Abstract
Mesencephalic dopamine neurons form synapses with acetylcholine (ACh)-containing interneurons in the nucleus accumbens (NAcc). Although their involvement in drug reward has not been systematically investigated, these large aspiny interneurons may serve an important integrative function. We previously found that repeated activation of nicotinic cholinergic receptors enhanced cocaine intake in rats but the role of muscarinic receptors in drug reward is less clear. Here we examined the impact of local changes in muscarinic receptor activation within the NAcc on cocaine and food self-administration in rats trained on a progressive ratio (PR) schedule of reinforcement. Animals were given a minimum of 9 continuous days of drug access before testing in order to establish a stable breaking point (BP) for intravenous cocaine infusions (0.75 mg/kg/infusion). Rats in the food group acquired stable responding on the PR schedule within 7 days. On the test day, rats were bilaterally infused in the NAcc with the muscarinic receptor agonist oxotremorine methiodide (OXO: 0.1, 0.3 or 1 nmol/side), OXO plus the M1 selective antagonist pirenzepine (PIRENZ; 0.3 nmol/side) or aCSF 15 min before cocaine or food access. OXO dose dependently reduced BP values for cocaine reinforcement (-17%, -44% [p<0.05] and -91% [p<0.0001] for 0.1, 0.3 and 1.0 nmol, respectively) and these reductions dissipated by the following session. Pretreatment with PIRENZ blocked the BP-reducing effect of 0.3 nmol OXO. Notably, OXO (0.1, 0.3 and 1.0 nmol/side) injection in the NAcc did not affect BP for food reward. The results suggest that muscarinic ACh receptors in the caudomedial NAcc may play a role in mediating the behavior reinforcing effects of cocaine.
Section: Neuropharmacology, Neuropharmacology and other forms of Intracellular Communication
Keywords: Muscarinic receptors, Mesolimbic dopamine system, Progressive-Ratio, Addiction, Cholinergic system, Acetylcholine, Pirenzepine
1. Introduction
The mesolimbic dopamine (DA) system has been the primary focus of research on the neurochemical substrates of drug reward and addiction. Terminals of DA cells projecting from the ventral midbrain are susceptible to the action of psychostimulants that potentiate DA transmission by acting as substrates for (e.g. amphetamines) or inhibitors of (e.g. cocaine) the DA reuptake transporter (Gulley and Zahniser 2003). A key projection site of DA cells from the ventral tegmental area (VTA) is the nucleus accumbens (NAcc), where DA terminals synapse on medium spiny gamma-aminobutyric acid (GABA)-containing cells and a smaller population of large, aspiny acetylcholine (ACh) -containing interneurons (Smith and Bolam 1990). Cholinergic interneurons have large dendritic arbors and an extensive network of axons that contact many cell bodies and terminals within the NAcc (Kawaguchi et al. 1995; Meredith and Chang 1994). In conjunction with DA inputs from VTA, ACh interneurons can modulate the activity of the GABA projection neurons, the primary output neurons of the NAcc (de Rover et al. 2002; Di Chiara et al. 1994).
Several lines of evidence suggest that stimulation of both nicotinic and muscarinic cholinergic receptors can affect mesolimbic DA levels and modify the reinforcing value of self-administered drugs. In this regard, the action of nicotine (the prototypical agonist at nicotinic ACh receptors) on the mesolimbic DA system has been studied extensively (Mansvelder et al. 2003; Mansvelder and McGehee 2002) as has its interaction with other drugs of abuse such as alcohol (Le et al. 2003; Soderpalm et al. 2000) and cocaine (Bechtholt and Mark 2002; Corrigall et al. 2002; Zachariou et al. 2001). These studies demonstrate that nicotinic activation increases extracellular DA in the NAcc, stimulates locomotor activity with repeated exposure and can potentiate the rewarding value of cocaine.
In comparison to the rather clear-cut situation for nicotinic activation, the role of muscarinic receptors in mediating reward processes is less clear. Muscarinic M5 receptors in the VTA are necessary for slow onset depolarization of dopamine neurons, increased dopamine levels in the NAcc and brain stimulation reward (Yeomans et al. 2001). In addition, there is evidence that systemic administration of muscarinic agonists can decrease amphetamine-induced hyperactivity (Shannon and Peters 1990) and inhibit amphetamine-induced DA release in the NAcc (Ichikawa et al. 2002). Conversely, muscarinic antagonists enhance the locomotor stimulating effects of amphetamine and cocaine (Bymaster et al. 1993; Hagan et al. 1987; Shannon and Peters 1990). Systemic administration of muscarinic agonists and partial agonists have been reported to decrease cocaine self-administration rates in mice (Rasmussen et al. 2000) whereas co-administration of the muscarinic antagonist scopolamine with cocaine decreases cocaine self-administration in rhesus monkeys (Ranaldi and Woolverton 2002). In addition, mice lacking the muscarinic M5 receptor subtype self-administer less cocaine and show reduced cocaine conditioned place preference (CPP) compared to their wild-type counterparts (Fink-Jensen et al. 2003), suggesting that M5 receptors potentiate cocaine reward.
Identifying the precise location of cholinergic effects on psychostimulant reward has been difficult because systemic administration of drugs and genetic deletion models affect receptors throughout the nervous system. However, recent work has focused attention on the NAcc as a site of interaction between cholinergic mechanisms and cocaine reinforcement. ACh interneurons in the NAcc are known to be responsive to cocaine self-administration (Berlanga et al. 2003; Mark et al. 1999) and ablation of cholinergic interneurons within the NAcc using immunotoxin-mediated cell targeting results in increased cocaine-induced locomotor activity and enhanced cocaine reward, as measured by CPP (Hikida et al. 2001). In contrast, augmentation of ACh levels with acetylcholinesterase inhibitors following cholinergic lesions has the opposite effect (Hikida et al. 2001; Hikida et al. 2003). These studies suggest that ACh in the NAcc may have an overall inhibitory effect on cocaine reward.
In humans, a clinical study of the acetylcholinesterase inhibitor, donepezil (Aricept) did not find a significant effect on the abuse of cocaine by addicts as measured by quantitative urinary benzoylecognine (Winhusen et al. 2005). However, this study had a limited number of subjects per group, perhaps insufficient (given the variability in quantitative cocaine metabolite levels) to detect a modest decrease in abuse. Still, the use of cholinergic drugs remains an appealing approach given the number of muscarinic, nicotinic and broad-spectrum cholinergic agents in clinical development as nootropic/cognitive enhancing agents (Narahashi et al. 2004). Cognitive deficits are believed to complicate stimulant abusers’ attempts at abstinence and rehabilitation, perhaps by increasing impulsivity (Goldstein et al. 2005). Medications that directly modulate reinforcement of self-administration may be appealing under these conditions. Pre-clinical studies provide strong evidence that cholinergic cells in the NAcc play an important role in modulating the reinforcing value of psychostimulants but the nature of the cholinergic receptors that are critical for this effect remains unclear. To address this question, the aim of the present study was to examine the role of muscarinic receptors on cocaine reinforcement within the NAcc using the self-administration model combined with microinjections of the muscarinic agonist oxotremorine in rats.
