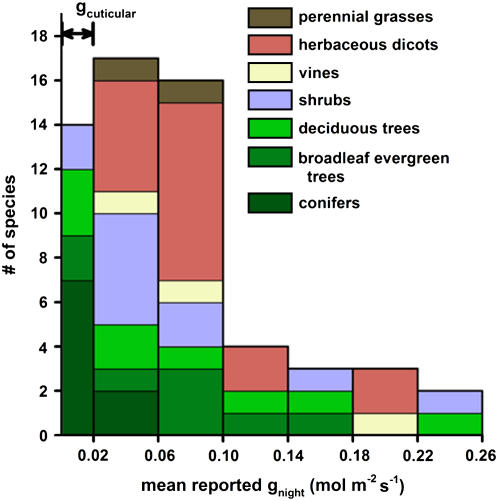
Incomplete stomatal closure during the night is observed in a diverse range of C3 and C4 species (Fig. 1; Supplemental Table S1) and can lead to substantial nighttime transpirational water loss. Although water loss is an inevitable consequence of stomatal opening for photosynthetic carbon gain, nighttime stomatal opening is unexpected because carbon gain is not occurring and the need to cool leaves is reduced or absent. Most species have the ability to close stomata more than is commonly observed at night, as demonstrated by reduced nighttime leaf conductance (gnight) in response to water stress, abscisic acid (ABA), and other treatments reviewed in this Update.
Figure 1.
Histogram summarizing reported gnight in species among different plant functional groups. For each species, gnight was averaged from all reported values with units in mol m−2 s−1 presented in Supplemental Table S1 and thus represents a mixture of field and greenhouse studies. The black two-headed arrow at the top left of the graph represents the range for reported gcuticular taken from many species, and reported gnight within this range may be largely due to gcuticular rather than gstomatal. A complete listing of species with references is provided in Supplemental Table S1.
The magnitude of water loss occurring during the night depends on both gnight and the vapor pressure difference (VPD) between leaves and the air, as well as canopy structure and atmospheric mixing. While gnight has been recorded at up to 90% of daytime conductance, nighttime VPD is typically much lower than daytime. Thus, nighttime transpiration rates (Enight) are typically 5% to 15% of daytime rates, although sometimes as high as 30%, based on gas exchange measurements of individual leaves, whole-plant sap flow, and field scale lysimetry (Benyon, 1999; Snyder et al., 2003; Bucci et al., 2004, 2005; Daley and Phillips, 2006; Scholz et al., 2007). While some methods are more accurate and/or have less uncertainty than others, a few studies have compared methods, generally finding agreement even across measurement scales (e.g. leaf versus whole plant; Green et al., 1989). Drawbacks for each method must be recognized, particularly when comparing species or environments. For example, leaf-level gas exchange typically includes cuticular as well as stomatal components of leaf conductance to water vapor, while sap flow methods typically have attendant uncertainties as to the proportion of the measured flux resulting in bole refilling rather than transpiration from the canopy. Nevertheless, there is broad agreement among the methods and scales that stomata of many species remain partially open during the night.
Measurements of minimum leaf conductance induced by ABA application and by drying excised leaves to wilting have been used to separate stomatal (gstomatal) and cuticular (gcuticular) conductance (Rawson and Clarke, 1988; Howard and Donovan, 2007). Conductance measured at maximal stomatal closure can be functionally defined as gcuticular because it is not under guard cell regulation. For a few species, this would include the effect of dust or stomatal plugs that prevent complete closure (Feild et al., 1998). In general, gcuticular estimates range from 0.004 to 0.020 mol m−2 s−1 (Rawson and Clarke, 1988; Nobel, 1991; Kerstiens, 1995; Boyer et al., 1997; Burghardt and Riederer, 2003; Howard and Donovan, 2007), far lower than most estimates of gnight (Fig. 1; Supplemental Table S1). Thus, most reported values of gnight are largely influenced by gstomatal.
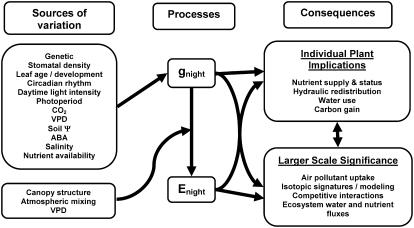
Although awareness of gnight and Enight has recently been growing, little is understood about the phenomena. In particular, the costs and benefits of high gnight and Enight remain largely unknown. However, patterns of occurrence and relationships of these processes with plant physiology are emerging. This Update reviews the occurrence of gnight in C3 and C4 species, plant and environmental factors that affect gnight, and both documented and hypothesized implications of gnight and Enight (Fig. 2).
