo minimal swelling or lateral movement during stomatal opening. Based on these characteristics, it is regarded as archetypal (Ziegler, 1987), perhaps closely resembling the first stomata. N. exaltata, like H. prolifera, is anomocytic, but fern stomata are structurally and functionally more advanced than those of Lycopods. Although the pattern of guard cell deformation during stomatal opening is similar to that of Lycopods, fern stomata of comparable guard cell dimensions achieve wider apertures, which assists with higher rates of gas exchange (N. exaltata exhibits photosynthetic rates more than 3 times that of H. prolifera under similar conditions; Franks, 2006). T. virginiana and T. aestivum are distinctive in that they both have a subsidiary cell running parallel to each guard cell. Their guard cells exhibit substantial lateral movement and physical interaction with the subsidiary cells during stomatal opening, resulting in wide stomatal pores that facilitate high rates of photosynthetic gas exchange. While stomata of the other species are variants on the common “kidney” form, those of T. aestivum exhibit the characteristic dumb-bell-shaped guard cells of grasses (Graminae). The functional significance of this uniquely different guard cell form remains unknown, although the capacity for rapid stomatal opening and closure in grasses is thought to be somehow linked to their unusual guard cell geometry (Johnsson et al., 1976; Hetherington and Woodward, 2003). It has further been proposed that superior dynamic performance of grass stomata could have facilitated the relatively recent spread and diversification of grasses during a period of global aridification 35 to 40 Ma ago (Hetherington and Woodward, 2003).
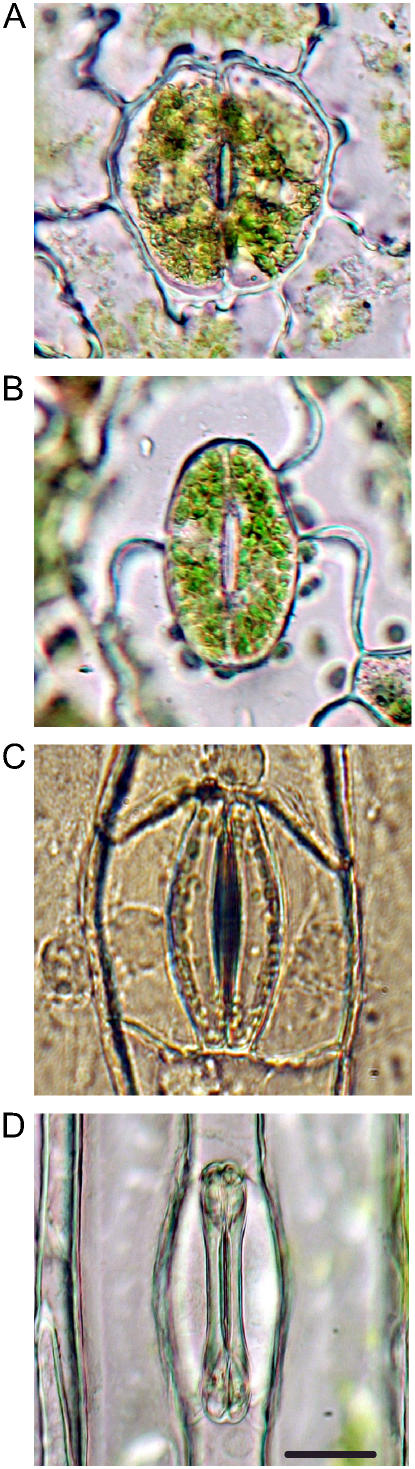
Figure 1.
Images of stomata (closed) from each of the four species examined. A, H. prolifers; B, N. exaltata; C, T. virginiana; D, T. aestivum. All are in epidermal peels, bathed in 25 mm MES, pH 6.5, 1.0 mm KCl, 0.1 mm CaCl2. Scale bar = 20 μm; all images to same scale.
The second objective of the study was to investigate the role of guard cell morphology and mechanics in stomatal function, particularly in relation to its potential influence on the speed of stomatal opening and closure. Stomatal response time is important because it determines the extent to which leaf gas exchange can be optimized under fluctuating environmental conditions. Fast and appropriately damped response to changes in the environmental drivers of photosynthesis or transpiration rate can lead to greater transpiration efficiency. Grasses have long been known for their capacity for rapid stomatal response (Raschke and Fellows, 1971; Brogardh and Johnsson, 1975; Johnsson et al., 1976; Karlsson and Assmann, 1990; Grantz and Assmann, 1991; Assmann et al., 1992), but the underlying mechanism for this has remained a mystery, due partly to the absence of data on the mechanical interactions between guard and subsidiary cells in grasses. Our approach to unraveling this centrally important aspect of stomatal function was to revisit the findings of several classical studies on guard cell solute transport and, with new information on guard cell mechanical and geometric properties, formulate an osmo-mechanical model of stomatal movement that could explain the superior performance of grass stomata under dynamic environmental conditions. To illustrate these characteristics, the stomatal properties of the grass T. aestivum were compared with those of the three non-grass species.
