Abstract
Neonatal (postnatal days 8-21) exposure of rats to the selective serotonin reuptake inhibitor (SSRI), citalopram, results in persistent changes in behavior including decreased sexual activity in adult animals. We hypothesized that this effect was a consequence of abnormal stimulation of serotonergic receptors 5- HT1A or/and 5-HT1B as a result of increased synaptic availability of serotonin during a critical period of development. We examined whether neonatal exposure to a 5-HT1A (8OH-DPAT) and/or a 5-HT1B (CGS 12066B) receptor agonist can mimic the effect of neonatal exposure to citalopram on adult sexual behavior. Results showed that neonatal treatment with 5-HT1B receptor agonist robustly impaired sexual behavior similar to the effect of citalopram whereas exposure to 5-HT1A receptor agonist only moderately influenced male sexual activity in adult animals. These data support the hypothesis that stimulation of serotonin autoreceptors during development contributes to the adult sexual deficit in rats neonatally exposed to citalopram.
Keywords: Neonatal exposure, Citalopram, 8OH-DPAT, CGS 12066B, Sexual behavior
Selective serotonin reuptake inhibitors (SSRIs) are the pharmacological treatment of choice for depression, anxiety and obsessive-compulsive disorder. Moreover, these drugs are specifically recommended for treatment of these disorders during pregnancy and lactation (Cohen et al., 2004; Wisner et al., 2000). This is largely due to their low perceived toxicity to both mother and fetus or infant. Moreover, the use of antidepressant drug treatments in children is a growing phenomenon in pediatric psychiatry (Ambrosini, 2000; Kaufman et al., 2001; Martin et al., 2000). Rarely prescribed to children as little as twenty years ago, antidepressants are now given to children 6 years old and younger (Delate et al., 2004; Martin et al., 2000). As a consequence, there is a significant and increasing likelihood that children will be exposed to SSRIs either in utero, via breast milk or directly in the course of treatment for childhood depression and obsessive-compulsive disorders.
A large fraction of children exposed in utero to SSRIs have been reported to display signs of antidepressant withdrawal in the first week or two of life (Laine et al., 2003; Nordeng et al., 2001; Sanz et al., 2005; Zeskind and Stephens, 2004) indicating that the fetus can be exposed to neurobiologically relevant doses of these drugs. In fact, significant SSRI and metabolite concentrations have been detected in both umbilical cord blood and amniotic fluid in women taking these medications during pregnancy. Mean ratios of cord to maternal serum concentrations ranged from 0.29 to 0.89 (Hendrick et al., 2003; Hostetter et al., 2000). Moreover, in a recent study of over 100,000 mothers and infants, Oberlander et al. noted that infants exposed to SSRIs displayed increased risk of low birthweight and incidence of respiratory distress, even when controlling for maternal depression (Oberlander et al., 2006)
Children exposed to SSRIs in utero have been followed for up to 72 months postnatally and have not been reported to display increased behavioral abnormalities compared to unexposed children although they have been reported to display subtle changes in motor development and in motor movement control (Casper et al., 2003; Costei et al., 2002; Nulman et al., 2002). However, no study has followed such children beyond early childhood. Thus, it remains possible that such exposure may result in later neurobehavioral abnormalities
Neonatal administration of serotonin reuptake inhibitors (citalopram, clomipramine, zimelidine, LU-10-134C) to rats during a critical period of brain development (from postnatal day 8 to 21) results in persistent behavioral changes in adult animals including reduced sexual behavior, increased locomotor activity, decreased shock induced aggression, increased ethanol consumption or increased rapid eye movement (REM) sleep time (Hilakivi et al., 1984; Maciag et al., 2006a; Maciag et al., 2006b; Mirmiran et al., 1981; Neill et al., 1990). Furthermore, neonatal administration of citalopram produces profound reductions in the immunohistochemical expression of both the rate-limiting serotonin synthetic enzyme (tryptophan hydroxylase) in dorsal raphe nucleus and in serotonin transporter expression in cortex that persist into adulthood (Maciag et al., 2006a; Maciag et al., 2006b). In contrast adult rats treated chronically with similar doses of antidepressants exhibit no persistent behavioral effects and/or changes in serotonergic indices after drug discontinuation and washout period. However, the possible mechanism(s) by which SSRI exposure during a critical period of development produces permanent changes in behavior and/or serotonin indices is unclear. Serotonin 1A and 1B receptors (5-HT1A and 5-HT1B, respectively) as autoreceptors and heteroreceptors have been implicated in regulation of serotonergic and non-serotonergic neuronal activity including cell firing or neurotransmitter release (Hoyer et al., 2002). We hypothesized that the long-term effects of neonatal SSRI exposure are a consequence of abnormal stimulation of 5-HT1A or/and 5-HT1B receptors as a result of increased synaptic concentrations of serotonin due to interference with the primary means of removing serotonin from the synapse. The purpose of the present study was to determine weather neonatal exposure to a 5-HT1A (8OH-DPAT) and/or 5-HT1B (CGS 12066B) receptor agonist at pharmacologically active doses can produce the deficit in adult sexual behavior similar to that observed after neonatal treatment with citalopram.