Figure 2.
Diagram summarizing sources of variation (internal and external) affecting gnight and transpiration (Enight), and consequences of gnight and Enight at the individual plant and larger scales.
SOURCES OF VARIATION AND FACTORS AFFECTING gnight
Variation Among and Within Species
Species in which gnight has been documented include a diverse range of genera and life forms (annuals and perennials; monocots, herbaceous dicots, shrubs, and trees; Fig. 1; Supplemental Table S1) native to a diversity of habitats: e.g. wetland (Loftfield, 1921), desert (Donovan et al., 2003; Snyder et al., 2003; Ludwig et al., 2006), neotropical savanna (Bucci et al., 2004, 2005; Domec et al., 2006; Scholz et al., 2007), temperate deciduous and evergreen forests (Benyon, 1999; Oren et al., 2001; Barbour et al., 2005; Daley and Phillips, 2006; Kavanagh et al., 2007), and subalpine forest (Herzog et al., 1998). Many horticultural and crop species have substantial gnight and/or Enight (England, 1963; Rosenberg, 1969; Rawson and Clarke, 1988; Green et al., 1989; Blom-Zandstra et al., 1995; Assaf and Zieslin, 1996; Musselman and Minnick, 2000). Although it has been suggested that sustained nocturnal stomatal opening is not a feature of grasses (Loftfield, 1921), substantial gnight has been observed in Distichlis spicata (C4; Snyder et al., 2003) and wheat (Triticum aestivum; C3; Rawson and Clarke, 1988), among others.
Substantial variation in magnitude of maximum gnight has been observed among closely related species (Supplemental Table S1); however, differences among some species are minimal and not biologically significant (see Helianthus species, Supplemental Table S1; Howard and Donovan, 2007). Multiple surveys have shown that gnight varies substantially among species within a particular environment or habitat type (Snyder et al., 2003; Bucci et al., 2004; Daley and Phillips, 2006; Kavanagh et al., 2007), and the relationship of species differences to source environment or habitat remains unclear. Additional studies investigating gnight in a phylogenetic context in native and common garden locations will be required to determine whether species differences in gnight are adaptive.
Many studies have also demonstrated genetic variation in magnitude of gnight among cultivars or accessions of single species (Supplemental Table S1). Arabidopsis (Arabidopsis thaliana) natural accessions had a 2.5-fold variation in magnitude of gnight when grown in a common environment, and the variation was correlated to mean annual VPD of the accessions native environment (M. Caird, unpublished data). Although correlative, this relationship suggests the potential for natural selection to have operated on gnight. In addition to genetic variation, there is also evidence for separate genetic control of gnight from gday. Three near-isogenic lines of Arabidopsis differed from their parental lines in either gnight or gday, but not both, providing evidence that these two traits can be regulated independently due to genetic factors alone (M. Caird, unpublished data). Future studies exploiting natural and mutant genotypes will likely play an important role in discovering the genetic factors that influence gnight in plants.
Although recent studies of nighttime water loss generally do not consider differences in stomatal density or adaxial and abaxial surface responses, these factors may contribute to within and among species variation in gnight. Not only does stomatal density often differ between adaxial and abaxial leaf surfaces, but the stomata on these surfaces can respond differently to environmental cues such as light. Stomata on the abaxial leaf surface, but not the adaxial surface, remained open at night in cotton (Gossypium hirsutum; Sharpe, 1973) and fava bean (Vicia faba; Aben et al., 1989). Future studies need to consider how these factors may affect gnight and Enight, particularly with regard to between and within species variation in gnight.