RESULTS
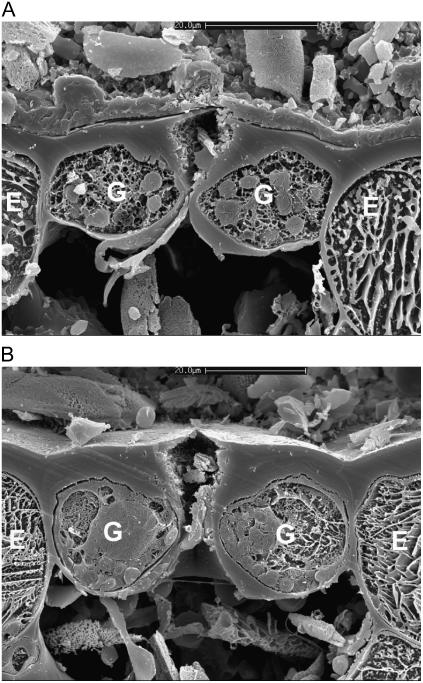
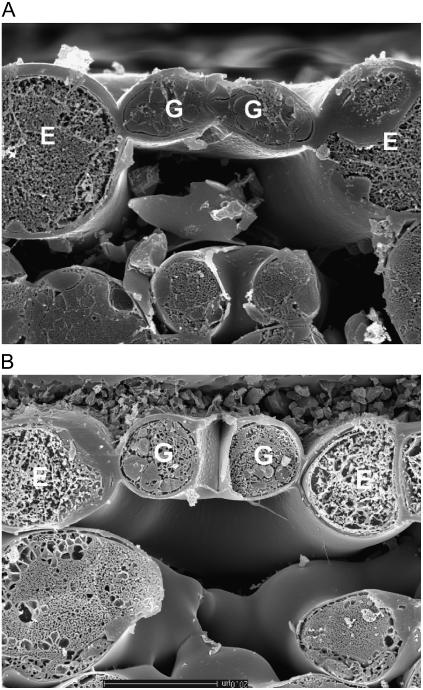
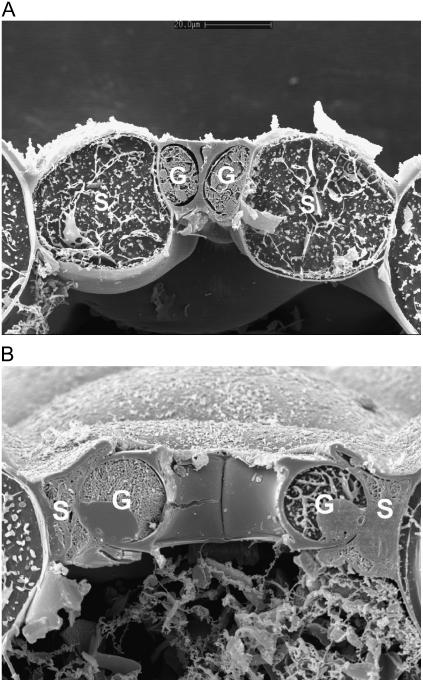
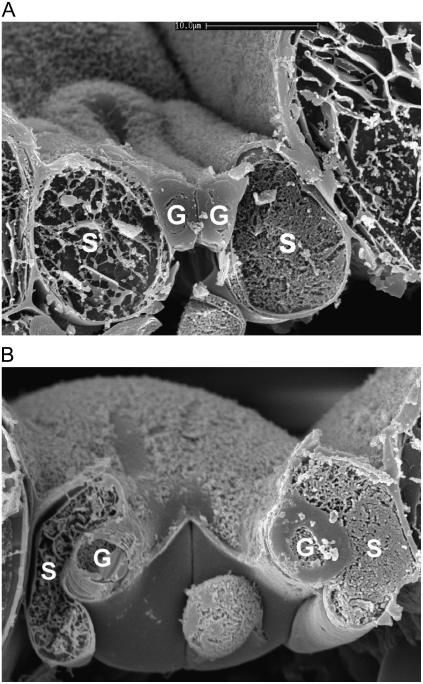
The mechanical diversity of the four stomatal types is illustrated dramatically in the scanning electron micrographs of cryo-sectioned leaf material in Figures 2 to 5. Here, the signature guard cell swelling and displacement characteristics of the different stomatal types are evident. In particular, H. prolifera and N. exaltata show little mechanical interaction between guard cells and epidermal cells as the guard cells swell to create the stomatal pore. H. prolifera displays the Psilotum-type guard cell deformation (Ziegler, 1987), whereby the guard cell lower (leaf inner) wall expands into the substomatal cavity during stomatal opening. N. exaltata shows the archetypal Adiantum-type guard cell deformation, whereby the guard cell upper and lower (leaf inner and outer) walls buckle outwards as the guard cells swell into a more rounded cross section to create the stomatal pore. By contrast, both T. virginiana and T. aestivum guard cells undergo substantial lateral displacement into their adjacent subsidiary cells during stomatal opening, to the extent that the subsidiary cells are almost squashed to accommodate the open stoma. Of great significance is the fact that, because these leaves were sampled at very high humidity, we know that rates of transpirational water loss were minimal, and therefore the water potential of all cells in the image was close to zero (the plants being also well watered). This means that turgor in all cells was maximal for the prevailing osmotic conditions. It is shown below that for the stomata in T. virginiana and T. aestivum to have reached the apertures indicated, turgor in the subsidiary cells had to be significantly reduced, as full or even partial subsidiary cell turgor simply would not have allowed such wide apertures. In the “Discussion,” we propose how this might be achieved.
Figure 2.
Cross section of H. prolifera stoma sampled by snap freezing an intact leaf. A, Closed; B, open under full sunlight, very high humidity. Note absence of guard cell mechanical interaction with adjacent epidermal cells. Cryo-SEM. Scale bar = 20 μm. G, Guard cell; E, epidermal cell.
Figure 3.
Cross section of N. exaltata stoma sampled by snap freezing an intact leaf. A, Closed; B, open under full sunlight, very high humidity. Note absence of guard cell mechanical interaction with adjacent epidermal cells. Cryo-SEM. Scale bar in B = 20 μm. Both images are to the same scale. G, Guard cell; E, epidermal cell.
Figure 4.
Cross section of T. virginiana stoma sampled by snap freezing an intact leaf. A, Closed; B, open under full sunlight, very high humidity. Note in B the strong guard cell mechanical interaction with adjacent subsidiary cells. Cryo-SEM. Scale bar in A = 20 μm. Both images are to the same scale. G, Guard cell; S, subsidiary cell.
Figure 5.
Cross section of T. aestivum stoma sampled by snap freezing an intact leaf. A, Closed; B, open under full sunlight, very high humidity. Note in B the strong guard cell mechanical interaction with adjacent subsidiary cells. Cryo-SEM. Scale bar in A = 10 μm. Both images are to the same scale. G, Guard cell; S, subsidiary cell.
