Abstract
Objective
Warangal district in Andhra Pradesh, southern India, records over one thousand pesticide poisoning cases each year and hundreds of deaths. We aimed to describe the frequency, distribution, and assess quality of management and subsequent outcomes from pesticide poisoning in one large hospital in the district.
Methods
We reviewed data on all patients admitted with pesticide poisoning to a district government hospital for the years 1997 to 2002. For 2002, details of the particular pesticide ingested and management were abstracted from the medical files.
Findings
During these six years, 8040 patients were admitted to the hospital with pesticide poisoning. The overall case fatality ratio was 22.6%. More detailed data from 2002 reveals two thirds of the patients were less than 30 years old, 57% were male and 96% had intentionally poisoned themselves. Two compounds, monocrotophos and endosulfan, accounted for the majority of deaths with known pesticides in 2002. Low fixed dose regimens were used in the majority of cases for the most commonly used antidotes (atropine and pralidoxime). Inappropriate antidotes were also used in some patients.
Conclusions
It is likely that these findings reflect the situation in many rural hospitals of the Asia Pacific region. Even without an increase in resources, there appear to be significant opportunities for reducing mortality by better medical management and further restrictions on the most toxic pesticides.
Keywords: endosulfan, monocrotophos, pesticides, pralidoxime iodide, case fatality ratio, India
Introduction
Over the last few decades agricultural pesticides have become a common household item in rural areas of the developing world. Due to their easy availability, pesticides have also become commonly used for intentional self-poisoning (Jeyaratnam 1990;Eddleston 2000;Eddleston & Phillips 2004). Acute pesticide poisoning is now an important cause of morbidity and mortality worldwide (Jeyaratnam 1990). According to World Health Organization (WHO) estimates published in 1990 (World Health Organization 1990), around 3 million poisoning cases with 220,000 deaths occur annually. About 99% of these deaths occur in developing countries. Although these numbers were extrapolated from a few small studies and are therefore much debated (Karalliedde et al. 2001), more recent studies suggests that the number of deaths may actually be around 300,000 (Buckley et al. 2004;Gunnell & Eddleston 2003), with the annual number of deaths in China alone around 175,000 (Phillips et al. 2002a;Phillips et al. 2002b).
Pesticide poisoning is a significant problem in India. Organophosphorus (OP) compounds cause most self-poisoning deaths in southern and central India (Thomas et al. 2000;Atul & Sharma 2002;Batra et al. 2003). In parts of northern India, aluminium phosphide causes most deaths in an epidemic that started two decades ago (Siwach & Gupta 1995;Singh & Tyagi 1999;Atul & Sharma 2002). All these pesticides are highly toxic, with one hospital reporting a case fatality ratio (CFR) for aluminium phosphide of over 90% (Hanif et al. 2002). Other pesticides used for self-poisoning include carbamates, organochlorines, and pyrethroids (Eddleston 2000). Medical management is difficult because there is little evidence with which to determine the best strategies for treatment and there is often intermittent supply of antidotes.(Buckley, Karalliedde, Dawson, Senanayake, & Eddleston 2004;Eddleston et al. 2003)
The state of Andhra Pradesh, southern India, is an area of intensive agricultural production. Pesticide use is high, and the state has one of the highest reported rates of pesticide poisoning in India (Gautami et al. 2001). The resources for treating this number of cases in government hospitals are limited and likely have an impact on patient outcome. The present study was carried out to determine the impact, management and outcomes of pesticide poisoning in one hospital of the state.
Methods
We collected and reviewed data on all patients admitted with pesticide poisoning to the Mahatma Gandhi Memorial (MGM) Hospital, a district level government hospital in the city and district of Warangal, for the years 1997 to 2002.
The MGM Hospital is a 550-bed teaching hospital located in the northern Telangana region of Andhra Pradesh. It is the referral hospital for poisoning patients from Warangal district, although some poisoning patients come from outside the district (in this study, >90% of patients were from Warangal). Most patients presenting to other hospitals in the district are transferred to MGM Hospital. Total in-patient admissions to the hospital average about 300 per day with many patients (usually admitted with fever) discharged within one day.
After resuscitation in a casualty ward, poisoned patients are admitted to the acute medical care unit (AMCU), which contains 20 beds and is staffed by four doctors and four nurses. Patients requiring intubation are referred to the four-bedded respiratory intensive care unit (RICU), which has two doctors and two nurses.