2. Results
Oxotremorine effects on cocaine self-administration.
The initial analysis examined the effect of OXO, PIRENZ, or OXO + PIRENZ on the number of cocaine infusions across all 5 time points (Figures 2 & 3). There was a significant interaction between time and treatment [F(20,116) = 5.06, P < 0.001], indicating that treatment differentially altered cocaine infusions. Follow up ANOVAs across treatment at each time point confirmed that treatment significantly affected cocaine infusions only on the test day [F(5,31) = 11.5, P < 0.001]. Treatment did not significantly alter cocaine infusions on the two baseline or two post-test time points. Based on the significant interaction and our a priori hypothesis that use of an antagonist would block the effect of the agonist, separate analyses were conducted on the OXO dose response data and the PIRENZ co-administration data.
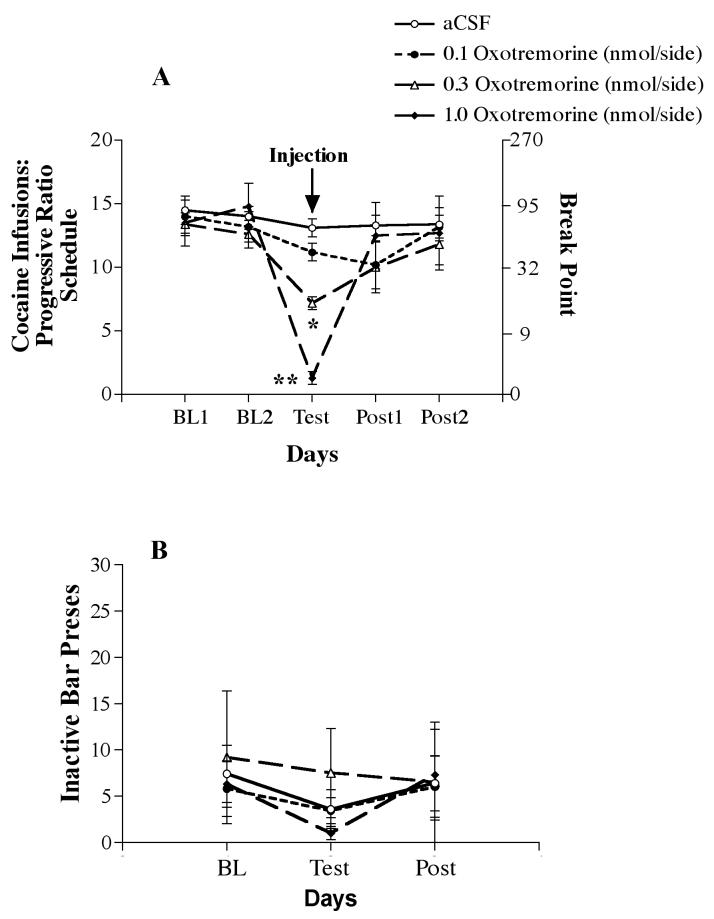
Figure 2.
A. Cocaine infusions per session for the last two days before test injections (Baseline: BL1 and BL2), the test day (Test: aCSF [N=8] or 0.1 [N=5], 0.3 [N=6], 1.0 nmol/side OXO [N=4]) and the two sessions following a test injection (Post 1 and Post 2) for rats responding on a progressive ratio reinforcement schedule. Values are mean (±SEM). Right Y-axis depicts the corresponding break point ratio for the number of cocaine infusions received. Asterisks indicate significant difference from aCSF: *P < 0.05; **P < 0.001. B. Number of bar presses on the inactive lever for the average of the two days before test injections (Baseline: BL), the test day (Test: aCSF or 0.1, 0.3, 1.0 nmol/side OXO) and the average of the two days following a test injection (Post). Values are mean (±SEM).
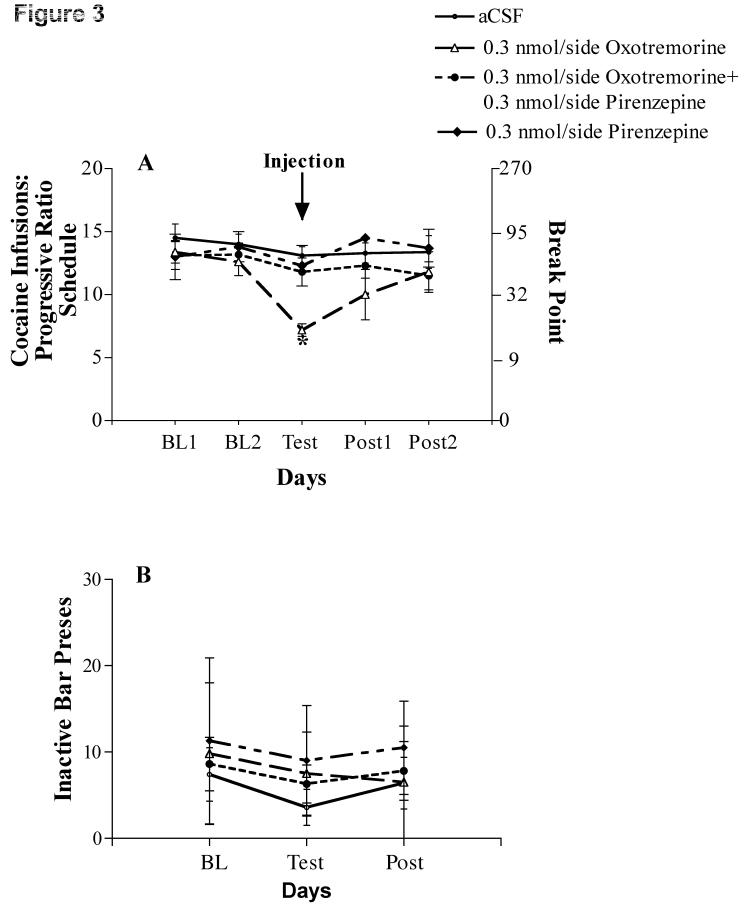
Figure 3.
A. Cocaine infusions per session on a progressive ratio schedule across two baseline days and post-injection days bracketing a test day where rats were given bilateral NAcc injections of 0.3 nmol/side PIRENZ [N=9] or a co-injection of 0.3 nmol/side OXO plus 0.3 nmol/side PIRENZ [N=4] prior to cocaine access. Results of aCSF [N=8] and 0.3 nmol/side OXO [N=6] injections are re-plotted from Figure 2A for comparison. Right Y-axis depicts the corresponding break point ratio for the number of cocaine infusions received. *P < 0.05 versus all other groups. B. Inactive lever presses per session across the average of two baseline days (BL) and the average of two post-injection days (Post) bracketing a test day where rats were given bilateral NAcc injections of 0.3 nmol/side OXO, 0.3 nmol/side PIRENZ or a co-injection of 0.3 nmol/side OXO plus 0.3 nmol/side PIRENZ prior to cocaine access. Values are mean (±SEM).