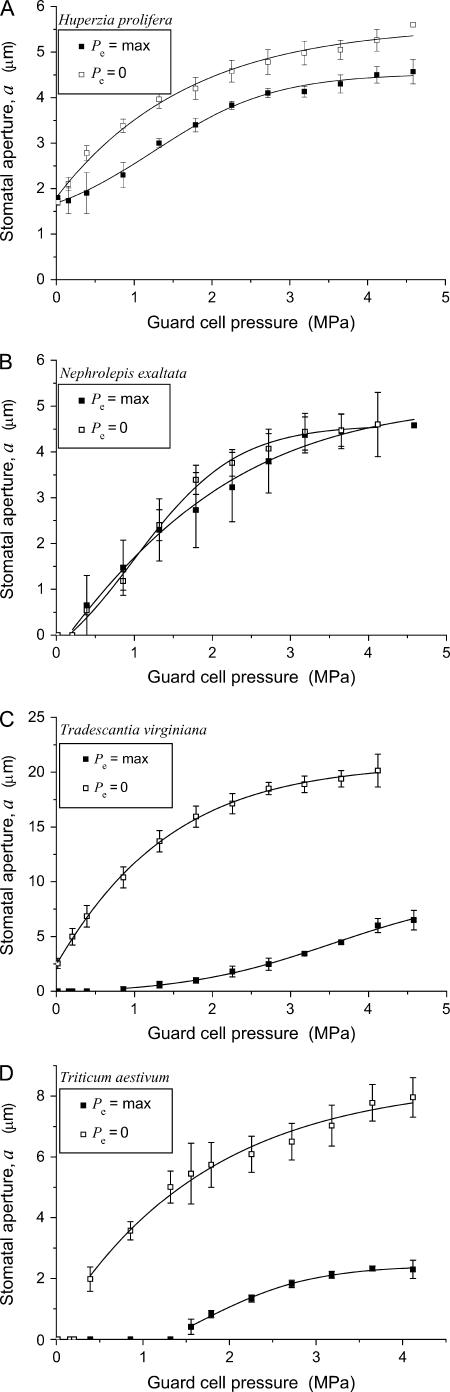
The relationship between Pg and a (as pore width in micrometers) at maximum and zero Pe is summarized for each species in Figure 6. A strong linear relationship was found between stomatal pore width and stomatal pore area in each species (data not shown), as was reported for T. virginiana by Franks and Farquhar (2001). Consistent with the evidence from the cryo-sections of open stomata (Figs. 2–5), the aperture corresponding to any given Pg was virtually unaffected by Pe in H. prolifera and N. exaltata, but substantially influenced by Pe in T. virginiana and T. aestivum. The small offset observed in H. prolifera (Fig. 6A) could be due to a small, generalized expansion of the epidermal cells at high turgor. At full Pe, T. virginiana stomata could not attain an aperture above about 7 μm, compared to its maximum of about 20 μm when Pe was zero. Similarly, T. aestivum a were on average not greater than 2.5 μm at full Pe, compared to about 8 μm at zero Pe. This massive mechanical counteraction of stomatal opening by Pe in T. virginiana and T. aestivum is a negative side effect arising from the need for greater lateral displacement of guard cells to create a larger stomatal pore. Known technically as the mechanical advantage of epidermal cells over guard cells (DeMichelle and Sharpe, 1973; Cooke et al., 1976; Wu et al., 1985; Franks et al., 1998), this epidermal impediment to stomatal opening would potentially eliminate much of the gain from a more mobile guard cell pair. As indicated in Figure 6, the maximum apertures obtained by T. virginiana and T. aestivum, as verified in Figures 2 to 5, cannot be reached under conditions of high Pe (as was the case for the leaves in Figs. 2–5) simply by maximizing Pg. Below, we propose a mechanism for overcoming the mechanical advantage and discuss some of the implications of such a mechanism in stomatal control.
Figure 6.
Relationship between a and Pg for the four species at full Pe and zero Pe (mean ± se). Note the massive impediment to stomatal opening (mechanical advantage) by Pe in T. virginiana and T. aestivum compared to little or no effect in H. prolifera and N. exaltata.
DISCUSSION
How to Meet the Need for Higher Stomatal Conductance
There are likely to have been two main selective pressures for increasing maximum operating leaf diffusive conductance (gs) in vascular plants, the first being the maintenance of a given rate of photosynthetic productivity as atmospheric CO2 concentrations declined over much of the Paleozoic era (Crowley and Berner, 2001) and the second being competition for higher rates of photosynthetic gas exchange at a given atmospheric CO2 concentration, which is facilitated by higher gs. There are several strategies through which gs can be increased, all aimed ultimately at increasing the sum of stomatal pore area/depth per unit leaf area (diffusive conductance of a given stomatal pore being roughly proportional to the ratio of pore area to depth; Brown and Escombe, 1900). The simplest and possibly the most accessible option is to increase the number of stomata per unit leaf area, i.e. stomatal density (SD). Within limitations, this seems to be a widely utilized strategy. There are numerous examples of plasticity in SD within species, with changes readily induced through exposure of developing leaves to changed atmospheric CO2 concentrations (Woodward et al., 2002; Hetherington and Woodward, 2003), or drought (Cutter et al., 1977; Quarrie and Jones, 1977), or the drought stress hormone abscisic acid (Bradford et al., 1983; Franks and Farquhar, 2001). However, in the case of increasing SD to obtain higher operating gs, there are practical limitations relating to space (maximum number of stomata of a given dimension per unit leaf area) and guard cell biochemistry (if the entire epidermis is guard cells, where will the guard cells import potassium from?).