The male offspring of timed-pregnant Long Evans rats were used in this experiment. All procedures were approved by the UMMC Animal Care and Use Committee and complied with AAALAC and NIH standards. Shortly after delivery (PN1-3) the pups were sexed and the males selected and cross-fostered as necessary to produce litters of 4-5 pups. Female pups were euthanized. On PN5-6, pups were tattooed for identification. Beginning at PN8, rat pups were injected subcutaneously with citalopram (CTM, Tocris, Ellisville, MO) at a dose 5 mg/kg, (±)-8-hydroxy-dipropylaminotetralin hydrobromide (8OH-DPAT, 5-HT1A agonist, Tocris, Ellisville, MO) at a dose 0.25 mg/kg, 7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]-quinoxaline dimaleate (CGS 12066B, 5-HT1B agonist, Tocris, Ellisville, MO) at a dose 5 mg/kg or saline (SAL) in a volume of 0.1 ml twice daily (0600 and 1200 h) from PN8-PN21. These doses were drawn from the extensive literature as resulting in ED50 or greater pharmacological response in vivo in rats with 10-fold or greater selectivity for the 5HT1A and 5HT1B receptor respectively (Frambes et al., 1990; Frances and Monier, 1991; Gonzalez et al., 1996; Tohyama et al., 2002). Each litter included at least one pup in each treatment group. Rats were weaned at PN28 and housed in groups of 2-3/cage under standard laboratory conditions with ad lib access to food and water. Except for weekly weighing, rats were left undisturbed until behavioral testing.
Sexual behavior was observed in adult rats (PN90 – PN104) during the dark phase of the light: dark cycle. Males were placed in a clear Plexiglas observation chamber (45 x 25 x 20 cm) for a 10-min adaptation period. The test was initiated by placing a female into the arena with the male. A group of ovariectomized females (stimulus females) were brought into estrous with estradiol benzoate (5 μg SC, 48 and 24 hr prior to testing) and progesterone (500 μg SC 4-6 hr prior to testing). Each test lasted 60 min and was conducted under dim red light. Each encounter was videotaped and analyzed for number of mounts, intromissions, ejaculations, latency (time) to first mount and latency (time) to first intromission. A mount was scored when the male mounted the female from the rear and grasped her flanks with his front feet. Intromission was scored when vaginal penetration was achieved by the male during a mount. Ejaculations were marked behaviorally by intromission with longer-lasting pelvic thrusts and accompanied by characteristic pelvic motor patterns. Ejaculations were followed by genital grooming and a period of disinterest in the female. Also, the postejaculatory refractory period was significantly longer than inter-intromission intervals (Sisk and Meek, 1997). An experienced technician, blind to treatment, observed and rated sexual behavior.
