Abstract
Drought induces stomatal closure, a response that is associated with the activation of plasma membrane anion channels in guard cells, by the phytohormone abscisic acid (ABA). In several species, this response is associated with changes in the cytoplasmic free Ca2+ concentration. In Vicia faba, however, guard cell anion channels activate in a Ca2+-independent manner. Because of potential differences between species, Nicotiana tabacum guard cells were studied in intact plants, with simultaneous recordings of the plasma membrane conductance and the cytoplasmic free Ca2+ concentration. ABA triggered transient rises in cytoplasmic Ca2+ in the majority of the guard cells (14 out of 19). In seven out of 14 guard cells, the change in cytoplasmic free Ca2+ closely matched the activation of anion channels, while the Ca2+ rise was delayed in seven other cells. In the remaining five cells, ABA stimulated anion channels without a change in the cytoplasmic Ca2+ level. Even though ABA could activate anion channels in N. tabacum guard cells independent of a rise in the cytoplasmic Ca2+ concentration, patch clamp experiments showed that anion channels in these cells are stimulated by elevated Ca2+ in an ATP-dependent manner. Guard cells thus seem to have evolved both Ca2+-independent and -dependent ABA signaling pathways. Guard cells of N. tabacum apparently utilize both pathways, while ABA signaling in V. faba seems to be restricted to the Ca2+-independent pathway.
The aperture of stomata is determined by two guard cells that surround the stomatal pore. Guard cells are devoid of plasmodesmata (Wille and Lucas, 1984) and thus function virtually autonomously. During drought, stomata close to reduce the loss of water via transpiration, a response that depends on the phytohormone abscisic acid (ABA; Assmann and Shimazaki, 1999; Schroeder et al., 2001; Roelfsema and Hedrich, 2005). ABA triggers the efflux of K+ salts from the guard cell, thereby reducing the osmotic potential of the cell and causing stomatal closure. The efflux of K+ salts is driven through depolarization of the plasma membrane, which allows K+ extrusion through outward rectifying K+ channels. Two mechanisms could mediate the ABA-induced depolarization of guard cells: activation of anion channels and/or inhibition of H+-ATPases in the plasma membrane.
In guard cells of Vicia faba studied in intact plants, ABA triggers a transient activation of R- and S-type anion channels (Roelfsema et al., 2001). A peak in anion channel activity is found after approximately 5 min; during prolonged stimulation, the activity of anion channels levels off, but a prestimulus value is only observed after removal of ABA. This led to the suggestion that the first phase of this ABA response induces fast stomatal closure, while the second phase inhibits reopening of the stomata (Roelfsema and Hedrich, 2005). The extent to which ABA may inhibit plasma membrane H+-ATPases is not known in detail, but ABA was shown to inhibit blue light-induced activation of H+-ATPases in guard cells (Zhang et al., 2004).
Various lines of evidence predicted an important role of the cytoplasmic Ca2+ concentration in ABA activation of anion channels in guard cells (Hetherington and Brownlee, 2004). Both R- and S-type anion channels in V. faba guard cells are stimulated by cytoplasmic Ca2+ (Schroeder and Hagiwara, 1989; Hedrich et al., 1990), and ABA can induce cytoplasmic Ca2+ transients in guard cells in isolated epidermal strips or epidermal fragments of Commelina communis (McAinsh et al., 1990; Gilroy et al., 1991) as well as in Arabidopsis (Arabidopsis thaliana; Allen et al., 1999, 2001). In contrast to these data, Levchenko et al. (2005) found that ABA activates anion channels without provoking cytoplasmic Ca2+ changes in guard cells of V. faba. The differences in results may be due to different experimental conditions, because Levchenko et al. (2005) measured guard cells in intact plants, while previous reports were mainly conducted with isolated epidermal peels. Alternatively, the differences may relate to differences between plant species. In some species, ABA-induced stomatal closure could involve alterations in the cytoplasmic Ca2+ concentration of guard cells, whereas in others, stomata close in a Ca2+-independent fashion.
In search of species-specific variation in the anion channel response to ABA, guard cell responses of Nicotiana tabacum were compared with those previously obtained for V. faba (Levchenko et al., 2005). In contrast to V. faba, this plant species can be transformed easily and thus has the advantage that it can be used for molecular biological approaches. The size of N. tabacum stomata is similar to that of V. faba and C. communis, which is a prerequisite for long-term intracellular recordings. For these reasons, N. tabacum and its close relative, Nicotiana benthamiana, already were the species of choice in several studies (Armstrong et al., 1995; Leyman et al., 1999; Hunt et al., 2003; von Caemmerer et al., 2004). We found that ABA activates anion channels in guard cells of intact plants, in a similar manner as previously observed for V. faba. However, in contrast to previous results with V. faba, the majority of N. tabacum guard cells showed an ABA-induced rise in the cytoplasmic Ca2+ concentration. Patch clamp experiments with N. tabacum guard cell protoplasts revealed that cytoplasmic Ca2+ as well as ABA enhance anion channel activity. These data suggest species-specific differences concerning the role of cytoplasmic Ca2+ changes in guard cell responses to ABA.