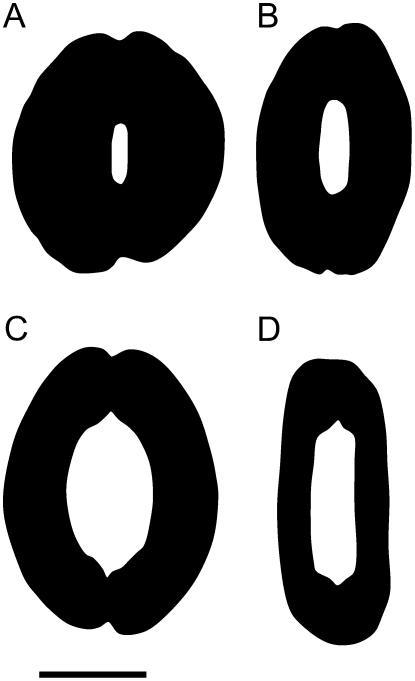
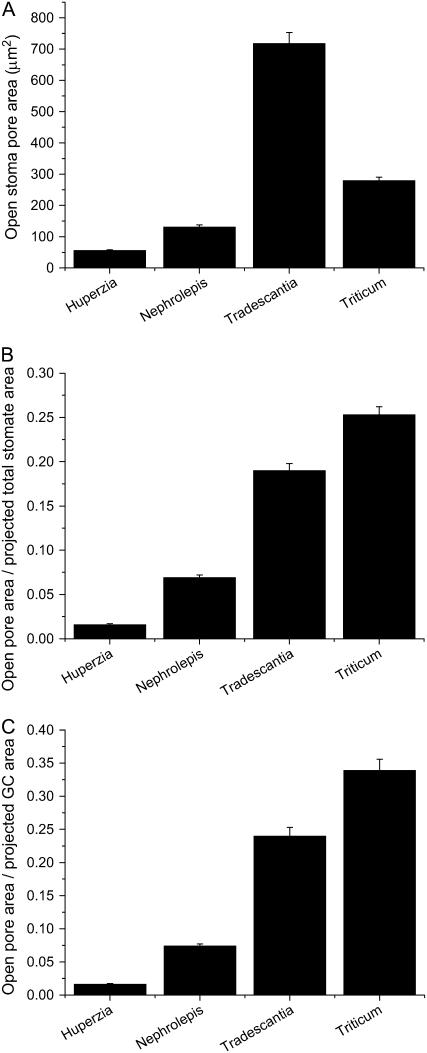
The spatial limitations on gs inherent to the four species in this study can be inferred from the illustrations in Figure 7, which show the relationship between projected maximum pore area (amax) and area of the whole stomatal complex (pore plus guard cells; asc) for each species, at maximum a. Clearly, on this basis, T. aestivum has the greater potential gs, being able to pack in greater pore area per unit leaf area. This ratio of amax to asc is quantified in Figure 8B, again showing the superiority of the T. aestivum guard cell design, with the difference in amax/asc being more than 20-fold between H. prolifera and T. aestivum. The values of amax/asc for T. virginiana and T. aestivum would decrease slightly if subsidiary cells were included as essential components of the stomatal complex, but the trend in Figure 8B would remain the same.
Figure 7.
A visual impression of the difference, across the four stomatal types, in amax per unit asc. This ratio, amax/asc, sets the upper limit for stomatal conductance per unit leaf area. A, H. prolifera; B, N. exaltata; C, T. virginiana; D, T. aestivum. Images were traced from stomata in which Pg was held at approximately 4 MPa with zero Pe. Scale bar = 20 μm.
Figure 8.
A, Maximum stomatal pore area; B, ratio of maximum stomatal pore area to projected total stomate area (pore plus guard cells); C, ratio of maximum stomatal pore area to projected guard cell area. All measurements were conducted on stomata in which Pg was held at approximately 4 MPa with zero Pe (mean ± se).
Another potential strategy for increasing gs is, rather than only multiplying the number of stomata, to multiply number of stomata and reduce stomatal size. While the genetically determined restrictions on this strategy might be greater than on only multiplying number of stomata per unit leaf area, the benefits are also greater. With a reduction in overall stomatal size comes a reduction in pore depth due to the smaller cross-sectional area of the guard cells, so with smaller stomata it is possible to achieve a greater gs per unit area occupied by stomata. Further analysis of this strategy is outside the scope of this article, but many high-gs species do exhibit high densities of very small stomata (Willmer and Fricker, 1996).
The Problem with the Mechanical Advantage
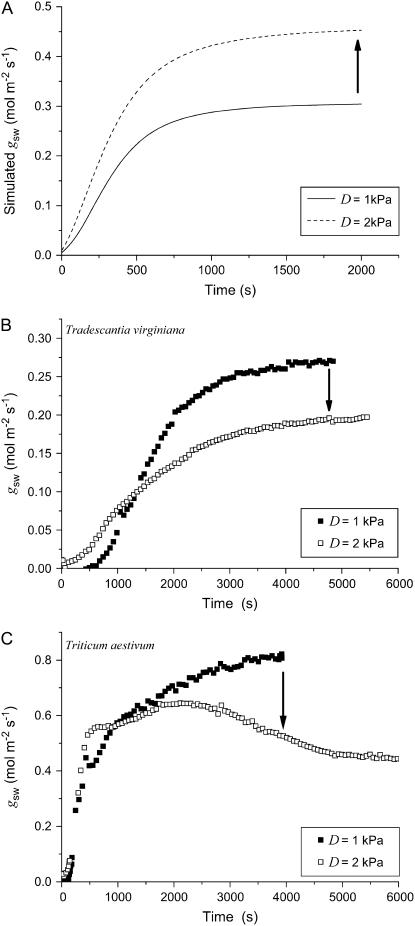
Stomata of T. virginiana and T. aestivum, while having the potential to provide the leaf with high gs, are also encumbered with the problem of the mechanical advantage of epidermal cells (see “Results”; Fig. 6). These stomatal types rely upon extensive lateral displacement of their guard cells into space occupied by adjacent subsidiary cells to achieve their substantial stomatal pore widths. The problem that this creates is 2-fold. First, there is the restriction that Pe places on stomatal opening, as outlined in the “Results” and illustrated in Figure 6. This cannot be overcome sufficiently by Pg alone. Second, there are adverse implications for the control of a under different evaporation potentials. Essentially, in the absence of an active compensating mechanism in the stomatal control system, the mechanical advantage dictates that a will open wider as evaporation potential increases, due to the increase in transpiration rate lowering Pe and facilitating the passive widening of the stomatal pores. Using the hydromechanical stomatal model described in Franks (2004), this effect is simulated for a stomatal opening sequence in Figure 9A for a plant with stomatal characteristics similar to T. virginiana and assuming no active compensation to counteract the increase in gs with increasing evaporative demand. Such a mode of operation would be highly destructive if it were to actually occur. However, it is almost universally observed that plants operate with lower (not higher) gs under higher evaporative demand (Lange et al., 1971; Schulze et al., 1972; Hall et al., 1976; Grantz, 1990; Franks and Farquhar, 1999). This is illustrated in Figure 9, B and C, for measured opening sequences on leaves of T. virginiana and T. aestivum, respectively, under two different values of leaf-to-air vapor pressure difference (D). In both cases, the final steady-state conductance to water vapor (gsw) was substantially lower at higher D. Note also that there appears to be little enhancement of the initial rate of increase in gsw at higher D compared to low D, suggesting a highly active compensating mechanism.