The dose-dependent effect of OXO microinjections on intravenous cocaine infusions is depicted in Figure 2A. When the analysis was conducted across all 5 time points, there was no main effect of treatment [F(3,19) = 1.56], but there was a significant effect of time [F(4,76) = 19.65, P < 0.001]. A significant interaction between time and treatment [F(12,76) = 5.44, P < 0.001] confirmed that OXO differentially altered cocaine infusions on the test day versus the other time points. This conclusion was supported by the significant main effect of treatment [F(3,19) = 3.95, P < 0.05] and the significant interaction between time and treatment [F(6,38) = 12.63, P < 0.001] when the analysis was conducted across 3 time points (2 baseline days and test day). The significant interactions were followed up with one-way ANOVAs across treatment at each time point. These analyses revealed a significant effect of treatment condition only on the test day [F(3,19) = 30.225, P < 0.001]. Neither session in the baseline period nor the 2-day post-test period showed a significant effect of treatment condition on the number of cocaine infusions. Pairwise comparisons (Tukey HSD) of cocaine infusions on the test day showed significant differences between aCSF and doses of OXO at 0.3 nmol (P < 0.05) and 1.0 nmol (P <0.001). Cocaine infusions following administration of the highest OXO dose also were significantly lower than those following the 0.1 and 0.3 nmol doses (P < 0.001).
There was no statistically significant difference in inactive lever responding between treatment conditions either before (baseline), during OXO treatment (test-day) or in the post-test sessions (Figure 2B). Thus, microinjection of OXO into the NAcc produced a dose-dependent selective reduction in the number of cocaine infusions.
Injection of the M1/4 receptor antagonist, PIRENZ (0.3 nmol/side) attenuated the effect of 0.3 nmol/side OXO, but it did not have an effect on cocaine infusions by itself (Figure 3A). This conclusion is supported by the lack of main effect of treatment [F(2,18) = 0.50] and lack of interaction between treatment and time [F(4,36) = 2.49]. In other words, the subtle but significant change in number of cocaine infusions across time points [F(2,36) = 6.29, P < 0.01] was not differentially altered by treatment. As with the OXO dose-response portion of the study, there were no statistically significant differences between the drug conditions (aCSF, PIRENZ or OXO + PIRENZ) in the number of presses on the inactive lever (Figure 3B).
Oxotremorine effects on food self-administration.
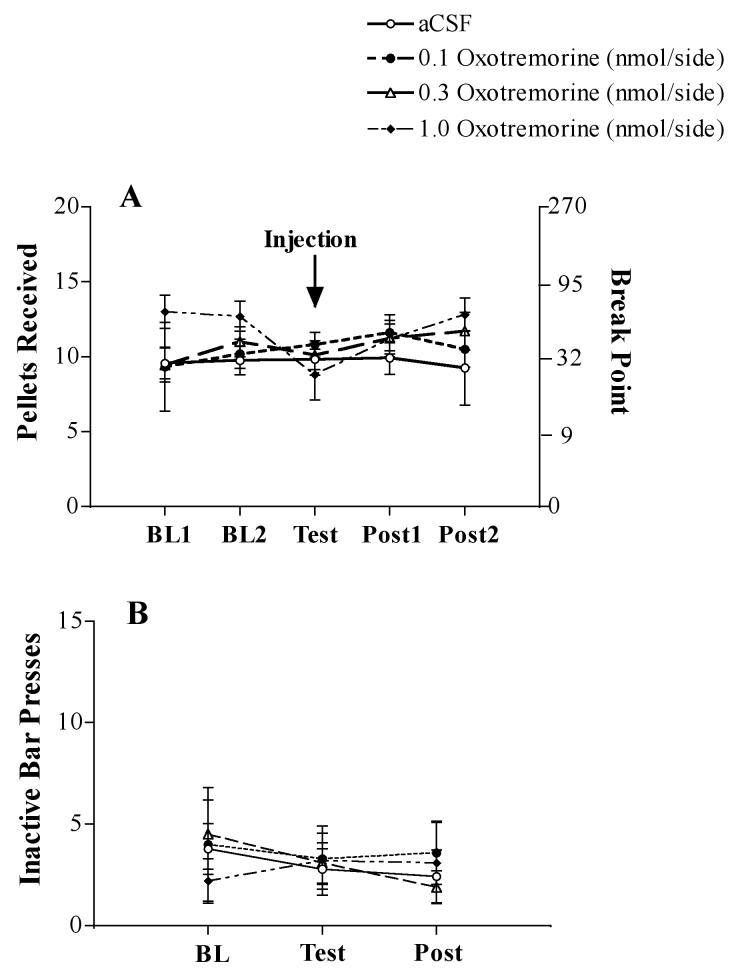
The number of food pellets received and break point on the PR reinforcement schedule following aCSF, 0.1, 0.3 and 1.0 nmol OXO are depicted in Figure 4A. None of the doses of OXO or aCSF affected the number of pellets rats self-administered or their BP ratios (all P’s >0.1). There were no statistically significant effects of treatment condition on activity on the inactive lever, represented in Fig 4B.
Figure 4.
A. Number of pellets received per session for the last two days (Baseline: BL1 and BL2) before test injections, the test day (Test: aCSF [N-12] or 0.1 [N=8], 0.3 nmol/side OXO [N=8]; 1.0 nmol/side OXO [N=5]) and the two sessions following a test injection (Post: P1 and P2) for rats responding on a PR reinforcement schedule. Values are mean (±SEM). Right Y-axis depicts the corresponding break point ratio for the number of food pellets received. B. Number of bar presses on the inactive lever for the average of the two days before test injections (Baseline: BL), the test day (Test: aCSF or 0.1, 0.3, 1.0 nmol/side OXO) and the average of the two days following a test injection (Post). Values are mean (±SEM).
3. Discussion
The major finding of this study was a dose-related reduction in cocaine self-administration in rats following intra-NAcc injections of the muscarinic receptor agonist oxotremorine. Because we measured these reductions in cocaine intake on a progressive ratio (PR) reinforcement schedule, we interpret the reduction in intake (i.e. lowered BP and fewer cocaine infusions per session) to reflect a reduction in reward value of cocaine in the presence of the muscarinic agonist. The interpretation is based on the widely documented finding that the self-administration of cocaine on PR schedules in a variety of species is linearly related to unit-dose (e.g. see Roberts et al. 1989), so a decrease in drug infusions can be interpreted as a decrease in drug reinforcement value.