RESULTS
Guard Cell Recordings in Intact N. tabacum Plants
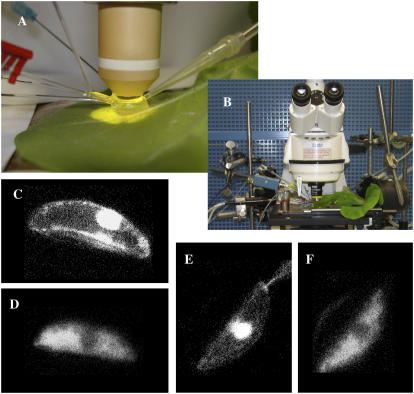
Guard cells of N. tabacum were studied in intact plants using double- and triple-barreled microelectrodes. The adaxial side of a mature leaf was attached to a plexiglass holder, enabling impalement of guard cells at the abaxial epidermis (Fig. 1, A and B). Two barrels of the microelectrodes were used to register and manipulate the membrane potential, while the Ca2+ reporter dye FURA2 could be injected with triple-barreled electrodes. After impalement, most electrodes were positioned in the cytoplasm, as judged from the distribution of FURA2 throughout the guard cell (Fig. 1C). In seven out of 49 cells, however, the dye did not spread trough the cell but remained in a confined compartment (Fig. 1D). These electrodes were probably placed in the vacuole based on the size and localization of the fluorescent compartment. In these cells, the voltage dependence of K+ channels was less steep compared to cells in which the electrode was located in the cytoplasm (data not shown). Most likely, the vacuolar membrane acts as a resistor connected in series with that of the plasma membrane. In some cells, the FURA2 initially loaded into the cytoplasm slowly appeared in the vacuole, indicating that N. tabacum guard cells are able to transport FURA2 from the cytoplasm to the vacuole (Fig. 1, E and F). The FURA2 ratio measurements therefore were only carried out from cytoplasm-rich areas.
Figure 1.
Guard cells in intact N. tabacum plants impaled with triple-barreled electrodes. A, Close up of an N. tabacum leaf impaled with triple-barreled electrode (left), guard cells in the abaxial epidermis were visualized with a water emersion objective (middle). Experimental solutions were perfused through a hypodermic needle (left back) over the cuticle to a suction pipette (right). B, Overview of the experimental setup with the upright microscope (middle), microelectrode amplifiers (left), and the N. tabacum plant (right). C, N. tabacum guard cell impaled with a triple-barreled electrode, through which FURA2 was injected, located with the tip in the cytoplasm. D, Guard cell injected with FURA2 through a triple-barreled electrode with the tip in the vacuole. E, Guard cell injected with FURA2 in the cytoplasm, but in which the FURA slowly appeared in the vacuole. F, Same guard cell as in E but 15 min later.
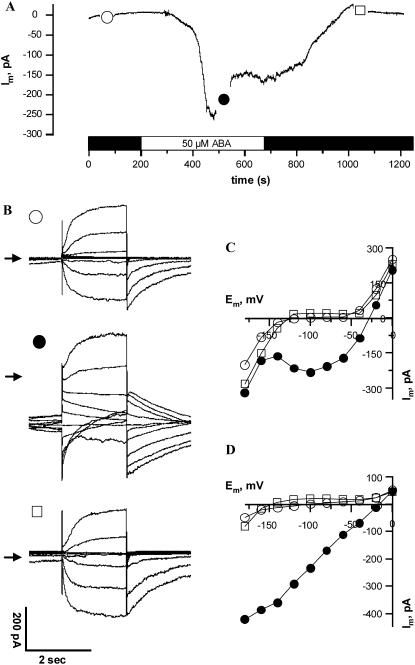
In the absence of ABA, the conductance of the guard cell plasma membrane in V. faba and Arabidopsis is dominated by two types of K+-selective channels, which were also recognized in guard cells of N. benthamiana (Armstrong et al., 1995). In N. tabacum guard cells in intact plants, outward rectifying channels activated at potentials positive of −60 mV, while inward channels were observed at potentials negative of −120 mV (Fig. 2B). At a holding potential of −100 mV, both time-dependent channels are not active, and, therefore, this potential was chosen to resolve ABA activation of anion channels (Roelfsema et al., 2004). ABA application triggered an inward current, after a lag time of 90 s (se = 12, n = 27) that peaked after 268 s (se = 26, n = 27) and partially deactivated again during prolonged stimulation with ABA. Before and during the application of ABA, the cells were clamped stepwise to potentials ranging from −180 mV to 0 mV with 20-mV increments (Fig. 2B). ABA stimulated the instantaneous conductance but had little effect on outward rectifying channels. The effect of ABA on inward rectifying channels was masked by the large activity of instantaneous currents (Fig. 2, C and D). The reversal potential of the instantaneous current in the presence of ABA shifted from values negative of −100 mV to −20 mV (Fig. 2D). The current voltage relation was linear, indicating that ABA activated S-type anion channels in these cells. The activation of R-type anion channels by ABA was not observed, even though this channel type was found in N. tabacum guard cells with patch clamp (data not shown). These data reveal that ABA stimulates S-type anion channels in guard cells of N. tabacum in a very similar manner as previously observed for V. faba (Roelfsema et al., 2004; Levchenko et al., 2005).
Figure 2.
ABA-induced changes in the plasma membrane conductance of an N. tabacum guard cell. A, Current trace of a guard cell clamped to a holding potential of −100 mV and exposed to 50 μm ABA (as indicated by the bar below the graph). Symbols indicate the time points at which voltage clamp step protocols were applied. B, Current traces from test pulses of the same cell as in A, symbols correlate. Arrows indicate the 0 pA level. Cells were clamped from a holding potential of −100 mV to potentials ranging from −180 to 0 mV with 20-mV increments. Note that the introduction of ABA (•) caused a dramatic increase of currents measured directly after the capacity compensation peak, while ABA had little effect on time-dependent outward currents. The currents virtually recovered to prestimulus values after a washout of ABA (□). C, Steady-state currents, sampled at the end of the 2-s test pulses, plotted against the clamp voltage (symbols correspond to A). Note that ABA increased inward current ranging from −40 to −140 mV and caused a large shift of the zero current potential to more positive values. D, Instantaneous currents, sampled directly after termination of the capacity compensation peak, plotted against the clamp voltage (symbols correspond to A). Note that ABA activated a channel with a linear instantaneous current-voltage relation and a reversal potential at −17 mV.
