Abstract
OBJECTIVE
To evaluate barriers to and strategies for medication adherence and predictors of adherence and the primary outcome in the Diabetes Prevention Program (DPP).
RESEARCH DESIGN AND METHODS
Within a randomized, controlled primary prevention study for type 2 diabetes, we collected data on study medication adherence, its predictors, and health outcomes in 27 clinical centers across mainland U.S. and Hawaii. Medication arm participants included 2,155 adults with impaired glucose tolerance randomly assigned to either metformin or matched placebo treatment arms. Structured interviews were used to promote medication adherence and to collect data regarding adherence. Adherence was measured by pill count. The primary DPP outcome of type 2 diabetes was assessed by fasting plasma glucose and oral glucose tolerance test.
RESULTS
Older age-groups were more adherent than the youngest group (P = 0.01) in the metformin group. The most frequently reported barrier to adherence was “forgetting” (22%). Women reported more adverse effects of metformin (15 vs. 10%, P = 0.002) in the metformin group. Odds of nonadherence increased as participants reported more than one barrier (odds ratio 19.1, P < 0.001). Odds of adherence increased as participants reported multiple strategies to take medication (2.69, P < 0.0001). There was a 38.2% risk reduction for developing diabetes for those adherent to metformin compared with those adherent to placebo (P < 0.0003).
CONCLUSIONS
DPP medication adherence results are unique in primary prevention for a chronic disease in a large multiethnic sample. Our finding that adherence was associated with risk reduction for diabetes supports the development of brief interventions in clinical settings where medication adherence is a challenge.
Abbreviations: DPP, Diabetes Prevention Program
The Diabetes Prevention Program (DPP) (1) was a multicenter study to evaluate the effect of two interventions, an intensive lifestyle intervention or metformin compared with a placebo medication, on delaying or preventing type 2 diabetes in high-risk individuals with impaired glucose tolerance. Results demonstrated that among 3,234 participants with impaired glucose tolerance who were followed for an average of 3.2 years, the intensive lifestyle intervention reduced the incidence of diabetes by 55% and the metformin intervention reduced the incidence by 30% compared with the placebo group (2).
Patient adherence to medical recommendations is a problem that has been studied over decades (3,4). Data from medication adherence studies (3,5,6) indicate that between 20 and 60% of patients fail to follow prescriptions. Since preventive medications do not provide the positive reinforcement of symptom control or relief, compared with effective therapeutic medications, adherence may be even more of a challenge, especially in primary prevention. Previous studies (7–9) have reported patient characteristics, such as age, sex, ethnicity, socioeconomic status, levels of social support, anxiety or depression, and past history of adherence to the medications, as moderators or predictors of medication adherence. Complexity of the therapeutic regimen and the characteristics of health care systems and providers are also important variables in understanding medication adherence (5,10).
The DPP cohort is a unique population for evaluating preventive medication adherence because of the large sample size, diversity of age and ethnic/racial groups, and the prospective design of the study. This report focuses on medication adherence and its predictors and health outcomes in the metformin and placebo groups during the DPP. In addition, it reports barriers to adherence and the relative effectiveness of strategies to improve medication adherence.
RESEARCH DESIGN AND METHODS
The DPP was a randomized, controlled clinical trial in which participants were randomly assigned to standard lifestyle recommendations plus metformin or placebo or to the intensive lifestyle intervention. The methods of the trial have been previously reported (1). The present manuscript focuses on the 2,155 participants who were assigned to the metformin or placebo interventions.
The DPP included 3,234 individuals who were at high risk of developing diabetes at 27 clinical centers across the mainland U.S. and Hawaii. To be eligible for inclusion, subjects had to be at least 25 years of age, with BMI ≥24 kg/m2 (≥22 kg/m2 in Asians), a fasting plasma glucose of 95–125 mg/dl (<126 mg/dl in American Indians), and a plasma glucose value of 140–199 mg/dl 2 h after a 75-g glucose load. Before randomization, participants had to complete an acceptable 3-week “run-in” period by performing some of the protocol activities, such as taking placebo medications and keeping a log of diet and physical activity. Ethnicity/race was self-reported by participants during the screening process. The study was approved by the institutional review boards at each center, and all participants provided written informed consent.