OXO is a relatively non-selective muscarinic receptor agonist (Garvey et al. 1992) but the methiodide derivative (which was used in this study) has been reported to stimulate phosphoinositide turnover (Messer et al. 1992), an effect that is consistent with activation of M1-like receptors. Coupled with the ability of PIRENZ, a potent M1 antagonist (Gil and Wolfe 1985; Noronha-Blob et al. 1988) to block oxotremorine’s reduction in the BP for cocaine, these findings suggest that the reduction was mediated through activation of the M1 receptor subtype. However, PIRENZ has a relatively strong affinity for M4 receptors (Moriya et al. 1999) in addition to M1 so the involvement of a combined M1/4 effect cannot be ruled out.
The ability of cholinergic systems to modulate psychostimulant reward has been the focus of a number of research efforts (for review, see Smith et al. 2004). Our findings are consistent with several other reports including the findings of Rasmussen et al. (2000) who reported a reduction in cocaine self-administration in mice following systemic administration of several muscarinic agents including OXO (Rasmussen et al. 2000). Beyond this however, the results of the present study have identified a target for the muscarinic suppression of cocaine reward in the NAcc and striatum, where muscarinic receptors of the M1-like subtype (M1, M3 and M5) and M2 subtype (M2 and M4) are located (Liste et al. 2002; Spencer et al. 1986; Tonnaer et al. 1988). The localization of a muscarinic-mediated inhibition of cocaine reward is also consistent with results of studies using immunotoxic techniques to destroy cholinergic interneurons in the NAcc. Hikida et al. demonstrated that ablation of ACh interneurons resulted in up-regulation of cocaine-induced locomotor activity and cocaine-induced CPP (Hikida et al. 2001). Conversely, enhancement of ACh activity in the NAcc has been reported to suppress cocaine reinforcement (Hikida et al. 2003). Their results suggest that ACh interneurons have an inhibitory effect on drug reward and locomotion. Our findings suggest that M1/4 receptor is a key component of this effect and that muscarinic activation selectively reduces cocaine seeking but not food seeking behavior.
Muscarinic receptors modulate dopamine output and intracranial stimulation reward (Yeomans et al. 2001). Rats will self-administer carbachol, a non-selective muscarinic agonist directly into the NAcc (Ikemoto et al. 1998). Muscarinic receptors also play a role in a variety of psychostimulant effects including the development of behavioral sensitization (Heidbreder and Shippenberg 1996), conditioned locomotion (Itzhak and Martin 2000) and sensitivity to locomotor activation (Gralewicz et al. 2003). In addition, there is evidence that muscarinic receptors mediate components of psychostimulant reward-related behaviors such as CPP (McIntyre et al. 1998; Schroeder and Packard 2002) and self-administration behavior in mice (Fink-Jensen et al. 2003; Thomsen et al. 2005) and monkeys (Ranaldi and Woolverton 2002). Muscarinic receptors have also been implicated in mediating learned components of drug seeking. Blockade of muscarinic receptors in the basolateral amygdala prevents the acquisition of drug-stimulus conditioning in a relapse model of cocaine self-administration (See et al. 2003). The results of the present study demonstrate that muscarinic receptors in the medial NAcc play a key role in determining the rewarding effect of cocaine but the definitive subtype and location of the receptors remain to be determined. Using perfused striatal slices in mice Zhang et al. (2002) have shown that multiple muscarinic receptors regulate dopamine output and that an interaction with GABA neurons is an important component of this regulation (Zhang et al. 2002). Therefore, it will be important to identify if muscarinic activation occurs upstream (i.e. pre-synaptically) or downstream (post-synaptically) from dopamine terminals in rat NAcc.
The studies reported here examined the effect of muscarinic activation in animals that had self-administered cocaine for a minimum of several weeks before testing. It is possible that repeated exposure to cocaine modified muscarinic function and/or density but the nature of this regulation is not entirely clear. It is known that acute exposure to cocaine inhibits muscarinic receptors (Sharkey et al. 1988) and with repeated exposure, muscarinic receptors have been reported to be up-regulated in the corpus striatum (Sousa et al. 1999). On the other hand, Macedo et al. have reported a down-regulation of M1- and M2-like receptors in striatum following repeated cocaine treatment (Macedo et al. 2004). In our studies, we found that co-administration of PIRENZ blocked the observed reduction in cocaine infusions following OXO injection in the NAcc, but it did not have a significant effect on cocaine intake by itself. This finding demonstrated that while PIRENZ was able to block the effect of an exogenous muscarinic agonist OXO, the endogenous activation of M1/4 receptors could not have been substantial enough for PIRENZ to affect on cocaine intake. This finding would also argue against an up-regulation of M1 receptors as a consequence of repeated cocaine exposure.
There has been considerable interest in medications that functionally antagonize cocaine’s dopaminergic effects by interacting with midbrain dopamine heteroreceptors (e.g. kappa and GABAB agonists; (Roberts 2005; Shippenberg et al. 1996). Studies decades ago by Janowsky and colleagues (Janowsky et al. 1973) found that an indirect cholinergic agonist (the aceytlcholinesterase inhibitor, physostigmine) blocked the subjective reinforcing effects of the dopaminergic stimulant methylphenidate. More recently, Price and colleagues found that administering oral benztropine (Cogentin) to cocaine addicts prior to cocaine use substantially augmented its euphoric properties (Larry Price, personal communication). Our study and these clinical data demonstrate the potential of cholinergic agents to functionally antagonize (muscarinic agonists) or increase (muscarinic antagonists) cocaine reinforcement mechanisms in the NAcc. Similar mechanisms may also contribute to the abuse potential of muscarinic antagonists (Buhrich et al. 2000).
Collectively, these data provide convergent evidence that cholinergic systems are instrumental in mediating psychostimulant reward. The results of the present study indicate that activation of muscarinic M1 receptors in the NAcc can suppress cocaine reward and suggest that these receptors may be a potential therapeutic target for the treatment of cocaine addiction.
4. Experimental Procedure
Animals
Thirty-three male Sprague-Dawley rats weighing 350-400 g were used in these studies and were maintained according to the guidelines set forth in the “Principles of Laboratory Animal Care” (National Research Council, 2003) and under the approval of the Oregon Health & Science University’s Institutional Animal Care and Use Committee. Animals were housed individually following surgery in a temperature-controlled environment (22° C) with a 12 hr day/night schedule (lights on 0700-1900). All animals had free access to food and water while in their home cages throughout the experiment.
Drug Preparation
Cocaine HCl was supplied by the Research Triangle Institute (Research Triangle Park, NC) under the National Institute on Drug Abuse Drug Supply Program and was dissolved in physiological saline (0.9%). Oxotremorine methiodide (OXO) and pirenzepine dihydrochloride (PIRENZ; Sigma, St Louis, MO) were dissolved in an artificial cerebrospinal fluid (aCSF) containing (in mM): 120 NaCl, 4.8 KCl, 2.5 CaCl2, 1.2 MgCl2, 25 NaHCO3, 1.2 KH2PO4, 10 glucose; pH 7.3. Muscarinic drug solutions were prepared no more than 1 hr before each experiment.