ABA-Induced Changes in Cytoplasmic Ca2+
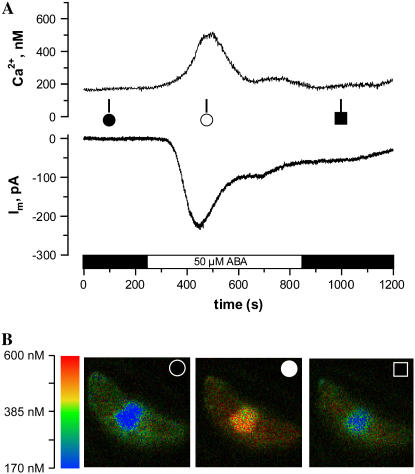
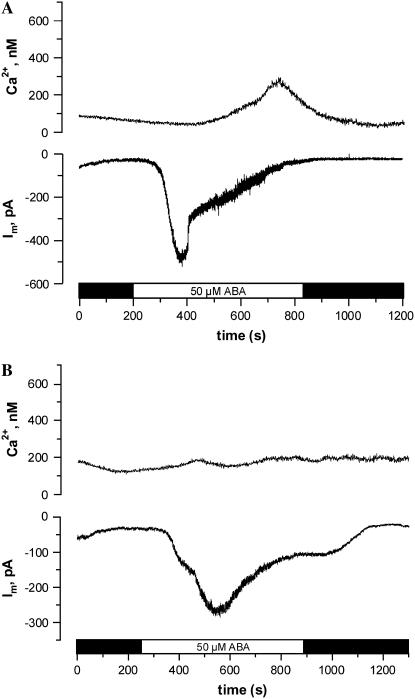
N. tabacum guard cells, loaded with the Ca2+ reporter dye FURA2, were exposed to 50 μm ABA applied at the cuticle as described above. Guard cells were clamped to −100 mV throughout the experiment to prevent voltage-dependent changes in the cytoplasmic Ca2+ concentration (Grabov and Blatt, 1998; Levchenko et al., 2005). Under these conditions, ABA induced a transient activation of anion channels just as in V. faba. Note that in contrast to the latter species, this response was accompanied by an increase in the cytoplasmic Ca2+ concentration in the majority of cells (14 out of 19). In one-half of the cells displaying a rise in cytoplasmic Ca2+, the increase of the Ca2+ concentration mirrored changes in the plasma membrane current (Fig. 3A). False color images of the 345:390 nm ratio of FURA2 fluorescence revealed that the free Ca2+ concentration rises throughout the cytoplasm, with the most obvious change in the proximity of the nucleus. Despite the close correspondence between the cytoplasmic Ca2+ concentration changes and changes in current observed for seven out of 19 cells, this correlation was absent in all other guard cells. In seven guard cells of N. tabacum, the ABA-induced rise in the cytoplasmic free Ca2+ concentration was delayed and did not match the activation state of inward current (Fig. 4A). In another subset of five cells, ABA did not trigger a change in the cytoplasmic Ca2+, even though ABA stimulated anion channels (Fig. 4B). The absence of an ABA-induced rise in the cytoplasmic Ca2+ concentration could not be linked to differences in Ca2+ buffering by FURA2. A comparison of the absolute fluorescence of FURA2 revealed a ratio of 0.99 for cells with or without Ca2+ changes, respectively. The magnitude of the current changes in cells that displayed a rise in cytoplasmic Ca2+ was not significantly different (t test, P > 0.05) from those that did not show changes in cytoplasmic Ca2+.
Figure 3.
N. tabacum guard cell in intact plant, with an ABA-induced rise of the cytoplasmic free Ca2+ concentration, matching an increase of anion currents. A, ABA responses of a guard cell clamped to −100 mV, loaded with FURA2, and exposed to 50 μm ABA as indicated by the bar below the graphs. Top trace, Cytoplasmic free Ca2+ concentration as calculated from the FURA2 F345:F390 fluorescent ratio. Note that ABA induces a transient rise in the free Ca2+ concentration. Lower trace, current trace at −100 mV. Note that ABA induces an inward current that matches the rise in the cytoplasmic free Ca2+ concentration. B, False color cytoplasmic free Ca2+ images of the same guard cell as in A, before, during, and after the response to ABA (symbols correspond to A). Color codes are linked to free Ca2+ concentration in the bar on the left. Note that ABA triggers a rise in the cytoplasmic free Ca2+ concentration throughout the cell, which is most obvious in the area surrounding the nucleus.
Figure 4.
N. tabacum guard cells in intact plants, in which ABA induced an increase in anion channel activity, accompanied by a delayed rise in the cytoplasmic free Ca2+ concentration (A) or without a change of the Ca2+ concentration (B). A and B, ABA responses of guard cells clamped to −100 mV, loaded with FURA2, and exposed to 50 μm ABA as indicated by the bar below the graphs. Top traces, cytoplasmic free Ca2+ concentration as calculated from the FURA2 F345:F390 fluorescent ratio. Note that ABA induces a transient rise in the free Ca2+ concentration that is delayed compared to the increase in current in A but no change in the Ca2+ concentration in B. Bottom traces, current trace at −100 mV. Note that ABA induces transient increases in inward current in both cells.