Data were collected retrospectively from medical files. Only patients who were hospitalized were included in the study. Patients with pesticide poisoning were identified from specific codes recorded at the time of admission. For patients admitted during 2002, information regarding gender, age, cause of intoxication, poison consumed, time of ingestion, presentation, and death, signs and severity of intoxication on admission, investigations, treatment and outcome was abstracted from the medical records onto a data sheet. The time to death (for those with a fatal outcome) was calculated. The data from all case sheets were entered into a database (MS Access) to calculate descriptive statistics on 2002 presentations.
Results
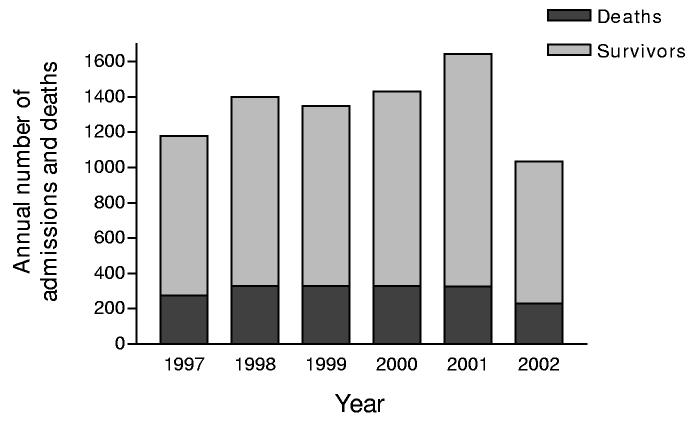
During 1997 to 2002, 8040 patients were admitted to the hospital with pesticide poisoning and 1819 of these died (figure 1). The most patients in one year was 1643 in 2001 with 326 deaths (CFR 20%), the lowest 1035 in 2002 with 230 deaths (CFR 22%). The highest CFR occurred in 1999 with 24% of patients dying.
Figure 1.
Annual number of pesticide admissions and deaths at the Mahatma Gandhi Memorial hospital (1997-2002).
In 2002, 1035 cases of poisoning were recorded. 653 patients were reported, or presumed from clinical signs, to have ingested OP pesticides, 213 had ingested organochlorines (1 patient ingested OP and organochlorine), and 170 had ingested other pesticides (table 1). The most commonly consumed OPs were monocrotophos (257 patients), chlorpyrifos (114), and quinalphos (78) (table 2). The most commonly consumed organochlorines were endosulfan (139), and endrin (74). The other commonly ingested pesticide was cypermethrin (58). Carbamates were uncommon with only 6 identified admissions. Some patients consumed an unknown pesticide - 144 patients were treated for OP poisoning due to the clinical signs at presentation and are counted above; in 83 cases, no clinical diagnosis was made.
Table 1.
Major treatments and outcomes according to pesticide ingested.
| Organophosphate N=653* |
Organochlorine N=212* |
Cypermethrin N=58 |
Carbamate N=6 |
Other pesticides N=106 |
|
|---|---|---|---|---|---|
| Atropine | 635 (97%) | 84 (40%) | 22 (38%) | 6 (100%) | 19 (18%) |
| Pralidoxime iodide | 593 (91%) | 63 (30%) | 11 (19%) | 2 (33%) | 4 (4%) |
| Anticonvulsants | 59 (9%) | 91 (43%) | 13 (22%) | 0 | 13 (12%) |
| Intubation | 211 (32%) | 53 (25%) | 7 (12%) | 2 (33%) | 11 (10%) |
| Duration of stay (median hours (IQR)) |
96 (72-144) | 70 (48-96) | 72 (48-96) | 84 (38-96) | 48 (36-72) |
| Deaths | 171 (26%) | 43 (20%) | 4 (7%) | 0 | 12 (11%) |
1 patient ingested organophosphate and organochlorine and is included with the organophosphate group only.
IQR - InterQuartileRange
Table 2.