Diurnal Patterns for gstomatal
For many species, gnight is not stable throughout the night period. Endogenous, gradual increases in stomatal opening during predawn hours have been reported in many species under natural field conditions as well as in controlled environments (Schwabe, 1952; Muchow et al., 1980; Anderson, 1982; Lasceve et al., 1997; Leymarie et al., 1998, 1999; Donovan et al., 2003; Bucci et al., 2004; Dodd et al., 2005; Howard and Donovan, 2007). In Arabidopsis accession Columbia, a mean minimum gnight of 0.117 mol m−2 s−1 slowly increased to a predawn mean of 0.161 mol m−2 s−1, amounting to a 38% increase in gstomatal during the night (Lasceve et al., 1997). Arabidopsis mutants with disrupted circadian rhythms do not have increased stomatal opening in predawn hours, indicating gnight has some component of circadian regulation (Dodd et al., 2004, 2005). Lasceve et al. (1997) also found starch-deficient Arabidopsis mutants do not have the increased endogenous predawn opening observed in wild-type plants, implying that starch metabolism, possibly through the formation of an osmoticant necessary for guard cell osmoregulation, is an important factor affecting stomatal opening during predawn.
Photoperiod length and light intensity can affect the speed and degree to which stomata close in the dark. Incomplete stomatal closure at night resulted from short-day as opposed to long-day photoperiods in Chrysanthemum (Schwabe, 1952). Higher light intensity during the day or longer supplementary lighting intervals (extending light period into the normal night) resulted in faster stomatal closure responses to lights turning off in roses, although closure was still incomplete (Blom-Zandstra et al., 1995). The spectrum of the low intensity supplementary light (25 μmol m−2 s−1) also affected gnight, with orange and blue supplementary light preventing complete stomatal closure 2.5 h into the night, while white and no (control) supplementary light resulted in gnight comparable to previously determined gcuticular (0.01 mol m−2 s−1; Blom-Zandstra et al., 1995). This evidence, together with evidence of starch-deficient Arabidopsis mutants having decreased nighttime stomatal opening (Lasceve et al., 1997), suggests that daytime conditions and photosynthetic rates can influence gnight. Although the exact mechanism is unclear, it is possible that a byproduct of starch metabolism may affect guard cell osmoregulation at night (Lasceve et al., 1997), causing greater stomatal opening when starch levels are high. Positive correlations have been observed for gnight and gday among species in Great Basin habitats (Snyder et al., 2003). Although only correlative data are available, the relationship may be the result of daytime conditions that allow high photosynthetic rates but also result in high gnight. Alternatively, leaf development and stomatal anatomy that affect gday could be a cause for correlation with gnight.
Responses to Atmospheric Water Demand
Atmospheric conditions can be important in driving Enight when stomata are open, as evidenced by canopy scale measurements of crop water loss on weighing lysimeters (England, 1963; Rosenberg, 1969). Advection was found to create sufficient evaporative demand to cause 20% to 30% of total daily transpiration to occur at night in alfalfa (Medicago sativa) in the field (Abdel-Aziz et al., 1964) and kiwifruit (Actinidia deliciosa) in orchards (Green et al., 1989). Seginer (1984) extended this concept to show energy requirements and conditions in greenhouses under which Enight occurs in roses using a modified version of the Penman model.
In natural systems, increased VPD has been correlated with greater Enight at the scale of sap flux for many tree species (Herzog et al., 1998; Benyon, 1999; Oren et al., 2001; Daley and Phillips, 2006; Kavanagh et al., 2007). A trend for lower gnight with increasing VPD has been observed in some species (Muchow et al., 1980; Oren et al., 2001; Bucci et al., 2004), yet some data indicate lack of any response (Barbour et al., 2005). However, correlative studies such as these do not control for possible variation due to inherent circadian regulated stomatal opening that might parallel decreasing VPD during the night. Nevertheless, similar correlations have been found when nighttime VPD around plants is experimentally manipulated, providing more direct evidence that some species do close stomata in response to higher VPD during the night just as during the daytime (Bakker, 1991). A more thorough understanding of whether VPD regulates gnight in a manner parallel to that of gday will require more studies that manipulate VPD while controlling for other potentially confounding factors, including circadian rhythms.
Responses to Water Availability and ABA
It is expected that at night stomata will be sensitive to decreased water availability, just as during the daytime, to conserve water. Lower gnight has been associated with decreased plant water status in Hibiscus cannabinus (Muchow et al., 1980), Pseudostuga menziesii (Running, 1976; Blake and Ferrell, 1977), and Helianthus anomalus (Ludwig et al., 2006). In a field experiment, gnight of unirrigated desert shrubs was lower than that of shrubs receiving surface irrigation (Donovan et al., 2003). In greenhouse studies, gnight decreased in response to a water stress treatment in wheat (Rawson and Clarke, 1988) and in Helianthus species (Howard and Donovan, 2007). Similar to drought, increased salinity also reduced gnight in desert shrubs (Donovan et al., 1999).