Compared to saline-treated rats, rats neonatally exposed to the 5-HT1B receptor agonist, CGS 12066B exhibited significantly lower sexual activity in adult animals. Kaplan-Meier analysis of mounting (Log-Rank = 8.25, df = 1, P = 0.004) and intromission (Log-Rank = 8.45, df = 1, P = 0.004) indicated that the percent of animals displaying these behaviors was lower in the CGS 12066B group. Moreover, the mount and intromission latencies were longer in the animal exposed to CGS-12066B (Fig. 1A, Fig. 2A and Table 1). Similarly, the numbers of mounts and intromissions were reduced (Fig. 1B, Fig. 2B and Table 1). CGS 12066B also decreased numbers of ejaculations when compared to control group but these differences were not significant (data not shown). The effect of a 5-HT1B agonist on sexual behavior was even more pronounced than that observed after neonatal CTM treatment (mounting behavior: Log-Rank = 1.57, df = 1, P = 0.21; intromitting behavior: Log-Rank = 3.7, df = 1, P = 0.05), (Fig. 1 and Fig. 2). In contrast, neonatal treatment with the 5-HT1A receptor agonist, 8OH-DPAT produced only moderate impairment of sexual behavior which was not significant (mounting behavior: Log-Rank = 0.005, df = 1, P = 0.941; intromitting behavior: Log-Rank = 1.81, df = 1, P = 0.178), (Fig. 1 and Fig. 2).
Fig. 1.
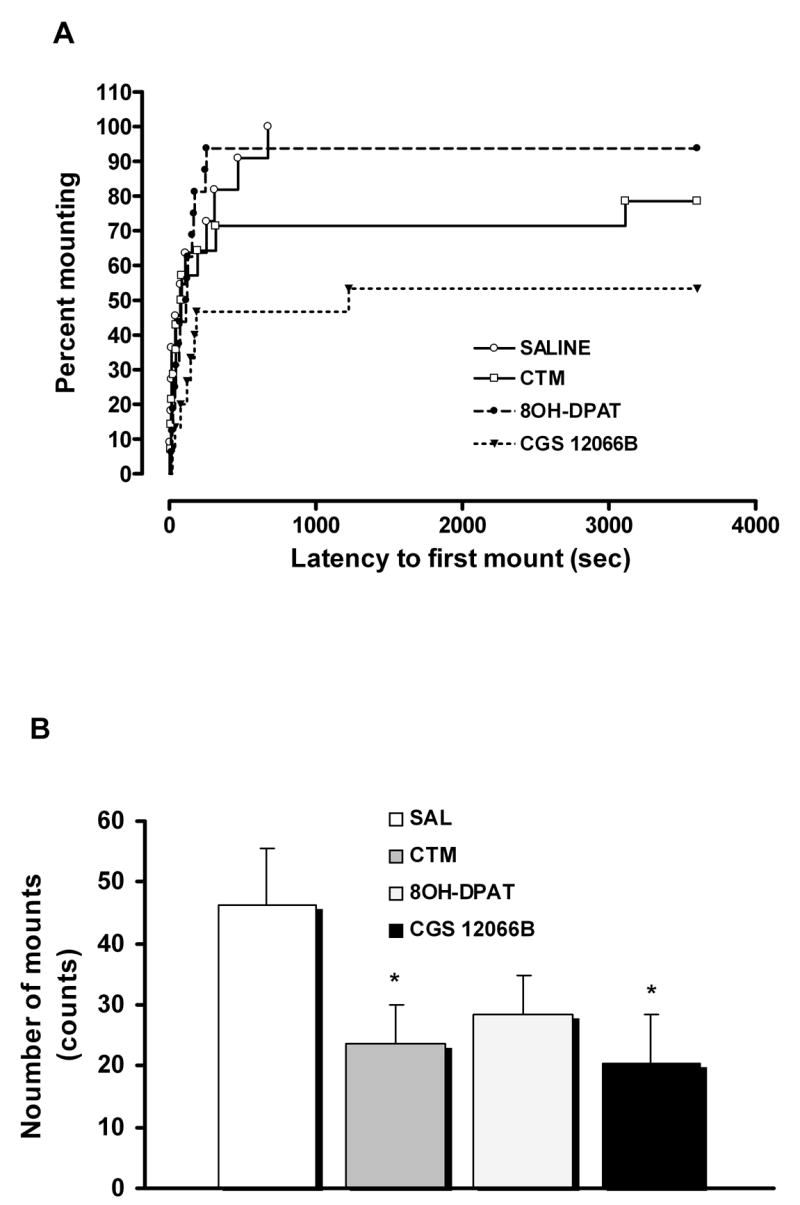
Effect of neonatal exposure to saline (SAL; n = 11), citalopram (CTM; n = 14), 8OH-DPAT (n = 16), and CGS 12066B (n = 15) on male sexual behavior. (A) Kaplan-Meier survival analysis of mounting behavior using Log-Rank statistic, overall comparisons: Log-Rank = 9.66, df = 3, P = 0.022. (B) Number of mounts; ANOVA (F3,52 = 2.079, P = 0.114) followed by LSD test (*P < 0.05 vs SAL).