Number of admissions and case fatality ratio for each pesticide.
| Name of pesticide | WHO Class |
Number of admissions * |
Deaths | Case fatality (& 95% confidence intervals) |
|---|---|---|---|---|
| Organophosphorus | ||||
| Methyl parathion | Ia | 5 | 3 | 60% (15-95) |
| Monocrotophos | Ib | 257 | 91 | 35% (30-42) |
| Acephate | III | 14 | 4 | 29% (8-58) |
| Malathion | III | 5 | 1 | 20% (5-72) |
| Phorate | Ia | 21 | 4 | 19% (5-42) |
| Triazophos | Ib | 6 | 1 | 17% (4-64) |
| Quinalphos | II | 78 | 9 | 12% (5-21) |
| Chlorpyrifos | II | 113 | 7 | 6% (3-12) |
| Unknown anticholinesterase |
144 | 48 | 33% (26-42) | |
| Organochlorines | ||||
| Endosulfan | II | 138 | 39 | 28% (21-37) |
| Endrin | Ib | 74 | 4 | 5% (1-13) |
| Other | ||||
| Indoxicarb | 7 | 1 | 14% (4-58) | |
| Cypermethrin | II | 58 | 4 | 7% (2-17) |
| Spinosad | 4 | 0 | 0% (0-60) | |
| Imadocloprid | II | 8 | 0 | 0% (0-38) |
| Unknown Pesticide | 83 | 9 | 11% (5-20) | |
Note - 1 patient survived after ingesting chlorpyrifos and endosulfan and is excluded from these comparisons. Pesticides ingested by 3 or less people are not shown (ethion (3), methomyl (3), phosalone (2), profenofos (2), dimethoate (1), mevinphos (1), fenvalerate (2), butachlor (2), carbofuran (1), carbaryl (1)).
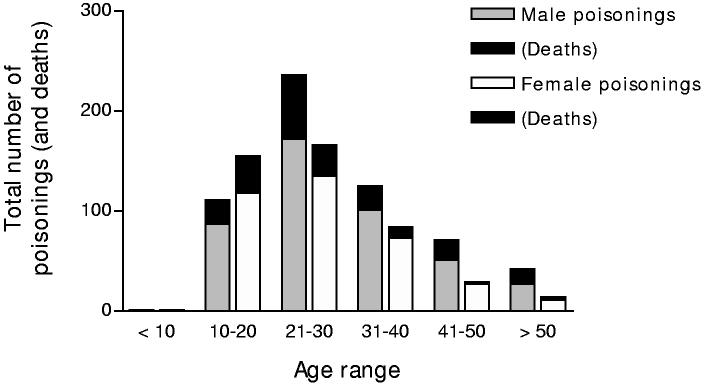
Men outnumbered women (57% vs. 43%) with all pesticide types. Two thirds of patients were aged less than 30 (figure 2). About 96% of cases were acts of intentional self-poisoning.
Figure 2.
Age and gender distribution of pesticide poisonings and deaths (2002).
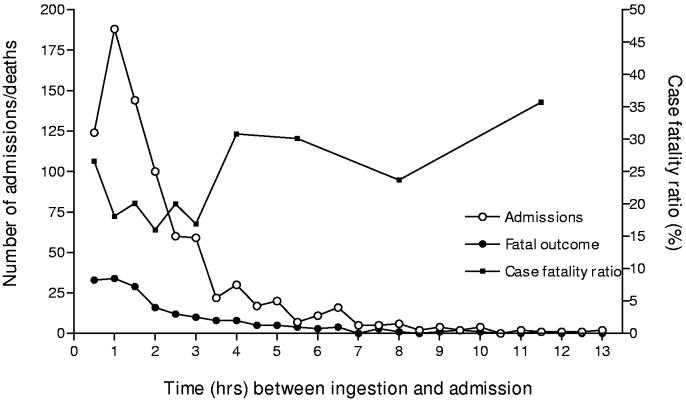
About 89% of patients were direct admissions to MGM Hospital; other patients were transferred from small rural hospitals surrounding Warangal city. The time between pesticide ingestion and admission to MGM was available in the records for 90% of the direct admissions (91% of the fatal cases). Median time to admission was 1.5hrs (interquartile range 1-3 hrs; figure 3). Patients transferred from other hospitals took significantly longer to arrive: median 6 hrs, interquartile range 3-11.5 hrs. The duration of hospitalization ranged from half an hour to 40 days. The total number of occupied bed-days was 3470. About 27% of patients were artificially ventilated (table 1).
Figure 3.
Time from ingestion to direct admission to Mahatma Gandhi Memorial hospital for all patients and deaths, with calculated Case Fatality Ratio.
* One patient was admitted more than 14hrs after ingestion (30hrs) and is not included in the admission numbers.
The overall mortality ratio was 22% and was similar for OP and other pesticides (table 1). However, there were marked differences between individual agents within classes. Methyl parathion and monocrotophos had a higher case fatality than other OPs, and endosulfan a much higher case fatality than endrin (table 2).