Operant training
Approximately three hours into their light phase animals were placed into standard operant conditioning chambers (30 x 24 x 29 cm) housed in sound-attenuating cubicles (Med Associates Inc.; St. Albans, VT). Each chamber contained a retracted active lever and an inactive lever. The start of a session was indicated by the illumination of the house light and extension of the retractable lever into the cage. Animals were trained to lever press for 45 mg Noyes food pellets (BioServe; Port Washington, NY) on a fixed-ratio-1 (FR-1) schedule of reinforcement during daily 1 hr sessions. Each bar press on the active lever resulted in the delivery of a food pellet and a 1 sec time-out signaled by the illumination of the stimulus light. Bar presses on the inactive lever were counted but had no consequence. Animals were not food deprived during operant conditioning or testing. Upon the completion of the food training, eighteen rats were implanted with intravenous (i.v.) catheters and bilateral intracerebral guide cannulae as described below. The rats in the food-reward group were implanted only with intracerebral guide cannulae.
Surgery
Animals were anesthetized with pentobarbital (20 mg/kg, IP) supplemented by ketamine (40 mg/kg, IP) and implanted with intrajugular catheters assembled based on the method described by Caine et al. (Caine et al. 1993). Briefly, the catheters were constructed of micro-renathane tubing (0.98 mm o.d. x .47 mm i.d.; Braintree Scientific Inc.; Braintree, MA) connected to an L-shaped external guide cannula (Plastics One Inc.; Roanoke, VA), which was fastened to a 1 cm diameter circular section of polypropylene mesh with cranioplastic cement. The tip of the catheter was inserted into the right or left jugular vein approximately 27 mm and the distal end was threaded subcutaneously to an exit point between the scapulae. Catheters were flushed with 0.2 ml of physiologic saline containing heparin (70 u/cc) and the antibiotic ticarcillin (Timentin®, 100 mg/cc; GlaxoSmithKline, Research Triangle Park, NC) daily. Catheter patency was tested when necessary by infusion of the fast-acting barbiturate Brevital® (methohexital sodium; 2.7 mg/kg in 0.1 ml; Lilly, Indianapolis, IN). Catheters were tested if response rates dropped dramatically (>50%) for two consecutive sessions or if responses declined consistently over four daily sessions. When indicated, Brevital tests were performed 1.5 hr after self-administration sessions and catheters were deemed patent if animals showed substantial loss of muscle tone within 5 sec of infusion.
To facilitate intracerebral microinjections of drugs, immediately after intrajugular catheterization animals were stereotaxically implanted with bilateral, 10 cm, 21 ga. stainless steel guide shafts positioned 4 mm above the shell of the NAcc according to the coordinates of Paxinos and Watson (Paxinos and Watson 1998): A +1.6, L ±1.0, V -3.5. Small burr holes were made in the skull and the dura mater was punctured with a needle. Guide shafts were lowered through the holes and secured to the skull via four, 5 mm long stainless steel screws (size 0-80) and dental acrylic. A small, U-shaped aluminum shield was placed 6-7 mm anterior to the guide shafts to provide protection for microinjectors and also an anchor point for a tether (via an alligator clip) to which PE20 infusion lines were attached. All coordinates are referenced to bregma, midsagittal suture and surface of the skull. Guide shafts were kept patent with 26 ga. stylets. Intravenous catheters and intracranial guide shafts were implanted during one surgical session. Animals were allowed a minimum of 7 days recovery after surgery.
Cocaine Self-administration Training and Microinjection Procedure
Following the post-operative recovery period each animal was placed in a test chamber, the IV catheter was flushed with saline and then attached to an infusion line. The beginning of each session was signaled by the illumination of the house light and extension of the active lever into the cage. The inactive lever remained extended at all times. Responses on this lever were counted but had no scheduled consequences. Active lever presses were accompanied by a 4 sec infusion of cocaine (0.75 mg/kg/infusion) on a fixed ratio (FR) 1; time out (TO) 20 s contingency schedule. Rats were trained on this schedule in daily 3 hr sessions until stable levels of responding were obtained (less than 15% variation in total responding over 3-4 days). The number of days required to reach the stability criterion ranged from 6-11 days. Average number of cocaine infusions (± SEM) per session at the end of the acquisition period was 43.3 ± 5.35. No priming infusions were given. When response levels stabilized, the reinforcement contingency was changed to a progressive ratio (PR) schedule similar to that described by Roberts and colleagues (McGregor and Roberts 1995; Roberts and Richardson 1992). Under this schedule, response requirements for each successive drug delivery increased progressively in the following series: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901. When ratios were completed, animals received 4 s infusions of cocaine with a 20 s TO as in the FR condition. The number of cocaine infusions earned (and the ratio achieved) before a 1 hr period of non-reinforcement was scored as the BP for that particular session. Previous work from this lab and others has shown that responding on PR schedules is linearly related to unit-dose of cocaine (Bechtholt and Mark 2002; Martin-Fardon and Weiss 2002; Roberts 1989). Once animals were placed on the PR schedule daily sessions continued until three consecutive days of stable responding (±2 break points) were obtained.
After a stable baseline had been established, rats were given bilateral infusions of aCSF, OXO or PIRENZ into the medial NAcc 15 min before being placed into test chambers for cocaine access. For injections, each rat was gently restrained, the NAcc stylets were removed and a microinjector was inserted into the guide shafts. The microinjector was made of 26 ga. stainless steel tubing, that extended 2 mm beyond the chronically implanted guide shaft when inserted, and a silica glass insert (0.15 mm o.d. x 0.037 mm i.d.; Polymicro Tech Inc.) which extended 2 mm beyond the 26 ga. tubing to reach the NAcc (final ventral coordinate = -7.5 mm). Silica inserts were used to minimize tissue damage in the NAcc. The silica was preloaded with drug and attached to PE20 tubing connected to a 10 μl gas-tight glass syringe (Hamilton;Fisher, St. Louis, MO) loaded onto a syringe pump (Razel; Braintree Inc., Braintree, MA). Injections or aCSF, OXO (0.1, 0.3 or 1.0 nmol/side) or PIRENZ (0.3 nmol/side) were given in a volume of 100 nl over 10 s and the injectors were left in place for an additional 30 s. In the OXO + PIRENZ group, the antagonist PIRENZ was given first (0.3 nmole/side; over 10 s followed by a 30 s diffusion time) followed immediately by OXO (0.3 nmole/side) injection. The stylets were replaced and each rat was placed in its home cage for 5 min before being transferred to cocaine self-administration chambers. Rats received either one (N=6), two (N=8) or three (N=4) injections on separate test days depending on the viability and longevity of the intravenous catheter. In animals that had more than one injection, drug condition was counter-balanced and injections days were separated by a minimum of 2 days of normal cocaine self-administration sessions without intracerebral injections.
Food self-administration and drug microinjections.