Fig. 2.
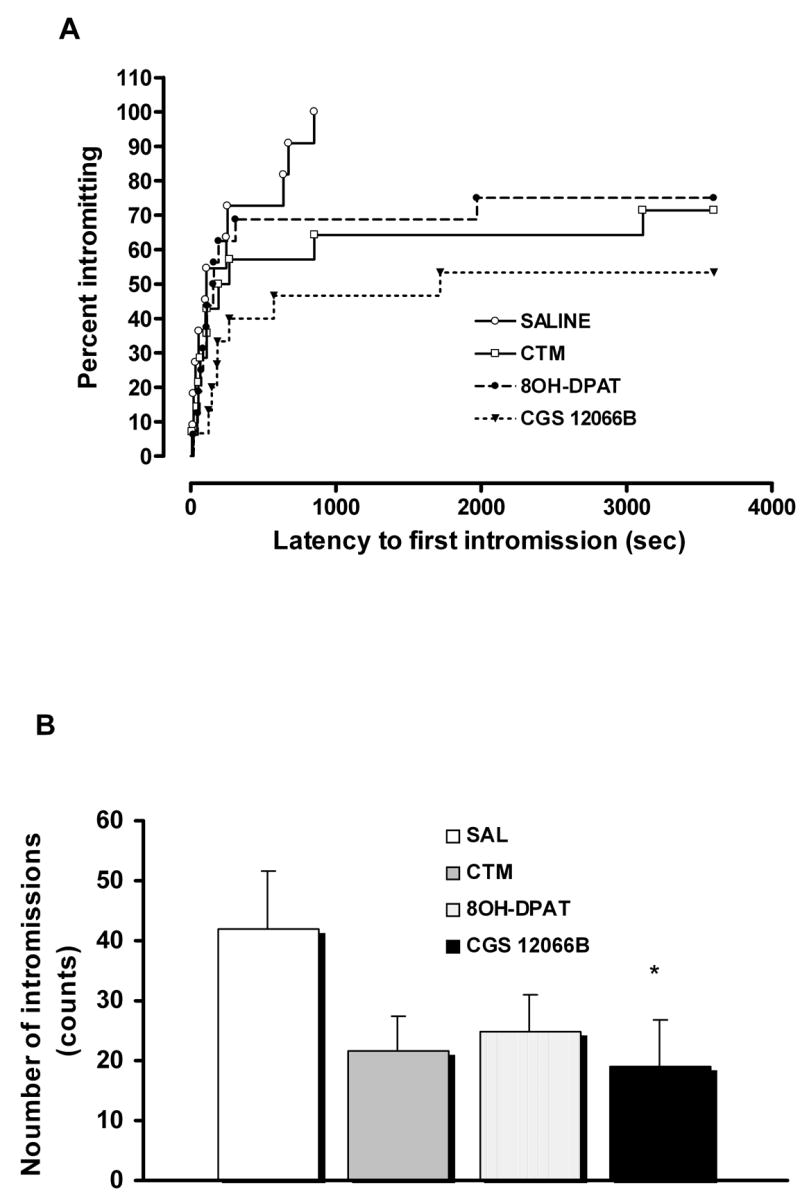
Effect of neonatal exposure to saline (SAL; n = 11), citalopram (CTM; n = 14), 8OH-DPAT (n = 16), and CGS 12066B (n = 15) on male sexual behavior. (A) Kaplan-Meier survival analysis of intromitting behavior using Log-Rank statistic, overall comparisons: Log-Rank = 8.26, df = 3, P = 0.041. (B) Number of intromissions; ANOVA (F3,52 = 1.773, P = 0.164) followed by LSD test (*P < 0.05 vs SAL).
Table 1.