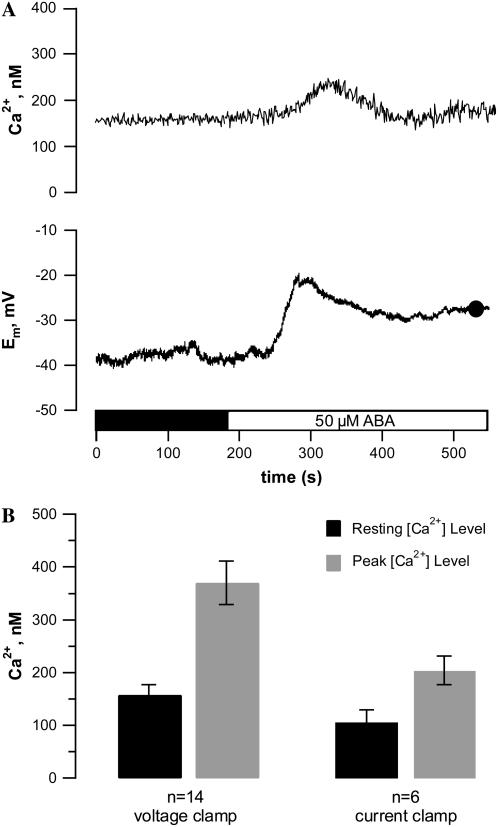
Guard cells of V. faba and Arabidopsis operate Ca2+-permeable plasma membrane channels that activate upon hyperpolarization (Grabov and Blatt, 1998; Pei et al., 2000). ABA may activate these channels through protein phosphorylation (Köhler and Blatt, 2002) or through an ABA-induced rise in reactive oxygen species (Pei et al., 2000). Based on the latter results, the ABA-induced rise in cytoplasmic Ca2+ should be voltage dependent and thus more pronounced at negative voltages. Hyperpolarization of the plasma membrane of N. tabacum to −250 mV induced a large rise in the cytoplasmic Ca2+ concentration (data not shown), indicating the guard cells of this species are equipped with the same Ca2+ uptake machinery as V. faba and Arabidopsis. Guard cells that were left at the free-running membrane potential (average Em = −55 mV, se = 5, n = 6) still displayed ABA-induced changes in the cytoplasmic Ca2+ concentration (Fig. 5A). In line with the properties of hyperpolarization-activated calcium uptake, the ABA-induced rise in cytoplasmic Ca2+ was smaller at the free-running potential compared to −100 mV (Fig. 5B).
Figure 5.
ABA-induced change in free-running membrane potential and cytoplasmic free Ca2+ concentration of a N. tabacum guard cell in an intact plant. A, ABA response of a guard cell at its free-running membrane potential, loaded with FURA2, and exposed to 50 μm ABA as indicated by the bar below the graphs. Top traces, cytoplasmic free Ca2+ concentration as calculated from the FURA2 F345:F390 fluorescent ratio. Note that ABA induces a transient rise in the free Ca2+ concentration that matches the change in free-running membrane potential. Bottom traces, free-running membrane potential of the same cell as in the top trace. B, Average free Ca2+ concentration of N. tabacum guard cells as calculated from the FURA2 F345:F390 fluorescent ratio before (black bar) or at the peak of the ABA response (gray bar); cells not showing an ABA-induced change in cytoplasmic free Ca2+ were not included. Guard cells were clamped at a holding potential of −100 mV (left bars) or at their free-running membrane potential (average Em = −55 mV, se = 5, n = 6, right bars). Error bars represent se, n is indicated below the bars. Note that the average free Ca2+ concentration is lower in cell at their free-running membrane potential and is raised by ABA irrespective of current- or voltage-clamp conditions.
Patch Clamp Experiments
Anion channels in guard cells were ABA activated through a Ca2+-independent pathway (Fig. 4), even though these channels were previously found to be stimulated by Ca2+ in V. faba (Schroeder and Hagiwara, 1989; Hedrich et al., 1990) and Arabidopsis (Allen et al., 1999). We therefore tested if anion channel activity in N. tabacum guard cells is also affected by cytoplasmic Ca2+. These studies were conducted with the patch clamp technique, which allows precise control of the cytoplasmic composition.
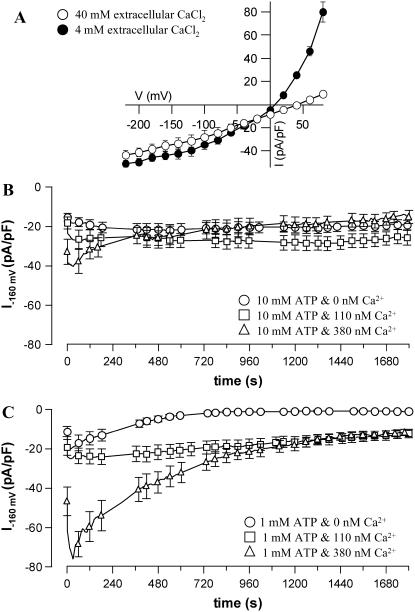
Protoplasts of N. tabacum guard cells were patched at conditions that block K+ channels and allow recordings of anion channels. The nature of the channels recorded under these conditions was tested with voltage clamp protocols from a holding potential of −158 mV to a preconditioning voltage of +62 mV and test potentials ranging from +82 to −218 mV. Lowering the extracellular Cl− concentration from 80 to 8 mm shifted the reversal potential of the plasma membrane (Fig. 6A) from 7.5 mV (se = 0.2, n = 4) to 41.3 mV (se = 3.5, n = 4). These data show that the channels recorded in these protoplasts have a high permeability for Cl− and a virtually linear current voltage relation, properties that are in line with those of S-type anion channels in other species (Linder and Raschke, 1992; Schroeder and Keller, 1992).
Figure 6.
Ca2+-dependent activation of plasma membrane anion channels in guard cell protoplasts of N. tabacum. A, Cl−-induced shift in reversal potential of anion channels. The current-voltage relation was obtained with voltage clamp step protocols from a holding potential of −158 mV to a preconditioning voltage of +62 mV and test potentials ranging from +82 to −218 mV. Measurements were carried out with a pipette solution containing 10 mm ATP, 110 nm free Ca2+, and 150 mm Cl−. A change of the extracellular Cl− concentration from 80 to 8 mm shifted the reversal potential from 7.5 mV (se = 0.2, n = 4) to 41.3 mV (se = 3.5, n = 4). B and C, Kinetics of activation of anion channels in guard cell protoplasts after establishing the whole cell configuration (t = 0) with 10 mm ATP (B) or 1 mm ATP (C) in the pipette solution. The plasma membrane was clamped to −158 mV and Ca2+ was given via the patch pipette either at a free Ca2+ concentration of 0 (○), 110 (□), or 380 (▵) nm. Error bars represent se, n = 6 to 12. Note that at 10 mm ATP, 380 nm Ca2+ activates anion channels during the first 30 s, but the current decays to the same value as with 0 or 110 nm during prolonged measurements. At 1 mm ATP, 110 and 380 nm Ca2+ activate anion channels during the first 30 s and inhibit slow inactivation.
