Abstract
The bean (Phaseolus vulgaris) stress-related gene number 2 (PvSR2) gene responds to heavy metals but not to other forms of environmental stresses. To elucidate its heavy metal-regulatory mechanism at the transcriptional level, we isolated and characterized the promoter region (−1623/+48) of PvSR2. Deletions from the 5′ end revealed that a sequence between −222 and −147 relative to the transcriptional start site was sufficient for heavy metal-specific induction of the promoter region of PvSR2. Detailed analysis of this 76-bp fragment indicated that heavy metal-responsive elements were localized in two regions (−222/−188 and −187/−147), each of which could separately confer heavy metal-responsive expression on the β-glucuronidase gene in the context of a minimal cauliflower mosaic virus 35S promoter. Region I (−222/−188) contains a motif (metal-regulatory element-like sequence) similar to the consensus metal-regulatory element of the animal metallothionein gene, and mutation of this motif eliminated the heavy metal-inducible function of region I. Region II (−187/−147) had no similarity to previously identified cis-acting elements involved in heavy metal induction, suggesting the presence of a novel heavy metal-responsive element. Transformed tobacco (Nicotiana tabacum) seedlings expressing β-glucuronidase under control of the PvSR2 promoter region (−687/+48) showed heavy metal-specific responsive activity that depended on the type and concentration of the heavy metal and the type of organ. These findings further our understanding of the regulation of PvSR2 expression and provide a new heavy-metal-inducible promoter system in transgenic plants.
In plants, a number of heavy metals are essential for normal growth as cofactors and as structural and catalytic components of proteins and enzymes. These micronutrients, as well as nonessential heavy metals such as cadmium, are toxic at high levels. Plants have evolved a suite of mechanisms that control and respond to the uptake and accumulation of both essential and nonessential heavy metals. These mechanisms include the chelation and sequestration of heavy metals by particular ligands including small peptides, organic acids, and amino acids, which bind free metal ions. They contribute to metal detoxification by buffering cytosolic metal ions (Clemens, 2001). The two best-characterized heavy metal-binding ligands in plant cells are the phytochelatins (PCs) and metallothioneins (MTs; for review, see Cobbett and Goldsborough, 2002). MTs are Cys-rich polypeptides encoded by a family of genes. In contrast, PCs are a family of enzymatically synthesized Cys-rich peptides. The induction of PC synthesis is based on the posttranscriptional activation of preformed PC synthase and is not under transcriptional control.
Several heavy metal-inducible genes have been reported in plants (Hagen et al., 1988; Lescure et al., 1991; Berna and Bernier, 1999), but surprisingly, little is known about the transcriptional regulation of gene expression in response to heavy metals. In particular, the cis-acting elements conferring heavy metal responsiveness are poorly characterized, and heavy metal-specific responsive promoters have not yet been reported in higher plants. One cis-acting element has been identified in the promoters of tobacco (Nicotiana tabacum) parA and soybean (Glycine max) GH2/4, two genes regulated by auxin and cadmium (Ellis et al., 1993; Kusaba et al., 1996). This element is related to the as-1 element previously identified in the cauliflower mosaic virus (CaMV) 35S promoter (Katagiri et al., 1989). In the green algae Chlamydomonas reinhardtii, the copper response element (CuRE) with consensus (5′-GTAC-3′) was identified in the promoter of CPX1 and CYC6. A copper response regulator, CRR1, binds to these sites and mediates target gene expression under copper-deficient conditions (Quinn and Merchant, 1995; Quinn et al., 2000). Recently, Quinn et al. (2003) showed that both CuRE and CRR1 are required for a response to nickel, which suggests that nickel interferes with a component in the nutritional copper signal transduction pathway.
In yeast (Saccharomyces cerevisiae) and animals, heavy metal-responsive elements (HMREs) and their corresponding metal-induced transcription factors have been characterized in detail. In animals, MT genes are regulated transcriptionally by metal-regulatory elements (MREs), which are present in multiple copies (Stuart et al., 1985). MREs consist of a highly conserved heptanucleotide core (5′-TGCRCNC-3′) and less conserved flanking nucleotides (Searle et al., 1987). A metal response element-binding transcription factor-1 (MTF-1) binds to MREs and regulates MT gene transcription. MTF-1 is a zinc-dependent, zinc-responsive transcription factor (Westin and Schaffner, 1988; Heuchel et al., 1994). In yeast, for example, it has been demonstrated that the copper MT gene is induced through a copper-responsive element, and this process is mediated by the copper-binding transcription factor ACEl (Fürst et al., 1988). ACE1 binds to a MRE upstream promoter of the target gene. The consensus sequence of this MRE is 5′-HTHNNGCTGD-3′ (D = A, G, or T; H = A, C, or T; N = any residue; Dixon et al., 1996).
We have previously isolated a novel heavy metal-specific responsive gene, Phaseolus vulgaris stress-related gene number 2 (PvSR2), from bean (Phaseolus vulgaris), which is different from previously identified plant MT-like genes (Zhang et al., 2001). The PvSR2 protein can enhance cadmium tolerance of Escherichia coli DH5α cells (Zhang et al., 2001) and transgenic tobacco plants (Chai et al., 2003). This prompted us to investigate the heavy metal-regulatory mechanism of PvSR2. In this study, we have characterized its promoter region both in a transient expression system and in stably transformed tobacco plants. We also have identified two promoter regions (−222/−188, −187/−147; relative to the transcriptional start site [TSS]) containing HMREs.
RESULTS
The PvSR2 Promoter Contains Regulatory Elements in Response to Environmental Signals
We have previously reported a 5′, 3′ truncated cDNA (GenBank accession no. U54704) of the PvSR2 gene (Zhang et al., 2001). In this study, we cloned the upstream genomic DNA region of this truncated PvSR2 cDNA and its 5′-end cDNA sequence. The 119-bp further upstream mRNA region of the truncated PvSR2 cDNA was obtained by 5′-RACE analysis (data not shown). The putative TSS mapped to the A residue located at position −47 with respect to the start codon (underlined in Fig. 1). About 1.7-kb upstream genomic DNA region of the PvSR2 cDNA corresponding to the ATG start codon (underlined in Fig. 1) was cloned from bean genomic DNA (data not shown). Thus, this identified 1.7-kb upstream genomic DNA region contains a 1,624-bp putative PvSR2 promoter sequence (GenBank accession no. DQ109992). The PvSR2 promoter region (−1,623/+50) is shown in Figure 1.
Figure 1.
The sequence of the putative promoter region (−1,623/+50) of PvSR2. The transcription start site is denoted +1, and the putative start codon is underlined. All potential transcription factor binding sites are boxed. HSE, Heat shock element; ABRE, abscisic acid-response element.