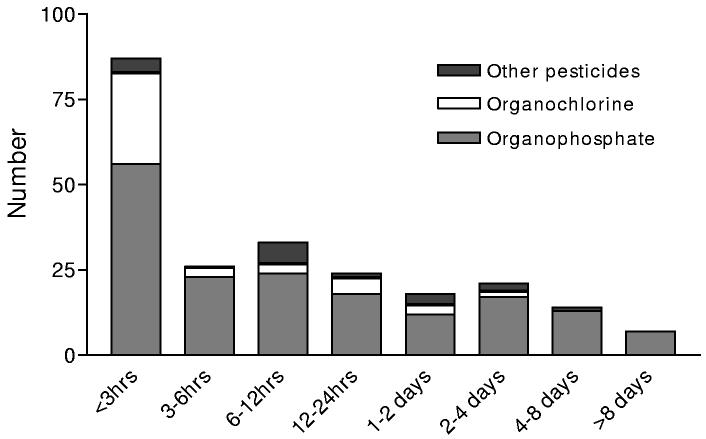
Data on time to death after poisoning showed that half the deaths occurred in the first six hours of admission, but that there were still many patients dying after 24 hours in hospital, particularly from OP poisoning (figure 4). There was no clear relationship between time from ingestion to admission and outcome - 26.6% of patients (33/124) who presented in the first thirty minutes died, while 25.8% (109/573) and 28.5% (39/137) of patients presenting in the next 3 hrs or after 4 hrs or more died, respectively (figure 3). About 29.8% of patients transferred from other hospitals died.
Figure 4.
Time from presentation until death with each pesticide type.
Of the 653 patients with confirmed or suspected OP poisoning, 593 received pralidoxime (table 1). The doses used were generally much lower than those recommended.(Johnson et al. 2000) Only 15 patients received a 2-gram bolus of pralidoxime; 80% received a low dose of 0.5g every 6-12 hours.
About 635 patients received atropine infusions but only half received an initial large bolus of atropine (typically 3-6mg) to obtain atropinisation (table 1). Typical maintenance doses of atropine were 2.4mg every 15 min with the interval between doses increased to 1-4 hours over the first day. Antibiotics (75%) and H2 antagonists (96%) were also frequently used. No patients had cholinesterase measurements taken since the assay was not available.
The use of anticonvulsants for treating patients poisoned with non-OP, pro-convulsant pesticides such as organochlorines and pyrethroids were low and some patients received antidotes for non-OP poisoning (table 1).
Discussion
Pesticide poisoning is a major clinical problem in the Warangal district with thousands of poisonings and hundreds of deaths every year. Discussions with forensic pathologists in the district suggest that the number of deaths occurring prior to hospital may be substantially more than the number that we observed in our hospital-based study. If the data from Warangal district is representative of all 23 districts in the state, then deaths from pesticide poisoning may exceed 5,000 per year in this state alone. These extrapolations suggest the magnitude of the problem of pesticide poisoning may be seriously underestimated in some quarters. One set of official figures for the years 1997-2001 recorded a total of 805 poisonings (varying between 0 and 573 per year) in the whole state of Andhra Pradesh.(Ministry of Agriculture 2001) This is in stark contrast with the 7005 recorded in just the MGM hospital in this state during this time.
Case fatality was high. Despite the limitations of a retrospective study without laboratory confirmation of the ingested poison, two major reasons are clear: highly toxic pesticides being used for self-harm and difficulties with patient management.
Availability of highly toxic pesticides
Pesticides are currently classified by the WHO on the basis of their toxicity in untreated animals from Class Ia (extremely hazardous) to Class III (slightly hazardous), and compounds unlikely to cause ill health (World Health Organization 2001). It is not yet clear whether this system is applicable for self-poisoning. The data from our case series suggest that it may not be applicable.
Although the most commonly fatal OPs (monocrotophos & methyl parathion) are Class I substances, the correlation between WHO class (World Health Organization 2001) and CFR for other pesticides was poor. Chlorpyrifos (Class II) had the lowest toxicity and appeared safer than the class III substances. Similarly, the most toxic organochlorine was endosulfan, which although having only Class II toxicity, is practically untreatable in humans and associated with high mortality (Roberts et al. 2003). Endrin (Class Ib) had a much lower CFR. These observations should be interpreted conservatively as this is a retrospective study without laboratory identification or inclusion of out of hospital deaths. Nor should it be dismissed, however; work in Sri Lanka has shown that identification of the pesticide by patient or relatives is confirmed by laboratory analysis in over 80% of cases (Eddleston, unpublished data). A further complication may be differences in the commonly ingested formulations or concentrations of each pesticide.