The effect of cytoplasmic Ca2+ on S-type channels was tested in experiments with patch pipettes containing 10 mm ATP and either 0, 110, or 380 nm free Ca2+. With pipette concentrations of 0 or 110 nm, the activity of anion channels remained virtually constant (Fig. 6B). An increase of the Ca2+ concentration to 380 nm activated anion channels during the first 30 s, but during prolonged recordings, the activity leveled off to the same value as with 110 or 0 nm Ca2+ (Fig. 6B) Apparently, Ca2+ stimulates anion channel activity during the first 30 s after establishing the whole cell configuration, but this effect is lost during prolonged recordings. This suggests that Ca2+ diffusing from the patch pipette into the cell initially stimulates anion channels. However, during prolonged recordings, cytoplasmic components provoking this response may diffuse from the cytoplasm into the pipette, causing the effect of Ca2+ to diminish in time.
Just as with R- and S-type anion channels in V. faba and Arabidopsis (Hedrich et al., 1990; Schwarz and Schroeder, 1998; Allen et al., 1999), the activity of anion channels in N. tabacum was modulated by nucleotides. At a reduced ATP concentration of 1 mm, anion channels were activated with 0 nm Ca2+ in the pipette during the first 30 s, but their activity decayed thereafter (Fig. 6C). Increasing the cytoplasmic Ca2+ concentration to 110 nm inhibited the decay of anion current in time. A further rise to 380 nm enhanced the peak activity of S-type anion channels after 30 s, but afterward, the channels reached the same steady state as with 110 nm Ca2+. These data show that depending on the ATP concentration and the free Ca2+ level, Ca2+ ions activate anion channels either through stimulation of the peak activity (t approximately 30 s) or through inhibition of slow inactivation (half time approximately 6 min).
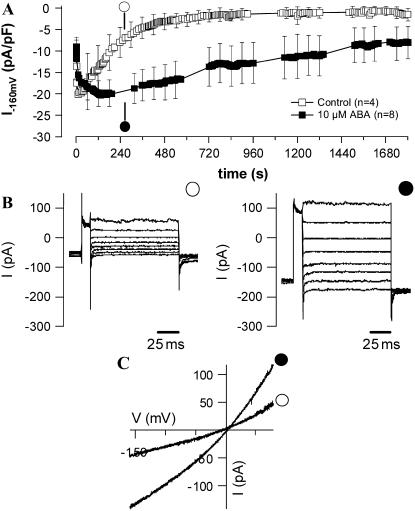
ABA probably activates anion channels through an intracellular receptor, because injection of ABA in V. faba guard cells of intact plants activates anion channels right away, while external application delayed this response by 1 to 2 min (Levchenko et al., 2005). In line with these results, guard cell anion channels in V. faba were activated by ABA applied through the patch clamp pipette (Levchenko et al., 2005). In guard cell protoplasts of N. tabacum, ABA did not enhance the peak anion channel activity but delayed the inactivation of anion channels in time (Fig. 7A). The delayed inactivation of anion channels was not accompanied with changes in voltage-dependent gating (Fig. 7B), nor did ABA alter the reversal potential of whole cell current voltage relation (Fig. 7C). The effect of ABA in patch clamp experiments thus was limited to inhibition of S-type anion channel inactivation in N. tabacum, while it also enhanced the peak activity of these channels in V. faba. These data and the ABA-induced changes in the cytoplasmic free Ca2+ concentration point to species-specific differences in ABA signaling of guard cells.
Figure 7.
Kinetics of ABA-induced activation of anion channels in guard cell protoplasts from N. tabacum. A, Average plasma membrane currents at −158 mV of N. tabacum guard cell protoplasts, patched with pipettes containing 1 mm ATP and 0 mm free Ca2+. ABA was omitted (○) or applied through the patch pipette at a concentration of 10 μm (•). Note that ABA inhibits the time-dependent deactivation of anion channels. B, Current traces obtained with voltage clamp step protocols from a holding potential of −158 mV to a preconditioning voltage of +60 mV and test potentials ranging from +82 to −218 mV. For clarity only current traces of every second voltage step (ΔV = −40 mV) are shown. C, Current-voltage relation of representative protoplasts recorded 4.5 min after establishing the whole cell configurations in the presence or absence of 10 μm ABA in the pipette. The protoplasts were challenged with fast voltage clamp ramps from a holding potential of −158 mV to +82 mV in 1,500 ms.
DISCUSSION
The role of Ca2+ in ABA signaling of guard cells has been discussed ever since DeSilva et al. (1985) showed synergism between ABA and extracellular applied Ca2+. This theory gained support by a number of publications, showing that ABA can trigger a single rise or sustained oscillations in the cytoplasmic free Ca2+ concentration of guard cell in several species (McAinsh et al., 1990; Gilroy et al., 1991; Allen et al., 1999; Webb et al., 2001). However, ABA did not trigger a rise of the Ca2+ concentration in every guard cell recorded (McAinsh et al., 1990; Gilroy et al., 1991; Allen et al., 1999). Guard cells that did not display a rise in Ca2+ still lost turgor (Gilroy et al., 1991) and thus apparently were able to activate anion channels in a Ca2+-independent manner. Such a Ca2+-independent ABA signaling pathway is supported by recent studies, showing that ABA inhibits inward rectifying K+ channels (Romano et al., 2000) and stimulates anion channels (Levchenko et al., 2005) independent of changes in the cytoplasmic Ca2+ concentration.
