Abstract
Objective
The OP9-DL1 culture system is an in vitro model for T cell development in which activation of the Notch pathway by Delta-like 1 promotes differentiation of mature T cells from progenitors. The roles of specific cytokines in this culture system have not been well defined, and controversy regarding the role of IL7 has recently emerged. We examined the roles played by IL7, Flt3 ligand, and stem cell factor (SCF) in differentiation of adult bone marrow cells in the OP9-DL1 culture system.
Methods
Hematopoietic progenitor cells isolated from mouse bone marrow were cultured with OP9 or OP9-DL1 stromal cells and evaluated for T and B lymphocyte differentiation using immunofluorescent staining.
Results
IL-7 provided both survival/proliferation and differentiation signals in a dose-dependent manner. T cell development from the CD4/CD8 double negative (DN) stage to the CD4/CD8 double positive (DP) stage required IL-7 provided by the stromal cells, while differentiation from the DP to the CD8 single positive (SP) stage required addition of exogenous IL-7. SCF favored the proliferation of DN lymphoid progenitors and inhibited differentiation to the DP stage in a dose-dependent manner. Conversely, blocking the function of SCF expressed endogenously by OP9-DL1 cells inhibited proliferation of lymphoid progenitors and accelerated T lineage differentiation. Flt3 ligand promoted proliferation without affecting differentiation.
Conclusion
These results validate the OP9-DL1 model for the analysis of T cell development from bone marrow-derived progenitor cells, and demonstrate specific roles of SCF, IL-7, and Flt3L in promoting efficient T lineage differentiation.
Introduction
Notch receptors and their ligands and modulators are important regulators of T lineage commitment during lymphocyte development. Among the four Notch receptors, Notch1 has been shown to be a critical component in the process of T cell development [1]. Stromal cells expressing the Notch ligand Delta-like1 promoted T/natural killer cell differentiation while inhibiting B cell differentiation from both human and mouse hematopoietic progenitors [2–4]. A culture system in which Delta-like 1 is expressed by the OP9 stromal cell line (OP9-DL1) has emerged as a valuable in vitro model for T cell development [3].
In addition to Notch signaling, lymphoid development is also regulated by a variety of cytokines. Three cytokines, Flt3 ligand (Flt3L), IL-7, and stem cell factor (SCF, also known as steel factor, mast cell growth factor and kit ligand) have been of particular interest with respect to both T and B lymphocyte development. Flt3L and SCF synergize with IL-7 to promote the growth of immature thymocytes [5–7], and signaling through the IL-7α and Flt3 receptors accounts for the generation of almost all mouse B lymphocytes [8]. Flt3-deficient mice showed a moderate decrease in the number of CD4/CD8 double negative (DN) thymocytes, while combination of a Flt3 null mutation with a hypomorphic allele of the SCF receptor (c-kit, W/Wv mutant) showed severely impaired lymphoid development [9]. IL-7 and IL-7 receptor knockout mice showed reduced thymocyte numbers and lack γδ T cells, suggesting that IL-7 plays an important role in γδ T cell differentiation [10–12]. IL-7 has also been shown to exert a dose-dependent effect on T cell development [13].
Balciunaite et al. have recently used to OP9-DL1 culture model to investigate the role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation [14]. This study concluded that the transition from DN to CD4/CD8 double positive (DP) thymocytes is IL-7-independent, and that IL-7 actually inhibits DP development of progenitors derived from adult tissues. A second study addressed the propensity of adult lymphoid progenitors to arrest at the DN2/DN3 stage of development in the OP9-DL1 system [15], and concluded that high levels of IL-7 combined with frequent passages and Notch receptor ligation are responsible for the failure of the culture model to allow efficient T cell differentiation from adult-derived lymphoid progenitors. To resolve the discrepancies between targeted mutant studies and the OP9-DL1 model, we evaluated the effects of SCF, Flt3L, and IL-7 on differentiation of adult progenitors in the culture system.
Materials and Methods
Animals
The murine strains C57BL/6 (B6) and B6.Cg-Thy1.1-Ly-5.1 were bred and maintained at the Animal Resource Center facility of the University of Utah. Mice used were between 4 and 12 weeks of age and were maintained on autoclaved, acidified water (pH 2.5) and autoclaved chow.
Antibodies
Monoclonal antibodies against CD8 (53-6.7), CD11b (M1/70), erythrocytes (TER119), Ly-6G (RB6-8C5), CD3 (KT3-1.1), CD5 (53-7.3), CD2 (Rm2.2), CD45R (B220; RA3-6B2), Thy-1.1 (19XE5), and CD19 (1D3) were purified from media of cultured hybridoma cell lines and were conjugated with biotin, phycoerythrin (PE), fluorescein isothiocyanate (FITC) in our laboratory. Biotinylated antibodies were secondarily stained with either PE-streptavidin (PE-Sav; Biomedia, Foster City, CA) or streptavidin-ECD (Beckman Coulter, Fullerton, CA). PE-conjugated monoclonal antibodies to Sca-1 and CD19, allophycocyanin-conjugated c-kit (APC-c-kit) antibody and biotin-conjugated NK1.1 antibody were purchased from PharMingen (San Diego, CA). APC-conjugated CD4 and CD44, PE-conjugated TCRβ and CD25, biotinylated TCRγδ, and APC-Cy7-conjugated CD8 were purchased from eBioscience (San Diego, CA). The neutralizing antibody against murine IL-7 was purchased from PeproTech (Rocky Hill, New Jersey). The hybridomas producing anti-c-kit mAbs, ACK2 (IgG2b) and ACK4 (IgG2a), were generous gifts from Dr. Shin-Ichi Nishikawa (Kyoto University, Kyoto, Japan). The tyrosine kinase inhibitor imatinib was recovered from crushed Gleevec tablets (Novartis Pharma, Basel, Switzerland) by methanol extraction, dried, and dissolved in dimethylsulfoxide for use. The effective dose was determined by growth inhibition of SCF-dependent EML cells.
Preparation of bone marrow cells and isolation of hematopoietic progenitor cell populations
The procedure for the preparation of BM cells for sorting was performed as previously described [16]. Bone marrow cells were incubated in a cocktail of antibodies to CD2, CD3, CD5, CD8, CD11b, Ly-6G, TER119, CD45R, and CD19. Lineage depletion was carried out by 2 successive incubations in sheep anti-rat Ig-coupled magnetic beads (Dynal, Oslo, Norway). Lineage-negative (Linneg) cells were stained with PE-Sca-1, APC-c-kit, biotin-Thy1.1 and streptavidin-ECD. Flow cytometry and sorting utilized a FACS Vantage instrument (Becton Dickinson Immunocytometry Systems, San Jose, CA). An aliquot of each sorted cell population was taken for reanalysis to test purity.
Cytokines
Recombinant mouse SCF was expressed in bacteria and purified from lysates as previously described [17]; we obtained similar results using SCF obtained commercially from Peprotech (Rocky Hill, NJ). Flt3L was kindly provided by Immunex (Seattle, WA). Recombinant murine IL-7 was purchased from Peprotech. Unless otherwise noted, the cytokines were used at the following concentrations: Flt3L, 5 ng/mL; IL-7, 5 ng/mL; SCF, 100 ng/mL.