Figure 9.
A, Simulation of the effect of D on the rate and magnitude of stomatal opening in a plant having stomatal mechanical characteristics similar to T. virginiana (high epidermal mechanical advantage) but no mechanism to actively compensate for the effect. B, Comparison of the opening sequence of T. virginiana stomata at D = 1 kPa and D = 2 kPa, following a change in photosynthetically active radiation from 0 to 1,000 μmol m−2 s−1. Plant well watered; leaf temperature 25°C. C, As for B, with T. aestivum. Arrows indicate the direction of the difference in gsw at D = 2 kPa relative to D = 1 kPa.
Overcoming the Mechanical Advantage
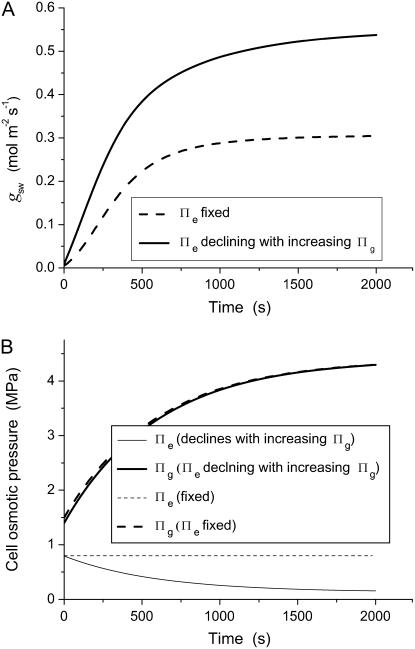
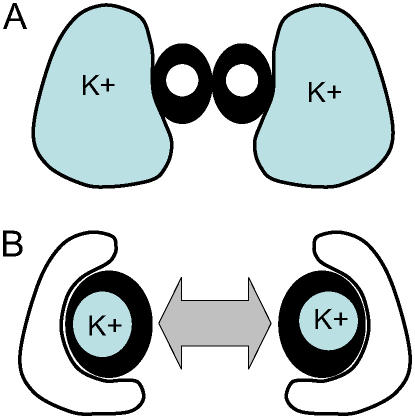
Based on the pressure probe results in Figure 6, it seems virtually impossible for T. virginiana and T. aestivum stomata to reach the apertures observed in Figures 4 and 5 under humid conditions, yet clearly they did. The simplest explanation, suggested by the degree of deformation of T. aestivum subsidiary cells (Fig. 5) is that subsidiary cell turgor is much lower during maximum a, such as occurs under high humidity. The only way that this can be achieved at high cellular water potentials is if the osmotic pressure of subsidiary cells declines substantially, thus reducing the effect of the mechanical advantage and allowing maximum lateral displacement of guard cells and maximum a. This effect is simulated in Figure 10, using the same hydromechanical feedback model as for the simulations in Figure 9A but allowing Πe (effectively the osmotic pressure of subsidiary cells) to decline in association with the increase in guard cell osmotic pressure (Πg; see “Materials and Methods”). This “see-sawing” of relative osmotic and turgor pressures between subsidiary cells and guard cells, illustrated schematically in Figure 11, overcomes the problem of the mechanical advantage and would allow T. aestivum to attain full apertures under high humidity, where it otherwise could not. Transfer of solutes (namely, K+) to guard cells via subsidiary and epidermal cells has long been recognized (Fujino, 1967; Fischer, 1968; Humble and Hsiao, 1969; Sawhney and Zelitch, 1969; Humble and Raschke, 1971; Pallaghy, 1971; Penny and Bowling, 1974; Dayanandan and Kaufman, 1975; Macrobbie and Lettau, 1980; Outlaw, 1983), but here we make the case for mandatory and opposite changes in osmotic and turgor pressure of guard and subsidiary cells where epidermal or subsidiary cells have a large mechanical advantage over guard cells. This requirement would diminish in species with diminished mechanical advantage (e.g. H. prolifera and N. exaltata in this study). A desirable side effect arising from this mechanism is that the rate of stomatal opening is increased (compare Fig. 10A solid line with dashed line), as is the rate of closure in a reversal of the same process. Thus, the mechanical advantage, which arose as an unavoidable consequence of increased guard cell lateral movement to create wider stomatal pores, may have been harnessed in the manner described above to ultimately facilitate, rather than impede, the operation of stomata. This facilitation enabled higher steady-state stomatal conductances to be achieved and increased the rate at which a can change, thereby supporting increased rates of photosynthetic gas exchange and increased transpiration efficiency. Of course we cannot be sure which of increased lateral movement and shuttling of osmotica came first, and evolution may well have occurred in parallel.
Figure 10.
A, Simulated stomatal opening sequence; and B, associated changes in guard cell (Πg) and epidermal (Πe) osmotic pressures for a plant with (solid lines) a mechanism by which epidermal osmotic pressure declines in association with increasing guard cell osmotic pressure during stomatal opening (see “Methods and Materials” for model details). All other stomatal functional characteristics, as well as environmental conditions, are constant. Dotted lines correspond to the condition in which Πe remains constant during stomatal opening. A decline in Πe during stomatal opening could allow the epidermal mechanical advantage to be overcome under conditions of low D, allowing the observed maximum a to be achieved at low D. Note also that not only does the mechanism allow gws to reach its full potential, but the rate of opening is also greatly increased.