Intervention
DPP medication case managers dispensed the assigned medications at each quarterly visit. Both participant and staff were masked to medication treatment assignment. The protocol dose of metformin or placebo was an 850-mg tablet twice each day. Dose was titrated up to the protocol dose during the 1st month. If a participant could not tolerate the full dose, generally because of gastrointestinal side effects, they were prescribed a once-daily dose of 850 mg. Participants returned any unused pills at the subsequent study visit. Case managers were trained to count returned pills and calculate adherence to the prescribed dose without emphasizing the pill count to the participant, with a goal to minimize the potential of participants discarding pills.
Case managers promoted adherence to the DPP study medications using a brief structured interview of five items regarding medication adherence (available at http://www.bsc.gwu.edu/dpp/index.htmlvdoc). The following items were included: what strategies helped them take their DPP pills as prescribed; what might be the main problem, if any, in taking DPP pills as prescribed; and what strategies might be helpful in dealing with the identified problem. Using this collaborative approach, the case manager collected data regarding barriers to and strategies used for promoting adherence from the previous 3-month period and encouraged participants to choose alternate adherence strategies as needed.
Main outcomes and measurements
Adherence data as measured by the pill count were documented as either <80 or ≥80% of the prescribed dose at each quarterly visit. Information about self-reported barriers to and strategies for adherence to DPP medications were asked using open-ended techniques but were later coded. This medication adherence interview survey was pilot tested with DPP participants (n = 40) and showed adequate test-retest reliability. The primary outcome measure for the main study was development of diabetes assessed semiannually (1,2).
Statistical analyses
The subgroup of participants for the analyses described in this article are the 2,155 who were assigned to metformin or placebo arms. For those who developed diabetes, observations regarding adherence were used in analyses from baseline up to and including the visit when diabetes was diagnosed. For those who did not develop diabetes, all observations were included. Medication adherence, barriers to adherence, and strategies to promote adherence were measured quarterly; therefore, repeated-measures data analysis using generalized estimating equations were performed (10). The step-down Bonferonni method was used to adjust for multiple comparisons in the association of barriers and strategies with demographic factors (11). Cox proportional hazards models were used to assess risk reduction in diabetes. All analyses were conducted using SAS software (SAS Institute, Cary, NC).
RESULTS
Patterns of adherence
The overall adherence rates, reported as the proportion of participants taking ≥80% of the prescribed dose over time in the study, were lower in the metformin (71%) than the placebo (77%) group (P < 0.001). Between treatment groups, there was no significant difference in the proportion of participants taking at least some portion of pills over time in the study (89.2% of the metformin group and 91.1% of the placebo group).
Table 1 is a comparison of adherence to medications and its univariate association with six demographic factors: age-group, sex, ethnicity/race, annual income, marital status, and level of education completed. The middle-aged and older age-groups (aged 45–59 and ≥60 years) had significantly greater adherence in both metformin and placebo groups than younger participants (aged 25–44 years). The metformin group, as expected, had lower adherence than the placebo group in each age category, presumably because of its gastrointestinal side effects. The largest differences in adherence between the metformin and placebo groups were in the oldest age-group. Women were significantly less adherent than men in the metformin group only (68 vs. 74%, P = 0.01). Among ethnicity/racial groups in Table 1, there were significant differences in rates of adherence, with Caucasians having the highest rates of adherence in both arms. Finally, among the groups for level of annual household income, there were significant differences in each treatment arm. In the metformin group, the lowest income group had the lowest adherence to metformin (60%), while the highest income group had the highest level of adherence (77%, P < 0.0001). Neither marital status nor level of education was significantly related to medication adherence.