Ca2+-Independent ABA Signaling
Anion channels in the plasma membrane of N. tabacum guard cells can be activated by ABA through a pathway that does not involve a rise in the cytoplasmic Ca2+ concentration. This pathway is evident from cells that do not display an ABA-dependent increase in cytoplasmic Ca2+ but still activate anion channels (Fig. 4B). ABA did induce a rise in the cytoplasmic Ca2+ concentration of other cells (Fig. 3), but the change of Ca2+ level did not necessarily match that of anion channel activation (Fig. 4A). Even though these data suggest that ABA activation of anion channels occurs through a Ca2+-independent mechanism, it could be inhibited in V. faba by high concentrations of 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA; Levchenko et al., 2005). Apparently, ABA activation of anion channels in V. faba required a basic level of cytoplasmic Ca2+. ABA may thus stimulate anion channel activity by enhancing the affinity for Ca2+ of components in the pathway that lead to anion channel activation (Levchenko et al., 2005).
Open Stomata 1 (OST1) of Arabidopsis (Mustilli et al., 2002) and its V. faba homolog AAPK (Li et al., 2000) resemble Ca2+-independent protein kinases. A close homolog of OST1, SRK2C was transiently stimulated by drought stress with a very similar time course as the stimulation of guard cell anion channels by ABA (Yoshida et al., 2002). The OST1 kinase interacts with the ABI1 protein kinase, which is also not Ca2+ sensitive and acts as a negative regulator of ABA signaling (Bertauche et al., 1996; Merlot et al., 2001). These proteins may thus provide ABA-sensitive, but Ca2+-independent, key elements for activation of anion channels in guard cells.
Ca2+-Dependent ABA Signaling
Even though the ABA-induced activation of plasma membrane anion channels occurs through a Ca2+-independent pathway, the anion channel activity in N. tabacum guard cell protoplasts was stimulated by Ca2+ (Fig. 6, B and C). This is in line with early reports showing that the activity of R- and S-type anion channels in guard cell protoplasts depends on Ca2+ and nucleotides (Schroeder and Hagiwara, 1989; Hedrich et al., 1990; Schmidt et al., 1995). Both channel types, however, seem to be stimulated by nucleotides through different mechanisms. Whereas nonhydrolysable ATP analogs stimulate R-type channels in V. faba guard cells (Hedrich et al., 1990) as well as in Arabidopsis hypocotyl cells (Thomine et al., 1997), the activity of S-type channels strictly depends on hydrolysable ATP (Schmidt et al., 1995; Allen et al., 1999). This suggests that S-type channels are stimulated through phosphorylation, which is supported by a reduced activation in presence of protein kinase inhibitors (Allen et al., 1999) and a delayed down-regulation by the protein phosphatase inhibitor okadaic acid (Schmidt et al., 1995).
Protein phosphorylation may also stimulate S-type anion channels in N. tabacum guard cell protoplasts, because Ca2+ activates these channels in a nucleotide-sensitive mechanism. S-type anion channels were stimulated by an elevated Ca2+ concentration of 380 nm in the patch pipette during the first 30 s. During prolonged measurement, the effect of Ca2+ was lost, which hints to the diffusion of cytoplasmic proteins that mediate this response. The highest degree of activation was found with 1 mm ATP in the pipette solution, while 10 mm ATP leads to a smaller activation of S-type anion channels. This indicates that apart from a Ca2+-dependent activation mechanism, anion channels are also subject to down-regulation by nucleotides at high concentrations.
It is likely that Ca2+-dependent signaling proteins, such as protein kinases associated with the plasma membrane, forward the Ca2+ signal to anion channels. Calcium-dependent protein kinases (CDPK; Hrabak et al., 2003) may represent such regulators, because they seem to be required for the activation of an anion channel in the vacuolar membrane of Arabidopsis guard cells (Pei et al., 1996). Furthermore, these protein kinases are also involved in ABA- and Ca2+-dependent regulation of S-type anion channels and hyperpolarization activated Ca2+ channels (Mori et al., 2006). Previously, a CDPK has been identified in V. faba guard cells, which was able to phosphorylate the KAT1 channel of Arabidopsis (Li et al., 1998).
Alternatively to CDPKs, calcineurin B-like proteins (CBL) may be involved in Ca2+-dependent regulation of ion channels (Batistic and Kudla, 2004; Hedrich and Kudla, 2006). These Ca2+ sensors bind to CBL-interacting protein kinases, which were found to stimulate the AKT1 channel in root cells (Xu et al., 2006). In guard cells of V. faba, similar proteins may inhibit the inward K+ channels, because this response was repressed by inhibitors of calcineurin (Luan et al., 1993). However, an interaction between CBL-interacting protein kinases proteins and plasma membrane anion channels in guard cells has not yet been shown.
Species-Specific ABA-Signaling Pathways
During evolution, two signaling pathways seem to have developed that link perception of ABA to guard cell responses. We found that guard cells of V. faba exclusively utilize the Ca2+-independent pathway (Levchenko et al., 2005), while both Ca2+-dependent and -independent responses were found in N. tabacum. Based on experiments with the Ca2+ reporters FURA2 (Allen et al., 1999; Hetherington and Brownlee, 2004) and cameleon (Allen et al., 2000), ABA also induces Ca2+ signals in guard cells of Arabidopsis and C. communis, suggesting that Ca2+-dependent processes also play a role in these species.
Apart from the activation of anion channels, ABA also inhibits blue light-induced activation of H+-ATPases in guard cells (Goh et al., 1996). This ABA response depends on the protein phosphatases ABI1 and 2 (Roelfsema et al., 1998) and may be mediated through hydrogen peroxide (Zhang et al., 2004). So far, it is not known whether this ABA response, or ABA responses in other cell types, utilizes Ca2+-dependent or -independent signaling pathways. Additional information obtained with other species or different cell types may reveal the evolutionary origin of both pathways and hint to ecological advantages for plants to use one or the other signaling mechanism.
MATERIALS AND METHODS
Plant Material
Nicotiana tabacum L. cv SR1 were grown in a greenhouse under HQL-pressure lamps (Philips, Powerstar HQI-E, 400 W) with a day/night cycle of 12/12 h. For patch clamp experiments, the youngest fully unfolded leaves of 3- to 4-week-old plants were used, while the second or third pair of leaves was used for impalement studies.