Fifteen male Sprague-Dawley rats were used in this experiment. Following the post-operative recovery period, animals were placed in a test chamber and a head clip was attached to the shield. The beginning of each session was signaled by the illumination of the house light and extension of the active lever into the cage. Responses on the inactive lever were counted but had no scheduled consequences. Responses on the active lever resulted in delivery of a 45 mg food pellet (BioServe; Port Washington, NY) using the same PR schedule as described above for rats in the cocaine group. When the ratios were completed animals received a food pellet followed by a 20s time out. The number of food pellets earned (and the ratio achieved) before a 1 hr period of non-reinforcement was scored as the break point for that particular session. Animals placed on the PR schedule continued daily sessions until two consecutive days of stable responding were obtained. Rats were not food restricted during training or testing.
After a stable baseline had been established, rats were given bilateral infusions of aCSF or OXO into the medial NAcc before being placed into test chambers for food access. For injections, each rat was gently restrained, the NAcc stylets were removed and microinjectors were inserted into the guide shafts. Injections of aCSF or OXO (0.1, 0.3 and 1.0 nmol/side) were given in a volume of 100 nl over 10 s with the injectors being left in place for an additional 30 s. The stylets were replaced and rats were placed in self-administration chambers. Rats received OXO (0.1 nmol/side [N=8], 0.3 nmol/side [N=8], 1.0 nmol/side [N=5]) or aCSF (N=12) on separate test days that were counter-balanced and separated by a minimum of 2 days of normal food administration sessions without injections.
Statistical Analyses
A mixed effects analysis of variance (ANOVA) was used to analyze the number of cocaine infusions (and inactive lever presses) per test session for the effects of treatment (aCSF, OXO, PIRENZ, or OXO + PIRENZ), with time as a repeated measures factor. Due to the significant interactions between treatment and time and our a priori hypothesis that use of an antagonist would block the effect of the agonist, separate analyses were conducted on the OXO dose response data (aCSF, N=8; 0.1 nmol OXO, N=5; 0.3 nmol OXO, N=6; 1.0 nmol OXO, N=4) and the PIRENZ co-administration data (aCSF, N=8; 0.3 nmol OXO + 0.3 nmol PIRENZ, N=9; 0.3 nmol PIRENZ, N=4). Five levels were analyzed for time (baseline 1, baseline 2, test day, post-test day 1 and post-test day 2). Data from two pre-injection (baseline) days were included to ensure stability of baseline responding and two post-test time points were analyzed in order to determine if intracerebral injections had any long-lasting effects on BP for cocaine self-administration. Significant interactions were followed up with simple effects analysis (the equivalent of separate one-way ANOVAs) and Tukey HSD post-hoc tests (Maxwell and Delaney 2004). The effect of OXO on food reward was tested in a separate group of rats and the data were analyzed using a two-way ANOVA to examine the effect of treatment (aCSF, N=12; 0.1 nmol OXO-M, N=8 and 0.3 nmol OXO-M, N=8; 1.0 nmol OXO-M, N=5), with time as a repeated measures factor (baseline, test day & post-test day). Alpha level for statistical significance was set at 0.05 for all tests.
Histology
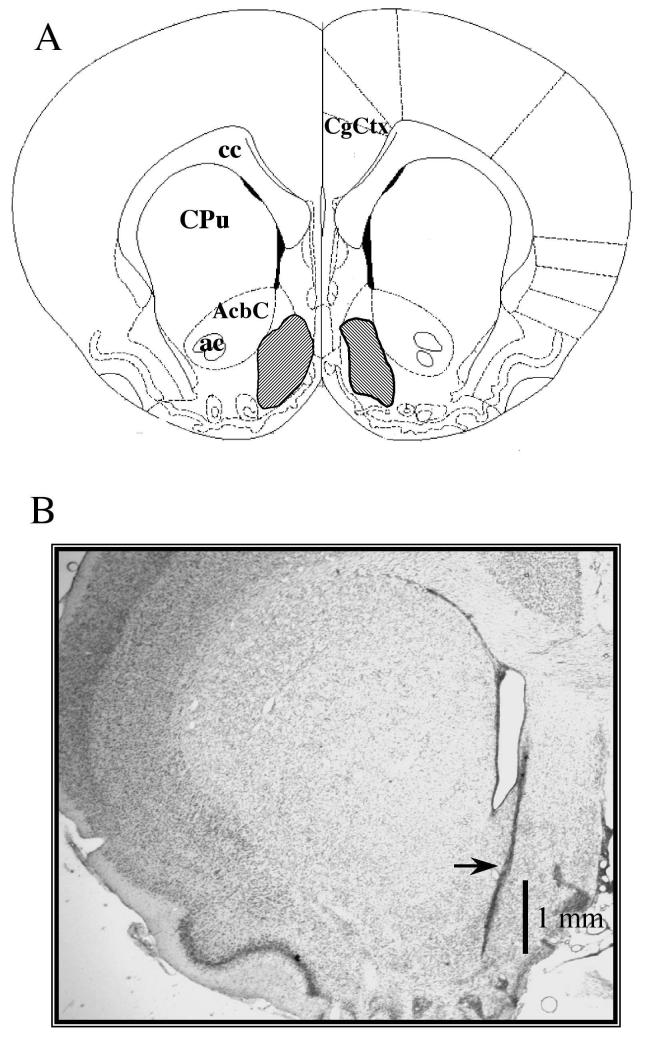
Following the final test session, animals were perfused transcardially with phosphate buffered saline (PBS) followed by 10% formalin. The brain was extracted, post-fixed and placed in 30% sucrose in PBS solution overnight. Forty μm sections were cut on a cryostat, slide mounted and stained with thionin. Using light microscopy, sections were examined for histological verification of microinjection placement according to the atlas of Paxinos and Watson (Paxinos and Watson 1998). Figure 1A shows a diagram of the area encompassing the injections in the medial NAcc for all animals used in this study. Animals where placement of either injector was outside of the hatched region were not included in the analyses. Fig 1B depicts a photomicrograph of a tract for the 2 mm section of the silica injector. The guide cannula is not visible in this section.
Figure 1.
A. Schematic drawing of the extent of bilateral injections within the NAcc shell. The diagram is taken from the atlas of Paxinos and Watson (1998) at the level of bregma +1.7 mm. All injector tips in rats used for analysis in these studies were located within the shaded area. B. Photomicrograph showing the track of an injector (arrow) in a representative section. Vertical bar indicates 1 mm. Abbreviations: AcbC, nucleus accumbens core; ac, anterior commissure; cc, corpus callosum, CgCtx, cingulate cortex; CPu, caudate-putamen.
Acknowledgements
This research was supported by NIH grants DA11203 and DA14639. M.C.G. was supported by individual NRSA F32 DA05965. A.J.B. was supported by grant T32 DA07262. We thank James Welch for excellent technical assistance with histological preparations.