The magnitude of gnight can additionally vary seasonally. For Chrysothamnus nauseosus, gnight was reduced at the end of growing season when soils were dry, while the cooccurring Sarcobatus vermiculatus had higher gnight (relative to gday) by the end of the season (Donovan et al., 2003). The Sarcobatus response may be related to its capacity to accumulate high concentrations of leaf apoplastic solutes (James et al., 2006), which could in turn affect stomatal regulation. Seasonal changes in gnight were also found for Pinus ponderosa, with stomata more open during the night in early summer, like Chrysothamnus (Grulke et al., 2004).
ABA can induce stomatal closure during the dark (Rawson and Clarke, 1988; Howard and Donovan, 2007). Similar to responses observed during the daytime, increased concentrations of exogenous ABA resulted in greater stomatal closure at night in Arabidopsis, and stomatal closure in response to ABA was more prominent at higher CO2 (Leymarie et al., 1998, 1999). In P. menziesii seedlings, nighttime leaf resistance was sensitive to the ABA content of leaves (Blake and Ferrell, 1977), indicating ABA induced stomatal closure in response to water stress at night just as during the daytime.
Nutrient Availability
Typically, higher nutrient availability, particularly nitrogen (N), is correlated with higher daytime photosynthesis (Lambers et al., 1998). However, varying results have been found for correlations of nutrient supply and gday (Meinzer et al., 1988; Toft et al., 1989). Similarly, species show different responses of gnight to limited nutrient supply. In two field studies with nutrient treatments, high nutrient plants had lower gnight, but the experimental designs do not allow unambiguous separation of direct effects due to reduced plant demand for nutrient acquisition regulating gnight from indirect effects of plant size or water status (Ludwig et al., 2006; Scholz et al., 2007). Other nutrient response experiments that controlled for plant water status have found differing effects of N supply on gnight. For example, reduced gnight was observed in N-limited Arabidopsis, but higher gnight was found in N-limited D. spicata and Populus balsamifera subsp. trichocarpa (M. Caird and A. Howard, unpublished data). Still other species showed no gnight response to soil nutrient limitations (Helianthus species; Howard and Donovan, 2007). The relationship between varying gnight responses to nutrients and particular life forms or ecological strategies is unknown and may be related to the underlying causes of nutrient status effects on gday.
IMPLICATIONS OF gnight AND Enight
Air Pollution Uptake
The occurrence of high gnight in many C3 and C4 plant species has important implications for air pollutant uptake (Goknur and Tibbitts, 1984; Segschneider et al., 1995; Musselman and Minnick, 2000; Takahashi et al., 2005). gstomatal is a major factor affecting ozone (O3) uptake in plants (Wieser and Havranek, 1993). Tree species in areas with high levels of O3 exposure can have stomata open at night (Wieser and Havranek, 1993; Matyssek et al., 1995), and nocturnal O3 uptake can be a significant proportion of daily O3 uptake (up to 9%; Grulke et al., 2004). Stomatal responsiveness may be reduced after exposure to O3 (Keller and Hasler, 1984; Skarby et al., 1987). Whole-plant production and carbon allocation in Betula pendula were also more sensitive to nighttime compared to daytime O3 exposure (Matyssek et al., 1995). Thus, O3 damage resulting from nighttime uptake may be an important factor for plants. However, gnight may also prove to be useful in areas of high air pollution. For example, H. cannabinus may be useful as a phytoremediator of NO2 because this species has high gnight and gday (Takahashi et al., 2005).
Isotopic Signatures and Modeling
Nighttime stomatal opening may influence oxygen isotope signatures of within-canopy CO2 (Barbour et al., 2005). This has important implications for models describing ecosystem respiratory CO2 flux and its partitioning into above- and below-ground components. 18O enrichment of leaves will also be affected by gnight, complicating the use of such signatures in detecting genetic or environmental effects on transpiration rate. Variation in magnitude of gnight among species and the regulation and responses of gnight to environmental factors (i.e. VPD) are important considerations in determining how large an impact nighttime stomatal opening will have on oxygen isotope signatures. More research on these topics is required and will need to be incorporated into models.