Current UN Food & Agriculture Organization (FAO) guidelines suggest that all Class I and II compounds should be withdrawn from agricultural practice. Class I pesticides and endosulfan have been banned in Sri Lanka (Roberts, Karunarathna, Buckley, Manuweera, Sheriff, & Eddleston 2003). Although the effect of these bans is not yet clear, mortality from pesticide poisoning in some Sri Lankan hospitals is below 15% rather than the 22% reported here. Targeted restrictions of the most commonly fatal pesticides, particularly monocrotophos and endosulfan, may bring down the number of deaths in Warangal (Konradsen et al. 2003;Eddleston et al. 2002a).
Timing of admission and outcome
Patients presented to the hospital remarkably quickly, with 89% being admitted directly to MGM hospital and 55% of these patients arriving within 1.5 hrs. This contrasts with the situation in the Christian Medical College, Vellore, in which most patients presented to hospital 10 (Cherian et al. 1997) or 12 (Johnson et al. 1996) hrs post-ingestion. This delay may explain the lack of pralidoxime effectiveness in their studies.
There was increased mortality amongst patients who presented to MGM hospital within 45 minutes of ingestion. It is possible that this is because some severely poisoned patients who lived very close to the hospital were able to get to hospital before their death. Severely poisoned patients living further away may have died before reaching the hospital, reducing mortality amongst patients who made it to hospital after 45 minutes.
Use of antidotes
A large amount of hospital resources went into treating pesticide poisoning and yet the overall mortality was high. Optimal treatment for OP poisoning should involve pralidoxime and atropine (Ballantyne & Marrs 1992;Eddleston, Singh, & Buckley 2003;Johnson, Jacobsen, Meredith, Eyer, Heath, Ligtenstein, Marrs, Szinicz, Vale, & Haines 2000). There were a number of difficulties with the way these antidotes were used in the hospital.
Oximes are cholinesterase reactivators that also increase the rate of elimination of OP (Bismuth et al. 1992). The WHO recommends much higher doses of pralidoxime than those used here – a bolus of at least 30mg/kg of pralidoxime chloride followed by an infusion of 8mg/kg per hour(Johnson, Jacobsen, Meredith, Eyer, Heath, Ligtenstein, Marrs, Szinicz, Vale, & Haines 2000). These doses of pralidoxime chloride are equivalent to 45mg/kg bolus (2 to 3 g) and 12mg/ kg/hour (0.6 to 1.0 g/hour) infusion of the iodide salt. Thus patients received pralidoxime doses as much as ten-fold lower than the WHO recommended dose. Furthermore, the use of a bolus dosing regimen will result in subtherapeutic pralidoxime concentrations for more than half of the time(Thompson et al. 1987;Johnson, Jacobsen, Meredith, Eyer, Heath, Ligtenstein, Marrs, Szinicz, Vale, & Haines 2000). However, it must be noted that, as yet, there is no hard evidence that oximes produce clinical benefit (Eddleston et al. 2002b). Furthermore, recent Indian studies suggest that low doses of oximes may be harmful to patients (Samuel et al. 1996;Cherian, Peter, Samuel, Jaydevan, Peter, Joel, Jeyaseelan, & Thomas 1997), although the results of these studies are subject to debate (Eddleston, Szinicz, Eyer, & Buckley 2002b;Lotti 2003).
The only oxime available in the district during the study was pralidoxime iodide. In most other areas of the world, chloride or sulfonate salts are the standard pralidoximes used (Bismuth, Inns, & Marrs 1992). Administration of WHO recommended doses of pralidoxime (Johnson, Jacobsen, Meredith, Eyer, Heath, Ligtenstein, Marrs, Szinicz, Vale, & Haines 2000) as an iodide salt would provide around twenty times the average daily intake of iodine. While this has not been reported to cause problems in this setting, adverse effects have been reported at these doses of iodine.(Risher et al. 2004)
The doses of atropine used may have been appropriate in many cases - it is not possible to determine retrospectively from the notes how successful the atropine dosing was. However, there are two reasons to suspect that it may be possible to improve use of atropine. Firstly, the doses used were similar in most patients, suggesting that titration to an appropriate level of atropinisation was not generally done. Secondly, the overall death rate was higher than in some other series (Eddleston 2000) and early deaths were common (figure 3). This may partly reflect the high toxicity of the substances but also suggests possibilities for improvement in the stabilization of patients. Further studies are required to determine whether clinical features come on particularly quickly with monocrotophos poisoning, resulting in patients being sicker on admission and more difficult to manage.