Figure 11.
Schematic of the proposed osmotic see-saw (combined opposite changes in both osmotic and turgor pressure between guard and subsidiary cells) in T. aestivum and other grass-type stomata. The mechanism reduces the large mechanical advantage that would otherwise prevent stomatal opening under high humidity, even if maximum turgor were generated in guard cells. A corollary is that the mechanism also greatly accelerates the rate of stomatal opening, helping to explain why T. aestivum has rates of stomatal opening more than an order of magnitude faster than any of the other species (Fig. 12). A, Cross-section of guard cells (thick dark walls) and subsidiary cells (thin walls) of closed stoma; B, open stoma, showing highly displaced subsidiary cells which have transferred most of their potassium to the guard cells, undergoing a significant reduction in turgor as a consequence. Compare SEM images in Figure 5.
Potassium shuttling between guard and subsidiary cells in relation to rapid stomatal opening was first discussed by Raschke and Fellows (1971) in their study on Zea mays and later by Brogardh and Johnsson (1975). However, these authors considered only the speed of solute transfer and the potentially rapid rate of change of guard cell osmotic pressure due to the large reservoir of potassium in subsidiary cells immediately adjacent to guard cells of grasses. The requirement for, and advantages of, a substantial reduction in both the osmotic and turgor pressure of subsidiary cells during opening of graminoid stomata was not considered in detail at that time. Later work, which focused on rapid responses in grass stomata exposed to a blue light stimulus (Johnsson et al., 1976; Karlsson and Assmann, 1990; Grantz and Assmann, 1991; Assmann et al., 1992), also did not consider, from a quantitative perspective, the role of the mechanical advantage of subsidiary cells. Our measurements of the Pg-Pe-a relationship in the graminoid stomatal complex of T. aestivum and the integration of this information into a mechanistic model of stomatal movement has revealed how the combination of osmotic shuttling, high epidermal mechanical advantage, and unique guard cell geometry in graminoid stomata allows them to open or close at a substantially faster rate than perhaps any other stomatal type.
Significance of Graminoid Stomatal Mechanics in the Rise of Grasses
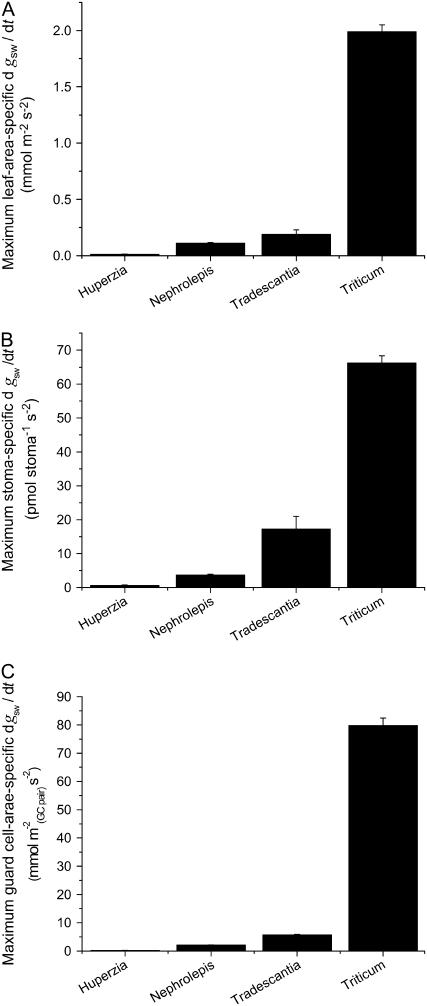
Several authors have postulated that the spread and diversification of grasses, which likely began in tropical forest understoreys between 55 to 70 Ma ago (Kellogg, 2001) but peaked during a period of global aridification approximately 30 to 45 Ma ago, could have been assisted by them having faster and therefore more transpiration-efficient stomata (Hetherington and Woodward, 2003, and refs. therein). However, the significance of the unique mechanical properties of graminoid-type stomata in relation to this superior performance has not until now been quantified. To illustrate just how superior their performance can be, we quantified the maximum rate of stomatal opening in response to light, under standardized environmental conditions, in each of the four species in this study (Fig. 12). Regardless of how the rate of opening was defined, the graminoid stomata of T. aestivum were about an order of magnitude faster than any of the other species.
Figure 12.
Maximum rate of increase in stomatal conductance to water vapor (dgsw/dt), quantified in relation to (A) leaf area, (B) individual stomata, or (C) projected area of the inflated guard cell pair, as a proxy for guard cell size. The grass T. aestivum was substantially faster in all categories. (Mean ± se. See “Methods and Materials” for an explanation of the units.)
CONCLUSION
The results demonstrate that the morphological diversity of stomata translates into considerable mechanical and, ultimately, functional diversity. This study examined only the stomata of four species, and, although it spanned a considerably broad morphological and evolutionary spectrum, there are many more stomatal forms yet to be examined in this way and the picture is far from complete. By the same measure, we do not wish to generalize too extensively about the function of grass stomata from our examination of just one species, but the results do provide a compelling case for the role of stomatal mechanics in the distinctive capabilities of this special group of plants. The findings highlight the importance of integrating mechanical and quantitative physical information about guard and adjacent cells in models of stomatal function to better describe and predict gas-exchange regulation in diverse vegetation types.
MATERIALS AND METHODS
Plant Material
Huperzia prolifera (Blume) Trevis, Tradescantia virginiana, and Nephrolepis exaltata L. Schott were propagated vegetatively (H. prolifera by layering; T. virginiana and N. exaltata by division of parent plants). Triticum aestivum was grown from seed, with all measurements performed on the third, fully expanded leaf. Plants were grown in 1-L pots in a glasshouse (30°C/25°C day/night temperature, full sun, high humidity, well watered) and fertilized with a slow-release fertilizer (Osmocote; Grace-Sierra Pty).