Potential for Increased Daytime Carbon Gain
Plants may be able to increase their photosynthetic carbon gain by preopening stomata before dawn. This might be especially advantageous in water-limited environments because of a higher potential for early morning carbon gain when temperatures and VPD are lower. Although stomatal responses to light are typically fast, there is some evidence to support the hypothesis that maintaining open stomata at night affects daytime opening. In Xanthium pennsylvanicum, the rate of stomatal opening in light was greater when stomata were open during the night (Mansfield and Heath, 1961). However, there is no evidence for an effect of experimentally lowering gnight on carbon gain or gday during the subsequent day. Additional research is necessary to directly test whether high gnight influences early morning and total daily carbon gain, and if so how much and by what mechanism.
Effects on Water Relations
Plant water potential is expected to equilibrate with the wettest soil layer in the rooting zone overnight. However, substantial Enight can prevent equilibration from occurring, resulting in soil-plant predawn water potential disequilibrium, or predawn disequilibrium (Donovan et al., 2001), which complicates interpretation of soil moisture availability based on plant water potential measurements. Enight has been observed to contribute to predawn disequilibrium in many species (Donovan et al., 1999, 2001, 2003; Sellin, 1999; Bucci et al., 2004, 2005; Kavanagh et al., 2007).
Substantial Enight may additionally reduce a plant's ability to conduct hydraulic redistribution (HR, also referred to as hydraulic lift; Richards and Caldwell, 1987). HR occurs when some roots are absorbing water from wet soil locations and other roots of the same plant are losing water to relatively dry soil locations. When stomata are open and the atmospheric conditions allow Enight to occur, the water loss through the shoot should decrease the amount released to drier soil layers because of impacts on plant water potentials. Plants in natural populations can simultaneously have both HR and high Enight, although these two processes may vary in magnitude through the growing season (Donovan et al., 2003; Domec et al., 2006).
Nutrient Supply and Distribution
Significant water loss without simultaneous photosynthetic carbon gain could constitute a major cost to a plant. However, it is possible that Enight may provide a benefit that outweighs this cost. Mobile mineral nutrients are moved into the immediate vicinity of plant roots (i.e. the rhizosphere) by transpiration-driven mass flow of the soil solution (Barber, 1995). Thus, the maintenance of a continuous water stream through the plant during both day and night could potentially result in enhanced nutrient availability to the plant. McDonald et al. (2002) showed that CO2-induced stomatal closure reduced transpiration and N acquisition by Populus deltoides.
Using the Barber-Cushman model, the effect of increased water flux on nitrate uptake and nutrient concentration in the rooting zone can be predicted (Barber and Cushman, 1981). The general result is that increasing water flux eliminates or minimizes the depletion zone, which develops in the rhizosphere, by maintaining a supply of nitrate to the root (Barber and Cushman, 1981; Barber, 1995). However, under low nitrate or high root length density conditions, the effect is reduced and total nitrate uptake is not affected dramatically. We are experimentally testing this hypothesis.
In addition to supply of nutrients to roots, the distribution of nutrients within plants, particularly phloem-immobile nutrients such as calcium, depends on the xylem flow rate and duration of transpiration (Marschner, 1995). An increase in the total amount of water flowing through the xylem may improve nutrition when organs are Ca deficient. Daley and Phillips (2006) also suggest that gnight may enhance nutrient transport within trees such as paper birch by providing oxygen to sapwood parenchyma cells that function in nutrient transport and storage.
Implications for Growth and Plant Fitness
Implications for plant water and nutrient relations suggest that Enight may also impact plant productivity and growth, although experimental evidence on the subject is scarce. It is intuitive that Enight poses costs to plants under water-limiting conditions as evidenced by reduction in gnight in response to water stress. However, more research is necessary to determine what benefits, if any, may either balance or outweigh these costs.
CONCLUSION
Although research dating back to the late 1800's describes stomata of many C3 and C4 plant species as incompletely closing during the night, very little is understood about this phenomenon. We have summarized a growing body of evidence showing that gnight is regulated, in many ways similar to daytime stomatal regulation, and that nighttime stomatal opening and transpiration have implications for plant growth and physiology. Nevertheless, more research will be necessary to fully appreciate the significance of gnight and Enight. Future research on plant regulation of gnight and the consequences of substantial Enight for water and nutrient relations will be key for understanding the ecological and evolutionary consequences of gnight and Enight in C3 and C4 plants.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. A summary of C3 and C4 plant species reported in the literature as having significant gnight and/or nighttime transpirational water loss or incomplete stomatal closure at night.