OP9 and OP9-DL1 co-cultures
OP9 and OP9-DL1 cell lines were maintained in αMEM (nucleotide-supplemented formulation) containing 20% FCS, HEPES, sodium pyruvate, 2-ME, and antibiotics. To determine the ability of progenitor cell populations to differentiate into lymphoid lineages in vitro, we co-cultured 1000–3000 cells/well in 24-well plates with the stromal cell lines OP9 and OP9-DL1, respectively, for B and T lineage differentiation. Co-cultures were maintained in αMEM supplemented as above, except 10% heat-inactivated FCS was substituted and cultures were supplemented with purified recombinant Flt3L, IL-7, and/or SCF as described in Results. At different time points, cells were harvested by forceful pipetting. The frequency and number of T and B lineage cells in each culture was determined by flow cytometry, using expression of TCRβ and TCRγδ to define different T cell lineages, expression of CD44 and CD25 to define the DN1-DN4 stages of T cell development, expression of both CD4 and CD8 to define the DP stage of T cell development, and expression of CD19 to define B lineage development. We detected natural killer cell differentiation using the NK1.1 antibody in combination with anti-CD3.
Statistical analysis
Data sets were evaluated for significance using the unpaired one-tailed T-test with unequal variance function in Microsoft Excel.
Results
OP9-DL1 co-culture promotes T lineage specification by adult bone marrow progenitors in the absence of exogenous cytokines
To evaluate and optimize the OP9-DL1 culture system for growth of T lineage cells from adult bone marrow-derived progenitors, we first tested co-culture of freshly isolated cells with either OP9 or OP9-DL1 stroma in the absence of exogenous cytokines. We have previously characterized lymphoid progenitor cells in adult bone marrow based on the phenotype LinnegSca-1posc-kitposThy1.1neg, which we here refer to as Thy1.1neg cells [18]. This population of cells includes a spectrum of lymphoid progenitors, including those termed early lymphocyte progenitors [19], common lymphoid progenitors [20], and the lymphoid progenitors identified by expression of the Flt3 receptor [21], but, importantly, lacks hematopoietic stem cells (HSC) [22] as well as a subset of common lymphoid progenitors characterized by expression of the CD45R molecule [23]. Bone marrow transplantation experiments have shown that Thy1.1neg cells generate B and T lineage cells more rapidly post-transplant in comparison to Thy1.1low HSC [18,24]. When Thy1.1neg cells were isolated and co-cultured with OP9 and OP9-DL1 stromal cells in the absence of exogenous cytokines, we observed limited proliferation (around 20-fold expansion) of Thy1.1neg cells for up to 20 days (Figure 1). OP9-DL1 promoted T cell differentiation of Thy1.1neg cells to the DP stage while inhibiting B cell differentiation. In contrast, OP9 supported B-lineage differentiation to the CD19+ pre-B cell stage. These results show that stimulation of bone marrow-derived lymphoid progenitor cells by Delta-like1, along with cytokines produced endogenously by OP9 cells, is necessary for T cell specification and early T cell differentiation but not sufficient for supporting differentiation beyond the DP stage.
Figure 1.
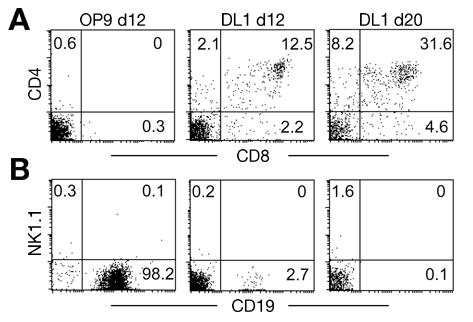
Differentiation of Thy1.1neg cells in OP9 and OP9-DL1 co-cultures without added cytokines. Panel (A) T cell differentiation on OP9 and OP9-DL1 cell lines. Purified Thy1.1neg cells were cultured on OP9 or OP9-DL1 stromal cell lines in the absence of exogenous cytokines. T cell markers (CD4 and CD8, respectively) were analyzed by flow cytometry on day 12 and day 20 after co-culture. (B) NK cell and B cell development on OP9 and OP9-DL1 cell lines. The same samples as shown in (A) were stained with NK1.1 and CD19. Each culture was initiated with 1,200 cells, which expanded to about 23,000 cells by day 12; no further increase in cell number was noted at day 20. Numbers in the plots represent the percentage of cells within the indicated quadrants. The data is a representative example of three independent experiments.
IL-7 is necessary for T cell differentiation in a stage-specific manner
In order to determine the effects of specific cytokines on T cell development, we added Flt3L, IL-7 and SCF into the co-culture system. Figure 2A shows the growth kinetics of Thy1.1neg cells cultured on OP9-DL1 monolayers in the presence of Flt3L alone, Flt3L plus IL-7, or all three cytokines.
Figure 2.

Effects of exogenous cytokines on T cell development. (A) Growth curve of Thy1.1neg cells in OP9-DL1 co-cultures with different combinations of cytokines. Thy1.1neg cells were cultured on OP9-DL1 stromal cells with different combinations of Flt3L, IL-7 and SCF. Cell numbers were counted at the indicated time points, and fold cell expansion was calculated. Error bars indicate standard error of the mean values of fold cell expansion obtained in separate experiments. Differences between Flt3L alone (n = 3) and Flt3L + IL-7 (n = 6) are significant at all time points (p < 0.03). Differences between Flt3L + IL-7 (n = 6) and Flt3L + IL-7 + SCF (n = 5) are significant at days 12, 16, 20, and 23 (p < 0.03). (B) Effect of IL-7 on T cell development. Thy1.1neg cell progeny were analyzed at different time points after co-culture with OP9-DL1. Expression of CD4 and CD8 was analyzed by flow cytometry. (C) Effect of IL-7 on αβ and γδ T cell development. Expression of αβ TCR and γδ TCR was analyzed at indicated time points. Numbers in the plots represent the percentage of cells within the indicated quadrants. (D) IL-7 promotes differentiation from the DP stage to the CD8 SP stage. Cultures of Thy.1.neg cells maintained on OP9-DL1 in the presence of Flt3L were split on day 23 and replated on OP9-DL1 in the presence of Flt3L or with Flt3L plus IL-7. Cultures were evaluated for CD4 and CD8 expression 3 days later.