Table 1.
Overall comparison of medication adherence by demographic factors
| Metformin
|
Placebo
|
|||
|---|---|---|---|---|
| Demographic characteristics | n | Percent adherent | n | Percent adherent |
| Age (years) | ||||
| 25–44 | 293 | 65.9 | 302 | 70.1 |
| 45–59 | 519 | 72.5 | 530 | 77.3 |
| ≥60 | 208 | 70.6 | 192 | 81.0 |
| P value | 0.01 | 0.0001 | ||
| Sex | ||||
| Male | 348 | 73.7 | 316 | 78.4 |
| Female | 672 | 68.4 | 708 | 74.9 |
| P value | 0.01 | 0.07 | ||
| Ethnicity/race | ||||
| Caucasian | 577 | 73.4 | 550 | 80.7 |
| African American | 209 | 66.4 | 211 | 67.0 |
| Hispanic | 150 | 65.0 | 158 | 73.0 |
| American Indian | 52 | 67.5 | 59 | 72.1 |
| Asian | 32 | 68.5 | 46 | 75.3 |
| P value | 0.008 | <0.0001 | ||
| Income* | ||||
| <$20,000 | 126 | 60.1 | 146 | 76.8 |
| $20,000 to <$35,000 | 165 | 66.1 | 181 | 75.6 |
| $35,000 to <$50,000 | 203 | 74.7 | 206 | 74.6 |
| $50,000 to <$75,000 | 212 | 70.0 | 201 | 78.6 |
| ≥$75,000 | 231 | 77.2 | 210 | 78.6 |
| Refused | 82 | 64.7 | 80 | 65.1 |
| P value | <0.0001 | 0.01 | ||
| Marital status | ||||
| Never married | 126 | 67.4 | 127 | 77.8 |
| Living together | 29 | 62.4 | 44 | 75.7 |
| Married | 632 | 72.5 | 636 | 75.5 |
| Separated | 31 | 64.6 | 27 | 68.9 |
| Divorced | 152 | 67.3 | 145 | 77.2 |
| Widowed | 50 | 65.8 | 45 | 77.3 |
| P value | 0.09 | 0.72 | ||
| Education (years) | ||||
| ≤12 | 258 | 68.8 | 270 | 76.2 |
| 13–16 | 484 | 69.6 | 497 | 75.1 |
| ≥17 | 278 | 72.6 | 257 | 77.2 |
| P value | 0.33 | 0.66 | ||
The adherence difference between metformin and placebo is consistent across age, sex, ethnicity, marital status, and education. However, heterogeneity is observed for income levels (P = 0.01). Those with <$20,000 income had the biggest gap in adherence between metformin and placebo.
Multivariate models were used to evaluate which of the four factors showing significant univariate differences were associated with adherence independently. Backward selection was used to remove factors that were not significant, and the final models containing the significant predictors are reported. Age and income were associated with adherence in both the metformin (P = 0.01 and P < 0.0001, respectively) and placebo (P = 0.0002 and P = 0.004) groups. However, race/ethnicity was only associated with medication adherence in the placebo group (P < 0.0001). When sex differences in adherence to metformin were adjusted for age, income, and race/ethnicity, the significance was lost.
Logistic regression was used to predict subsequent adherence at 1 and 3 years after randomization by the level of adherence measured 3 months after the DPP treatment began. Among participants who were ≥80% adherent to medication at 3 months, the odds of being adherent at 1 year were 4.53 (95% CI 3.61–5.69; P < 0.0001) and at 3 years were 3.13 (2.31–4.25; P < 0.0001).