Cryo-SEM
Leaves from plants kept either in darkness overnight (closed stomata) or exposed to full sun for several hours in an enclosed, water-saturated greenhouse environment (open stomata) were snap frozen in liquid nitrogen. Frozen leaf fragments were then mounted on a stub in low temperature Tissue Tek and planed in transverse section in a cryo-microtome according to Huang et al. (1994). The planed specimens were then transferred to the cold stage of a scanning electron microscope (SEM; JEOL 6400), etched for several minutes at −90°C, cooled to −170°C, and coated with gold for observation at 15 kV.
Pressure Probe
Measurements of a at controlled Pg at maximum and zero Pe were carried out on epidermal preparations using the equipment and procedures described in Franks et al. (1998) and Franks and Farquhar (2001). Peels were maintained in 25 mm MES, pH 6.5 (adjusted with NaOH), 1 mm KCl, 0.1 mm CaCl2, either without (full Pe conditions) or with (zero Pe conditions) 450 mm mannitol. Measurements were obtained for n = 3 to 9 stomata per treatment in each species.
Gas Exchange
Well-watered plants were kept in darkness overnight in the lab, and then leaves were clamped into the chamber of an open-flow leaf gas-exchange analyzer (LI-6400; LI-COR). Chamber conditions were controlled at the following levels, initially keeping the leaf in darkness: ambient CO2 concentration 350 μmol mol−1, leaf temperature 30°C; D either 1 kPa or 2 kPa. When conditions stabilized, photosynthetically active radiation was increased instantly to 1,000 μmol m−2 s−1, and leaf gas-exchange parameters were logged at 60-s intervals for 90 min. Maximum rates of stomatal opening for D = 1 kPa were measured on n = 3 leaves from three different plants per species by measuring the slope of a line fitted through the steepest 6-min interval on the gws versus time plot. Rates in Figure 12 were calculated on the basis of three different reference points: (A) moles per unit leaf area per second per second; (B) moles per stoma per second per second, to remove any bias due to SD; and (C) moles per guard cell pair projected area per second per second, to correct for different guard cell sizes.
Stomatal Simulation Model
All simulations used the simple steady-state hydro-mechanical feedback model presented in detail in Franks (2004). Briefly, steady-state maximum guard cell osmotic pressure (Πg(max)) is preset to a typical value, together with epidermal osmotic pressure (Πe), hydraulic conductances from soil to epidermal cells (ks-l) and from soil to guard cells (ks-g), and guard cell Pg versus a characteristics calibrated according to results in Franks et al. (1998). Actual Πg is a function of Πg(max) and subsidiary cell turgor. For a given D, the solution for the simultaneous equations in the feedback loop comprising transpiration rate (E), guard cell water potential (Ψg), Πg, Pg, epidermal water potential (Ψe), Pe, a, and finally gsw is obtained by iteration. Stomatal aperture is scaled to gsw using a multiplier to account for SD and pore depth. The only structural modifications made here were: (1) for the simulation in Figure 9A, Πg was held constant to demonstrate the effect of the mechanical advantage in the absence of osmotic compensation; (2) for the simulation in Figure 11, Πe was either fixed or made a function of Πg, such that Πe = a(b − Πg)/b, i.e. as Πg approaches b, Πe approaches zero; and (3) to generate time series for stomatal opening, the model was solved in discrete time steps for Πg increasing from zero according to
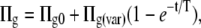 |
(1) |
where Πg0 is guard cell osmotic pressure at zero aperture (here 1.4 MPa), Πg(var) is the difference between Πg0 and Πg(max) (here 3.0 MPa), t is time from zero, and T is the time constant (here 600 s).
Acknowledgments
We thank S.C. Wong and C. Huang for excellent technical assistance, A. Hardham for help with microscope facilities, M.J. Canny for useful advice on cryo-SEM, and B.E.S. Gunning for generous help and advice in many aspects of the pressure probe work.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Peter J. Franks (peter.franks@jcu.edu.au).
Open Access articles can be viewed online without a subscription.
References
- Allaway WG, Milthorpe FL (1976) Structure and functioning of stomata. In TT Kozlowski, ed, Water Deficits and Plant Growth, Vol 4. Soil Water Measurement, Plant Responses, and Breeding for Drought Resistance. Academic Press, New York, pp 57–102
- Assmann SM, Lee DM, Malkus P (1992) Rapid stomatal response to red-light in Zea mays. Photochem Photobiol 56 685–689 [Google Scholar]
- Bradford KJ, Sharkey TD, Farquhar GD (1983) Gas exchange, stomatal behaviour, and d13C values of the flacca tomato mutant in relation to abscisic acid. Plant Physiol 72 245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogardh T, Johnsson A (1975) Regulation of transpiration in Avena: responses to white light steps. Physiol Plant 35 115–125 [Google Scholar]
- Brown HT, Escombe F (1900) Static diffusion of gases and liquids in relation to the assimilation of carbon and translocation in plants. Philos Trans R Soc Lond B 193 223–291 [Google Scholar]
- Cooke JR, DeBaerdemaeker JG, Rand RH, Mang HA (1976) A finite element shell analysis of guard cell deformation. Trans ASAE (Am Soc Agric Eng) 19 1107–1121 [Google Scholar]
- Crowley TJ, Berner RA (2001) Paleoclimate: CO2 and climate change. Science 292 870–872 [DOI] [PubMed] [Google Scholar]
- Cutter JM, Rains DW, Loomis RS (1977) The importance of cell size in the water relations of plants. Physiol Plant 40 255–260 [Google Scholar]
- Dayanandan P, Kaufman PB (1975) Stomatal movements associated with potassium fluxes. Am J Bot 62 221–231 [Google Scholar]
- DeMichelle DW, Sharpe PJH (1973) An analysis of the mechanics of guard cell motion. J Theor Biol 41 77–96 [DOI] [PubMed] [Google Scholar]