Adding exogenous cytokines supported the survival, proliferation, and differentiation of Thy1.1neg cells. After 12 days of co-culture, Flt3L (5 ng/ml) supported a 100-fold expansion of progenitors. Flt3L combined with IL-7 (5 ng/ml) supported a 1200-fold expansion, while the combination of Flt3L, IL-7 and SCF (100 ng/ml) supported a 2400-fold expansion. Flt3L as a single cytokine favored the proliferation of lymphoid progenitors, but did not support efficient progression beyond the DP stage (Figure 2B). On 20th day of co-culture in the presence of Flt3L, 55.9% of the cells had differentiated to the DP stage, while only 2.2% were CD8 single positive (SP) T cells. Addition of IL-7 with Flt3L into the co-culture medium resulted in a 10-fold enhancement of cell expansion at day 15 (Figure 2A); the apparent inhibition of DP cells seen at day 15 in the presence of both Flt3L and IL-7 is in reality due to a greater expansion of DN cells, since the total number of DP cells in the two culture conditions is similar (30.1% of 102 cells versus 2.2% of 103 cells). On the 20th day of co-culture in Flt3L plus IL-7, 13.6% of cells were DP and 22.6% of cells were CD8+ SP (Figure 2B). Comparison of the growth curves seen in Flt3L alone versus Flt3L plus IL-7 shows a 100-fold increase in cell number after 23 days of culture when both cytokines were present. Applying this difference in cell numbers to the phenotypic analysis of the cultures (Figure 2B) indicates that 100-fold more DP cells and 1000-fold more CD8 SP cells were observed in cultures containing both cytokines relative to those containing only Flt3L.
Since Flt3L is a potent stimulator of dendritic cell growth, some of which express CD8, we confirmed T lineage development in these cultures by evaluating expression of T cell receptor αβ and γδ complexes by antibody staining. As shown in Figure 2C, we observed a pronounced expression of TCR γδ after 16 days of culture only when both Flt3L and IL-7 were provided. By day 20, either αβ or γδ TCR expression was observed on about 20% of the cells in these cultures. In contrast, Flt3L alone supported outgrowth of predominantly αβ TCR-expressing cells, representing about 10% of the cells recovered at day 23 of culture. These results are consistent with the data from IL-7 receptor knockout mice, indicating that IL-7 is required for development of the γδ T cell lineage [12,25–29].
We also correlated TCR expression with CD8 expression. Cells cultured with OP9-DL1, Flt3L, and IL-7 were analyzed by multicolor flow cytometry for TCRβ, TCRγδ and CD8 expression on day 23 of culture. The results showed that about 40% of CD8 SP cells express TCRβ and 12% express TCRγδ (data not shown), indicating that these cultures include both mature CD8 SP T cells as well as immature CD8 SP intermediates lacking the TCR.
Animals lacking either the high affinity α chain of the IL-7 receptor or the common cytokine receptor γ chain exhibit a block in thymopoiesis prior to the DP stage, at the DN2 stage [11,30]. In contrast, Figure 2 shows a block at the DP stage in OP9-DL1 cultures maintained the absence of exogenous IL-7. This apparent discrepancy may be explained by the production of a small amount of IL-7 by the OP9 cell line [31]. To test this possibility, we used a neutralizing antibody against murine IL-7 to block the function of IL-7 produced endogenously by OP9-DL1 cells (Figure 3). In OP9-DL1 cultures supplemented only with Flt3L, addition of anti-IL-7 inhibited the transitions from DN1 (CD44+CD25−) to DN2 (CD44+CD25+) and from DN2 to DN3 (CD44−CD25+) compared to parallel cultures lacking anti-IL7 (Figure 3A). After 23 days of co-culture, a dramatic block in the transition from DN2 to DN3, as well as in the number of DP cells (Figure 3D), was evident in the presence of anti-IL-7. Withdrawal of anti-IL-7 with the addition of exogenous IL-7 on day 23 promoted the transition from DN2 to DN3 (Figure 3A, days 27 and 31). Analysis of growth curves under these conditions is shown in Figure 3B, which indicates a 2-to 10-fold inhibition of cellular expansion in the presence of 0.1 μg/ml anti-IL-7, with a maximal effect at day 15 of culture. In contrast, very little effect on cell numbers was observed in 23 day cultures maintained in the presence of 0.1 μg/ml of anti-IL7. The inhibition of the DN2 to DN3 transition was dose-dependent, increasing with higher concentrations of anti-IL-7 (Figure 3C). In the presence of 0.5 μg/ml of blocking antibody, 83% of DN cells remained in the DN1 stage after 12 days of culture, while in the absence of blocking antibody 34 to 39% of DN cells progressed to DN2 and 43 to 46% to DN3 (Figure 3C). Exogenous IL-7 promoted differentiation to the DN4 stage by day 12, with 10% of DN cells progressing to this stage compared to 2 to 4% in the absence of exogenous IL-7 and 1 to 3% in the presence of anti-IL-7 (Figures 3A and 3C).
Figure 3.

Antibody blocking of endogenous IL-7 inhibits T cell development. (A) Effect of neutralizing antibody against IL-7 on DN cell development. Multicolor analysis of differentiating cells cultured on OP9-DL1 cells with Flt3L was performed at the indicated times. CD4 and CD8 DN cells were gated and analyzed for the expression of CD44 and CD25. On day 23, the cells cultured on OP9-DL1 cells in the presence of anti-IL-7 were washed and returned to culture either under the original conditions or in the presence of exogenous Flt3L and IL-7. (B) Growth curves of Thy1.1neg cells plated on OP9-DL1 cells in the presence of Flt3L (5 ng/ml), either alone or in the presence of exogenous IL-7 (5 ng/ml) or of the indicated concentrations of rabbit anti-IL-7. (C) Dosage effect of neutralizing antibody against IL-7 on T cell differentiation. Cells were co-cultured with OP9-DL1 cells and Flt3L with exogenous IL-7, without exogenous IL-7, or in the presence of control immunoglobulin or the indicated concentrations of anti-IL-7. At day 12, 16, 20, and 23, multicolor analysis was performed as indicated in (A). (D) Effect of anti-IL-7 on DP T cell development. Cells were co-cultured with OP9-DL1 cells and Flt3L in the presence of exogenous IL-7, in the absence of exogenous IL-7, or in the presence of control immunoglobulin or anti-IL-7. CD4 and CD8 expression was analyzed at the indicated time points. The data is representative of two independent experiments.
We also evaluated the differentiation of DP cells in these experiments. Endogenous IL-7 promoted differentiation from the DN to the DP stage, resulting in a depletion of DN cells and an accumulation of DP cells over the course of 23 days in culture as shown in Figure 3D. Addition of exogenous IL-7 promoted further development, resulting in the appearance of CD8 SP cells as well as γδ T cells which lacked expression of CD4 and CD8 (Figure 3D and data not shown). Consistent with inhibition of the DN2 to DN3 transition in the presence of anti-IL7, we observed an increased number of DN cells and a corresponding decrease in DP cells in these cultures (Figure 3D). Since the absolute number of cells in cultures with or without anti-IL-7 was within a factor of 2 at day 23, the inhibition of DP cells in cultures containing anti-IL-7 reflects an absolute decrease in DP cell number as well as percentage. Experiments using Bcl-2 transgenic donor animals partially reversed the inhibitory effect (data not shown), demonstrating that at least part of IL-7’s role at this stage of development is to promote DP cell survival as previously shown in vivo [32,33]. We also tested the effect of anti-IL-7 on B cell development. At a concentration of 0.1ug/ml, anti-IL7 strongly inhibited B cell development from Thy1.1neg cells on day 12 of OP9 co-culture in the presence of Flt3L (data not shown). These data indicate that biologically active amounts of IL-7 produced by the OP9 stromal cells promote cell survival and differentiation to the DP stage, but that further differentiation requires addition of exogenous IL-7 to the cultures.
