Abstract
Chloramphenicol resistance in Haemophilus influenzae occurs most frequently via plasmid-mediated chloramphenicol acetyltransferase production. We studied four strains with high-level chloramphenicol resistance (MIC greater than 20 micrograms/ml) which did not have detectable chloramphenicol acetyltransferase activity. The chloramphenicol resistance determinant was transformed into a chloramphenicol-susceptible laboratory H. influenzae strain from each of the four wild-type strains, enabling isogenic comparisons. By thin-layer chromatography and a bioassay, there was no evidence of non-chloramphenicol acetyltransferase modification of chloramphenicol. In vitro protein synthesis in the presence of chloramphenicol was equivalently inhibited in the chloramphenicol-resistant transformants and in the susceptible recipient. Chloramphenicol uptake by these strains during logarithmic growth was compared by high-pressure liquid chromatographic quantitation; at chloramphenicol concentrations of 5, 10, and 20 micrograms/ml the four transformants showed a decreased rate of uptake of chloramphenicol compared with the isogenic chloramphenicol-susceptible recipient. Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of outer membrane proteins revealed a markedly diminished 40-kilodalton protein in the resistant transformants. We propose that the mechanism of chloramphenicol resistance in these strains is a relative permeability barrier due to the loss of an outer membrane protein.
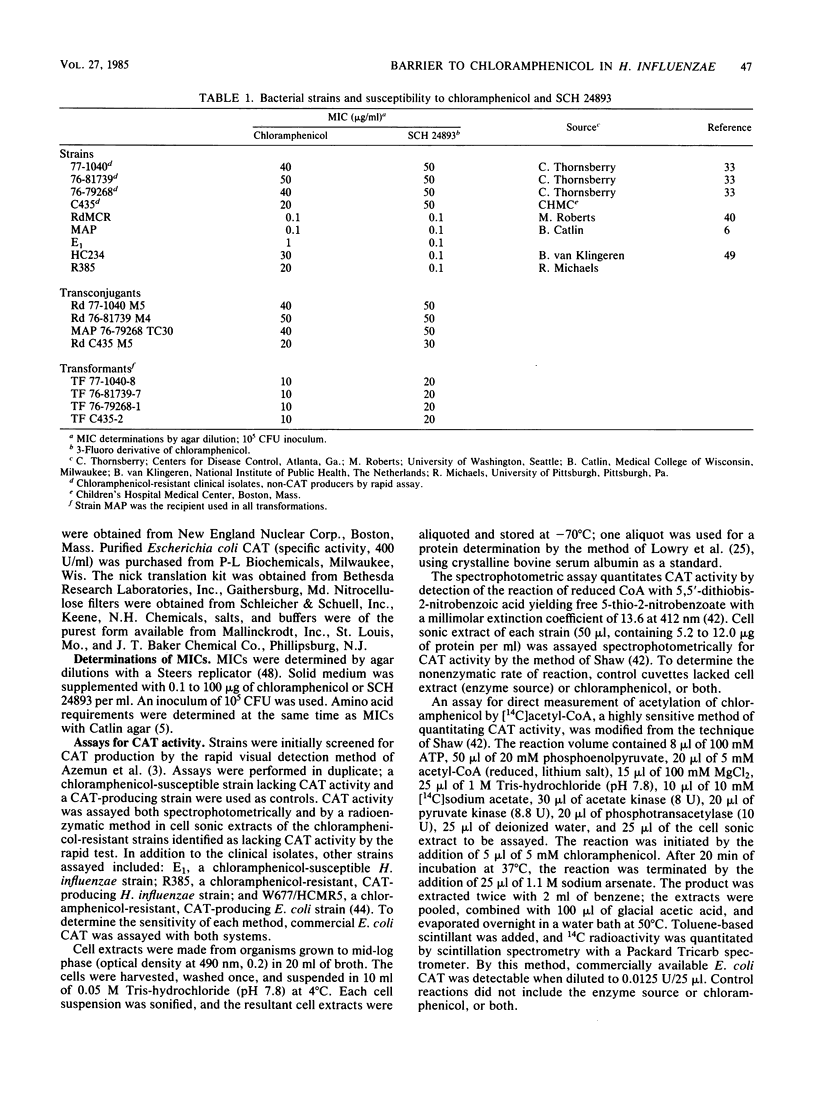
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albritton W. L., Setlow J. K., Slaney L. Transfer of Haemophilus influenzae chromosomal genes by cell-to-cell contact. J Bacteriol. 1982 Dec;152(3):1066–1070. doi: 10.1128/jb.152.3.1066-1070.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. M., Henkin T. M., Chambliss G. H., Bott K. F. New chloramphenicol resistance locus in Bacillus subtilis. J Bacteriol. 1984 Apr;158(1):386–388. doi: 10.1128/jb.158.1.386-388.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azemun P., Stull T., Roberts M., Smith A. L. Rapid detection of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Aug;20(2):168–170. doi: 10.1128/aac.20.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman G. A., Fahnestock S. R. Chloramphenicol resistance mutation in Escherichia coli which maps in the major ribosomal protein gene cluster. J Bacteriol. 1979 Mar;137(3):1315–1323. doi: 10.1128/jb.137.3.1315-1323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Chopra I., Eccles S. J. Diffusion of tetracycline across the outer membrane of Escherichia coli K-12: involvement of protein Ia. Biochem Biophys Res Commun. 1978 Jul 28;83(2):550–557. doi: 10.1016/0006-291x(78)91025-2. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- De Graaff J., Elwell L. P., Falkow S. Molecular nature of two beta-lactamase-specifying plasmids isolated from Haemophilus influenzae type b. J Bacteriol. 1976 Apr;126(1):439–446. doi: 10.1128/jb.126.1.439-446.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwell L. P., Saunders J. R., Richmond M. H., Falkow S. Relationships among some R plasmids found in Haemophilus influenzae. J Bacteriol. 1977 Jul;131(1):356–362. doi: 10.1128/jb.131.1.356-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. M., Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Irvin J. E., Ingram J. M. Chloramphenicol-resistant variants of Pseudomonas aeruginosa defective in amino acid transport. Can J Biochem. 1980 Oct;58(10):1165–1171. doi: 10.1139/o80-156. [DOI] [PubMed] [Google Scholar]
- Istre G. R., Conner J. S., Glode M. P., Hopkins R. S. Increasing ampicillin-resistance rates in Hemophilus influenzae meningitis. Am J Dis Child. 1984 Apr;138(4):366–369. doi: 10.1001/archpedi.1984.02140420032012. [DOI] [PubMed] [Google Scholar]