Administering pralidoxime and atropine to patients poisoned with organochlorines and pyrethroids may be harmful, is unlikely to provide any beneficial effect, and wastes resources. It may be a widespread problem in the overstretched developing world hospitals that globally see the majority of the patients. Further education and training of doctors may be able to reduce this problem.
General management
Diazepam or phenobarbitone should usually be sufficient to control seizures from pesticide poisoning, although patients with severe organochlorine poisoning may require general anesthesia. The value of prophylactic anticonvulsant therapy is not yet clear.
Gastrointestinal decontamination usually involved gastric lavage using normal saline. Activated charcoal was never administered. Although some patients (possibly those who present within 1 hour of ingestion) may benefit from decontamination, there is currently no evidence for clinical benefit (American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists 1997;American Academy of Clinical Toxicology and European Association of Poison Centres and Clinical Toxicologists 1997). Furthermore, gastric lavage can be dangerous when it is performed without intubation in patients at risk of seizures or rapid loss of consciousness, or in patients who do not consent to the procedure (American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists 1997) and Eddleston, submitted).
The lack of good evidence for the management of poisoned patients, and the consequent conflicting recommendations of many textbooks (Eddleston et al. 2004), do not help the situation. There are also probably too few doctors and nursing staff to adequately care for patients, and increasing the doctor to patient ratio may lead to better care.
However, there may also be opportunities for improved care using existing resources. The development of national, regional and/or institutional guidelines for early diagnosis of pesticide poisoning and appropriate use of antidotes may be helpful. More attention to the problem during undergraduate medical training is also warranted given the importance of the problem in Indian and other Asian hospitals. A better understanding of the pathophysiology of pesticides might stop doctors using inappropriate antidotes. There may also be considerable benefit from the development of postgraduate training programmes in clinical toxicology. This would potentially provide the clinical leadership and high-level expertise necessary to aid in the development of guidelines and to promote the training of junior doctors throughout the Asia-Pacific region. More clinical research on the natural history of pesticide poisoning and the effectiveness of antidotes should also help guide therapy (Buckley, Karalliedde, Dawson, Senanayake, & Eddleston 2004).
Investigations
Acetylcholinesterase and butyrylcholinesterase levels were not monitored, in part because facilities were not available in the hospital. These may be useful biomarkers to more accurately determine which of the unknown symptomatic poisonings might benefit from OP antidotes. Instead, clinical symptoms such as pulse rate and pupillary size were used for diagnosing OP poisoning, and all therapeutic decisions were based on such observations. The uncertainty about the diagnosis may have contributed to some of the apparently inappropriate choices of antidotes. While there is little likelihood of harm from such investigations, a study would be required to confirm these speculative benefits and assess the cost-effectiveness of using cholinesterase values to diagnose OP poisoning.
Possible avenues to reduce deaths
The limited resources in government hospitals in terms of trained doctors, nurses, support personnel, laboratory facilities and finance, may be partially responsible for the high number of deaths. There is a need for a prospective study to determine whether dedicated staff, training, and evidence-based protocols can lead to significant decreases in in-hospital mortality. At the same time, discussions should be opened with the state Agricultural Ministry concerned targeted bans of pesticides.
Improving the availability of antidotes is an important factor that could improve outcomes. The doses of PAM iodide were low, perhaps because of concerns about adverse effects of the iodide. Availability of PAM chloride or other oximes may mean that WHO recommended doses are used. Increasing availability and affordability of antidotes would be a significant public-health measure in the Asia Pacific region. The WHO/IPCS and the Asia Pacific Association of Medical Toxicology (APAMT) are the logical groups to take the leadership in this area.
Finally, we believe a prospective study is required to more accurately identify the total number of deaths and cases in the district and in the state of Andhra Pradesh, India. Such a study could be used to assess the impact of instituting the simple measures we have outlined above on the mortality and morbidity from pesticide poisoning.
Acknowledgements
we thank the staff members of MRD section of MGM Hospital, Warangal, for their help, and Steve Bird for his critical review. CHSR thanks CSIR, New Delhi, for providing financial assistance in the form of a SRF. ME is a Wellcome Trust Career Development Fellow, funded by grant GR063560MA from the Wellcome Trust's Tropical Interest Group. The South Asian Clinical Toxicology Research Collaboration is funded by a Wellcome Trust/National Health and Medical Research Council International Collaborative Research Grant GR071669MA.