SCF promotes proliferation of T progenitors but inhibits T cell differentiation
Analysis of SCF and c-kit mutations has established that SCF is an important positive regulator of early T cell differentiation [34–36]. However, in vivo experiments have not revealed whether there is differential effect of SCF on expansion of prothymocytes versus differentiation into later stages of the T lineage. In order to study the function of SCF in T cell development, we added exogenous soluble SCF into the progenitor-OP9-DL1 co-culture system. Addition of exogenous SCF (100 ng/ml) to cultures containing Flt3L and IL-7 had an additive effect on cell proliferation, as progenitors were expanded 2400-fold on day 12 under these conditions (Figure 2A). However, exogenous SCF dramatically inhibited T cell development (Figure 4A). After 16 days of co-culture on OP9-DL1, cultures with Flt3L+IL-7 included 19.2% cells of DP phenotype. In contrast, parallel cultures grown in Flt3L, IL-7 and SCF contained about 2-fold more cells, of which 5.4% were DP. After 20–23 days of culture, the presence of SCF in the cultures resulted in about 5-fold more cells relative to cultures containing only Flt3L+IL-7 (Figure 2A), and a dramatic inhibition of DP cells (Figure 4A). Analysis of TCR expression showed that cultures containing SCF had fewer αβ- and γδ-positive cells, although TCR-positive cells were observed at approximately 50% of the numbers found in Flt3L+IL-7 cultures when total cell numbers were taken into account (Figure 4B). Morphological analysis of the cells grown in the presence of SCF showed that the majority of the cells in these cultures were lymphoblasts and not mast cells (data not shown). These results indicate that SCF is a potent negative regulator of the DN-to-DP conversion when provided in the soluble form.
Figure 4.

Exogenous soluble SCF inhibits T lineage differentiation of Thy1.1neg cells. (A) Effect of SCF on αβ T cell differentiation. Upper panel: T cell differentiation of Thy1.1neg cells on OP9-DL1 with the addition of Flt3L and IL-7. Lower panel: T cell differentiation of Thy1.1neg cells on OP9-DL1 with addition of Flt3L, IL-7 and SCF. CD4 and CD8 expression was analyzed at different time points. (B) Effect of SCF on αβ and γδ T cell differentiation. αβ TCR and γδ TCR expression was analyzed as described for Panel A. (C) Effect of SCF on DN cell development. Multicolor analysis of differentiating cells cultured on OP9-DL1 cells was performed at the indicated times. CD4 and CD8 DN cells were gated and analyzed for the expression of CD44 and CD25. (D) Effect of SCF withdrawal on DN cell development. SCF was withdrawn from the culture medium on day 23, and expression of CD44 and CD25 was analyzed on gated DN cells. (E) Dose effect of SCF on T cell differentiation. Cells were co-cultured with OP9-DL1 cells without SCF, or in the presence of SCF at the indicated concentrations. At day 23, expression of CD44 and CD25 was analyzed on gated CD4 and CD8 DN cells. The data are representative examples of three to six independent experiments except panel E, which is from one experiment.
SCF inhibits T cell differentiation by subverting the transition between the DN2 and DN3 stages
We next studied the effect of soluble SCF on the developmental progression of DN T cells in OP9-DL1 cultures. As shown in Figure 4C, we saw no phenotypic differences between cell populations cultured with or without exogenous SCF during the first 2 weeks of co-culture on OP9-DL1. About 90% of lymphoid progenitors expressed CD25 on day 12. Most differentiating cells were CD44+CD25+CD4−CD8− (Figure 4C) and also c-kit+TCRαβ−TCRγδ− (data not shown), which is characteristic of the DN2 stage. The majority of cells grown in Flt3L and IL-7 subsequently differentiated to the DN3 stage, as indicated by the down-regulation of CD44 expression at day 16 (Figure 4C). On days 20 and 23, these cells developed through the DN4 stage (Figure 4C) and gave rise to DP and CD8 SP cells (Figure 4A). In contrast, cultures containing exogenous SCF began to accumulate differentiating T cells at the DN2 stage by day 16, and this accumulation was more significant at day 20 and day 23. As shown in Figure 4C and 4D, cells present in cultures containing SCF exhibited decreased levels of both CD25 and CD44 expression, similar to a cell population present at lower frequencies in cultures lacking SCF. To test whether withdrawal of SCF would release the accumulation of these cells and allow progression to the DN3 stage, we withdrew SCF from the co-culture medium at day 23 (Figure 4D). This experiment showed that the SCF-mediated inhibition of the DN2 to DN3 transition is largely irreversible. We observed a dose-dependent increase in the proportion of these cells (Figure 4E) in the presence of increasing concentrations of SCF. These results demonstrate that exogenous SCF affects T cell differentiation by subverting the DN2-DN3 transition and preventing further T lineage maturation. A similar result of DN2/DN3 arrest when adult lymphoid progenitors were cultured on OP9-DL1 cells was recently reported [15], with a suggestion that low levels of IL-7 (1 ng/ml) along with infrequent passages could promote progression to the DP stage. However, total cellular expansion in those studies was low compared to that reported here.
Blocking SCF function inhibits proliferation but accelerates T cell differentiation
Based on the well-defined role of SCF as a potent co-stimulator of proliferation in hematopoietic progenitors, [37] we hypothesized that SCF supports proliferation of primitive thymocytes while inhibiting differentiation. This hypothesis predicts that blocking the function of endogenous SCF expressed by OP9-DL1 cells would repress proliferation and promote differentiation. In order to test this hypothesis, we used the anti-c-kit antibodies ACK4 and ACK2. ACK4 does not interfere with the binding of SCF to c-kit, while ACK2 interferes with the function of SCF by inhibiting its binding to c-kit [38]. As a second approach, we utilized the tyrosine kinase inhibitor imatinib to block downstream signal transduction in the c-kit-SCF signaling pathway [39]. As shown in Figure 5A, both the inhibitory ACK2 antibody as well as the tyrosine kinase inhibitor imatinib significantly inhibited the proliferation of Thy1.1neg cells on OP9-DL1 cells in the presence of Flt3L and IL-7. In contrast, the non-inhibitory antibody ACK4 had no effect on the growth of progenitor cells. Cultures grown for 12 days in the presence of ACK2 had about 63-fold fewer cells compared to ACK4 controls (p < 0.01), while imatinib inhibited proliferation of progenitors by about 18-fold (p < 0.02). Importantly, inhibiting the function of endogenous SCF in the cultures reduced the proliferation of lymphoid progenitors but did not prevent differentiation of γδ and αβ T cell lineages (Figure 5B) or expression of CD4 and CD8 (Figure 5C). It is important to note that the absolute number of DP and TCR+ cells is lower in cultures maintained with ACK2 or imatinib compared to control cultures, due to the overall 100-fold decrease in cellular expansion at day 23 (Figure 5A). These data indicate that interfering with the function of endogenous SCF expressed by the OP9-DL1 cells inhibits expansion of lymphoid progenitor cells at the DN stage without preventing differentiation to the DP stage or expression of TCR.
Figure 5.