- Jahn G., Laufs R., Kaulfers P. M., Kolenda H. Molecular nature of two Haemophilus influenzae R factors containing resistances and the multiple integration of drug resistance transposons. J Bacteriol. 1979 May;138(2):584–597. doi: 10.1128/jb.138.2.584-597.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., O'Hara K. Mechanism of chloramphenicol-resistance mediated by kR102 factor in Pseudomonas aeruginosa. J Antibiot (Tokyo) 1976 Feb;29(2):176–180. doi: 10.7164/antibiotics.29.176. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laufs R., Kaulfers P. M. Molecular characterization of a plasmid specifying ampicillin resistance and its relationship to other R factors from Haemophilus influenzae. J Gen Microbiol. 1977 Dec;103(2):277–286. doi: 10.1099/00221287-103-2-277. [DOI] [PubMed] [Google Scholar]
- McDade R. L., Jr, Johnston K. H. Characterization of serologically dominant outer membrane proteins of Neisseria gonorrhoeae. J Bacteriol. 1980 Mar;141(3):1183–1191. doi: 10.1128/jb.141.3.1183-1191.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Mitsuhashi S. New type of R factors incapable of inactivating chloramphenicol. J Bacteriol. 1972 Jan;109(1):1–7. doi: 10.1128/jb.109.1.1-7.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nierhaus D., Nierhaus K. H. Identification of the chloramphenicol-binding protein in Escherichia coli ribosomes by partial reconstitution. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2224–2228. doi: 10.1073/pnas.70.8.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S., Takata R., Tanaka K., Tamaki M. Chloramphenicol resistant mutants of Bacillus subtilis. Mol Gen Genet. 1973 Dec 20;127(2):163–173. doi: 10.1007/BF00333664. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Characteristics of some single-step mutants to chloramphenicol resistance in Escherichia coli K12 and their interactions with R-factor genes. Genet Res. 1966 Apr;7(2):281–286. doi: 10.1017/s0016672300009708. [DOI] [PubMed] [Google Scholar]
- Reeve E. C. Genetic analysis of some mutations causing resistance to tetracycline in Escherichia coli K12. Genet Res. 1968 Jun;11(3):303–309. doi: 10.1017/s0016672300011484. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Swenson C. D., Owens L. M., Smith A. L. Characterization of chloramphenicol-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1980 Oct;18(4):610–615. doi: 10.1128/aac.18.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M., Stull T. L., Smith A. L. Comparative virulence of Haemophilus influenzae with a type b or type d capsule. Infect Immun. 1981 May;32(2):518–524. doi: 10.1128/iai.32.2.518-524.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H., Averill D. R., Jr, Marino J., Moxon E. R. Production of Haemophilus influenzae b meningitis in infant rats by intraperitoneal inoculation. Infect Immun. 1973 Aug;8(2):278–290. doi: 10.1128/iai.8.2.278-290.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Smith D. H. Improved enzymatic assay of chloramphenicol. Clin Chem. 1978 Sep;24(9):1452–1457. [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- VAZQUEZ D. ANTIBIOTICS WHICH AFFECT PROTEIN SYNTHESIS: THE UPTAKE OF 14C-CHLORAMPHENICOL BY BACTERIA. Biochem Biophys Res Commun. 1963 Aug 14;12:409–413. doi: 10.1016/0006-291x(63)90115-3. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber A. F., Opheim K. E., Koup J. R., Smith A. L. Comparison of enzymatic and liquid chromatographic chloramphenicol assays. Antimicrob Agents Chemother. 1981 Feb;19(2):323–325. doi: 10.1128/aac.19.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Klingeren B., van Embden J. D., Dessens-Kroon M. Plasmid-mediated chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Mar;11(3):383–387. doi: 10.1128/aac.11.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]