Analysis of the PvSR2 promoter using TRANSFAC (http://www.transfac.gbf.de/cgi-bin/) showed that a number of potential cis-acting elements to respond to environmental signals were present. An MRE-like sequence (5′-TGCAGGC-3′), similar to the conserved core MRE sequence involved in heavy metal-induced expression of MT genes in animals (Stuart et al., 1985), was located at position −212/−206. Two putative GATA boxes (−859/−856 and −807/−804) and one I box (−1,480/−1,475) thought to be involved in light response were also found (Giuliano et al., 1988; Lam and Chua, 1989). Nine putative basic motifs (5′-NGAAN-3′) of heat shock elements were present in −1,501/−1,496, −1,373/−1,368, −1,219/−1,214, −1,072/−1,067, −1,019/−1,014, −993/−988, −463/−458, −259/−254, and −191/−186. However, a functional heat shock element contains at least three basic repeats that must be arranged in alternating orientations (Amin et al., 1988; Xiao and Lis, 1988).Thus, none of them appears to be functional. Lastly, an abscisic acid-response element-like motif (5′-ACGTG-3′) required for gene expression during dehydration stress (Simpson et al., 2003) was located at −134/−130. These putative regulatory elements suggest that the promoter region of PvSR2 (P-PvSR) may respond to a variety of environmental signals, including heavy metal stress, dehydration, and light. The sequence also contains a putative TATA box (TATAAATT) at −24/−31 relative to the TSS and a putative CAAT box at −226/−223.
Deletion Analysis Identifies a 76-bp Promoter Region (−222/−147) Required for Heavy Metal Induction
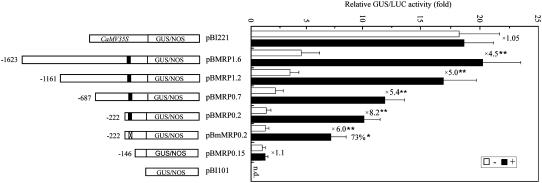
To test the heavy metal-inducible activity of the PvSR2 promoter and localize the heavy metal-regulatory region, the 1,671-bp promoter fragment (−1,623/+48) was transcriptionally fused to the promoterless reporter gene β-glucuronidase (GUS) and a series of 5′ deletions was prepared (Fig. 2). Each construct was introduced into tobacco protoplasts and tested for GUS activity in the presence (black bars) and absence (white bars) of 15 μm HgCl2. A CaMV 35S:luciferase (Luc) construct (pBI221-Luc) was used as an internal control by cotransformation to normalize the transformation efficiency in each experiment. The CaMV 35S promoter (pBI221) showed no significant induction by HgCl2 stress (1.05-fold). The negative control (pBI101), which contains a promoterless GUS gene, had undetectable levels of GUS activity in this assay. The highest levels of GUS activity were detected using the pBMRP1.6 construct, and GUS activity was significantly induced by approximately 4.5-fold in the presence of Hg2+ (one-sided paired t test; P < 0.01). The constructs containing deletions to −222 (pBMRP0.2) showed a decrease in total GUS activity both with and without heavy metal induction, although heavy metals were still able to induce between 5- and 8-fold greater levels of GUS (one-sided paired t test; P < 0.01). However, a deletion up to position −146 (pBMRP0.15) resulted in a significant loss of heavy metal-induced GUS expression (1.1-fold induction by Hg2+). These data indicated that the region between positions −222 and −147 was able to confer heavy metal-specific induction of the reporter gene and suggested that the HMREs were present in this 76-bp region.
Figure 2.
Deletion analysis of P-PvSR. Schematic diagrams of the constructs used for transient expression assays are shown at left. The black areas indicate the MRE-like sequence (−212/−206), while the crosshatched area indicates a mutated MRE-like sequence. The numbers on the far left indicate the 5′ end points of the promoter fragments relative to the transcription start site. In each experiment, protoplasts were transformed with each of the plasmids shown and a CaMV 35S:Luc construct, pBI221-Luc, was incubated for 24 h in heavy metal-free MS medium either with (+) or without (−) 15 μm HgCl2. The plasmid containing a promoter-less GUS (pBI101) was used as a negative control, while the CaMV 35S:GUS-containing plasmid (pBI221) was used as a positive control. Activity, given as relative GUS/LUC units (fold), is averaged from three independent experiments. sd is indicated. The ratio of the promoter activity with HgCl2 stress compared to the promoter activity without HgCl2 stress (induction ratio) is shown at the far right. Significant difference between −Hg and +Hg conditions was analyzed using one-sided paired t test (P < 0.01 [**]). Numbers above the bar of pBmMRP0.2 sample indicate the percentage of heavy metal-inducible GUS activity relative to that of pBMRP0.2 sample, and its significant difference test was assessed by one-sided paired t test (P < 0.05 [*]). No statistical differences of GUS activity existed between these two samples in absence of heavy metal (P > 0.05; Student's t test). n.d., Not detectable.
To test whether the MRE-like sequence present in this 76-bp promoter region was responsible for heavy metal induction, we also tested the chimeric construct pBmMRP0.2, in which the MRE-like sequence was mutated with G to T or A to C. Mutation of the MRE-like sequence significantly decreased heavy metal-inducible GUS expression level by approximately 27% compared to the wild-type version (based on one-sided paired t test; P < 0.05), while GUS activity in the absence of heavy metals was reduced by only approximately 2%, showing no statistical difference between the MRE-like sequence and its mutated version (P > 0.05; Student's t test). The result indicated that the MRE-like sequence could contribute to efficient heavy metal induction, other important elements must also be present in the −222/−147 region of the promoter.
Two HMREs Lie in the −222/−147 Promoter Region
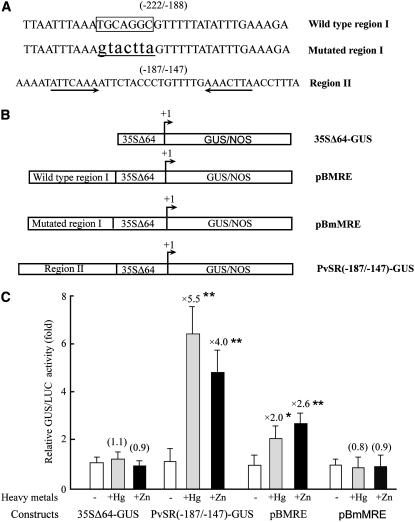
To test this, the 76-bp promoter fragment was subdivided into two pieces, one from −222/−188 (termed region I) and the other from −187/−147 (termed region II). A third construct, in which the MRE-like sequence in region I was mutated as before, was also prepared (Fig. 3A). All three sequences were fused to a CaMV 35S minimal promoter (35SΔ64, −64/+8 from the TSS) upstream of the GUS gene to generate pBMRE, pBmMRE, and PvSR[−187/−147]-GUS (Fig. 3B). The 35SΔ64-GUS was used as a control. These constructs were all tested for induction of GUS activity in heavy metal-supplemented tobacco protoplasts cotransformed by pBI221-Luc (Fig. 3C). The 35SΔ64 showed no significant induction by heavy metals (1.1-fold Hg2+ induction; 0.9-fold Zn2+ induction). Furthermore, in heavy metal-deficient medium (white bars), there was no significant difference in the level of GUS activity in any of the transformants tested. The two promoter regions do not function to repress GUS expression in heavy metal-deficient protoplasts. The MRE-like sequence was able to provide a modest induction in the presence of heavy metals (2.0-fold Hg2+ induction, P < 0.05; 2.6-fold Zn2+ induction, P < 0.01), thus confirming the suggestion from the data in Figure 2 that the MRE-like sequence could contribute to heavy metal induction. No significant induction of GUS activity was observed when protoplasts transformed with pBmMRE were treated with heavy metals (0.8-fold with 20 μm HgCl2; 0.9-fold with 50 μm ZnSO4). Thus, we conclude that the MRE-like sequence in region I is required for heavy metal-inducible activity in this region. More importantly, heavy metals were observed to provide a strong induction of GUS activity from the region II-containing promoter (4- to 5.5-fold induction by 50 μm ZnSO4 or 20 μm HgCl2, P < 0.01, respectively). These data demonstrate that a novel and relatively strong HMRE is found within region II.