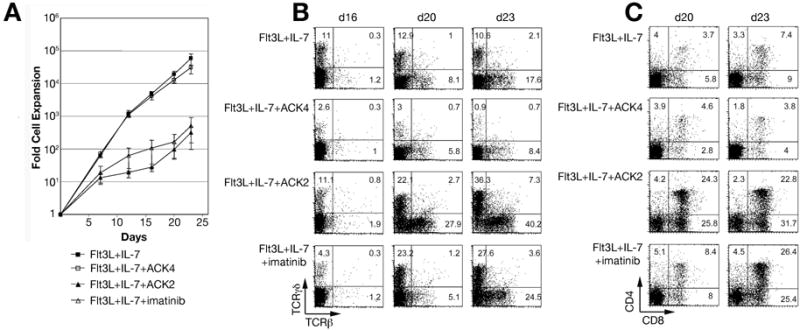
Blocking endogenous SCF function promotes T lineage development. (A) Growth curve of Thy1.1neg cells on OP9-DL1 with different combinations of cytokines and inhibitors. Cell numbers were counted at each time point, and the average fold expansion was calculated. Error bars indicate standard error of the mean values of fold cell expansion obtained in separate experiments. Significant differences (p < 0.04) were observed between Flt3L + IL-7 + ACK4 (n = 4) and Flt3L + IL-7 + ACK2 (n = 3) and between Flt3L + IL-7 (n = 4) and Flt3L + IL-7 + imatinib (n = 3) at all times shown, while differences between Flt3L + IL-7 and Flt3L + IL-7 + ACK4 or between Flt3L + IL-7 + ACK2 and Flt3L + IL-7 + imatinib were not statistically significant at any time (p > 0.1). (B) Blocking of SCF function does not prevent αβ and γδ T cell development. Thy1.1neg cells were cultured on OP9-DL1 with and without SCF inhibitors. Expression of αβ TCR and γδ TCR was analyzed as indicated. (C) Blocking of SCF function does not inhibit DP T cell development. Kinetic expression of T cell surface markers CD4 and CD8 was analyzed at the indicated times after co-culture.
Discussion
Notch signaling plays a critical role in the specification of the T lineage. In the present study, we describe in vitro B and T cell lineage differentiation from adult bone marrow-derived Thy1.1neg lymphoid progenitor cells, using the OP9 and OP9-DL1 co-culture system. These studies demonstrate that while Notch signaling is necessary for T lineage specification, cytokines play important regulatory roles during T cell differentiation. Notch signaling interplays with cytokines to promote T cell differentiation to the DP and CD8+ SP stages of T cell development. One mechanistic aspect to this interplay was recently identified, as Notch signaling was shown to upregulate c-kit expression by lymphoid progenitor cells [40].Although it has been suggested that the lack of CD4+ SP cells in OP9-DL1 cultures may be due to the absence of MHC class II-mediated selection [3], it is worthwhile noting that both Notch [41] as well as IL-7 [42] stimulation favors differentiation of CD8+ SP cells.
Based on RT-PCR analysis, OP9 cells express high levels of SCF, low levels of IL-7, and very little Flt3L [31]. We have shown here that OP9-DL1 cultures established in the absence of exogenous cytokines effectively block B lineage development and support limited growth and differentiation of T cells to the DP stage. Addition of exogenous Flt3L and IL-7 to OP9-DL1 co-cultures results in both qualitative and quantitative improvements in T lineage development. Thus, while Notch signaling is required for specification of the T lineage, cytokine signals are also needed for optimal proliferation, TCR expression, and for the DP to SP transition.
Flt3L, SCF and IL-7 are all known to be important in the regulation of lymphoid differentiation. Flt3L has potent effects on hematopoietic stem and progenitor cells [43], dendritic cell differentiation [44], and B lymphopoiesis [45,46]. However, the effect of Flt3L on T cell differentiation has not been well defined. Flt3−/− mice have relatively normal thymopoiesis [9] with a moderate reduction in DN2 thymocytes [47]. It is possible that other functionally redundant cytokines limit the T lineage phenotype observed in Flt3−/− mice. In support of this interpretation, Flt3 and c-kit double deficient mice showed severely defective hematopoiesis and lymphoid development [9]. We found that addition of exogenous Flt3L into OP9-DL1 cultures promoted moderate expansion of progenitor T cells (Figure 2A) along with differentiation to the DP stage (Figure 2B). However, expression of TCRγδ (Figure 2C) and progression to the SP stage of development was poorly supported in OP9-DL1 cultures supplemented with Flt3L alone. These results indicate that the function of Flt3L in thymopoiesis is primarily to support the survival and proliferation of progenitors. A similar conclusion was drawn from studies of Flt3L supplementation of cytokine-driven liquid cultures and fetal thymic organ culture models [48], although in those experiments the effect of Flt3L as a single cytokine was not reported.
IL-7 has important roles in both B and T lymphopoiesis [49–51]. It is a key regulator for lymphoid progenitor survival and expansion [11,52,53]. IL-7 signal disruption models have shown that IL-7 promotes TCR γ gene rearrangement as well as progression of differentiation through the DN2 stage [11,12,27]. IL-7 signals also plays a role during positive selection and favors CD8+ SP differentiation [42,54,55]. Our in vitro studies are in agreement with previous studies defining the function of IL-7 in γδ T cell development, and also show that IL-7 has an important effect on later stages of T cell differentiation. In the absence of exogenous IL-7, T cell differentiation was blocked at the DP stage in our in vitro model. In contrast, antibody-mediated inhibition of endogenous IL-7 function resulted in a repression of the DN2 to DN3 transition (Figure 3). These experiments indicate that the low level of endogenous IL-7 produced by OP9-DL1 cells is sufficient to promote T cell development through the DN2 stage and into the DP stage, but not to optimally promote TCR rearrangement and subsequent progression to the SP stage of development. A dosage effect of IL-7 on thymic development has also recently been reported in an in vivo model [13]. We observed additional dosage effects of IL-7 in the OP9-DL1 culture system, as increasing the dose of IL-7 resulted in a higher percentage of CD8+ SP T cells (data not shown). Furthermore, when OP9-DL1 cultures initiated in the presence of Flt3L reach the DP stage of development, addition of exogenous IL-7 into the co-culture system promoted differentiation of the arrested DP cells to the CD8+ SP stage (Figure 2D). These data indicate stage- and dosage-specific roles for IL-7 in T cell development, and demonstrate that the OP9-DL1 culture model provides and effective means by which to investigate the dosage effects of cytokines in T cell development.