This work was supported by the National Science Foundation (through a graduate research fellowship to M.A.C., and grant nos. IBN–0416581 and DEB–0419969 [to J.H.R.], and IBN–0131078 and IBN–0416627 [to L.A.D.]) and the California Agricultural Experiment Station.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mairgareth A. Caird (macaird@ucdavis.edu).
The online version of this article contains Web-only data.
References
- Abdel-Aziz MH, Taylor SA, Ashcroft GL (1964) Influence of advective energy on transpiration. Agron J 56 139–142 [Google Scholar]
- Aben J, Thiel F, Boekestein A (1989) Are the stomata of Vicia faba L. closed in the dark? Ultramicroscopy 31 457 [Google Scholar]
- Anderson JE (1982) Factors controlling transpiration and photosynthesis in Tamarix chinensis. J Ecol 63 48–56 [Google Scholar]
- Assaf G, Zieslin N (1996) Night water consumption by rose plants. J Hortic Sci 71 673–678 [Google Scholar]
- Bakker JC (1991) Leaf conductance of four glasshouse vegetable crops as affect by air humidity. Agric For Meteorol 55 23–36 [Google Scholar]
- Barber SA (1995) Soil Nutrient Bioavailability: A Mechanistic Approach. John Wiley & Sons, New York
- Barber SA, Cushman JH (1981) Nitrogen uptake model for agronomic crops. In JK Iskander, ed, Modeling Waste Water Renovation-Land Treatment. Wiley-Interscience, New York
- Barbour MM, Cernusak LA, Whitehead D, Griffin KL, Turnbull MH, Tissue DT, Farquhar GD (2005) Nocturnal stomatal conductance and implications for modeling δ18O of leaf-respired CO2 in temperate tree species. Funct Plant Biol 32 1107–1121 [DOI] [PubMed] [Google Scholar]
- Benyon R (1999) Nighttime water use in an irrigated Eucalyptus grandis plantation. Tree Physiol 19 853–859 [DOI] [PubMed] [Google Scholar]
- Blake J, Ferrell WK (1977) Association between soil and xylem water potential, leaf resistance, and abscisic acid content in droughted seedlings of Douglas fir (Pseudotsuga menziesii). Physiol Plant 39 106–109 [Google Scholar]
- Blom-Zandstra M, Pot CS, Maas FM, Schapendonk HCM (1995) Effects of different light treatments on the nocturnal transpiration and dynamics of stomatal closure of two rose cultivars. Sci Hortic (Amsterdam) 61 251–262 [Google Scholar]
- Boyer JS, Wong SC, Farquhar GD (1997) CO2 and water vapor exchange across leaf cuticle (epidermis) at various water potentials. Plant Physiol 114 185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Campanello P, Scholz FG (2005) Mechanisms contributing to seasonal homeostasis of minimum leaf water potential and predawn disequilibrium between soil and plant water potential in neotropical savanna trees. Trees (Berl) 19 296–304 [Google Scholar]
- Bucci SJ, Scholz FG, Goldstein G, Meinzer FC, Hinojosa JA, Hoffman WA, Franco AC (2004) Processes preventing nocturnal equilibration between leaf and soil water potential in tropical savanna woody species. Tree Physiol 24 1119–1127 [DOI] [PubMed] [Google Scholar]
- Burghardt M, Riederer M (2003) Ecophysiological relevance of cuticular transpiration of deciduous and evergreen plants in relation to stomatal closure and leaf water potential. J Exp Bot 54 1941–1949 [DOI] [PubMed] [Google Scholar]
- Daley MJ, Phillips NG (2006) Interspecific variation in nighttime transpiration and stomatal conductance in a mixed New England deciduous forest. Tree Physiol 26 411–419 [DOI] [PubMed] [Google Scholar]
- Dodd AN, Parkinson K, Webb AAR (2004) Independent circadian regulation of assimilation and stomatal conductance in the ztl-1 mutant of Arabidopsis. New Phytol 162 63–70 [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR (2005) Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science 309 630–633 [DOI] [PubMed] [Google Scholar]