Protoplast Isolation and Patch Clamp Experiments
Guard cell protoplasts were isolated according to Raschke and Hedrich (1989). Anion currents were studied in the whole-cell configuration of the patch clamp technique (Hamill et al., 1981) using patch pipettes prepared from Kimax-51 glass (Kimble Products) and coated with silicone (Sylgard 184 silicone elastomer kit, Dow Corning). Currents were recorded with an EPC-7 patch clamp amplifier (HEKA) and low-pass filtered with an eight-pole Bessel filter at a cutoff frequency of 2 kHz and sampled at 2.5-fold filter frequency. Data were digitized (ITC-16; Instrutech), stored on hard disc, and analyzed with HEKA and Wavemetrics software (HEKA Elektronik). The pipette solution contained 150 mm tetraethylammonium chloride, 2 mm MgCl2, 10 mm EGTA, 1 mm MgATP, 10 mm HEPES-Tris, pH 7.2. The bath solution was composed of 40 mm CaCl2, 10 mm MES-Tris, pH 5.6. Protoplasts were characterized by a mean membrane capacitance of 4.4 ± 0.7 pF (n = 140). For ABA experiments, (±)-cis, trans ABA (Lancaster) was used from methanol stocks.
Recordings on Guard Cells in Intact Leaves
Guard cells in intact plants were impaled and recorded with double- and triple-barreled electrodes as described (Roelfsema et al., 2001; Levchenko et al., 2005). Two barrels were filled with 300 mm KCl for membrane potential measurements and voltage clamp, the third barrel was filled with 2 mm FURA2 (Fluka) for current injection, and with an additional 50 mm BAPTA for calibration of FURA2 in the intact plant (Levchenko et al., 2005). The three barrels of the intracellular electrode were connected to microelectrode amplifiers (VF-102, BioLogic), and the membrane potential was clamped by using a differential amplifier (CA-100, BioLogic). Data were filtered at 250 Hz and sampled at 1 kHz during short pulses, or filtered at 10 Hz and sampled at 33 Hz for long-term registration. The solution on the leaf surface contained 5 mm KCl, 5 mm potassium citrate, pH 6, 0.1 mm CaCl2, and 1 mm MgCl2.
Ratiometric Fluorescence Spectroscopy
The dual-excitation wavelength of the Ca2+-dependent fluorescent dye FURA2 was used to monitor the cytoplasmic free Ca2+ concentration. FURA2 was loaded into the guard cell cytoplasm or vacuole by iontophoretic microinjection from the third microelectrode barrel. A loading current up to −350 pA was applied to inject the dye into the cell, during which the holding potential was kept at −100 mV. The injection current therefore was automatically compensated for by a current in the opposite direction through the current injection barrel. Ratiometric fluorescence spectroscopy measurements were carried out by using Metafluor software (Universal Imaging). FURA2 was excited with 200-ms flashes of UV light at 345 and 390 nm with a time interval of 1 s (VisiChrome High speed Polychromator System, Visitron). The emission signal was filtered with a 510-nm bandpass filter (D510/40 m, AF Analysentechnik) and captured with a cooled CCD camera (CoolSNAP HQ, Roper Scientific). Background fluorescence levels of both wavelengths were taken from a reference region placed within a part of the unloaded neighboring guard cell or neighboring epidermal cells. The intracellular free Ca2+ concentration was calculated according to Grynkiewicz et al. (1985) by using the equation:
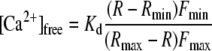 |
where Kd represents the binding constant of FURA2 for Ca2+, R represents the 345:390 nm excitation ratio, and Rmin and Rmax correspond to Ca2+-free and Ca2+-saturated FURA2, respectively. Fmin and Fmax give the fluorescence intensity measured at 390 nm with Ca2+-free and Ca2+-saturated FURA2, respectively. We adapted a Kd of 270 nm, determined in vitro by Levchenko et. al (2005). Rmin and Fmin were defined as the values obtained after simultaneously injecting FURA2 and BAPTA at 0 mV into guard cells of intact plants. The values for Rmax and Fmax were obtained by clamping the plasma membrane from 0 mV to −250 mV, inducing a saturating influx of Ca2+ through hyperpolarization-activated Ca2+ channels.
This work was supported by the Deutsche Forschungsgemeinschaft (grants to R.H., M.R.G.R., and P.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Rainer Hedrich (hedrich@botanik.uni-wuerzburg.de).