Figure 3.
Identification of two promoter regions responsible for heavy metals. A, The sequence of the two promoter regions. Region I (−222/−188) contains the MRE-like sequence (boxed). The mutated MRE-like sequence is underlined and shown in lowercase. An inverted repeat sequence ATTCAAA is indicated by arrows in the region II. B, Schematic diagrams of the test constructs. 35SΔ64-GUS contains a minimal CaMV 35S promoter (35SΔ64; sequence −64/+8 from the TSS) fused to GUS gene. The wild-type region I, mutated region I, and region II were in the context of 35SΔ64 fused to GUS gene to construct pBMRE, pBmMRE, and PvSR[−187/−147]- GUS, respectively. The transcription start site is designed as +1. C, GUS activity of protoplasts transformed with the various constructs and an internal control construct pBI221-Luc in heavy metal-free MS medium (−) or the same medium also containing 20 μm HgCl2 (+Hg) or 50 μm ZnSO4 (+Zn). The 35SΔ64-GUS was used as a control. The ratio of the promoter activity with heavy metals to the promoter activity without heavy metals is indicated above the bar for each construct (induction ratio). Activity is given as relative GUS/LUC units (fold) and represents an average from three independent experiments. sd is indicated. Significant difference between without heavy metal and heavy metal stress conditions was analyzed using one-sided paired t test (P < 0.05 [*]; P < 0.01 [**]).
The PvSR2 Promoter (−687/+48) Drives Heavy Metal-Inducible GUS Expression in Transgenic Tobacco Seedlings
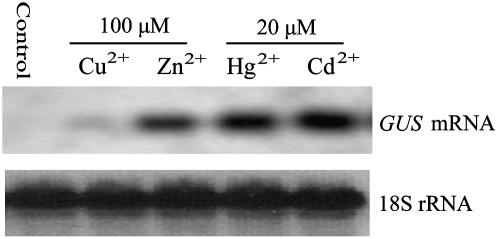
To assess further heavy metal-inducible promoter activity in stably transformed plants, the PPvSR2 (−687/+48):GUS chimeric construct (pMRP0.7-GUS) was introduced into tobacco. T-DNA inheritance was scored by kanamycin segregation analysis in the T1 generation. The T1 seedlings of six T0 lines (T0-3, T0-5, T0-6, T0-8, T0-12, and T0-17) segregated as a single, dominant, Mendelian character, and the data is presented in Table I. Among six lines, line T0-8 was randomly selected and its kanamycin-resistant T1 seedlings were employed to analyze GUS transcript accumulation following heavy metal stress by northern-blot analysis. The data showed significant levels of GUS mRNA in Cu2+-, Zn2+-, Cd2+-, and Hg2+-treated seedlings compared to the control seedlings grown in the absence of heavy metals (Fig. 4). This pattern of induction is consistent with previous northern-blot assay of the PvSR2 gene (Zhang et al., 2001) and indicates that the PvSR2 promoter fragment (−687/+48, designated here as P-PvSR[−687/+48]) transcriptional activity is specifically induced by heavy metal in transgenic tobacco seedlings.
Table I.
Genetic analysis of transgenic tobacco
Sterile T1 seeds were plated on MS medium supplemented with 200 μg/mL kanamycin. Two weeks after sowing, the seedlings were scored for their resistance or sensitivity to kanamycin. R, Resistant; S, sensitive seedlings. χ2 values were calculated with 1 degree of freedom.
| Lines | R | S | Ratio | χ2 | P |
|---|---|---|---|---|---|
| T0-3 | 86 | 27 | 3:1 | 0.012 | 0.90 |
| T0-5 | 63 | 19 | 3:1 | 0.017 | 0.90 |
| T0-6 | 82 | 26 | 3:1 | 0.012 | 0.90 |
| T0-8 | 75 | 26 | 3:1 | 0.013 | 0.90 |
| T0-12 | 58 | 18 | 3:1 | 0.017 | 0.90 |
| T0-17 | 65 | 21 | 3:1 | 0.013 | 0.90 |
Figure 4.
Northern-blot analysis of one tobacco line transformed with the PvSR2 promoter (−687/+48). Total RNA was isolated from the seedling leaves of line T0-8 grown without heavy metals (control) or with either 20 μm CdCl2, 20 μm HgCl2, 100 μm CuSO4, or 100 μm ZnSO4. RNA (15 μg) was electrophoresed on a 1.2% (w/v) formaldehyde agarose gel, transferred onto nylon membranes, and probed with 32P-dCTP-labeled GUS gene fragment. 18S rRNA was used as a loading control.
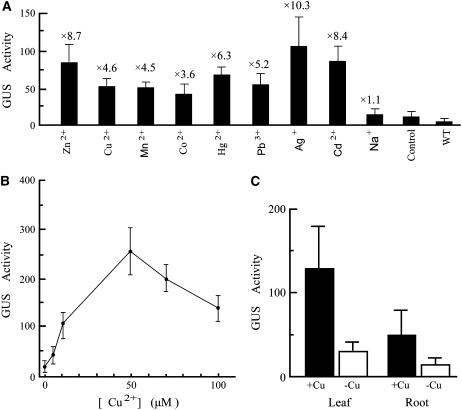
The transformed seedlings of the above six lines were selected to further analyze the effects of type and concentration of the heavy metal or the type of organ on the promoter activity. The P-PvSR[−687/+48] responded to various heavy metal ions by a 4- to 10-fold induction of GUS activity (Fig. 5A). After treatment with 20 μm toxic nonessential heavy metal ions for 24 h, the highest inducible GUS activity was observed in the seedlings treated with Ag+ (10.3-fold induction), followed by Cd2+ (8.4-fold induction), Hg2+ (6.3-fold induction), and Pb3+ (5.2-fold induction). After treatment with 100 μm essential heavy metals for 24 h, Zn2+ (8.7-fold induction) was found to be the most potent inducer of GUS activity, followed by Cu2+ (4.6-fold induction), Mn2+ (4.5-fold induction), and Co2+ (3.6-fold induction). The seedlings treated with 1% (w/v) NaCl showed no significant induction (1.1-fold) compared with the control seedlings (without heavy metal stress).