Effect of neonatal exposure to saline, citalopram, 8OH-DPAT and CGS 12066B on male sexual behavior
| Sexual behavior | SAL (n = 11) | CTM (n = 14) | 8OH-DPAT (n = 16) | CGS 12066B (n = 15) |
|---|---|---|---|---|
| Mounts (counts)a | 46.2 ± 9.4 | 23.6 ± 6.2* | 28.3 ± 6.4 | 20.47 ± 7.9* |
| Intromissions (counts)b | 42.1 ± 9.5 | 21.5 ± 6.0 | 24.8 ± 6.1 | 19.1 ± 7.6* |
| Mount latency (sec)c | 179.4 ± 67.4 | 1053.0 ± 427.0‡ | 327.7 ± 219.0 | 1812.0 ± 452.9** |
| Intromission latency (sec)d | 272.8 ± 91.4 | 1373.0 ± 444.6‡ | 1106 ± 389.3 | 1894.0 ± 438.4** |
Data represent the mean ± SEM. Single factor analysis of variance – ANOVA
F3,52 = 2.079, P = 0.114
F3,52 = 1.773, P = 0.164
F3,52 = 4.66, P = 0.006
F3,52= 2.63, P = 0.06) followed by LSD test
P < 0.01
P < 0.05
0.10 > P > 0.05.
Inhibition serotonin reuptake in neonatal rats would be expected to increase synaptic concentrations of serotonin and result in increased stimulation of serotonin autoreceptors (5-HT1A and/or 5-HT1B) during a critical period of development. The data presented here demonstrate that neonatal treatment with CGS 12066B (5-HT1B receptor agonist) mimics the effect of neonatal exposure to the SSRI, citalopram, on adult sexual behavior. Although similar trends may be evident, our data indicate that neonatal administration of 8OH-DPAT (5-HT1A receptor agonist) did not affect significantly male sexual activity. Thus, our data supports the hypothesis that increased stimulation of serotonin autoreceptors, most distinctly 5-HT1B, receptors plays a significant role in producing the lasting deficit in sexual behavior in adult animals exposed as neonates to SSRIs. Moreover, these data suggest an inhibitory role of the signaling cascade initiated by stimulation of 5-HT1 receptors on the neonatal organization of male sexual activity and competence. For example, Feng et al., have reported that early exposure to clomipramine, a serotonin reuptake inhibitor, results in impairment of ERK signal transduction, which is a major component of the intracellular cascade by which 5-HT1 receptors regulate TPH expression (Feng et al., 2003).
It is well known that serotonin can either inhibit or facilitate the expression of sexual behavior in adult rat, depending on which subtypes of serotonergic receptors are stimulated. Stimulation of central 5-HT1B receptors appears to inhibit male sexual behavior primarily by an increase in number of intromissions preceding ejaculation and an increase in ejaculation latency. Similarly, disruption of copulatory behavior has been observed following administration of a 5-HT2 receptor agonist such as (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI) (Fernandez-Guasti and Rodriguez-Manzo, 1992; Foreman et al., 1989; Gorzalka et al., 1990; Klint et al., 1992; Larsson and Ahlenius, 1999). On the other hand, activation of 5-HT1A receptors exerts the opposite effect on male sexual behavior, producing a significant facilitation of ejaculatory behavior (Ahlenius et al., 1981; Gorzalka et al., 1990; Larsson and Ahlenius, 1999). As noted above, early exposure to an SSRI would be expected to result in abnormal stimulation of 5HT1B and/or 5-HT2 receptors. This could play an inhibitory role in organizing male sexual behavior and result in the permanent deficit in that behavior in adult animals. In fact, it has been previously reported that neonatal administration (from postnatal day 8 to 16) of 5-HT2 receptor agonist (DOI) also decreased sexual activity in adult, male rats. The lack of significant changes in sexual behavior in adult rats neonatally exposed to 8OH-DPAT is in line with a previous study of the long term effects of early exposure to 5-HT1A receptor agonists and suggest that activation of 5-HT1A receptors does not play as prominent a role in the organization of sexual behavior during development (Gonzalez et al., 1996).
In our previous study, we have reported that neonatal treatment with citalopram produced not only behavioral disturbances but also significant changes in serotonin indices including reduction in immunoreactivity for tryptophan hydroxylase in dorsal raphe nucleus and serotonin transporter in cortex which seems to be permanent and persisted into adulthood. Thus, it is possibility that neonatal exposure to 5-HT1B agonist can also produce long term changes in serotonin indices and that these changes could be related to the alterations in behavior. Studies to resolve this question are in progress.