References
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411 1053–1057 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Schumacher K, Shimazaki CT, Vafeados D, Kemper A, Hawke SD, Tallman G, Tsien RY, Harper JF, et al (2000) Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 289 2338–2342 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Kuchitsu K, Chu SP, Murata Y, Schroeder JI (1999) Arabidopsis abi1-1 and abi2-1 phosphatase mutations reduce abscisic acid-induced cytoplasmic calcium rises in guard cells. Plant Cell 11 1785–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR (1995) Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA 92 9520–9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki K (1999) The multisensory guard cell: stomatal responses to blue light and abscisic acid. Plant Physiol 119 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batistic O, Kudla J (2004) Integration and channeling of calcium signaling through the CBL calcium sensor/CIPK protein kinase network. Planta 219 915–924 [DOI] [PubMed] [Google Scholar]
- Bertauche N, Leung J, Giraudat J (1996) Protein phosphatase activity of abscisic acid insensitive 1 (ABI1) protein from Arabidopsis thaliana. Eur J Biochem 241 193–200 [DOI] [PubMed] [Google Scholar]
- DeSilva DLR, Hetherington AM, Mansfield TA (1985) Synergism between calcium ions and abscisic acid in preventing stomatal opening. New Phytol 100 473–482 [Google Scholar]
- Gilroy S, Fricker MD, Read ND, Trewavas AJ (1991) Role of calcium in signal transduction of Commelina guard cells. Plant Cell 3 333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh CH, Kinoshita T, Oku T, Shimazaki K (1996) Inhibition of blue light-dependent H+ pumping by abscisic acid in Vicia guard-cell protoplasts. Plant Physiol 111 433–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabov A, Blatt MR (1998) Membrane voltage initiates Ca2+ waves and potentiates Ca2+ increases with abscisic acid in stomatal guard cells. Proc Natl Acad Sci USA 95 4778–4783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260 3440–3450 [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp technique for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv Euro J Physiol 391 85–100 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K (1990) Calcium ion and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J 9 3889–3892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Kudla J (2006) Calcium signaling plant K+ uptake networks channel. Cell 125 1221–1223 [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55 401–427 [DOI] [PubMed] [Google Scholar]
- Hrabak EM, Chan CWM, Gribskov M, Harper JF, Choi JH, Halford N, Kudla J, Luan S, Nimmo HG, Sussman MR, et al (2003) The Arabidopsis CDPK-SnRK superfamily of protein kinases. Plant Physiol 132 666–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L, Mills LN, Pical C, Leckie CP, Aitken FL, Kopka J, Mueller-Roeber B, McAinsh MR, Hetherington AM, Gray JE (2003) Phospholipase C is required for the control of stomatal aperture by ABA. Plant J 34 47–55 [DOI] [PubMed] [Google Scholar]
- Köhler B, Blatt MR (2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32 185–194 [DOI] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R (2005) Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA 102 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyman B, Geelen D, Quintero FJ, Blatt MR (1999) A tobacco syntaxin with a role in hormonal control of guard cell ion channels. Science 283 537–540 [DOI] [PubMed] [Google Scholar]
- Li J, Lee YR, Assmann SM (1998) Guard cells possess a calcium-dependent protein kinase that phosphorylates the KAT1 potassium channel. Plant Physiol 116 785–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Wang XQ, Watson MB, Assmann SM (2000) Regulation of abscisic acid-induced stomatal closure and anion channels by guard cell AAPK kinase. Science 287 300–303 [DOI] [PubMed] [Google Scholar]
- Linder B, Raschke K (1992) A slow anion channel in guard cells, activating at large hyperpolarization, may be principal for stomatal closing. FEBS Lett 313 27–30 [DOI] [PubMed] [Google Scholar]
- Luan S, Li WW, Rusnak F, Assmann SM, Schreiber SL (1993) Immunosuppressants implicate protein phosphatase regulation of K+ channels in guard-cells. Proc Natl Acad Sci USA 90 2202–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Brownlee C, Hetherington AM (1990) Abscisic acid-induced elevation of guard cell cytosolic Ca2+ precedes stomatal closure. Nature 343 186–188 [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25 295–303 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang Y-F, Andreoli S, Tiriac H, Alonso J, Harper JF, Ecker JR, et al (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4 e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustilli AC, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406 731–734 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Ward JM, Harper JF, Schroeder JI (1996) A novel chloride channel in Vicia faba guard cell vacuoles activated by the serine/threonine kinase, CDPK. EMBO J 15 6564–6574 [PMC free article] [PubMed] [Google Scholar]
- Raschke K, Hedrich R (1989) Patch clamp measurements on isolated guard-cell protoplasts and vacuoles. Methods Enzymol 174 312–330 [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into “the Watergate”. New Phytol 167 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Levchenko V, Hedrich R (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37 578–588 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Staal M, Prins HBA (1998) Blue light-induced apoplastic acidification of Arabidopsis thaliana guard cells: inhibition by ABA is mediated through protein phosphatases. Physiol Plant 103 466–474 [Google Scholar]
- Roelfsema MRG, Steinmeyer R, Staal M, Hedrich R (2001) Single guard cell recordings in intact plants: light-induced hyperpolarization of the plasma membrane. Plant J 26 1–13 [DOI] [PubMed] [Google Scholar]
- Romano LA, Jacob T, Gilroy S, Assmann SM (2000) Increases in cytosolic Ca2+ are not required for abscisic acid-inhibition of inward K+ currents in guard cells of Vicia faba L. Planta 211 209–217 [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao YJ, Schroeder JI (1995) Strong regulation of slow anion channels and abscisic-acid signaling in guard-cells by phosphorylation and dephosphorylation events. Proc Natl Acad Sci USA 92 9535–9539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Allen GJ, Hugouvieux V, Kwak JM, Waren D (2001) Guard cell signal transduction. Annu Rev Plant Physiol Plant Mol Biol 52 627–658 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Hagiwara S (1989) Cytosolic calcium regulates ion channels in the plasma membrane of Vicia faba guard cells. Nature 338 427–430 [Google Scholar]
- Schroeder JI, Keller BU (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA 89 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz M, Schroeder JI (1998) Abscisic acid maintains S-type anion channel activity in ATP-depleted Vicia faba guard cells. FEBS Lett 428 177–182 [DOI] [PubMed] [Google Scholar]
- Thomine S, Guern J, BarbierBrygoo H (1997) Voltage-dependent anion channel of Arabidopsis hypocotyls: nucleotide regulation and pharmacological properties. Journal of Membrane Biology 159 71–82 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55 1157–1166 [DOI] [PubMed] [Google Scholar]
- Webb AA, Larman MG, Montgomery LT, Taylor JE, Hetherington AM (2001) The role of calcium in ABA-induced gene expression and stomatal movements. Plant J 26 351–362 [DOI] [PubMed] [Google Scholar]
- Wille AC, Lucas WJ (1984) Ultrastructural and histochemical studies on guard cells. Planta 160 129–142 [DOI] [PubMed] [Google Scholar]
- Xu J, Li HD, Chen LQ, Wang Y, Liu LL, He L, Wu WH (2006) A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell 125 1347–1360 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43 1473–1483 [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang HB, Takemiya A, Song CP, Kinoshita T, Shimazaki K (2004) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136 4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]