- Domec J-C, Scholz FG, Bucci SJ, Meinzer FC, Goldstein G, Villalobos-Vega R (2006) Diurnal and seasonal variation in root xylem embolism in neotropical savanna woody species: impact on stomatal control of plant water status. Plant Cell Environ 29 26–35 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Grise DJ, West JB, Pappert RA, Alder NN, Richards JH (1999) Predawn disequilibrium between plant and soil water potentials in two cold-desert shrubs. Oecologia 120 209–217 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Linton MJ, Richards JH (2001) Predawn plant water potential does not necessarily equilibrate with soil water potential under well-watered conditions. Oecologia 129 328–335 [DOI] [PubMed] [Google Scholar]
- Donovan LA, Richards JH, Linton MJ (2003) Magnitude and mechanisms of disequilibrium between predawn plant and soil water potentials. Ecology 84 463–470 [Google Scholar]
- England CB (1963) Water use by several crops in a weighing lysimeter. Agron J 55 239–242 [Google Scholar]
- Feild TS, Zwieniecki MA, Donoghue MJ, Holbrook NM (1998) Stomatal plugs of Drimys winteri (Winteraceae) protect leaves from mist but not drought. Proc Natl Acad Sci USA 95 14256–14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goknur AB, Tibbitts TW (1984) Dark opening of stomata as related to SO2 sensitivity of potatoes. HortScience 19 548 [Google Scholar]
- Green SR, McNaughton KG, Clothier BE (1989) Observations of night-time water use in kiwifruit vines and apple trees. Agric For Meteorol 48 251–261 [Google Scholar]
- Grulke NE, Alonso R, Nguyen T, Cascio C, Dobrowolski W (2004) Stomata open at night in pole-sized and mature ponderosa pine: implications for O3 exposure metrics. Tree Physiol 24 1001–1010 [DOI] [PubMed] [Google Scholar]
- Herzog KM, Thum R, Kronfub G, Heldstab H-J, Hasler R (1998) Patterns and mechanisms of transpiration in a large subalpine Norway spruce (Picea abies (L.) Karst.). Ecol Res 13 105–116 [Google Scholar]
- Howard AR, Donovan LA (2007) Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiol 143 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James JJ, Alder NN, Muhling KH, Lauchli AE, Shackel KA, Donovan LA, Richards JH (2006) High apoplastic solute concentrations in leaves alter water relations of the halophytic shrub, Sarcobatus vermiculatus. J Exp Bot 57 139–147 [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Pangle R, Schotzko A (2007) Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiol (in press) [DOI] [PubMed]
- Keller T, Hasler R (1984) The influence of a fall fumigation with ozone on the stomatal behavior of spruce and fir. Oecologia 64 284–286 [DOI] [PubMed] [Google Scholar]
- Kerstiens G (1995) Cuticular water permeance of European trees and shrubs grown in polluted and unpolluted atmospheres, and its relation to stomatal response to humidity in beech (Fagus sylvatica L.). New Phytol 129 495–503 [Google Scholar]
- Lambers H, Chapin FS, Pons TL (1998) Plant Physiological Ecology. Springer, New York
- Lasceve G, Leymarie J, Vavasseur A (1997) Alterations in light-induced stomatal opening in a starch-deficient mutant of Arabidopsis thaliana L. deficient in chloroplast phosphoglucomutase activity. Plant Cell Environ 20 350–358 [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A (1998) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25 785–791 [Google Scholar]
- Leymarie J, Lasceve G, Vavasseur A (1999) Elevated CO2 enhances stomatal responses to osmotic stress and abscisic acid in Arabidopsis thaliana. Plant Cell Environ 22 301–308 [Google Scholar]
- Loftfield JVG (1921) The Behavior of Stomata, Vol 314. Carnegie Institution, Washington, DC
- Ludwig F, Jewitt RA, Donovan LA (2006) Nutrient and water addition effects on day- and night-time conductance and transpiration in a C3 desert annual. Oecologia 148 219–225 [DOI] [PubMed] [Google Scholar]
- Mansfield TA, Heath OVS (1961) Photoperiodic effects on stomatal behaviour in Xanthium pennsylvanicum. Nature 191 974–975 [Google Scholar]
- Marschner H 1995. Mineral Nutrition of Higher Plants, Ed 2. Academic Press, San Diego