References
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology. 2002;162:178–85. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–56. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Buhrich N, Weller A, Kevans P. Misuse of anticholinergic drugs by people with serious mental illness. Psychiatr Serv. 2000;51:928–9. doi: 10.1176/appi.ps.51.7.928. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Heath I, Hendrix JC, Shannon HE. Comparative behavioral and neurochemical activities of cholinergic antagonists in rats. Journal of Pharmacology & Experimental Therapeutics. 1993;267:16–24. [PubMed] [Google Scholar]
- Caine SB, Lintz R, Koob GF. Intravenous drug self-administration techniques in animals. In: Sahgal A, editor. Behavioral Neuroscience; A Practical Approach. Oxford University Press; New York: 1993. pp. 117–43. [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson L. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology. 2002;160:198–205. doi: 10.1007/s00213-001-0965-2. [DOI] [PubMed] [Google Scholar]
- de Rover M, Lodder JC, Kits KS, Schoffelmeer AN, Brussaard AB. Cholinergic modulation of nucleus accumbens medium spiny neurons. European Journal of Neuroscience. 2002;16:2279–90. doi: 10.1046/j.1460-9568.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends in Neurosciences. 1994;17:228–33. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Fedorova I, Wortwein G, Woldbye DP, Rasmussen T, Thomsen M, Bolwig TG, Knitowski KM, McKinzie DL, Yamada M, Wess J, Basile A. Role for M5 muscarinic acetylcholine receptors in cocaine addiction. Journal of Neuroscience Research. 2003;74:91–6. doi: 10.1002/jnr.10728. [DOI] [PubMed] [Google Scholar]
- Garvey DS, Wasicak JT, Chung JY, Shue YK, Carrera GM, May PD, McKinney MM, Anderson D, Cadman E, Vella-Rountree L, et al. Synthesis and in vitro characterization of novel amino terminally modified oxotremorine derivatives for brain muscarinic receptors. Journal of Medicinal Chemistry. 1992;35:1550–7. doi: 10.1021/jm00087a008. [DOI] [PubMed] [Google Scholar]
- Gil DW, Wolfe BB. Pirenzepine distinguishes between muscarinic receptor-mediated phosphoinositide breakdown and inhibition of adenylate cyclase. Journal of Pharmacology & Experimental Therapeutics. 1985;232:608–16. [PubMed] [Google Scholar]
- Goldstein RZ, Alia-Klein N, Leskovjan AC, Fowler JS, Wang GJ, Gur RC, Hitzemann R, Volkow ND. Anger and depression in cocaine addiction: association with the orbitofrontal cortex. Psychiatry Res. 2005;138:13–22. doi: 10.1016/j.pscychresns.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Gralewicz S, Lutz P, Wiaderna D, Tomas T. Alteration in behavioral sensitivity to amphetamine after treatment with oxotremorine. Brain Research. 2003;147:163–73. doi: 10.1016/s0166-4328(03)00152-9. [DOI] [PubMed] [Google Scholar]
- Gulley JM, Zahniser NR. Rapid regulation of dopamine transporter function by substrates, blockers and presynaptic receptor ligands. European Journal of Pharmacology. 2003;479:139–52. doi: 10.1016/j.ejphar.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Tonnaer JADM, Rijk H, Broekkamp CLE, van Delft AML. Facilitation of amphetamine-induced rotation by muscarinic antagonists is correlated with M2 receptor affinity. Brain Research. 1987;410:69–73. doi: 10.1016/s0006-8993(87)80021-5. [DOI] [PubMed] [Google Scholar]
- Heidbreder CA, Shippenberg TS. Evidence for an involvement of muscarinic cholinergic systems in the induction but not expression of behavioral sensitization to cocaine. Synapse. 1996;24:182–92. doi: 10.1002/(SICI)1098-2396(199610)24:2<182::AID-SYN10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proceedings of the National Academy of Sciences. 2001;98:13351–4. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proceedings of the National Academy of Sciences. 2003;100:6169–73. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa J, Chung YC, Li Z, Dai J, Meltzer HY. Cholinergic modulation of basal and amphetamine-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Brain Research. 2002;958:176–84. doi: 10.1016/s0006-8993(02)03692-2. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Glazier BS, Murphy JM, McBride WJ. Rats self-administer carbachol directly into the nucleus accumbens. Physiology & Behavior. 1998;63:811–4. doi: 10.1016/s0031-9384(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Scopolamine inhibits cocaine-conditioned but not unconditioned stimulant effects in mice. Psychopharmacology. 2000;152:216–23. doi: 10.1007/s002130000537. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. Antagonistic effects of physostigmine and methylphenidate in man. Am J Psychiatry. 1973;130:1370–6. doi: 10.1176/ajp.130.12.1370. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Wilson CJ, Augood SJ, Emson PC. Striatal interneurons: chemical, physiological and morphological characterization. Trends in Neuroscience. 1995;12:527–35. doi: 10.1016/0166-2236(95)98374-8. [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology. 2003;168:216–21. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Liste I, Bernard V, Bloch B. Acute and chronic acetylcholinesterase inhibition regulates in vivo the localization and abundance of muscarinic receptors m2 and m4 at the cell surface and in the cytoplasm of striatal neurons. Molecular and Cellular Neurosciences. 2002;20:244–56. doi: 10.1006/mcne.2001.1083. [DOI] [PubMed] [Google Scholar]
- Macedo DS, Correia EE, Vasconcelos SM, Aguiar LM, Viana GS, Sousa FC. Cocaine treatment causes early and long-lasting changes in muscarinic and dopaminergic receptors. Cellular and Molecular Neurobiology. 2004;24:129–36. doi: 10.1023/B:CEMN.0000012718.08443.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, De Rover M, McGehee DS, Brussaard AB. Cholinergic modulation of dopaminergic reward areas: upstream and downstream targets of nicotine addiction. European Journal of Pharmacology. 2003;480:117–23. doi: 10.1016/j.ejphar.2003.08.099. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Cellular and synaptic mechanisms of nicotine addiction. Journal of Neurobiology. 2002;53:606–17. doi: 10.1002/neu.10148. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Weiss F. BTCP is a potent reinforcer in rats: Comparison of behavior maintained on fixed- and progressive-ratio schedules. 2002;72:343–53. doi: 10.1016/s0091-3057(01)00764-x. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. 2nd Ed. Lawrence Erlbaum Assoc, Lawrence Erlbaum Assoc; 2004. [Google Scholar]
- McGregor A, Roberts DC. Effect of medial prefrontal cortex injections of SCH 23390 on intravenous cocaine self-administration under both a fixed and progressive ratio schedule of reinforcement. Behavioural Brain Research. 1995;67:75–80. doi: 10.1016/0166-4328(94)00106-p. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Ragozzino ME, Gold PE. Intra-amygdala infusions of scopolamine impair performance on a conditioned place preference task but not a spatial radial maze task. Behavioral Brain Research. 1998;95:219–26. doi: 10.1016/s0166-4328(97)00161-7. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Chang HT. Synaptic relationships of enkephalinergic and cholinergic neurons in the nucleus accumbens of the rat. Brain Research. 1994;667:67–76. doi: 10.1016/0006-8993(94)91714-0. [DOI] [PubMed] [Google Scholar]