Our experiments showed that expansion of pro-T cells and DN to DP progression requires a low level of IL-7, while a higher level of IL-7 is required for TCR rearrangement and transition to the CD8 SP stage. This result contrasts with several recent reports showing that the differentiation of DN1 and DN2 thymocytes obtained from the adult mice is inhibited by IL-7 [14,15]. Several aspects of the experiments reported previously differ from those reported here, including the source of cultured cells (thymocytes versus bone marrow cells), specific culture conditions, and the source and concentration of cytokines. Our experiments agree with those of Balciunaite et al. and Huang et al. with respect to a relative inhibition of the DN to DP conversion in the presence of IL-7, in that at days 15 to 23 of culture we observed fewer DP cells in cultures supplemented with Flt3L plus IL-7 relative to those supplemented with Flt3L alone (Figures 2B and 3D). However, our results differ from the previous studies in that we observed progression through the DP stage to the CD8 SP stage in the presence of IL-7, while in the absence of exogenous IL-7 differentiation was reversibly blocked at the DP stage (Figure 2D). Interestingly, inclusion of an anti-IL-7 antibody in cultures supplemented with exogenous Flt3L inhibited the accumulation of DP cells relative to cultures maintained in Flt3L without the blocking antibody (Figure 3D). This result is consistent with data obtained in reaggregate thymic organ culture experiments [56], in which IL-7 signaling in response to pre-TCR activation was shown to be required for efficient generation of DP thymocytes. In the presence of the anti-IL-7 antibody, T cell development was blocked at both the DN1-DN2 and the DN2-DN3 transitions, consistent with previous studies in IL-7- and IL-7Rα-deficient mice [32,57,58]. The relative increase in the DN1 compartment in the presence of anti-IL-7 antibody (Figure 3C) is due to the accumulation of bone marrow-derived lineages of cells that are not dependent on IL-7 for growth, including mostly myeloid cells, some dendritic cells, and few NK cells (data not shown). Thus, while the data reported by Balciunaite et al. and Huang et al. raise concerns regarding the use of the OP9-DL1 system to model T cell development from adult-derived progenitor cells, our data show that the inclusion of 5 ng/ml IL-7 with adult bone marrow-derived progenitors in the OP9-DL1 system did not lead to an inhibition of CD4 and/or CD8 expression. As in B cell development [59], the dose and timing of IL-7 used in OP9-DL1 as well as fetal thymic organ culture systems [60] dictates whether the DN to DP transition is inhibited. Importantly, neither Balciunaite et al. nor Huang et al. demonstrated efficient DP conversion or selective differentiation of CD8 SP cells in their experiments, even when IL-7 concentrations were reduced. This is in contrast to the results reported here, as well as to the original description of the OP9-DL1 system [3] and suggests that lowering the concentration of exogenous IL-7 plays only a compensatory role in overcoming other, currently unknown, factors that limit T lineage differentiation in this culture model. Identification of the factors necessary for efficient T cell differentiation in vitro will require further direct comparison of stromal cell lines, serum batches, and culture conditions between laboratories, as previously suggested by Huang et al. [15].
SCF and its receptor c-kit play important roles in multiple developmental processes, including hematopoiesis [61]. Null mutations in either SCF or c-kit result in embryonic lethality [62]. Recently, the lethal effect of a c-kit null mutation has been shown to be reversible, both genetically [63] and by transgenic expression of erythropoietin [64]. Rescue from c-kit lethality results in a severe block in T and B cell development, reduced thymic cellularity, and a lack of common lymphoid progenitors. As in IL-7Rα and common cytokine γ receptor mutants, thymic development is arrested at the DN1 stage in mice lacking functional c-kit. These and other data have established a critical role for SCF in expansion of early thymic progenitors. Our data suggest that in addition to promoting the expansion of early lymphoid progenitors, SCF may also function to inhibit T cell differentiation in a concentration-dependent pattern (Figures 4 and 5). Blocking SCF function inhibited proliferation without blocking T cell differentiation (Figure 5). In order to exclude the possibility that c-kit signaling inhibitors (ACK2 and imatinib) are toxic for c-kit positive progenitors and thus result in a comparatively high percentage of c-kitlow/neg differentiating T cells, we analyzed the expression of c-kit by progenitors during the co-culture. These experiments showed that progenitors grown in medium containing ACK2 or imatinib were c-kit positive and viable (data not shown). While RT-PCR studies have shown that OP9 cells strongly express SCF mRNA [31], ELISA analysis of medium conditioned by OP9 cells detects less than 15 pg/ml of SCF protein (data not shown). By fluorescent microscopy, we mainly detect SCF in permeabilized OP9 cells, indicating that the majority of SCF expressed by these cells remains intracellular rather than being expressed on the cell surface or secreted into the medium (data not shown). However, since blocking endogenous SCF activity in OP9-DL1 co-cultures by two different approaches resulted in arrested proliferation without inhibition of differentiation, we conclude that SCF-c-kit interactions play a regulatory role in this model system. Thus, OP9-DL1 variant cell lines that express more or less SCF, or produce differential ratios of the secreted versus the membrane-bound forms of SCF [65], will perform differently in the in vitro culture model. This may explain discrepancies in results from different laboratories.
The addition of soluble SCF at a concentration typically utilized in culture models (100 ng/ml) profoundly inhibited T cell differentiation without affecting proliferation. The arrest in T cell differentiation occurred at the DN2-DN3 transition, exactly as reported by Huang et al. [15]. It is likely that the biologically relevant form of SCF is membrane-bound (mSCF), which not only activates the c-kit receptor, but also mediates cell-cell adhesion in OP9 co-culture models [66] and promotes upregulation of adhesion molecules [67,68]. Excess soluble SCF would therefore be expected to inhibit mSCF-c-kit interactions between developing progenitors and OP9-DL1 cells. Consistent with this hypothesis, we observed stronger inhibition of progenitor proliferation in the presence of ACK2, which blocks mSCF-mediated cell adhesion, compared to imatinib, which does not (Figure 5A). However, since Huang et al. and Balciunaite et al. were both able to enhance DP conversion by decreasing IL-7 concentrations in OP9-DL1 cultures, it is unlikely that excess soluble SCF derived from their stromal cell lines can explain the inefficiency of DP conversion seen at higher IL-7 concentrations in their experiments. One approach to evaluating the efficiency of individual OP9 and OP9-DL1 subclones for supporting lymphoid development may be to assess differentiation of T and B lineages in the absence of exogenous cytokines (Figure 1).
Taken together, our findings suggest that IL-7 and SCF have differential effects during T cell development. Imbalance of the Notch, IL-7 and SCF signaling pathways will result in differentiation defects, and it is likely that quantitative differences between these and other signaling pathways, including the TCR, strongly influence differentiation of the T lineage. Further studies of the expression patterns of Notch ligands, IL-7, and mSCF within the thymus and the corresponding receptors on differentiating T cells will help to clarify the mechanism(s) by which Notch signal interplays with different cytokines and TCR ligation during T cell development.
Acknowledgments
We thank Dr. Juan Carlos Zúñiga-Pflücker for providing mouse stromal cell lines OP9 and OP9-DL1. This work was supported by NIH grant DK57899 and by the Brian Rooney Fund of the Lymphoma Foundation.