- Fischer RA (1968) Stomatal opening in isolated epidermal strips of Vicia faba. I. Response to light and to CO2-free air. Plant Physiol 43 1947–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ (2004) Stomatal control and hydraulic conductance, with special reference to tall trees. Tree Physiol 24 865–878 [DOI] [PubMed] [Google Scholar]
- Franks PJ (2006) Higher rates of leaf gas exchange are associated with higher leaf hydrodynamic pressure gradients. Plant Cell Environ 29 584–592 [DOI] [PubMed] [Google Scholar]
- Franks PJ, Buckley TN, Shope JC, Mott KA (2001) Guard cell volume and pressure measured concurrently by confocal microscopy and the cell pressure probe. Plant Physiol 125 1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks PJ, Cowan IR, Farquhar GD (1998) A study of stomatal mechanics using the cell pressure probe. Plant Cell Environ 21 94–100 [Google Scholar]
- Franks PJ, Farquhar GD (1999) A relationship between humidity response, growth form and photosynthetic operating point in C-3 plants. Plant Cell Environ 22 1337–1349 [Google Scholar]
- Franks PJ, Farquhar GD (2001) The effect of exogenous abscisic acid on stomatal development, stomatal mechanics, and leaf gas exchange in Tradescantia virginiana. Plant Physiol 125 935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino M (1967) Role of adenosinetriphosphate and adenosinetriphosphatase in stomatal movement. Sci Bull Fac Ed Nagasaki Univ 18 1–47 [Google Scholar]
- Grantz DA (1990) Plant response to atmospheric humidity. Plant Cell Environ 13 667–679 [Google Scholar]
- Grantz DA, Assmann SM (1991) Stomatal response to blue-light: water-use efficiency in sugarcane and soybean. Plant Cell Environ 14 683–690 [Google Scholar]
- Haberlandt G (1884) Physiological Plant Anatomy. Jayyed Press, Delhi
- Hall AE, Schulze E-D, Lange OL (1976) Current perspectives of steady state stomatal response to environment. In OL Lange, L Kappen, E-D Schulze, eds, Water and Plant Life. Ecological Studies 19. Springer, Berlin, pp 169–185
- Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424 901–908 [DOI] [PubMed] [Google Scholar]
- Huang CX, Canny MJ, Oates K, McCully ME (1994) Planing frozen hydrated plant specimens for SEM observation and EDX microanalysis. Microsc Res Tech 28 67–74 [DOI] [PubMed] [Google Scholar]
- Humble GD, Hsiao TC (1969) Specific requirement of potassium for light-activated opening of stomata in epidermal strips. Plant Physiol 44 230–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humble GD, Raschke K (1971) Stomatal opening quantitatively related to potassium transport. Evidence from electron probe analysis. Plant Physiol 48 447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsson M, Issaias S, Brogardh T, Johnsson A (1976) Rapid, blue-light-induced transpiration response restricted to plants with grass-like stomata. Physiol Plant 36 229–232 [Google Scholar]
- Karlsson PE, Assmann SM (1990) Rapid and specific modulation of stomatal conductance by blue-light in ivy (Hedera helix): an approach to assess the stomatal limitation of carbon assimilation. Plant Physiol 94 440–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg EA (2001) Evolutionary history of the grasses. Plant Physiol 125 1198–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange OL, Lösch R, Schulze E-D, Kappen L (1971) Responses of stomata to changes in humidity. Planta 100 76–86 [DOI] [PubMed] [Google Scholar]
- Macrobbie EAC, Lettau J (1980) Potassium content and aperture in intact stomatal and epidermal cells of Commelina communis L. J Membr Biol 56 249–256 [Google Scholar]
- Meidner H, Bannister P (1979) Pressure and solute potentials in stomatal cells of Tradescantia virginiana. J Exp Bot 30 255–265 [Google Scholar]
- Meidner H, Mansfield TA (1968) Physiology of Stomata. McGraw Hill, London
- Outlaw WH (1983) Current concepts on the role of potassium in stomatal movement. Physiol Plant 59 302–311 [Google Scholar]
- Pallaghy CK (1971) Stomatal movement and potassium transport in epidermal strips of Zea mays: the effect of CO2. Planta 101 287–295 [DOI] [PubMed] [Google Scholar]
- Penny MG, Bowling DJF (1974) A study of potassium gradients in the epidermis of intact leaves of Commelina communis L. in relation to stomatal opening. Planta 119 17–25 [DOI] [PubMed] [Google Scholar]
- Quarrie SA, Jones HG (1977) Effect of abscisic acid and water stress on development and water stress of wheat. J Exp Bot 28 192–203 [Google Scholar]
- Raschke K, Fellows M (1971) Stomatal movement in Zea mays: shuttle of potassium and chloride between guard cells and subsidiary cells. Planta 101 296–316 [DOI] [PubMed] [Google Scholar]
- Sawhney BL, Zelitch I (1969) Direct determination of potassium ion accumulation in guard cells in relation to stomatal opening in light. Plant Physiol 44 1350–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E-D, Lange OL, Buschbom U, Kappen L, Evanari M (1972) Stomatal responses to changes in humidity in plants growing in the desert. Planta 108 259–270 [DOI] [PubMed] [Google Scholar]
- Stubblefield S, Banks HP (1978) The cuticle of Drepanophycus spinaeformis, a long-ranging Devonian Lycopod from New York and Eastern Canada. Am J Bot 65 110–118 [Google Scholar]
- Sun T-X, Edwards D, Li C-S (2005) The stomatal apparatus of Lycopodium japonicum and its bearing on the stomata of the Devonian lycophyte Drepanophycus spinaeformis. Bot J Linn Soc 149 209–216 [Google Scholar]
- Willmer CM, Fricker M (1996) Stomata, Ed 2. Chapman and Hall, London
- Woodward FI, Lake JA, Quick WP (2002) Stomatal development and CO2: ecological consequences. New Phytol 153 477–484 [DOI] [PubMed] [Google Scholar]
- Wu H, Sharpe PJH, Spence RD (1985) Stomatal mechanics. III. Geometric interpretation of the mechanical advantage. Plant Cell Environ 8 269–274 [Google Scholar]
- Ziegler H (1987) The evolution of stomata. In E Zeiger, GD Farquhar, IR Cowan, eds, Stomatal Function. Stanford University Press, Stanford, CA, pp 29–57