Figure 5.
The PvSR2 promoter (−687/+48) activity in transgenic tobacco seedlings. For each treatment, GUS activities were measured in the leaves of 10 10-d-old seedlings from each of six lines (see Table I) at 24 h posttreatment. GUS activity is expressed as nanomoles p-nitrophenol per minute per milligram protein. The bars denote the sd of the average of GUS activity for six lines (n = 6). A, The PvSR2 promoter responded to various heavy metals. The seedlings were treated without heavy metal stress (control) or treated with 20 μm each HgCl2, CdCl2, AgNO3, or Pb(NO)3, or 100 μm each ZnSO4, CoCl2, CuSO4, or MnCl2, or 1% (w/v) NaCl. The wild-type (WT) tobacco seedlings were included as a negative control. Numbers above the bars show the fold induction of heavy metal over untreated control seedling. B, The dose response of GUS activity in the seedling leaves to different Cu2+concentrations. C, GUS activity in leaves and roots of seedlings treated with or without 50 μm CuSO4.
Because Cu2+ is readily taken up by plants and is easy to apply, it was chosen for studying the effects of increasing heavy metal concentration on GUS activity. GUS activity increased as a function of Cu2+ concentration from 0 (control, untreated with Cu2+) to 50 μm and decreased thereafter (Fig. 5B). This decrease presumably reflects the toxic effect of Cu2+ at high concentrations. Thus, to compare the inductive effects of heavy metals in leaves and roots, seedlings were treated with copper at the optimal concentration (50 μm) for 24 h. The GUS activity in the young leaves was 3-fold higher than that in the young roots in response to copper. GUS activity in both young leaves and young roots was induced roughly 4-fold by 50 μm Cu2+, indicating the leaf is not more metal responsive than the root (Fig. 5C). We conclude that the P-PvSR[−687/+48] responds to various heavy metals, but its activity varies with the kind and concentration of heavy metal.
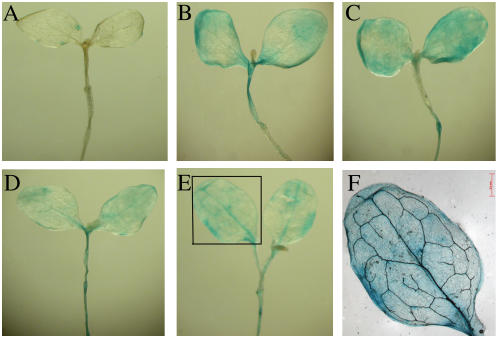
To acquire more detailed information on the location of P-PvSR[−687/+48] expression, histochemical GUS staining was performed with seedlings of line T0-8 treated by several heavy metal ions. Compared to untreated seedlings, which showed little GUS staining (Fig. 6A), GUS expression were strongly induced by Hg2+ (Fig. 6B), Cd2+ (Fig. 6C), Cu2+ (Fig. 6D), and Zn2+ (Fig. 6E). A magnified view of GUS staining after treatment by Zn2+ (Fig. 6F) clearly shows the distribution through the vasculature.
Figure 6.
Histochemical assay for GUS activity in the seedlings of line T0-8. The seedlings were treated without heavy metal stress (A) or for 24 h with HgCl2 (B), CdCl2 (C), CuSO4 (D), and ZnSO4 (E), respectively. Ten seedlings at least were examined and typical results are presented. A magnified view of the leaf shown boxed in E is shown in F. Bar = 0.5 mm.
DISCUSSION
Two HMREs Contribute to the Heavy Metal Responsiveness of the PvSR2 Promoter
In this study, we have characterized a heavy metal-specific promoter both in a transient expression system and transgenic tobacco plants. Our analysis indicated HMREs were found localized in two regions (−222/−188 and −187/−147), each of which could separately confer heavy metal-responsive expression on the GUS reporter gene. The 35-bp region I contains a MRE-like sequence that exhibited a weak heavy metal induction (about 2-fold) in the context of a minimal CaMV 35S promoter (Fig. 3C). The PvSR2 MRE-like sequence differs from animal MRE core sequence in the fifth position, in which a G is replaced by a C and by flanking sequences that are AT rich rather than GC rich. Since mutation of the MRE-like sequence attenuated the heavy metal-inducible function of region I, we conclude that the MRE-like sequence is required for the heavy metal-inducible function of this region.
Of greater interest, however, was the 41-bp region II, which showed a greater heavy metal induction (4- to 5.5-fold) in the context of a minimal CaMV 35S promoter (Fig. 3C). Previously identified cis-acting elements involved in heavy metal-induced gene expression include MRE of animal MT genes (Stuart et al., 1985), MRE of rat SOD1 (Kim et al., 1993; Yoo et al., 1999), the zinc-responsive elements of yeast (Zhao et al., 1998), the CuRE of the photosynthetic algae C. reinhardtii (Quinn et al., 2000), and the iron regulatory element of soybean (Wei and Theil, 2000). HMRE-containing region II did not show similarity to any known metal-responsive elements, suggesting it contains a novel cis-acting element conferring responsiveness to heavy metal. Intriguingly, it contains an inverted repeat of the sequence 5′-ATTCAAA-3′, separated by a 16-bp spacer (Fig. 3A). These two elements are necessary for the heavy metal induction of P-PvSR. However, whether or not a synergistic interaction is present between these two elements remains unclear. A detailed mutagenesis study is necessary for further elucidation and definition of the core sequence of PvSR2-associated HMRE(s). Another question left unanswered by these studies is whether HMRE-binding factors directly sense heavy metals or whether they are only indirectly activated by heavy metals. Identification of the HMRE-binding transcription factor will be a first step toward distinguishing between two models.
The PvSR2 Promoter May Be Useful in Transgenic Plant Studies
The choice of promoter is often of great importance in regulating transgene in plants. The use of a heavy metal-inducible promoter to control the target gene expression could avoid potential harmful effects due to overexpression of the target gene under control of constitutive promoters in transgenic plants. For example, the yeast copper-inducible promoter system has been well characterized in transgenic tobacco plants for controlling GUS (Mett et al., 1993) and cytokinin synthase (ipt) genes (McKenzie et al., 1998). Several promoters have failed to induce gene expression in response to cadmium in transgenic plants. The mouse MT gene promoter, normally induced by Zn2+ and Cd2+, was found to be inactive in tobacco (Maiti et al., 1991). The CaMV 35S promoter was not affected by cadmium exposure in tobacco, whereas the ribulose bisphosphate carboxylase promoter was repressed at high cadmium concentration (Stefanov et al., 1997). We show here that P-PvSR can respond to a variety of heavy metal ions, especially Ag+, Zn2+, Cd2+, Hg2+, and Pb3+, in transgenic tobacco (Fig. 5A). Furthermore, P-PvSR can sense low concentrations of heavy metal ions (Fig. 5B). Therefore, P-PvSR represents a novel heavy metal-specific responsive promoter of higher plants that could be used in controlling target genes for increasing heavy metal tolerance of plants or accumulating heavy metal in transgenic plants. P-PvSR could also be engineered into reporter gene constructs to provide sensitive in vitro assays for bioreporting of environmental heavy metal contamination.