Several limitations must be acknowledged in this initial study. First, 5-HT1 receptor agonists were used only at single doses and additional studies will be required to determine the dose response function for these agonists to disrupt adult male sexual behavior. It is possible that higher doses of 8OH-DPAT will have effects on male sexual behavior that were not observed in the present study. Likewise, complete dose-response studies will be needed to directly compare the effects of CGS-12066B to CTM on this index. Finally, the effect of CTM in the present study was somewhat more variable than in previous studies. Nonetheless, these preliminary data support the hypothesis that stimulation of serotonin autoreceptors, particularly 5HT1B receptors, is involved in the lasting behavioral effects of early SSRI exposure.
In toto, these data provide preliminary evidence that stimulation of serotonin autoreceptors, particularly the 5HT1B subtype during a critical period of development can result in lasting impairment of male sexual behavior. These data further indicate that the mechanism by which early SSRI exposure results in lasting alterations in neurobehavioral indices involves abnormal stimulation of serotonin autoreceptors by increased synaptic cleft concentrations of serotonin.
References
- Ahlenius S, et al. Effects of a new type of 5-HT receptor agonist on male rat sexual behavior. Pharmacol Biochem Behav. 1981;15:785–92. doi: 10.1016/0091-3057(81)90023-x. [DOI] [PubMed] [Google Scholar]
- Ambrosini PJ. A review of pharmacotherapy of major depression in children and adolescents. Psychiatr Serv. 2000;51:627–33. doi: 10.1176/appi.ps.51.5.627. [DOI] [PubMed] [Google Scholar]
- Casper RC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142:402–8. doi: 10.1067/mpd.2003.139. [DOI] [PubMed] [Google Scholar]
- Cohen LS, et al. Diagnosis and treatment of depression during pregnancy. CNS Spectr. 2004;9:209–16. doi: 10.1017/s1092852900009007. [DOI] [PubMed] [Google Scholar]
- Costei AM, et al. Perinatal outcome following third trimester exposure to paroxetine. Arch Pediatr Adolesc Med. 2002;156:1129–32. doi: 10.1001/archpedi.156.11.1129. [DOI] [PubMed] [Google Scholar]
- Delate T, et al. Trends in the use of antidepressants in a national sample of commercially insured pediatric patients, 1998 to 2002. Psychiatr Serv. 2004;55:387–91. doi: 10.1176/appi.ps.55.4.387. [DOI] [PubMed] [Google Scholar]
- Feng P, et al. Impairments of ERK signal transduction in the brain in a rat model of depression induced by neonatal exposure of clomipramine. Brain Res. 2003;991:195–205. doi: 10.1016/j.brainres.2003.08.018. [DOI] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Rodriguez-Manzo G. Further evidence showing that the inhibitory action of serotonin on rat masculine sexual behavior is mediated after the stimulation of 5-HT1B receptors. Pharmacol Biochem Behav. 1992;42:529–33. doi: 10.1016/0091-3057(92)90150-e. [DOI] [PubMed] [Google Scholar]
- Foreman MM, et al. The role of the 5-HT2 receptor in the regulation of sexual performance of male rats. Life Sci. 1989;45:1263–70. doi: 10.1016/0024-3205(89)90128-8. [DOI] [PubMed] [Google Scholar]
- Frambes NA, et al. 5-HT1A, 5-HT1B and 5-HT2 receptor agonists induce differential behavioral responses in preweanling rat pups. Eur J Pharmacol. 1990;182:9–17. doi: 10.1016/0014-2999(90)90488-r. [DOI] [PubMed] [Google Scholar]
- Frances H, Monier C. Tolerance to the behavioural effect of serotonergic (5-HT1B) agonists in the isolation-induced social behavioural deficit test. Neuropharmacology. 1991;30:623–7. doi: 10.1016/0028-3908(91)90082-m. [DOI] [PubMed] [Google Scholar]
- Gonzalez MI, et al. Neonatal organizational effects of the 5-HT2 and 5-HT1A subsystems on adult behavior in the rat. Pharmacol Biochem Behav. 1996;54:195–203. doi: 10.1016/0091-3057(95)02134-5. [DOI] [PubMed] [Google Scholar]
- Gorzalka BB, et al. Serotonin receptor subtypes and sexual behavior. Ann N Y Acad Sci. 1990;600:435–44. doi: 10.1111/j.1749-6632.1990.tb16900.x. discussion 445–6. [DOI] [PubMed] [Google Scholar]