Barriers to adherence
Among both metformin and placebo arms, the most commonly reported barriers to taking DPP medication as prescribed were forgetting to take doses (22%), adverse effects (including gastrointestinal) (8.0%), and disruption of routines (8.0%). On average, almost 60% of participants reported “no barriers” to taking DPP pills as prescribed. Figure 1 (top half) compares the metformin group and placebo groups for participant-reported barriers to adherence, including indications of significant time and treatment effects. The metformin group reported adverse effects more frequently than the placebo group (P < 0.0001), while a greater proportion of placebo participants reported no barriers to adherence than metformin participants (P < 0.0001). There was a significant change seen over time in the reporting of adverse effects, with both groups reporting this barrier less frequently over time. Table 2 (top half) shows the odds ratios for predicting nonadherence to medication by self-reported barriers in the metformin group only. The odds of nonadherence were greatly increased as participants report more than one barrier, with an odds ratio of 19.2 (95% CI 5.1–72.8; P < 0.001) for the small subsample of participants who reported a combination of barriers for taking metformin as prescribed.
Figure 1.
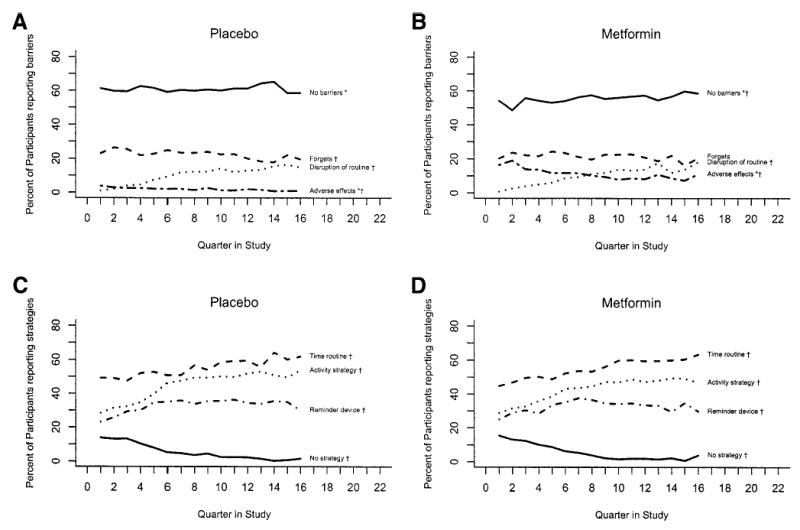
Comparison of barriers and strategies to adherence in DPP medication groups, placebo (A and C) and metformin (B and D). Significant difference between the two groups in barriers exists in “No barriers” and “Adverse effects.” Percent of barriers changed over time except “Forgets” in the metformin group. There were no significant differences in the strategies between the two groups. However, over time, percentages of each strategy changed (P < 0.05). *P < 0.001 treatment effect; †P < 0.05 time effect.
Table 2.
Odds ratios and 95% CIs from logistic regression models predicting nonadherence to metformin by reported barriers and predicting adherence to metformin by reported strategies
| Odds ratio (95% CI)* | P value* | |
|---|---|---|
| Reported barriers | ||
| No barriers | 1.00 | — |
| Disruption of routines | 3.62 (2.82–4.64) | <0.0001 |
| Forgets | 5.11 (4.30–6.07) | <0.0001 |
| Adverse effects | 9.69 (7.86–11.95) | <0.0001 |
| Forgets and disruption of routines | 8.76 (6.30–12.18) | <0.0001 |
| Adverse effects and disruption of routines | 9.66 (5.42–17.24) | <0.0001 |
| Forgets, adverse effects, and disruption of routines† | 19.21 (5.07–72.79) | <0.0001 |
| Reported strategies | ||
| No strategies | 1.00 | — |
| Time routine | 1.15 (0.90–1.46) | 0.25 |
| Activity strategy | 1.36 (1.06–1.76) | 0.02 |
| Reminder device | 1.57 (1.19–2.07) | 0.0015 |
| Both time and activity | 1.66 (1.29–2.15) | 0.0001 |
| Both time and reminder device | 1.68 (1.25–2.27) | 0.0006 |
| Both activity and reminder device | 1.74 (1.24–2.42) | 0.0012 |
| Time, activity, and reminder device | 2.69 (1.67–4.33) | <0.0001 |
Barriers and strategies were modeled separately. P values are based on repeated-measures modeling using generalized estimating equations.