The Function of the MRE-Like Sequence in Plants
Although originally identified in the promoter of all mammalian MT genes, MRE or MRE-like sequences have also been found in the promoters of plant metal-responsive or regulatory genes such as some MT-like genes (Evans et al., 1990; Whitelaw et al., 1997; Yu et al., 2000; Siti Nor Akmar et al., 2002), the maize (Zea mays) Bz2 gene (Bodeau and Walbot, 1996), the zinc-inducible gene SHMT1 (Perry et al., 2005), and the Mn2+-regulated peroxidase genes mnp2 (Mayfield et al., 1994) and mnp3 (Cohen et al., 2002). It has thus been suggested that these MRE or MRE-like sequences may be involved in heavy metal responsiveness. However, to our knowledge, to date there is no evidence showing MRE or MRE-like sequence confers heavy metal responsiveness to these plant genes (Evans et al., 1990; Whitelaw et al., 1997; Fukuzawa et al., 2004; Perry et al., 2005). Thus, MRE-like sequences in plants may be involved in other forms of regulation. For example, MRE sequence is a strong enhancer of cSHMT transcription and contributes to the mimosine responsiveness of the cSHMT gene (Perry et al., 2005). It is also interesting that MREs are also present in mammalian MT genes that are nonresponsive to metal induction and appear necessary for the basal MT gene expression in the absence of exposure to exogenous metal ions in animal (Haq et al., 2003).
The MRE-like sequence (5′-TGCAGGC-3′) present in −212/−206 of P-PvSR, can, in the context of a minimal CaMV 35S promoter, induce GUS expression by about 2-fold in the presence of heavy metals. This is in agreement with the findings that a single copy of MRE from mouse MT gene (Culotta and Hamer, 1989) or three copies of MREs of rat SOD1 (Yoo et al., 1999) in the context of a minimal promoter can provide a 3- to 4-fold heavy metal induction. Thus, the MRE-like sequence of P-PvSR is a functional element in response to heavy metal.
A comprehensive understanding of the detailed mechanisms for metal-regulated transcription will ultimately require an understanding of how eukaryotic cells sense, transport, distribute, and remove metals from their environment. Metal-regulated transcription in higher plants is not well documented. In higher eukaryotes, MT genes are the most intensively studied and best-understood examples of metal-regulated transcription units. An MTF-1 binds to MREs and regulates animal MT gene transcription (Westin and Schaffner, 1988). A model for the induction of MT gene expression has been proposed (Haq et al., 2003). Although MTF-1 responds only to zinc, nonzinc heavy metals can displace zinc from intra- and/or extracellular storage proteins, increasing the pool of free zinc available for the activation of MTF-1.
Although similar MRE core elements were found in the upstream region of some plant genes, no heavy metal-responsive functions of them were reported so far. Thus, heavy metal-responsive transcription factors binding to MRE or MRE-like sequences is not a main pathway in plants. Therefore, alternative mechanisms of sensing, such as signal transduction pathways, that involve protein kinases may be involved. Mitogen-activated protein kinase (MAPK) pathways are modules involved in the transduction of extracellular signals to intracellular targets in all eukaryotes. In plants, it has been shown that MAPKs play a role in the signaling of biotic and abiotic stresses, plant hormones, and cell cycle cues. Recently, the effect of cadmium on plant MAPKs in rice suggests that a MAPK cascade may function in the cadmium-signaling pathway (Yeh et al., 2004). Copper and cadmium stress activate two distinct MAPK pathways in alfalfa (Medicago sativa) seedlings (Jonak et al., 2004). The regulation of PvSR2 expression by heavy metal represents a novel metalloregulatory system for investigation, particularly because it is one of the very few, if not the only, that focuses on specific responsiveness to heavy metal.
MATERIALS AND METHODS
Plant Materials and Bacterial Strains
Tobacco (Nicotiana tabacum L. cv W38) plants were grown in Murashige and Skoog (MS) medium under a 16-h-light (25°C)/8-h-dark (20°C) cycle. Surface-sterilized bean (Phaseolus vulgaris L. cv Saxa) seeds were grown in pots (in soil) with a photoperiod of 16 h at 22°C during the day and at 18°C during the night in the greenhouse. When two primary leaves were fully expanded, the plants were sprayed with 0.2% (w/v) HgCl2. Escherichia coli strain DH5α cells were used for the cloning and propagation of all the recombinant plasmid vectors. Agrobacterium tumefaciens strain LBA4404 was used for tobacco leaf-disc transformations.
Genomic DNA Walking
The 5′-flanking region of PvSR2 was isolated from bean genomic DNA according to the instructions of TaKaRa LA PCR in vitro cloning kit (TaKaRa). Briefly, genomic DNA isolated from young bean leaves was digested with restriction enzymes PstI and EcoRI and then ligated to a cassette DNA with corresponding restriction sites. A primary PCR to amplify the 5′-regulatory region was performed with a PvSR2 gene-specific primer (GSP; 5′-CTCCACTGTGTTAACGCCGGGCTTC-3′) and cassette primer C1. Diluted primary PCR products were then amplified using a nested PvSR2 GSP (5′-GTTCCTTGGCGTAAGAGTAGAGGATGG-3′) and cassette primer C2. The PCR and nested PCR amplifications were performed as described by the manufacturer. The nested PCR products were cloned into a pMD18-T vector (TaKaRa), sequenced, and designated as pUC-MRP.
5′ RACE
Total RNA was isolated from the leaves of bean 6 h after spraying with 0.2% (w/v) HgCl2 using RNAgent Total RNA Isolation system (Promega). 5′ RACE was carried out using a SMART RACE cDNA Amplification kit (CLONTECH) on adapter-ligated cDNA, synthesized from 1 μg of total RNA. 5′-RACE reaction was performed using a PvSR2-specific primer1 (GSP1; 5′-AGATGGAACCTGTCGTACACCGGA-3′) by touchdown PCR program, as described by the manufacturer. The PCR fragments were cloned into a pMD18-T vector and sequenced. Four separated positive clones were sequenced for accurately mapping the transcription start site.