Reference List
- 1.American Academy of Clinical Toxicology and European Association of Poison Centres and Clinical Toxicologists Position statement: single-dose activated charcoal. J.Toxicol.Clin.Toxicol. 1997;35:721–741. doi: 10.3109/15563659709162569. [DOI] [PubMed] [Google Scholar]
- 2.American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists Position statement: gastric lavage. J.Toxicol.Clin.Toxicol. 1997;35:711–719. doi: 10.3109/15563659709162568. [DOI] [PubMed] [Google Scholar]
- 3.Atul M, Sharma GK. A comparative study of poisoning cases autopsied in LHMC, New Delhi, and JIPMER, Pondicherry. J.Forensic Med.Toxicol. 2002;XIX http://www.jfmt.org/ARTICLE16.htm. [Google Scholar]
- 4.Ballantyne B, Marrs TC. Overview of the biological and clinical aspects of organophosphates and carbamates. In: Ballantyne B, Marrs TC, editors. Clinical and experimental toxicology of organophosphates and carbamates. 0 edn. Butterworth heinemann; Oxford: 1992. pp. 3–14. [Google Scholar]
- 5.Batra YK, Keoliya AN, Jadhav GU. Poisoning: an unnatural cause of morbidity and mortality in rural india. J.Assoc.Physicians India. 2003;51:955–959. [PubMed] [Google Scholar]
- 6.Bismuth C, Inns RH, Marrs TC. Efficacy, toxicity and clinical uses of oximes in anticholinesterase poisoning. In: Ballantyne B, Marrs TC, editors. Clinical and experimental toxicology of organophosphates and carbamates. 0 edn. Butterworth Heinemann; Oxford: 1992. pp. 555–577. [Google Scholar]
- 7.Buckley NA, Karalliedde L, Dawson A, Senanayake N, Eddleston M. Where is the evidence for the management of pesticide poisoning - is clinical toxicology fiddling while the developing world burns? J.Toxicol.Clin.Toxicol. 2004;42:1–4. doi: 10.1081/clt-120028756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherian AM, Peter JV, Samuel J, et al. Effectiveness of P2AM (PAM -pralidoxime) in the treatment of organophosphrus poisoning. A randomised, double blind placebo controlled trial. J.Assoc.Physicians India. 1997;45:22–24. [Google Scholar]
- 9.Eddleston M. Patterns and problems of deliberate self-poisoning in the developing world. Q.J.Med. 2000;93:715–731. doi: 10.1093/qjmed/93.11.715. [DOI] [PubMed] [Google Scholar]
- 10.Eddleston M, Buckley NA, Mohamed F, et al. Speed of initial atropinisation in significant organophosphorus pesticide poisoning - a comparison of recommended regimens. Journal of Toxicology - Clinical Toxicology. 2004;362 doi: 10.1081/clt-200035223. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eddleston M, Karalliedde L, Buckley N, et al. Pesticide poisoning in the developing world - a minimum pesticides list. Lancet. 2002a;360:1163–1167. doi: 10.1016/s0140-6736(02)11204-9. [DOI] [PubMed] [Google Scholar]
- 12.Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328:42–44. doi: 10.1136/bmj.328.7430.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eddleston M, Singh S, Buckley N. Acute organophosphorus poisoning. Clinical Evidence. 2003;10:1652–1663. [PubMed] [Google Scholar]
- 14.Eddleston M, Szinicz L, Eyer P, Buckley N. Oximes in acute organophosphorus pesticide poisoning: a systematic review of clinical trials. Q.J.Med. 2002b;95:275–283. doi: 10.1093/qjmed/95.5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gautami S, Sudershan RV, Bhat RV, et al. Chemical poisoning in three Telengana districts of Andhra Pradesh. Forensic Sci.Int. 2001;122:167–171. doi: 10.1016/s0379-0738(01)00493-5. [DOI] [PubMed] [Google Scholar]
- 16.Gunnell D, Eddleston M. Suicide by intentional ingestion of pesticides: a continuing tragedy in developing countries. Int.J.Epidemiol. 2003;32:902–909. doi: 10.1093/ije/dyg307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanif SA, Rizvi SJ, Husain M. Aluminium phosphide : a profile of preferred “hemlock” in District Aligarh, U.P. J.Forensic Med.Toxicol. 2002;XIX http://www.jfmt.org/ARTICLE1.HTM. [Google Scholar]