There were very few participants in this category, 11 throughout the study and only 1 or 2 at each quarterly visit.
We also examined the proportion of metformin and placebo participants who reported specific barriers to adherence by categories of demographic characteristics. Notably, the older age-group was more likely to report no barriers (62.7 vs. 57.7% in the middle-aged group and 51.5% in the younger-aged group, P < 0.0001) and least likely to report “forgetting” as a barrier to DPP medication adherence (21.7 vs. 27.8 and 34.9% in the middle- and younger-aged groups, P <0.0001). Men were more likely to report no barriers (60 vs. 51% in women, P = 0.001), and women were more likely to report adverse effects (15 vs. 10% in men, P = 0.002) among the metformin group only. By ethnic/racial group, there were significant differences among groups in reporting forgetting as a barrier to adherence, with American Indians reporting this barrier the most (36%) and Asian Americans reporting it the least (18%) (P < 0.0001). This difference remained significant after being adjusted for the age effect. Reports of adverse effects were also significantly different by ethnic/racial category in the placebo group, with American Indians reporting them most (5.9%) and Asians reporting them least (1.4%) (P = 0.03). There were no significant differences in barriers reported for the metformin group by annual family income, marital status, or level of education.
Strategies to improve adherence
Overall, the main strategies reported as helpful for medication adherence were time of day routines, e.g., “8 a.m. before breakfast,” (52%); a reminder device, such as a day-of-the-week pill box (30%); and an activity strategy, such as association with brushing teeth (40%). Overall, only 8% of participants reported using no adherence strategy. Figure 1 (bottom half) compares the placebo and metformin groups for adherence strategies reported over time in the study. There were no significant treatment effects between study groups for adherence strategies, but there were significant changes over time since randomization (P < 0.0001), with more participants reporting the use of adherence strategies to promote adherence over time. Table 2 (bottom half) shows the odds ratios for predicting adherence in the metformin group by strategies used to improve adherence. The odds of being adherent to ≥80% of the prescribed dose increased as individuals identified more strategies to improve adherence. Use of reminder devices, mainly day-of-the-week pill boxes, was the most powerful single strategy to improve adherence (odds ratio 1.57, P = 0.001).
There were few significant differences in the proportions of either metformin and placebo participants who reported specific adherence strategies that were helpful to improve adherence when analyzed by categories of demographic characteristics. In the metformin group, African Americans were most likely to report the use of time-related strategies (62.4%), while Caucasians reported it the least (46.6%) (P < 0.0001). There were no significant differences in strategies reported by the metformin group participants by annual family income, marital status, or level of education.
Medication adherence and development of diabetes
Cox proportional hazards models were used to predict the development of diabetes by the dichotomous variable for adherence, that is, <80 or ≥80% adherent to their prescribed study medication during at least 80% of their clinic visits. For those who took half of the full dose (one 850-mg pill a day instead of two), a weighted 80% of their visits was calculated. Each visit at which participants took the full dose was counted as 100%, and each visit taking half dose was counted as 50%. These visits were averaged over the course of the study. Taking metformin per protocol was important in preventing diabetes. Among metformin participants, there was a significant 24.8% risk reduction (95% CI 1.1–42.9%; P ≤0.04) for developing diabetes for those who were adherent to metformin compared with those nonadherent to metformin. In the placebo group, however, there was no significant reduction in risk seen for those who were adherent versus nonadherent (9% [−15.4 to 28.2]; P = 0.44). Finally, there was a risk reduction of 38.2% (19.8–52.3; P = 0.0003) for the metformin group when we modeled diabetes risk for the adherent participants in the metformin group compared with those adherent in the placebo group.