Construction of Recombinant Plasmids
A series of nested 5′ deletions of P-PvSR fragments (1.6, 1.2, 0.7, 0.2, and 0.15 kb) were amplified by PCR from pUC-MRP using the common antisense primer (ASP; 5′-GCTCTAGATGATGGAACTGTGAAGATTGT-3′) and either the sense primers SP1 (5′-CCCAAGCTTCTGCAGACATCGTTTTGTATT-3′), SP2 (5′-CCCAAGCTTTGTTTTGAAATAGGAAAAAGTAAC-3′ ), SP3 (5′-CCCAAGCTTTTCTTCCTACATCTCACCCA-3′), SP4 (5′-CCCAAGCTTAATTTAAATGCAGGCGTTT-3′), or SP5 (5′-CCCAAGCTTAGGAAGCAATAACGTGGAAAAT-3′), respectively. The 5′-deletion derivatives were cloned into the HindIII/XbaI sites of pBI221 (CLONTECH) upstream of GUS instead of the CaMV 35S promoter, producing constructs pBMRP1.6, pBMRP1.2, pBMRP0.7, pBMRP0.2, and pBMRP0.15, respectively. Similarly, the promoter fragment (−222/+48) with mutation of the MRE-like sequence was obtained by PCR from pBMRP0.2 using the ASP and the sense primers mSP4 (5′-CCCAAGCTTAATTTAAAGTACTTAGTTTTTATATTTGAAAGA-3′). Above PCR amplification was completed using 0.25 units of LA Taq DNA Polymerase (TaKaRa) and 1 ng template in a 25-μL reaction mixture for 25 cycles. PCR cycling parameters were denatured at 94°C for 30 s, primer annealing at 55°C for 30 s, and primer extension at 72°C for 1 min, with a final elongation at 72°C for 10 min. The PCR products were inserted to the HindIII/XbaI sites of pBI221 to yield pBmMRP0.2. The promoter region of all constructs was confirmed by sequencing. The underlined sequences on all above primers indicate the HindIII and XbaI restriction endonuclease sequences that were included to facilitate the cloning into pBI221.
The modified firefly gene (luc+) derived from pGL3-Basic (Promega) was amplified by PCR with the primer LucSP (5′-GCTCTAGAGCCACCATG GAAGACGCCA-3′; XbaI restriction site underlined) and the LucASP (5′-TACCGAGCTCTTACACGGCGATCTTTCCGC-3′; SacI restriction site underlined). The PCR amplification was performed at an annealing temperature of 58°C for 25 cycles. The PCR products were cloned into a pMD18-T vector, sequenced, and designated as pUC-Luc. The XbaI-SacI fragment containing the luc+ from pUC-Luc was inserted into the same sites of pBI221 instead of GUS to yield the plasmid pBI221-Luc.
To generate construct pMRP0.7-GUS for the transformation of tobacco, a HindIII-EcoRI fragment containing the P-PvSR[−687/+48]-GUS cassette from pBMRP0.7 was inserted into the HindIII/EcoRI sites of pBI121 (CLONTECH).
The 35-bp region I promoter sequence (−222/−188) or its derivative containing a mutated MRE-like sequence was introduced upstream from the minimal CaMV 35S promoter (−64/+8, 35SΔ64) by PCR using the common antisense primer 35S-R (5′-GCTCTAGAGTCCCCCGTG-3′) and either sense primer 35S-64F1 (5′-CCCAAGCTTTTAATTTAAATGCAGGCGTTTTTATATTTGAAAGACCCACTATCCTTCGCAA-3′) or 35S-64mF1 (5′-CCCAAGCTTTTAATTTAAAGTACTTAGTTTTTATATTTGAAAGA CCCACTATCCTTCGCAA-3′), respectively. Similarly, the 41-bp region II promoter sequence (−187/−147) was fused to the 35SΔ64 by PCR using the antisense primer 35S-R and the sense primer 35S-64F2 (5′-CCCAAGCTTAAATATTCAAAATTCTACCCTGTTTTGAAACTTAACCTTTACCCACTATCCTTCGCAA-3′). The 72-bp (−64/+8) minimal CaMV 35S promoter fragment was also obtained by PCR using the antisense primer 35S-R and the sense primer 35S-F (5′-CCCAAGCTTCCCACTATCCTTCGCAA-3′). The underlined sequences on all above primers indicate the HindIII and XbaI restriction endonuclease sequences. The pBI221 (1 ng) was used as a template for all above PCR amplifications. The amplification began with 94°C for 2 min, followed by 26 cycles of 94°C for 10 s, 52°C for 15 s, and 72°C for 10 s, with a final elongation at 72°C for 10 min. The PCR products were cloned into the HindIII/XbaI sites of pBI221 to yield pBMRE, pBmMRE, PvSR[−187/−147]-GUS, and 35SΔ64-GUS. The promoter region of all constructs was confirmed by sequencing.
Transient Expression Assay
Isolation of tobacco mesophyll protoplasts and protoplasts were transiently transformed with polyethylene glycol as described by Negrutiu et al. (1987). Typically, 0.5 mL of tobacco protoplast suspension (1 × 106/mL) was cotransfected with 20 μg of a test construct and 5 μg of a CaMV 35S:Luc control vector, pBI221-Luc. For analysis of the 5′-deletion constructs, the protoplasts transfected with either construct were then divided into two aliquots, one resuspended in 2 mL heavy metal-free liquid MS medium (MnSO4-, ZnSO4-, Na2MoO4-, CuSO4-, and FeSO4-deficient MS medium supplemented with 30 g/L Suc, 72.8 g/L mannitol) and the other in the same medium containing 15 μm HgCl2. For functional analysis of the promoter region I or II in the context of a minimal CaMV 35S promoter, the transfected protoplasts cotransfected with 30 μg of a test construct and 5 μg of pBI221-Luc were divided into three aliquots. Each aliquot was cultured in 2 mL heavy metal-free liquid MS medium or in the same medium supplemented with 20 μm HgCl2 or 50 μm ZnSO4. The samples were incubated in the dark at 25°C in 3-cm-diameter petri dishes, and transient gene expression was assayed at 24 h posttransformation. A total of 2 mL cultured protoplast was divided into two portions (1.5 mL and 0.5 mL), and then protoplasts were collected by centrifugation at 100g for 1 min. One (1.5 mL) was resuspended in 150 μL GUS buffer (50 mm phosphate buffer, pH 7.0, 10 mm EDTA, 0.1% [v/v] Triton X-100, 0.1% [w/v] Sarcosyl, and 10 mm β-mercaptoethanol) and the other (0.5 mL) was resuspended in 100 μL 1× Luc cell culture lysis reagent (Promega) and then lysed by vortexing briefly. After incubation for 10 min on ice, cell debris was removed by centrifugation at 10,000g for 5 min at 4°C. Protein concentrations were determined by the method of Bradford using bovine serum albumin as a standard for two samples.
GUS activity was quantified as described by Jefferson (1987) using p-nitrophenyl-β-d-glucuronide (Sigma) as substrate. A Sephadex G-25 column was previously equilibrated in GUS buffer and employed for the removal of chlorophyll molecules. The protein was collected and kept temporarily on ice until the start of the assays. For each assay, the protein extract and assay buffer (50 mm phosphate buffer, pH 7.0, 1 mm EDTA, 0.1% [v/v] Triton X-100, 1 mm p-nitropheny1-β-D glucuronide) were prewarmed to 37°C, and a 50-μL protein sample was added to 400 μL assay buffer. After 0, 30, or 60 min of incubation, 150 μL of aliquots were removed and added to 150 μL stop solution (1.4 m 2-amino-2-methyl-1, 3-propanediol) to terminate reaction. Absorbance at 415 nm was read with a SmartSpec 3000 spectrophotometer (Bio-Rad Laboratories) against a stopped blank reaction (0 min) using p-nitrophenol as a standard. GUS values were expressed as nanomoles p-nitrophenol per minute per milligram protein. LUC assays were performed using the Promega LUC assay system according to the manufacturer's instructions. Luminescence was measured (2-s delay and 10-s integration time) in a GENios plus (TECAN). LUC-specific activity is expressed as light units per milligram protein. GUS activity was normalized against LUC activity and expressed as a ratio of GUS/LUC units. The data are presented as mean ± sd from three independent experiments.