- Matyssek R, Gunthardt-Goerg MS, Maurer S, Keller T (1995) Nighttime exposure to ozone reduces whole-plant production in Betula pendula. Tree Physiol 15 159–165 [DOI] [PubMed] [Google Scholar]
- McDonald EP, Erickson JE, Kruger EL (2002) Can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct Plant Biol 29 1115–1120 [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Sharifi MR, Nilsen ET, Rundel PW (1988) Effects of manipulation of water and nitrogen regime on the water relations of the desert shrub Larrea tridentata. Oecologia 77 480–486 [DOI] [PubMed] [Google Scholar]
- Muchow RC, Ludlow MM, Fisher MJ, Myers RJK (1980) Stomatal behaviour of kenaf and sorghum in a semiarid tropical environment. I. During the night. Aust J Plant Physiol 7 609–619 [Google Scholar]
- Musselman RC, Minnick TJ (2000) Nocturnal stomatal conductance and ambient air quality standards for ozone. Atmos Environ 34 719–733 [Google Scholar]
- Nobel PS (1991) Physiochemical and Environmental Plant Physiology. Academic Press, San Diego
- Oren R, Sperry JS, Ewers BE, Pataki DE, Phillips N, Megonigal JP (2001) Sensitivity of mean canopy stomatal conductance to vapor pressure deficit in flooded Taxodium distichum L. forest: hydraulic and non-hydraulic effects. Oecologia 126 21–29 [DOI] [PubMed] [Google Scholar]
- Rawson HM, Clarke JM (1988) Nocturnal transpiration in wheat. Aust J Plant Physiol 15 397–406 [Google Scholar]
- Richards JH, Caldwell MM (1987) Hydraulic lift: substantial nocturnal water transport between soil layers by Artemisia tridentata roots. Oecologia 73 486–489 [DOI] [PubMed] [Google Scholar]
- Rosenberg NJ (1969) Seasonal patterns in evapotranspiration by irrigated alfalfa in the Central Great Plains. Agron J 61 879–886 [Google Scholar]
- Running SW (1976) Environmental control of leaf water conductance in conifers. Can J For Res 6 104–112 [Google Scholar]
- Scholz FG, Bucci SJ, Goldstein G, Meinzer FC, Franco AC, Miralles-Wilhelm F (2007) Removal of nutrient limitations by long-term fertilization decreases nocturnal water loss in savanna trees. Tree Physiol (in press) [DOI] [PubMed]
- Schwabe WW (1952) Effects of photoperiodic treatment on stomatal movement. Nature 169 1053–1054 [DOI] [PubMed] [Google Scholar]
- Seginer I (1984) One the night transpiration of greenhouse roses under glass or plastic cover. Agric For Meteorol 30 257–268 [Google Scholar]
- Segschneider H-J, Wildt J, Forstel H (1995) Uptake of 15NO2 by sunflower (Helianthus annuus) during exposures in light and darkness: quantities, relationship to stomatal aperture and incorporation into different nitrogen pools within the plant. New Phytol 131 109–119 [DOI] [PubMed] [Google Scholar]
- Sellin A (1999) Does pre-dawn water potential reflect conditions of equilibrium in plant and soil water status? Acta Oecol 20 51–59 [Google Scholar]
- Sharpe PJH (1973) Adaxial and abaxial stomatal resistance of cotton in the field. Agron J 65 570–574 [Google Scholar]
- Skarby L, Troeng E, Bostrom CA (1987) Ozone uptake and effects on transpiration, net photosynthesis, and dark respiration in Scots pine. Forest Sci 33 801–808 [Google Scholar]
- Snyder KA, Richards JH, Donovan LA (2003) Night-time conductance in C3 and C4 species: do plants lose water at night? J Exp Bot 54 861–865 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Konaka D, Sakamoto A, Morikawa H (2005) Nocturnal uptake and assimilation of nitrogen dioxide by C3 and CAM plants. Z Naturforsch Sect C Biosci 60 279–284 [DOI] [PubMed] [Google Scholar]
- Toft NL, Anderson JE, Nowak RS (1989) Water use efficiency and carbon isotope composition of plants in a cold desert environment. Oecologia 80 11–18 [DOI] [PubMed] [Google Scholar]
- Wieser G, Havranek WM (1993) Ozone uptake in the sun and shade crown of spruce: quantifying the physiological effects of ozone exposure. Trees (Berl) 7 227–232 [Google Scholar]