CONCLUSIONS
The DPP implemented a structured interview at each quarterly visit, primarily to promote medication adherence through problem solving and, secondarily, to gain knowledge for the translation of effective preventive medication adherence interventions. Adherence as measured by pill count showed remarkably consistent high rates of adherence over time in the study. Because of the intention-to-treat analysis design of the main study, the overall adherence rates of 71% of metformin and 77% of placebo participants taking ≥80% of their prescribed dose include even those participants who had discontinued their coded medication for protocol reasons. Although metformin is known to have gastrointestinal side effects for some individuals (12), on average 84% of participants were taking at least some of their prescribed metformin dose during the study. Of those participants, 86% were prescribed the full protocol dose of 850 mg twice each day. In the DPP design, neither participants nor DPP staff were told medication group assignment. We believe this masking was maintained even though some participants had gastrointestinal symptoms. Approximately half of each group guessed wrong at the end of study when asked whether they had been in the metformin or placebo group.
We promoted a basic problem-solving approach to improve adherence using a brief structured interview. Case managers asked about barriers and facilitated the choosing of new strategies using a “tool box” approach (13). This brief interview may have contributed to the overall adherence rates sustained for >3 years.
The DPP medication adherence results are a unique contribution to preventive medication adherence literature, as the cohort included diverse age and ethnic/racial groups. The oldest age-group, which was 20% of the DPP sample, had a significantly higher proportion of participants adherent to metformin compared with the youngest age-group (aged 25–44 years) (Table 1). These DPP adherence data are in contrast to several studies in chronic diseases that generally showed either decreased adherence in older individuals or no effect of increasing age on adherence (14,15).
Caution should be used when interpreting the adherence results by ethnicity/race categories because sample sizes in these categories differ widely. Each minority group, however, was less adherent than Caucasian participants in both the placebo and metformin groups. This deserves further study with appropriate sample sizes for each group in order to evaluate whether the lower adherence is related to the adverse effects of the medication, a poor response to the collaborative approach used in the DPP, a lack of culturally appropriate reminder tools to promote adherence, or a difference in health beliefs about medication taking. Early adoption of medication adherence was predictive of continuing adherence. This emphasizes the need for early interventions to promote optimal adherence and supports previous findings (4,7) that early adoption of medication-taking behaviors predicts later adherence.
Because the adherence measure was a pill count, a possible explanation for these findings is that participants learned to bring back the appropriate number of pills to appear adherent. However, when we modeled the prediction of the development of diabetes by level of adherence, we found greater risk reduction among the adherent metformin group than the nonadherent group. Thus, the pill count measure of adherence, although crude because it was recorded as a dichotomous variable, was indeed related to risk reduction for diabetes. It is possible that some of the health benefits in the adherent cohort may reflect differences in behavioral characteristics of adherent individuals (e.g., 16); however, no such benefit was seen in the placebo group in the DPP. Our results were also in contrast to the β-Blocker Heart Attack Trial (17), in which individuals adherent to placebo had similar outcomes to those adherent to the β-blocker. Our data show a 38.2% risk reduction for those adherent to metformin compared with those adherent to placebo. It has been noted that adherence is often not strongly associated with health outcomes (18). Perhaps these conflicting study results reflect medication adherence measured for primary versus secondary prevention of a disease such as type 2 diabetes.
The relatively high proportion of participants reporting no barriers reflects those who also were adherent to medication. The metformin versus placebo group differences in reporting no barriers and adverse effects were not surprising, given the expected gastrointestinal effects of metformin for a small proportion (13%) of participants. There were no differences between treatment groups among the strategies reported helpful for improving adherence during the previous quarter (Fig. 1C and D).