- 18.Jeyaratnam J. Acute pesticide poisoning: a major global health problem. Wld Hlth Statist.Quart. 1990;43:139–144. [PubMed] [Google Scholar]
- 19.Johnson MK, Jacobsen D, Meredith TJ, et al. Evaluation of antidotes for poisoning by organophosphorus pesticides. Emergency Medicine. 2000;12:22–37. [Google Scholar]
- 20.Johnson S, Peter JV, Thomas K, Jeyaseelan L, Cherian AM. Evaluation of two treatment regimens of pralidoxime (1gm single bolus dose vs 12gm infusion) in the management of organophosphorus poisoning. J.Assoc.Physicians India. 1996;44:529–531. [PubMed] [Google Scholar]
- 21.Karalliedde L, Eddleston M, Murray V. The global picture of organophosphate insecticide poisoning. In: Karalliedde L, Feldman S, Henry J, Marrs T, editors. Organophosphates and health. 0 edn. Imperial College Press; London: 2001. pp. 431–471. [Google Scholar]
- 22.Konradsen F, van der Hoek W, Cole DC, et al. Reducing acute poisoning in developing countries - options for restricting the availability of pesticides. Toxicology. 2003;192:249–261. doi: 10.1016/s0300-483x(03)00339-1. [DOI] [PubMed] [Google Scholar]
- 23.Lotti M. A critical review of oximes in the treatment of acute organophosphate poisoning. J.Toxicol.Clin.Toxicol. 2003;41 [Google Scholar]
- 24.Ministry of Agriculture . Division of Medical Toxicology and Risk Assessment. Central Insecticides Laboratory. Directorate of Plant Protection, Quarantine & Storage. Ministry of Agriculture, Government of India; 2001. Report on Establishment of Harmonized Pesticide Poisoning Database in India. [Google Scholar]
- 25.Phillips MR, Li X, Zhang Y. Suicide rates in China, 1995-99. Lancet. 2002a;359:835–840. doi: 10.1016/S0140-6736(02)07954-0. [DOI] [PubMed] [Google Scholar]
- 26.Phillips MR, Yang G, Zhang Y, et al. Risk factors for suicide in China: a national case-control psychological autopsy study. Lancet. 2002b;360:1728–1736. doi: 10.1016/S0140-6736(02)11681-3. [DOI] [PubMed] [Google Scholar]
- 27.Risher J, Diamond G, Swarts SG, Amata R. Agency for Toxic Substances and Disease Registry (ATSDR) U.S. Department of Health and Human Services; 2004. Toxicological profile for Iodine. http://www.atsdr.cdc.gov/toxprofiles/tp158.html. [Google Scholar]
- 28.Roberts DM, Karunarathna A, Buckley NA, et al. Influence of pesticide regulation on acute poisoning deaths in Sri Lanka. Bull.World Health Organ. 2003;81:789–798. [PMC free article] [PubMed] [Google Scholar]
- 29.Samuel J, Peter JV, Thomas K, Jeyaseelan L, Cherian AM. Evaluation of two treatment regimens of pralidoxime (1gm single bolus dose vs 12gm infusion) in the management of organophosphorus poisoning. J.Assoc.Physicians India. 1996;44:529–531. [PubMed] [Google Scholar]
- 30.Singh D, Tyagi S. Changing trends in acute poisoning in Chandrigah zone. A 25-year autopsy experience from a tertiary care hospital in northern India. Am.J.Forensic Med.Pathol. 1999;20:203–210. doi: 10.1097/00000433-199906000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Siwach SB, Gupta A. The profile of acute poisoning in Harayana-Rohtak Study. J.Assoc.Physicians India. 1995;43:756–759. [PubMed] [Google Scholar]
- 32.Thomas M, Anandan S, Kuruvilla PJ, Singh PR, David S. Profile of hospital admissions following acute poisoning - experiences from a major teaching hospital in south India. Adv.Drug React.Toxicol.Rev. 2000;19:313–317. [PubMed] [Google Scholar]
- 33.Thompson DF, Thompson GD, Greenwood RB, Trammel HL. Therapeutic dosing of pralidoxime chloride. Drug Intell.Clin.Pharm. 1987;21:590–593. doi: 10.1177/1060028087021007-804. [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization . Public health impact of pesticides used in agriculture. 0 edn. WHO; Geneva: 1990. [Google Scholar]
- 35.World Health Organization . WHO recommended classification of pesticides by hazard and guidelines to classification 2000-2001. 0 edn. WHO; Geneva: 2001. WHO/PCS/01.4. [Google Scholar]