References
- 1.Radtke F, Wilson A, Mancini SJ, MacDonald HR. Notch regulation of lymphocyte development and function. Nat Immunol. 2004;5:247–253. doi: 10.1038/ni1045. [DOI] [PubMed] [Google Scholar]
- 2.Jaleco AC, Neves H, Hooijberg E, et al. Differential effects of Notch ligands Delta-1 and Jagged-1 in human lymphoid differentiation. J Exp Med. 2001;194:991–1002. doi: 10.1084/jem.194.7.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 4.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 5.Moore TA, Zlotnik A. Differential effects of Flk-2/Flt-3 ligand and stem cell factor on murine thymic progenitor cells. J Immunol. 1997;158:4187–4192. [PubMed] [Google Scholar]
- 6.Morrissey PJ, McKenna H, Widmer MB, et al. Steel factor (c-kit ligand) stimulates the in vitro growth of immature CD3-/CD4-/CD8- thymocytes: synergy with IL-7. Cell Immunol. 1994;157:118–131. doi: 10.1006/cimm.1994.1210. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey DI, Kennedy J, Mombaerts P, Tonegawa S, Zlotnik A. Onset of TCR-beta gene rearrangement and role of TCR-beta expression during CD3-CD4-CD8-thymocyte differentiation. J Immunol. 1994;152:4783–4792. [PubMed] [Google Scholar]
- 8.Voβhenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003;4:773–779. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- 9.Mackarehtschian K, Hardin JD, Moore KA, Boast S, Goff SP, Lemischka IR. Targeted disruption of the flk2/flt3 gene leads to deficiencies in primitive hematopoietic progenitors. Immunity. 1995;3:147–161. doi: 10.1016/1074-7613(95)90167-1. [DOI] [PubMed] [Google Scholar]
- 10.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peschon JJ, Morrissey PJ, Grabstein KH, et al. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maki K, Sunaga S, Komagata Y, et al. Interleukin 7 receptor-deficient mice lack gammadelta T cells. Proc Natl Acad Sci U S A. 1996;93:7172–7177. doi: 10.1073/pnas.93.14.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Kassar N, Lucas PJ, Klug DB, et al. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 14.Balciunaite G, Ceredig R, Fehling HJ, Zuniga-Pflucker JC, Rolink AG. The role of Notch and IL-7 signaling in early thymocyte proliferation and differentiation. Eur J Immunol. 2005;35:1292–1300. doi: 10.1002/eji.200425822. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Garrett KP, Pelayo R, Zuniga-Pflucker JC, Petrie HT, Kincade PW. Propensity of adult lymphoid progenitors to progress to DN2/3 stage thymocytes with Notch receptor ligation. J Immunol. 2005;175:4858–4865. doi: 10.4049/jimmunol.175.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spangrude GJ, Brooks DM, Tumas DB. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: in vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–1016. [PubMed] [Google Scholar]
- 17.Yee NS, Langen H, Besmer P. Mechanism of kit ligand, phorbol ester, and calcium-induced down-regulation of c-kit receptors in mast cells. J Biol Chem. 1993;268:14189–14201. [PubMed] [Google Scholar]
- 18.Perry SS, Pierce LJ, Slayton WB, Spangrude GJ. Characterization of thymic progenitors in adult mouse bone marrow. J Immunol. 2003;170:1877–1886. doi: 10.4049/jimmunol.170.4.1877. [DOI] [PubMed] [Google Scholar]
- 19.Igarashi H, Gregory SC, Yokota T, Sakaguchi N, Kincade PW. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity. 2002;17:117–130. doi: 10.1016/s1074-7613(02)00366-7. [DOI] [PubMed] [Google Scholar]
- 20.Kondo M, Weissman IL, Akashi K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell. 1997;91:661–672. doi: 10.1016/s0092-8674(00)80453-5. [DOI] [PubMed] [Google Scholar]
- 21.Adolfsson J, Mansson R, Buza-Vidas N, et al. Identification of Flt3+ lymphomyeloid stem cells lacking erythro-megakaryocytic potential: A revised road map for adult blood lineage commitment. Cell. 2005;121:295–306. doi: 10.1016/j.cell.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Spangrude GJ, Brooks DM. Phenotypic analysis of mouse hematopoietic stem cells shows a Thy-1-negative subset. Blood. 1992;80:1957–1964. [PubMed] [Google Scholar]
- 23.Martin CH, Aifantis I, Scimone ML, et al. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat Immunol. 2003;4:866–873. doi: 10.1038/ni965. [DOI] [PubMed] [Google Scholar]
- 24.Perry SS, Wang H, Pierce LJ, Yang AM, Tsai S, Spangrude GJ. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood. 2004;103:2990–2996. doi: 10.1182/blood-2003-09-3030. [DOI] [PubMed] [Google Scholar]
- 25.Cao X, Shores EW, Hu-Li J, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 26.Park SY, Saijo K, Takahashi T, et al. Developmental defects of lymphoid cells in Jak3 kinase-deficient mice. Immunity. 1995;3:771–782. doi: 10.1016/1074-7613(95)90066-7. [DOI] [PubMed] [Google Scholar]
- 27.Durum SK, Candeias S, Nakajima H, et al. Interleukin 7 receptor control of T cell receptor gamma gene rearrangement: role of receptor-associated chains and locus accessibility. J Exp Med. 1998;188:2233–2241. doi: 10.1084/jem.188.12.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of gamma delta T cell development and early thymocyte maturation in IL-7 -/- mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 29.Kang J, Coles M, Raulet DH. Defective development of gamma/delta T cells in interleukin 7 receptor-deficient mice is due to impaired expression of T cell receptor gamma genes. J Exp Med. 1999;190:973–982. doi: 10.1084/jem.190.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 31.Cho SK, Webber TD, Carlyle JR, Nakano T, Lewis SM, Zuniga-Pflucker JC. Functional characterization of B lymphocytes generated in vitro from embryonic stem cells. Proc Natl Acad Sci U S A. 1999;96:9797–9802. doi: 10.1073/pnas.96.17.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maraskovsky E, O'Reilly LA, Teepe M, Corcoran LM, Peschon JJ, Strasser A. Bcl-2 can rescue T lymphocyte development in interleukin-7 receptor- deficient mice but not in mutant rag-1-/- mice. Cell. 1997;89:1011–1019. doi: 10.1016/s0092-8674(00)80289-5. [DOI] [PubMed] [Google Scholar]
- 33.Kondo M, Akashi K, Domen J, Sugamura K, Weissman IL. Bcl-2 rescues T lymphopoiesis, but not B or NK cell development, in common gamma chain-deficient mice. Immunity. 1997;7:155–162. doi: 10.1016/s1074-7613(00)80518-x. [DOI] [PubMed] [Google Scholar]
- 34.Rodewald HR, Kretzschmar K, Swat W, Takeda S. Intrathymically expressed c-kit ligand (stem cell factor) is a major factor driving expansion of very immature thymocytes in vivo. Immunity. 1995;3:313–319. doi: 10.1016/1074-7613(95)90116-7. [DOI] [PubMed] [Google Scholar]
- 35.deCastro CM, Denning SM, Langdon S, et al. The c-kit proto-oncogene receptor is expressed on a subset of human CD3-CD4-CD8- (triple-negative) thymocytes. Exp Hematol. 1994;22:1025–1033. [PubMed] [Google Scholar]