The data in Table 2 for predicting nonadherence (<80% of prescribed dose) for metformin participants by reported barriers or predicting adherence by strategies are unique in that they portray odds ratios for single barriers or strategies that can be compared with each other and for combinations of two or three barriers or strategies. Participants were able to report up to three barriers and three strategies during structured interviews. While most reported only one primary barrier, for those who reported two or three, the odds of being nonadherent increased dramatically. However, this wide CI for the three-barrier combination (forgetting, adverse effects, and disruption of routines) is likely a reflection of the relatively few participants who reported this combination.
Similarly, the greater the number of strategies reported the greater the odds of a participant’s adherence to metformin. These data highlight the complexity of issues regarding medication adherence. What seems evident is that those who report multiple barriers are less adherent and those who report multiple helpful strategies to promote adherence are more adherent. These results support findings from a review of chronic disease adherence studies that successful adherence interventions usually have multiple components (19). The DPP findings show that multiple strategies predict medication adherence for prevention of diabetes.
When analyzed by selected demographic characteristics, the reported barriers and the adherence strategies provide information that can be used for tailoring preventive medication adherence interventions. For example, while men and women had significantly different reports of adverse effects from DPP medication, they seemed to find the same strategies useful to promote adherence. While there was a significant difference in the metformin arm among ethnic/racial groups regarding the barrier of forgetting to take medication, the reports of successful strategies were similar across groups. While each of the main barriers showed a significant difference by ethnic/racial groups, the small number of American Indians tended to report these barriers with greater frequency. American Indians may be more forthcoming in reporting barriers to adherence, or they may experience barriers with a greater frequency.
Several limitations of this research should be noted. First, the measure of adherence by pill count is subject to bias by virtue of participants’ ability to manipulate the measure by discarding pills before a visit. The training for DPP case managers, however, included periodic reviews of the rationale for not focusing on the pill count with the participant during the study visit. Second, participants in the DPP were part of a clinical trial, and so results may not be generalizable to other populations. Third, the small sample sizes for several ethnic/racial categories indicate the need for caution in interpreting these data. Finally, our adherence intervention was not tested by random assignment; all medication group participants received the brief intervention to maximize adherence. In the absence of a control group, we can only speculate regarding its effectiveness.
In conclusion, the DPP provided an opportunity to study preventive medication adherence and associated factors in a multicenter, randomized, controlled trial. To our knowledge, no such data have been published for a large primary prevention medication trial. While a study cohort is not a real-world sample, the DPP participants were diverse by age and race/ethnicity and they followed the protocol for ~3 years on average. The adherence interview was brief; it could be done in <3–5 min by health care professionals who were not physicians. Our findings that level of medication adherence predicted the primary outcome of diabetes lend support for future development and evaluation of brief, practical medication adherence interventions for primary care settings.
Acknowledgments
We are grateful to all the DPP medication case managers who worked diligently with participants to reach the goals of the study. Special thanks to Dr. Leslie Atler, Karwyn Gustafson, Salwa Fahmi, and Tonya Harroun for important contributions to the Medication Resource Workgroup during the DPP. We thank Dr. Jacqueline Dunbar-Jacob for expert advice during the implementation of the medication adherence protocol for the DPP.
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention. LifeScan, Health O Meter, Hoechst Marion Roussel, Merck-Medco Managed Care, Merck, Nike Sports Marketing, Slim Fast Foods, and Quaker Oats donated materials, equipment, or medicines for concomitant conditions. McKesson BioServices, Matthews Media Group, Inc., and the Henry M. Jackson Foundation provided support services under subcontract with the Coordinating Center. Supported by: the Diabetes Prevention Program, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Child Health and Human Development, and the National Institute on Aging; the Office of Research on Minority Health and Health Disparities, the Office of Women’s Health; the Indian Health Service; the Centers for Disease Control and Prevention; the General Clinical Research Program, the National Center for Research Resources; the American Diabetes Association; Bristol-Myers Squibb; Lipha Pharmaceuticals; and Parke-Davis.