- Messer WS, Jr., Ngur DO, Abuh YF, Dokas LA, Ting SM, Hacksell U, Nilsson BM, Dunbar PG, Hoss W. Stereoselective binding and activity of oxotremorine analogs at muscarinic receptors in rat brain. Chirality. 1992;4:463–8. doi: 10.1002/chir.530040802. [DOI] [PubMed] [Google Scholar]
- Moriya H, Takagi Y, Nakanishi T, Hayashi M, Tani T, Hirotsu I. Affinity profiles of various muscarinic antagonists for cloned human muscarinic acetylcholine receptor (mAChR) subtypes and mAChRs in rat heart and submandibular gland. Life Sciences. 1999;64:2351–8. doi: 10.1016/s0024-3205(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Narahashi T, Moriguchi S, Zhao X, Marszalec W, Yeh JZ. Mechanisms of action of cognitive enhancers on neuroreceptors. Biol Pharm Bull. 2004;27:1701–6. doi: 10.1248/bpb.27.1701. [DOI] [PubMed] [Google Scholar]
- Noronha-Blob L, Canning B, Costello D, Kinnier WJ. Selective agents for muscarinic receptors linked to phosphoinositide breakdown. European Journal of Pharmacology. 1988;154:161–7. doi: 10.1016/0014-2999(88)90093-3. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th Edition Academic Press, Academic Press; 1998. [Google Scholar]
- Ranaldi R, Woolverton WL. Self-administration of cocaine: scopolamine combinations by rhesus monkeys. Psychopharmacology. 2002;161:442–8. doi: 10.1007/s00213-002-1069-3. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Sauerberg P, Nielsen EB, Swedberg MD, Thomsen C, Sheardown MJ, Jeppesen L, Calligaro DO, DeLapp NW, Whitesitt C, Ward JS, Shannon HE, Bymaster FP, Fink-Jensen A. Muscarinic receptor agonists decrease cocaine self-administration rates in drug-naive mice. European Journal of Pharmacology. 2000;402:241–6. doi: 10.1016/s0014-2999(00)00442-8. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Breaking points on a progressive ratio schedule reinforced by intravenous apomorphine increase daily following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacology Biochemistry & Behavior. 1989;32:43–7. doi: 10.1016/0091-3057(89)90208-6. [DOI] [PubMed] [Google Scholar]
- Roberts DC. Preclinical evidence for GABAB agonists as a pharmacotherapy for cocaine addiction. Physiol Behav. 2005;86:18–20. doi: 10.1016/j.physbeh.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Loh EA, Vickers G. Self-administration of cocaine on a progressive ratio schedule in rats: dose-response relationship and effect of haloperidol pretreatment. Psychopharmacology. 1989;97:535–8. doi: 10.1007/BF00439560. [DOI] [PubMed] [Google Scholar]
- Roberts DCS, Richardson NR. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. In: Wu P, Boulton A, Baker GB, editors. Neuromethods: Animal Models of Drug Addiction. Humana Press; Clifton, NJ: 1992. pp. 263–9. [Google Scholar]
- Schroeder JP, Packard MG. Posttraining intra-basolateral amygdala scopolamine impairs food- and amphetamine-induced conditioned place preferences. Behavioral Neuroscience. 2002;116:922–7. doi: 10.1037//0735-7044.116.5.922. [DOI] [PubMed] [Google Scholar]
- See RE, McLaughlin J, Fuchs RA. Muscarinic receptor antagonism in the basolateral amygdala blocks acquisition of cocaine-stimulus association in a model of relapse to cocaine-seeking behavior in rats. Neuroscience. 2003;117:477–83. doi: 10.1016/s0306-4522(02)00665-6. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Peters SC. A comparison of the effects of cholinergic and dopaminergic agents on scopolamine-induced hyperactivity in mice. Journal of Pharmacology & Experimental Therapeutics. 1990;255:549–53. [PubMed] [Google Scholar]
- Sharkey J, Ritz MC, Schenden JA, Hanson RC, Kuhar MJ. Cocaine inhibits muscarinic cholinergic receptors in heart and brain. Journal of Pharmacology & Experimental Therapeutics. 1988;246:1048–52. [PubMed] [Google Scholar]
- Shippenberg TS, Heidbreder C, Lefevour A. Sensitization to the conditioned rewarding effects of morphine: pharmacology and temporal characteristics. Eur J Pharmacol. 1996;299:33–9. doi: 10.1016/0014-2999(95)00852-7. [DOI] [PubMed] [Google Scholar]
- Smith AD, Bolam JP. The neural netwark of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends in Neuroscience. 1990;13:259–65. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Yin X, Sizemore GM, Liguori A, Johnson WE, 3rd, Martin TJ. Involvement of cholinergic neuronal systems in intravenous cocaine self-administration. Neuroscience and Biobehavioral Reviews. 2004;27:841–50. doi: 10.1016/j.neubiorev.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behavioural Brain Research. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Sousa FC, Gomes PB, Macedo DS, Marinho MM, Viana GS. Early withdrawal from repeated cocaine administration upregulates muscarinic and dopaminergic D2-like receptors in rat neostriatum. Pharmacology, Biochemistry & Behavior. 1999;62:15–20. doi: 10.1016/s0091-3057(98)00142-7. [DOI] [PubMed] [Google Scholar]
- Spencer DGJ, Horvath E, Traber J. Direct autoradiographic determination of M1 and M2 muscarinic acetylcholine receptor distribution in the rat brain: relation to cholinergic nuclei and projections. Brain Research. 1986;380:59–68. doi: 10.1016/0006-8993(86)91429-0. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Woldbye DP, Wortwein G, Fink-Jensen A, Wess J, Caine SB. Reduced cocaine self-administration in muscarinic M5 acetylcholine receptor-deficient mice. J Neurosci. 2005;25:8141–9. doi: 10.1523/JNEUROSCI.2077-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnaer JA, Ernste BH, Wester J, Kelder K. Cholinergic innervation and topographical organization of muscarinic binding sites in rat brain: a comparative autoradiographic study. Journal of Chemical Neuroanatomy. 1988;1:95–110. [PubMed] [Google Scholar]
- Winhusen TM, Somoza EC, Harrer JM, Mezinskis JP, Montgomery MA, Goldsmith RJ, Coleman FS, Bloch DA, Leiderman DB, Singal BM, Berger P, Elkashef A. A placebo-controlled screening trial of tiagabine, sertraline and donepezil as cocaine dependence treatments. Addiction. 2005;100(Suppl 1):68–77. doi: 10.1111/j.1360-0443.2005.00992.x. [DOI] [PubMed] [Google Scholar]
- Yeomans J, Forster G, Blaha C. M5 muscarinic receptors are needed for slow activation of dopamine neurons and for rewarding brain stimulation. Life Sciences. 2001;68:2449–56. doi: 10.1016/s0024-3205(01)01038-4. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux J, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–89. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. Journal of Neuroscience. 2002;22:6347–52. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]