- 36.Godfrey DI, Zlotnik A, Suda T. Phenotypic and functional characterization of c-kit expression during intrathymic T cell development. J Immunol. 1992;149:2281–2285. [PubMed] [Google Scholar]
- 37.Zsebo KM, Wypych J, McNiece IK, et al. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990;63:195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa M, Matsuzaki Y, Nishikawa S, et al. Expression and function of c-kit in hemopoietic progenitor cells. J Exp Med. 1991;174:63–71. doi: 10.1084/jem.174.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heinrich MC, Griffith DJ, Druker BJ, Wait CL, Ott KA, Zigler AJ. Inhibition of c-kit receptor tyrosine kinase activity by STI 571, a selective tyrosine kinase inhibitor. Blood. 2000;96:925–932. [PubMed] [Google Scholar]
- 40.Massa S, Balciunaite G, Ceredig R, Rolink AG. Critical role for c-kit (CD117) in T cell lineage commitment and early thymocyte development in vitro. Eur J Immunol. 2006;36:526–532. doi: 10.1002/eji.200535760. [DOI] [PubMed] [Google Scholar]
- 41.Fowlkes BJ, Robey EA. A reassessment of the effect of activated Notch1 on CD4 and CD8 T cell development. J Immunol. 2002;169:1817–1821. doi: 10.4049/jimmunol.169.4.1817. [DOI] [PubMed] [Google Scholar]
- 42.Brugnera E, Bhandoola A, Cibotti R, et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 43.Haylock DN, Horsfall MJ, Dowse TL, et al. Increased recruitment of hematopoietic progenitor cells underlies the ex vivo expansion potential of FLT3 ligand. Blood. 1997;90:2260–2272. [PubMed] [Google Scholar]
- 44.Laouar Y, Welte T, Fu XY, Flavell RA. STAT3 is required for Flt3L-dependent dendritic cell differentiation. Immunity. 2003;19:903–912. doi: 10.1016/s1074-7613(03)00332-7. [DOI] [PubMed] [Google Scholar]
- 45.Veiby OP, Lyman SD, Jacobsen SE. Combined signaling through interleukin-7 receptors and flt3 but not c-kit potently and selectively promotes B-cell commitment and differentiation from uncommitted murine bone marrow progenitor cells. Blood. 1996;88:1256–1265. [PubMed] [Google Scholar]
- 46.McKenna HJ, Morrissey PJ. Flt3 ligand plus IL-7 supports the expansion of murine thymic B cell progenitors that can mature intrathymically. J Immunol. 1998;160:4801–4809. [PubMed] [Google Scholar]
- 47.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 48.Moore TA, Zlotnik A. Differential effects of Flk-2/Flt-3 ligand and stem cell factor on murine thymic progenitor cells. J Immunol. 1997;158:4187–4192. [PubMed] [Google Scholar]
- 49.Miller JP, Izon D, DeMuth W, Gerstein R, Bhandoola A, Allman D. The earliest step in B lineage differentiation from common lymphoid progenitors is critically dependent upon interleukin 7. J Exp Med. 2002;196:705–711. doi: 10.1084/jem.20020784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang J, Der SD. Cytokine functions in the formative stages of a lymphocyte's life. Curr Opin Immunol. 2004;16:180–190. doi: 10.1016/j.coi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 52.von Freeden-Jeffry U, Solvason N, Howard M, Murray R. The earliest T lineage-committed cells depend on IL-7 for Bcl-2 expression and normal cell cycle progression. Immunity. 1997;7:147–154. doi: 10.1016/s1074-7613(00)80517-8. [DOI] [PubMed] [Google Scholar]
- 53.Li WQ, Jiang Q, Khaled AR, Keller JR, Durum SK. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J Biol Chem. 2004;279:29160–29166. doi: 10.1074/jbc.M401656200. [DOI] [PubMed] [Google Scholar]
- 54.Varas A, Vicente A, Jimenez E, et al. Interleukin-7 treatment promotes the differentiation pathway of T-cell-receptor-alpha beta cells selectively to the CD8+ cell lineage. Immunology. 1997;92:457–464. doi: 10.1046/j.1365-2567.1997.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hare KJ, Jenkinson EJ, Anderson G. An essential role for the IL-7 receptor during intrathymic expansion of the positively selected neonatal T cell repertoire. J Immunol. 2000;165:2410–2414. doi: 10.4049/jimmunol.165.5.2410. [DOI] [PubMed] [Google Scholar]
- 56.Trigueros C, Hozumi K, Silva-Santos B, et al. Pre-TCR signaling regulates IL-7 receptor alpha expression promoting thymocyte survival at the transition from the double-negative to double-positive stage. Eur J Immunol. 2003;33:1968–1977. doi: 10.1002/eji.200323831. [DOI] [PubMed] [Google Scholar]
- 57.Moore TA, von Freeden-Jeffry U, Murray R, Zlotnik A. Inhibition of ©™ T cell development and early thymocyte maturation in IL-7 -/- mice. J Immunol. 1996;157:2366–2373. [PubMed] [Google Scholar]
- 58.Di Santo JP, Rodewald HR. In vivo roles of receptor tyrosine kinases and cytokine receptors in early thymocyte development. Curr Opin Immunol. 1998;10:196–207. doi: 10.1016/s0952-7915(98)80249-5. [DOI] [PubMed] [Google Scholar]
- 59.Carsetti R. The development of B cells in the bone marrow is controlled by the balance between cell-autonomous mechanisms and signals from the microenvironment. J Exp Med. 2000;191:5–8. doi: 10.1084/jem.191.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLuca D, Clark DR. Interleukin-7 negatively regulates the development of mature T cells in fetal thymus organ cultures. Dev Comp Immunol. 2002;26:365–384. doi: 10.1016/s0145-305x(01)00085-4. [DOI] [PubMed] [Google Scholar]
- 61.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–1364. [PubMed] [Google Scholar]
- 62.Russell ES. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- 63.Waskow C, Paul S, Haller C, Gassmann M, Rodewald H. Viable c-Kit(W/W) mutants reveal pivotal role for c-kit in the maintenance of lymphopoiesis. Immunity. 2002;17:277–288. doi: 10.1016/s1074-7613(02)00386-2. [DOI] [PubMed] [Google Scholar]
- 64.Waskow C, Terszowski G, Costa C, Gassmann M, Rodewald HR. Rescue of lethal c-KitW/W mice by erythropoietin. Blood. 2004;104:1688–1695. doi: 10.1182/blood-2004-04-1247. [DOI] [PubMed] [Google Scholar]
- 65.Anderson DM, Lyman SD, Baird A, et al. Molecular cloning of mast cell growth factor, a hematopoietin that is active in both membrane bound and soluble forms. Cell. 1990;63:235–243. doi: 10.1016/0092-8674(90)90304-w. [DOI] [PubMed] [Google Scholar]
- 66.Kodama H, Nose M, Niida S, Nishikawa S. Involvement of the c-kit receptor in the adhesion of hematopoietic stem cells to stromal cells. Exp Hematol. 1994;22:979–984. [PubMed] [Google Scholar]
- 67.Kovach NL, Lin N, Yednock T, Harlan JM, Broudy VC. Stem cell factor modulates avidity of alpha 4 beta 1 and alpha 5 beta 1 integrins expressed on hematopoietic cell lines. Blood. 1995;85:159–167. [PubMed] [Google Scholar]
- 68.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. J Exp Med. 1995;181:1805–1815. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]