- Hendrick V, et al. Placental passage of antidepressant medications. Am J Psychiatry. 2003;160:993–6. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- Hilakivi LA, et al. Effects of neonatal treatment with clomipramine on adult ethanol related behavior in the rat. Brain Res. 1984;317:129–32. doi: 10.1016/0165-3806(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Hostetter A, et al. Amniotic fluid and umbilical cord blood concentrations of antidepressants in three women. Biol Psychiatry. 2000;48:1032–4. doi: 10.1016/s0006-3223(00)00958-6. [DOI] [PubMed] [Google Scholar]
- Hoyer D, et al. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–54. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Kaufman J, et al. Are child-, adolescent-, and adult-onset depression one and the same disorder? Biol Psychiatry. 2001;49:980–1001. doi: 10.1016/s0006-3223(01)01127-1. [DOI] [PubMed] [Google Scholar]
- Klint T, et al. The selective 5-HT2 receptor antagonist amperozide attenuates 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane-induced inhibition of male rat sexual behavior. Eur J Pharmacol. 1992;212:241–6. doi: 10.1016/0014-2999(92)90336-3. [DOI] [PubMed] [Google Scholar]
- Laine K, et al. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen Psychiatry. 2003;60:720–6. doi: 10.1001/archpsyc.60.7.720. [DOI] [PubMed] [Google Scholar]
- Larsson K, Ahlenius S. Brain and sexual behavior. Ann N Y Acad Sci. 1999;877:292–308. doi: 10.1111/j.1749-6632.1999.tb09274.x. [DOI] [PubMed] [Google Scholar]
- Maciag D, et al. Neonatal antidepressant exposure has lasting effects on behavior and serotonin circuitry. Neuropsychopharmacology. 2006a;31:47–57. doi: 10.1038/sj.npp.1300823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag D, et al. Neonatal citalopram exposure produces lasting changes in behavior which are reversed by adult imipramine treatment. Eur J Pharmacol. 2006b;532:265–9. doi: 10.1016/j.ejphar.2005.12.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, et al. Pharmacotherapy of early-onset depression. Update and new directions. Child Adolesc Psychiatr Clin N Am. 2000;9:135–57. [PubMed] [Google Scholar]
- Mirmiran M, et al. Suppression of active sleep by chronic treatment with chlorimipramine during early postnatal development: effects upon adult sleep and behavior in the rat. Brain Res. 1981;204:129–46. doi: 10.1016/0006-8993(81)90657-0. [DOI] [PubMed] [Google Scholar]
- Neill D, et al. Diminished sexual activity in a new animal model of endogenous depression. Neurosci Biobehav Rev. 1990;14:73–6. doi: 10.1016/s0149-7634(05)80162-9. [DOI] [PubMed] [Google Scholar]
- Nordeng H, et al. Neonatal withdrawal syndrome after in utero exposure to selective serotonin reuptake inhibitors. Acta Paediatr. 2001;90:288–91. [PubMed] [Google Scholar]
- Nulman I, et al. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: a prospective, controlled study. Am J Psychiatry. 2002;159:1889–95. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- Oberlander TF, et al. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- Sanz EJ, et al. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: A database analysis. Lancet. 2005;365:482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Meek LR. Sexual and reproductive behaviors. In: Crawley JN, et al., editors. Current Protocols in Neuroscience. Vol. 3. John Wiley & Sons, Inc; New York: 1997. pp. 8.2.1–8.2.15. [DOI] [PubMed] [Google Scholar]
- Tohyama Y, et al. Effects of serotine receptors agonists, TFMPP and CGS12066B, on regional serotonin synthesis in the rat brain: an autoradiographic study. J Neurochem. 2002;80:788–98. doi: 10.1046/j.0022-3042.2002.00757.x. [DOI] [PubMed] [Google Scholar]
- Wisner KL, et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933–40. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- Zeskind PS, Stephens LE. Maternal selective serotonin reuptake inhibitor use during pregnancy and newborn neurobehavior. Pediatrics. 2004;113:368–75. doi: 10.1542/peds.113.2.368. [DOI] [PubMed] [Google Scholar]