Plant Transformation and Treatment Conditions
A. tumefaciens-mediated tobacco transformation with pMRP0.7-GUS was performed as described by Horsch et al. (1985). The primary transgenic plants lines (T0) were identified by PCR analysis followed by sequencing and then self pollinated to produce T1 seeds. T-DNA inheritance was scored by kanamycin segregation analysis in the T1 generation. The surface-sterilized seeds were germinated in MS agar medium supplemented with 200 μg/mL kanamycin. Segregation of the germinated progeny was scored 2 weeks after sowing. A χ2 calculation according to the hypothesis ratio (3:1) was performed. The six lines (T0-3, T0-5, T0-6, T0-8, T0-12, and T0-17) segregated as 3:1. The seedlings of these six lines were selected for further analysis.
The sterilized six lines seeds were germinated in heavy metal-free MS agar medium (MnSO4-, ZnSO4-, Na2MoO4-, CuSO4-, and FeSO4-deficient MS medium) containing 200 μg/mL kanamycin for 10 d. For each treatment, ten 10-d-old kanamycin-resistant seedlings from each line were analyzed. To analyze the responsive type of heavy metal ions, the seedlings were transferred to the same MS medium supplemented with either 20 μm each HgCl2, CdCl2, AgNO3, and Pb(NO)3 or 100 μm each ZnSO4, CoCl2, CuSO4, and MnCl2 or 1% (w/v) NaCl. In the control groups, the seedlings and wild-type tobacco seedlings were grown in heavy metal-free MS agar medium with no heavy metal stress. For dose response curves, different concentrations (0, 5, 10, 50, 70, and 100 μm) CuSO4 were applied. After induction by heavy metal for 24 h, the seedling leaves were homogenized in 1 mL of chilled GUS extraction buffer. GUS extraction and GUS assay were carried out as described above. In addition, seedlings were induced by 50 μm CuSO4 for 24 h, and GUS activity in the leaves and roots of the seedlings were also analyzed, respectively.
Histochemical localization of GUS activity in transgenic plant seedlings of line T0-8 treated without (control) or with 50 μm each of HgCl2, CdCl2, ZnSO4, and CuSO4 for 24 h was performed as previously described by Jefferson et al. (1987).
Northern-Blot Analysis
Total RNA was isolated from the leaves of 10-d-old seedlings of line T0-8 grown in heavy metal-free MS agar medium either alone or supplemented with 20 μm each of CdCl2 or HgCl2, or 100 μm each of CuSO4 or ZnSO4. Fifteen micrograms total RNA was separated on a 1.2% (w/v) formaldehyde agarose gel electrophoresis, transferred onto nylon membranes (Millipore), and hybridized with [α-32P]dCTP-labeled GUS gene fragment (corresponding to the entire GUS open reading frame) as described by Sambrook et al. (1989). Rehybridizations using a [α-32P]dCTP-labeled 18S rDNA probe were performed as a control for RNA loading.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers U54704 and DQ109992.
Acknowledgments
We are grateful to Professor Hans Lambers (University of Western Australia) and Professor David Morse (Université de Montréal) for comments on the manuscript.
This work was supported by the National Natural Science Foundation of China (grant nos. 39870078, 30370128, and 30570146) and by the National Program of Research and Development of Transgenic Plants of China (grant nos. J00–A–008–03 and JY–03–A–20–02) to T.C.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Tuanyao Chai (tychai@gucas.ac.cn).
References
- Amin J, Ananthan J, Voellmy R (1988) Key features of heat shock regulatory elements. Mol Cell Biol 8 3761–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berna A, Bernier F (1999) Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme. Plant Mol Biol 39 539–549 [DOI] [PubMed] [Google Scholar]
- Bodeau JP, Walbot V (1996) Structure and regulation of the maize Bronze2 promoter. Plant Mol Biol 32 599–609 [DOI] [PubMed] [Google Scholar]
- Chai TY, Chen Q, Zhang YX, Dong J, An CC (2003) Cadmium resistance in transgenic tobacco plants enhanced by expressing bean heavy metal-responsive gene PvSR2. Sci China Ser C Life Sci 46 623–630 [DOI] [PubMed] [Google Scholar]
- Clemens S (2001) Molecular mechanisms of plant metal tolerance and homeostasis. Planta 212 475–486 [DOI] [PubMed] [Google Scholar]
- Cobbett CS, Goldsborough PB (2002) Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol 53 159–182 [DOI] [PubMed] [Google Scholar]
- Cohen R, Yarden O, Hadar Y (2002) Lignocellulose affects Mn2+ regulation of peroxidase transcript levels in solid-state cultures of Pleurotus ostreatus. Appl Environ Microbiol 68 3156–3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta VC, Hamer DH (1989) Fine mapping of a mouse metallothionein gene metal response element. Mol Cell Biol 9 1376–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon WJ, Inouye C, Karin M, Tullius TD (1996) CUP2 binds in a bipartite manner to upstream activation sequence c in the promoter of the yeast copper metallothionein gene. J Biol Inorg Chem 1 451–459 [Google Scholar]
- Ellis JG, Tokuhisa JG, Llewellyn DJ, Bouchez D, Singh K, Dennis ES, Peacock WJ (1993) Does the ocs-element occur as a functional component of the promoters of plant genes? Plant J 4 433–443 [DOI] [PubMed] [Google Scholar]
- Evans IM, Gatehouse LN, Gatehouse JA, Robinson NJ, Croy RRD (1990) A gene from pea (Pisum sativum L.) with homology to metallothionein genes. FEBS Lett 262 29–32 [DOI] [PubMed] [Google Scholar]
- Fukuzawa H, Yu LH, Umeda-Hara C, Tagawa M, Uchimiya H (2004) The rice metallothionein gene promoter does not direct foreign gene expression in seed endosperm. Plant Cell Rep 23 231–235 [DOI] [PubMed] [Google Scholar]
- Fürst P, Hu S, Hackett R, Hamer D (1988) Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55 705–717 [DOI] [PubMed] [Google Scholar]
- Giuliano G, Pichersky E, Malik VS, Timko MP, Scolnik PA, Cashmore AR (1988) An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc Natl Acad Sci USA 85 7089–7093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen G, Uhrhammer N, Guilfoyle TJ (1988) Regulation of expression of an auxin-induced soybean sequence by cadmium. J Biol Chem 263 6442–6446 [PubMed] [Google Scholar]
- Haq F, Mahoney M, Koropatnick J (2003) Signaling events for metallothionein induction. Mutat Res 533 211–226 [DOI] [PubMed] [Google Scholar]
- Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W (1994) The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J 13 2870–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsch RB, Fry JE, Hoffman NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general-method for transferring genes into plants. Science 227 1229–1231 [DOI] [PubMed] [Google Scholar]
- Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep 5 387–405 [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonak C, Nakagami H, Hirt H (2004) Heavy metal stress activation of distinct mitogen-activated protein kinase pathways by copper and cadmium. Plant Physiol 136 3276–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri F, Lam E, Chua NH (1989) Two tobacco DNA-binding proteins with homology to the nuclear factor CREB. Nature 340 727–730 [DOI] [PubMed] [Google Scholar]
- Kim YH, Yoo HY, Jung G, Kim JY, Rho HM (1993) Isolation and analysis of the rat genomic sequence encoding Cu/Zn superoxide dismutase. Gene 133 267–271 [DOI] [PubMed] [Google Scholar]
- Kusaba M, Takahashi Y, Nagata T (1996) A multiple-stimuli-responsive as-1-related element of parA gene confers responsiveness to cadmium but not to copper. Plant Physiol 111 1161–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Chua NH (1989) ASF-2, a factor that binds to the cauliflower mosaic virus 35S promoter and a conserved GATA motif in cab promoters. Plant Cell 1 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescure AM, Proudhon D, Pesey H, Ragland M, Theil EC, Briat JF (1991) Ferritin gene transcription is regulated by iron in soybean cell cultures. Proc Natl Acad Sci USA 88 8222–8226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiti IB, Wagner GJ, Hunt AG (1991) Light-inducible and tissue-specific expression of a chimeric mouse metallothionein cDNA in tobacco. Plant Sci 76 94–107 [Google Scholar]
- Mayfield MB, Godfrey BJ, Gold MH (1994) Characterization of the mnp2 gene encoding manganese peroxidase isozyme 2 from the basidiomycete Phanerochaete chrysosporium. Gene 142 231–235 [DOI] [PubMed] [Google Scholar]
- McKenzie MJ, Mett V, Reynolds PHS, Jameson PE (1998) Controlled cytokinin production in transgenic tobacco using a copper-inducible promoter. Plant Physiol 116 969–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett VL, Lochhead LP, Reynolds PHS (1993) The copper-controllable gene expression system for whole plants. Proc Natl Acad Sci USA 90 4567–4571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrutiu I, Shillito R, Potrykus I, Biasini G, Sala F (1987) Hybrid genes in the analysis of transformation conditions. I. Setting up a simple method for direct gene transfer in plant protoplasts. Plant Mol Biol 8 363–373 [DOI] [PubMed] [Google Scholar]
- Perry C, Sastry R, Nasrallah IM, Stover PJ (2005) Mimosine attenuates serine hydroxymethyltransferase transcription by chelating zinc: implications for inhibition of DNA replication. J Biol Chem 280 396–400 [DOI] [PubMed] [Google Scholar]
- Quinn JM, Barraco P, Eriksson M, Merchant S (2000) Coordinate copper-and oxygen-responsive Cyc6 and Cpx1 expression in Chlamydomonas is mediated by the same element. J Biol Chem 275 6080–6089 [DOI] [PubMed] [Google Scholar]
- Quinn JM, Kropat J, Merchant S (2003) Copper response element and Crr1-dependent Ni2+-responsive promoter for induced, reversible gene expression in Chlamydomonas reinhardtii. Eukaryot Cell 2 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn JM, Merchant S (1995) Two copper-responsive elements associated with the Chlamydomonas Cyc6 gene function as targets for transcriptional activators. Plant Cell 7 623–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual, Ed 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Searle PF, Stuart GW, Palmiter RD (1987) Metal regulatory elements of the mouse metallothionein-I gene. Experientia Suppl 52 407–414 [DOI] [PubMed] [Google Scholar]
- Simpson SD, Nakashima K, Narusaka Y, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003) Two different novel cis-acting elements of erd1, a clpA homologous Arabidopsis gene function in induction by dehydration stress and dark-induced senescence. Plant J 33 259–270 [DOI] [PubMed] [Google Scholar]
- Siti Nor Akmar A, Cheah SC, Murphy DJ (2002) Isolation and characterization of two divergent type 3 metallothioneins from oil palm, Elaeis guineensis. Plant Physiol Biochem 40 255–263 [Google Scholar]
- Stefanov I, Frank J, Gedamu L, Misra S (1997) Effect of cadmium treatment on the expression of chimeric genes in transgenic tobacco seedlings and calli. Plant Cell Rep 16 291–294 [DOI] [PubMed] [Google Scholar]
- Stuart GW, Searle PF, Palmiter RD (1985) Identification of multiple metal regulatory elements in mouse metallothionein-I promoter by assaying synthetic sequences. Nature 317 828–831 [DOI] [PubMed] [Google Scholar]
- Wei JZ, Theil EC (2000) Identification and characterization of the iron regulatory element in the ferritin gene of a plant (soybean). J Biol Chem 275 17488–17493 [DOI] [PubMed] [Google Scholar]
- Westin G, Schaffner W (1988) A zinc-responsive factor interacts with a metal-regulated enhancer element (MRE) of the mouse metallothionein-I gene. EMBO J 7 3763–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw CA, Le Huquet JA, Thurman DA, Tomsett AB (1997) The isolation and characterization of type II metallothionein-like genes from tomato (Lycopersicon esculentum L.). Plant Mol Biol 33 503–511 [DOI] [PubMed] [Google Scholar]
- Xiao H, Lis JT (1988) Germline transformation used to define key features of heat-shock response elements. Science 239 1139–1142 [DOI] [PubMed] [Google Scholar]
- Yeh CM, Hsiao LJ, Huang HJ (2004) Cadmium activates a mitogen-activated protein kinase gene and MBP kinases in rice. Plant Cell Physiol 45 1306–1312 [DOI] [PubMed] [Google Scholar]
- Yoo HY, Chang MS, Rho HM (1999) Heavy metal-mediated activation of the rat Cu/Zn superoxide dismutase gene via a metal-responsive element. Mol Gen Genet 262 310–313 [DOI] [PubMed] [Google Scholar]
- Yu LH, Liu JY, Umeda M, Uchimiya H, Zhao NM (2000) Cloning and sequence characteristics of the genomic gene of a rice metallothionein. Chin Sci Bull 45 153–156 [Google Scholar]
- Zhang YX, Chai TY, Dong J, Zhao WM, Cheng AC, Chen ZL, Burkard G (2001) Cloning and expression analysis of the heavy metal-responsive gene PvSR2 from bean. Plant Sci 161 783–790 [Google Scholar]
- Zhao H, Butler E, Rodgers J, Spizzo T, Duesterhoeft S, Eide D (1998) Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements. J Biol Chem 273 28713–28720 [DOI] [PubMed] [Google Scholar]