Footnotes
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.The Diabetes Prevention Program Research Group. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Diabetes Prevention Program Research Group. Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effect of lifestyle intervention and metformin. Diabetes. 2005;54:2404–2414. doi: 10.2337/diabetes.54.8.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiMatteo MR. Variations in patients’ adherence to medical recommendations: a quantitative review of 50 years of research. Med Care. 2004;42:200–209. doi: 10.1097/01.mlr.0000114908.90348.f9. [DOI] [PubMed] [Google Scholar]
- 4.Dunbar-Jacob J, Erlen JA, Schlenk EA, Ryan CR, Sereika SM, Doswell WM. Adherence in chronic disease. Annu Rev Nurs Res. 2000;18:48–90. [PubMed] [Google Scholar]
- 5.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 6.Donnan PT, MacDonald TM, Morris AD the DARTS/MEMO Collaboration. Adherence to prescribed oral hypoglycemic medication in a population of patients with type 2 diabetes: a retrospective study. Diabet Med. 2002;19:279–284. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 7.Dunbar J. Predictors of patient adherence: patient characteristics. In: Shumaker S, Schon EB, Okene JK, editors. The Handbook of Health Behavior Change. New York: Springer; 1990. pp. 348–360. [Google Scholar]
- 8.Sherbourne CD, Hays RD, Ordway L, Di-Matteo MR, Kravitz RL. Antecedents of adherence to medical recommendations: results from the medical outcomes study. J Behav Med. 1992;15:447–468. doi: 10.1007/BF00844941. [DOI] [PubMed] [Google Scholar]
- 9.DiMatteo MR, Sherbourne CD, Hays RD, Ordway L, Kravitz RL, McGlynn EA, Kaplan S, Rogers WH. Physicians’ characteristics influence patients’ adherence to medical treatment: results from the medical outcomes study. Health Psych. 1983;12:93–102. doi: 10.1037/0278-6133.12.2.93. [DOI] [PubMed] [Google Scholar]
- 10.Diggle PJ, Liang K-Y, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- 11.Holm S. A simple sequentially rejective Bonferroni test procedure. Scandinavian J Statistics. 1979;6:65–70. [Google Scholar]
- 12.Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002;137:25–33. doi: 10.7326/0003-4819-137-1-200207020-00009. [DOI] [PubMed] [Google Scholar]
- 13.The Diabetes Prevention Program Research Group. The Diabetes Prevention Program: description of the lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vik SA, Maxwell CJ, Hogan DB. Measurement, correlates, and health outcomes of medication adherence among seniors. Ann Pharmacother. 2004;38:303–312. doi: 10.1345/aph.1D252. [DOI] [PubMed] [Google Scholar]
- 15.Ryan AA. Medication compliance and older people: a review of the literature. Int J Nurs Stud. 1999;36:153–162. doi: 10.1016/s0020-7489(99)00003-6. [DOI] [PubMed] [Google Scholar]
- 16.The Coronary Drug Research Project Research Group. Influence of adherence to treatment and response of cholesterol on mortality in the Coronary Drug Project. N Engl J Med. 1980;303:1038–1041. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 17.Horwitz RI, Viscoli CM, Berkman L, Donaldson RM, Horwitz SM, Murray CJ, Ransohoff DF, Sindelar J. Treatment adherence and risk of death after a myocardial infarction. Lancet. 1990;336:542–545. doi: 10.1016/0140-6736(90)92095-y. [DOI] [PubMed] [Google Scholar]
- 18.DiMatteo RM, Giordani PJ, Lepper H, Croghan TW. Patient adherence and medical treatment outcomes. Med Care. 2002;40:794–811. doi: 10.1097/00005650-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 19.McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA. 2002;288:2868–2879. doi: 10.1001/jama.288.22.2868. [DOI] [PubMed] [Google Scholar]


